Abstract
Our continuing effort in antifungal natural product discovery has led to the identification of five 6-acetylenic acids with chain lengths from C16 to C20: 6-hexadecynoic acid (compound 1), 6-heptadecynoic acid (compound 2), 6-octadecynoic acid (compound 3), 6-nonadecynoic acid (compound 4), and 6-icosynoic acid (compound 5) from the plant Sommera sabiceoides. Compounds 2 and 5 represent newly isolated fatty acids. The five acetylenic acids were evaluated for their in vitro antifungal activities against Candida albicans, Candida glabrata, Candida krusei, Candida tropicalis, Candida parapsilosis, Cryptococcus neoformans, Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, Trichophyton mentagrophytes, and Trichophyton rubrum by comparison with the positive control drugs amphotericin B, fluconazole, ketoconazole, caspofungin, terbinafine, and undecylenic acid. The compounds showed various degrees of antifungal activity against the 21 tested strains. Compound 4 was the most active, in particular against the dermatophytes T. mentagrophytes and T. rubrum and the opportunistic pathogens C. albicans and A. fumigatus, with MICs comparable to several control drugs. Inclusion of two commercially available acetylenic acids, 9-octadecynoic acid (compound 6) and 5,8,11,14-eicosatetraynoic acid (compound 7), in the in vitro antifungal testing further demonstrated that the antifungal activities of the acetylenic acids were associated with their chain lengths and positional triple bonds. In vitro toxicity testing against mammalian cell lines indicated that compounds 1 to 5 were not toxic at concentrations up to 32 μM. Furthermore, compounds 3 and 4 did not produce obvious toxic effects in mice at a dose of 34 μmol/kg of body weight when administered intraperitoneally. Taking into account the low in vitro and in vivo toxicities and significant antifungal potencies, these 6-acetylenic acids may be excellent leads for further preclinical studies.
The antifungal and antimicrobial properties of fatty acids have been known for centuries. Compared to saturated fatty acids, unsaturated fatty acids with double and/or triple bonds are, in general, more potent against fungal pathogens (16). The representative unsaturated fatty acid with a single double bond at C-10, undecylenic acid (UDA), is still on the market as a cost-effective antifungal agent and the active ingredient of many topical over-the-counter antifungal preparations (20). We have previously demonstrated that two acetylenic acids with a triple bond at C-6, 6-octadecynoic acid (compound 3) and 6-nonadecynoic acid (compound 4), isolated from the plant Pentagonia gigantifolia, show potent in vitro antifungal activities against Candida albicans and fluconazole-resistant C. albicans patient isolates (25). In our ongoing effort to search for prototype natural product antifungal leads (1), the plant Sommera sabiceoides, which, along with P. gigantifolia, is a member of the family Rubiaceae, was selected for bioassay-guided fractionation due to the potent antifungal activity of its crude methanol extract against C. albicans ATCC 90028 (50% inhibitory concentration [IC50], <20 μg/ml). As a result, five antifungal acetylenic acids, 6-hexadecynoic acid (compound 1), 6-heptadecynoic acid (compound 2), compound 3, compound 4, and 6-icosynoic acid (compound 5), were isolated. Compounds 2 and 5 are newly isolated natural products. This paper describes the isolation and structure elucidation of the new natural products and the antifungal activities of all the isolated compounds along with two commercially available analogs, 9-octadecynoic acid (compound 6) and 5,8,11,14-eicosatetraynoic acid (compound 7). A particular focus will be on the potential of these antifungal compounds as leads for the development of new treatments for topical fungal infections.
MATERIALS AND METHODS
General experimental procedures.
Melting points (mp) were measured with a Thomas Hoover capillary melting point apparatus and are uncorrected. Infrared (IR) spectra were recorded on an ATI Mattson Genesis Series FTIR spectrometer. Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance DRX-400 NMR spectrometer for the 1H- and 13C-NMR spectra and calibrated by residual CDCl3 for δC 77.4 and δH 7.24 ppm. Electrospray ionization (ESI)-Fourier transform mass spectrometry (FTMS) was measured on a Bruker-Magnex BioAPEX 30es ion cyclotron high-resolution high-performance liquid chromatography (HPLC)-FT spectrometer by direct injection into an electrospray interface. Liquid chromatography-mass spectrometry (LC-MS) was conducted on a ThermoFinnigan aQa LC/MS system employing ESI and atmospheric pressure chemical ionization, interfaced with a ThermoFinnigan HPLC system with photodiode array detector. Column chromatography was run using a reverse-phase silica gel (RP-18; 40 μm; J. T. Baker). Thin-layer chromatography was performed on silica gel sheets (Alugram Sil G/UV254; Macherey-Nagel, Germany) and reverse-phase plates (RP-18 F254S; Merck, Germany). The antifungal drugs amphotericin B (AMB; ICN Biomedicals, OH), fluconazole (FLU; Sequoia Research Products Ltd., United Kingdom), ketoconazole (KTC; Sigma-Aldrich, MO), UDA (Sigma-Aldrich, MO), terbinafine (TRB; Novartis, NJ), and caspofungin (CASPO; Merck, NJ) were used as positive controls for in vitro antifungal susceptibility testing. Also included in the assay were commercial products 9-octadecynoic acid (compound 6; Alfa Aesar, MD) and 5,8,11,14-eicosatetraynoic acid (compound 7; A. G. Scientific, Inc., CA).
Isolation of acetylenic acids 1 to 5 from Sommera sabiceoides.
The roots of Sommera sabiceoides Schuman were collected by Manuel Rimachi in Loreto, Peru, in April 1996 and identified by M. Rimachi and Sidney McDaniel. A voucher specimen is deposited at the Herbarium of Mississippi State University (voucher IBE-MR 11586). Ground dried roots (210 g) of S. sabiceoides were percolated with 95% ethanol (EtOH; 2.5 liters, three times). Removal of the solvent under vacuum at 40°C yielded an EtOH extract (8.3 g). An 8.1-g sample of the EtOH extract was chromatographed on a reverse-phase silica gel (C18; 200 g) using a gradient solvent system of aqueous CH3CN (80% CH3CN, 3 liters; 85% CH3CN, 2 liters; and finally 100% CH3CN, 2 liters). Each fraction was collected in about 20 ml and examined on a reverse-phase silica gel plate (RP-18). The fractions showing a single spot on thin-layer chromatography were combined. Five pure compounds (1 to 5) were obtained.
6-Hexadecynoic acid (compound 1).
White powder; mp, 40°C; IR (KBr) νmax, 3,200 (br, weak absorption), 2,917, 2,850, 1,707, 1,460, 1,409, 1,312, 1,263, 1,206, 1,138, 1,076, 899, 717 cm−1; 1H-NMR (CDCl3, 400 MHz) δ 0.87 (3H, t, J = 6.9 Hz, H-16), 1.25 to 1.39 (12H, m, H-10-15), 1.45 (2H, br quint, J = 6.9 Hz, H-9), 1.51 (2H, br quint, J = 7.1 Hz, H-4), 1.71 (2H, br quint, J = 7.2 Hz, H-3), 2.11 (2H, br t, J = 6.8 Hz, H-8), 2.16 (2H, br t, J = 6.8 Hz, H-5), 2.34 (2H, t, J = 7.4 Hz, H-2), 10.20 (1H, br s, COOH); 13C NMR (CDCl3, 100 MHz) δ 14.5 (C-16), 18.9 (C-5), 19.1 (C-8), 23.1 (C-15), 24.3 (C-3), 28.9 (C-4). 29.3, 29.5, 29.6, 29.7 and 29.9 (C-9 to C-13), 32.3 (C-14), 34.2 (C-2), 79.7 (C-6), 81.2 (C-7), 180.3 (C-1); ESI-MS m/z 253.2181 {calculated for [M(C16H28O2) + H]+, 253.2162}. To our knowledge, no NMR spectroscopic data for this compound are available in the literature (34).
6-Heptadecynoic acid (compound 2).
White powder; mp, 39°C; IR (KBr) νmax, 3,200 (br, weak absorption), 2,918, 2,850, 1,711, 1,470, 1,409, 1,261, 1,205, 1,075, 868, 716 cm−1; 1H NMR (CDCl3, 400 MHz) δ 0.87 (3H, t, J = 6.8 Hz, H-17), 1.20 to 1.40 (14H, m, H-10-16), 1.45 (2H, br quint, J = 6.8 Hz, H-9), 1.51 (2H, br quint, J = 7.0 Hz, H-4), 1.71 (2H, br quint, J = 7.2 Hz, H-3), 2.11 (2H, br t, J = 6.9 Hz, H-8), 2.16 (2H, br t, J = 6.8 Hz, H-5), 2.33 (2H, t, J = 7.4 Hz, H-2), 10.30 (1H, br s, COOH); 13C-NMR (CDCl3, 100 MHz) δ 14.6 (C-17), 18.9 (C-5), 19.2 (C-8), 23.1 (C-16), 24.4 (C-3), 28.9 (C-4), 29.4, 29.59, 29.63, 29.8, 30.02, and 30.06 (C-9 to C-14), 32.4 (C-15), 34.4 (C-2), 79.8 (C-6), 81.2 (C-7), 180.3 (C-1); ESI-MS m/z 267.2299 {calculated for [M(C17H30O2) + H]+, 267.2319}.
6-Octadecynoic acid (compound 3).
Reference 25 includes a report of the physical and spectral data for compound 3.
6-Nonadecynoic acid (compound 4).
Reference 25 includes a report of the physical and spectral data for compound 4.
6-Icosynoic acid (compound 5).
White powder; mp, 53°C; IR (KBr) νmax, 3,200 (br, weak absorption), 2,916, 2,849, 1,714, 1,539, 1,471, 1,409, 1,312, 1,250, 1,206, 1,076, 964, 899, 868, 717 cm−1; 1H NMR (CDCl3, 400 MHz) δ 0.87 (3H, t, J = 6.8 Hz, H-20), 1.20 to 1.40 (20H, m, H-10-19), 1.46 (2H, br quint, J = 7.2 Hz, H-9), 1.52 (2H, br quint, J = 7.2 Hz, H-4), 1.72 (2H, br quint, J = 7.2 Hz, H-3), 2.12 (2H, br t, J = 6.8 Hz, H-8), 2.17 (2H, br t, J = 6.8 Hz, H-5), 2.35 (2H, t, J = 7.4 Hz, H-2), 10.50 (1H, br s, COOH); 13C NMR (CDCl3, 100 MHz) δ 14.5 (C-20), 18.9 (C-5), 19.1 (C-8), 23.1 (C-19), 24.3 (C-3), 28.8 (C-4), 29.3, 29.5, 29.6, 29.8, 29.98, 30.07 (×4), and 30.12 (C-9 to C-18), 32.3 (C-19), 34.2 (C-2), 79.7 (C-6), 81.2 (C-7), 180.4 (C-1); ESI-MS m/z 309.2773 {calculated for [M(C20H36O2) + H]+, 309.2788}.
LC-MS analysis of the crude ethanol extract.
Analysis was done on an ODS PhenoSphere-Next column (C18; 125 by 4.0 mm) using a mobile phase of CH3CN-H2O (80:20) at a flow rate of 0.8 ml/min. Five major peaks at tR of 7.17, 9.97, 14.82, 19.81, and 26.84 min were identified from the total ion current chromatogram and corresponded to the mass units of 251, 265, 279, 293, and 307, respectively, in the negative ion ESI-MS mode. The peaks for tR of 14.82 and 19.81 min were identified as compounds 3 and 4, respectively, by using authentic compounds isolated from P. gigantifolia (25).
In vitro antifungal susceptibility testing.
CLSI (formerly NCCLS) methods were used for susceptibility testing (29, 30). Organisms were obtained from the American Type Culture Collection (Manassas, VA) and included Candida albicans ATCC 90028, C. albicans ATCC 14053, C. albicans ATCC 60193, C. albicans ATCC 32354, C. albicans ATCC 200955, Candida glabrata ATCC 90030, Candida krusei ATCC 6258, Candida parapsilosis ATCC 22019, Candida tropicalis ATCC 750, Cryptococcus neoformans ATCC 90113, Cryptococcus neoformans ATCC 66031, Aspergillus fumigatus ATCC 90906, A. fumigatus ATCC 204305, Aspergillus flavus ATCC 204304, Aspergillus niger ATCC 16404, Trichophyton mentagrophytes ATCC 9533, T. mentagrophytes ATCC MYA-4439, Trichophyton rubrum ATCC MYA-4438, and T. rubrum ATCC 10218. C. albicans patient isolates 1 (first isolate) and 17 (fluconazole resistant) were kindly provided by T. C. White and S. W. Redding (25, 35).
On the day of the assay, suspensions of test organisms were prepared either by suspending three to five colonies from 24- to 72-h Sabouraud dextrose (SD) agar plates in sterile normal saline (Candida spp. and Cryptococcus spp.) or by scraping the growth from 1- to 6-week-old potato dextrose (PD) slants and filtering through sterile Miracloth (Calbiochem, San Diego, CA) (Trichophyton spp. and Aspergillus spp.). Standard curves of the optical density (OD) at 630 nm versus CFU/ml of saline suspensions of test organisms were generated using linear regression in order to calculate target assay inocula. Since no significant differences were observed in OD readings between 630 nm and 530 nm (CLSI recommended wavelength), OD measurements for inoculum preparation were performed at 630 nm. Assay inocula were prepared by using the standard curves to appropriately dilute microbe suspensions in incubation broth (RPMI 1640; 0.2% dextrose, 0.03% glutamine, buffered with 0.165 M morpholinepropanesulfonic acid [MOPS] at pH 7.0; Cellgro, Herndon, VA) for Candida spp., SD broth (BD Diagnostic Systems, Sparks, MD) for C. neoformans, and 5% Alamar blue (Biosource International, Camarillo, CA)-RPMI 1640 broth (0.2% dextrose, 0.03% glutamine, buffered with 0.165 M MOPS at pH 7.0) for Aspergillus spp. and Trichophyton spp. to afford final target inocula of 1.5 × 103 CFU/ml for Candida spp. and Cryptococcus spp. or 2.7 × 104 CFU/ml for Aspergillus spp. and Trichophyton spp. Inocula were verified for each assay by plating onto SD or PD agar for colony enumeration.
For Cryptococcus neoformans, SD broth was used instead of RPMI broth due to low growth. Two separate experiments using RPMI broth with both C. neoformans strains afforded poor assay quality and poor Z prime analysis, as the OD difference at 530 nm between medium blanks and solvent controls was very small. Therefore, the test medium only for C. neoformans was changed to our standard natural product-screening medium of SD broth. However, all other CLSI conditions were used (inoculum size, incubation temperature, and incubation time). Therefore, this should be taken into consideration when comparing data.
Test samples were serially diluted in 20% dimethyl sulfoxide-saline and transferred in duplicate to 96-well flat-bottom microplates. The highest test concentrations of the compounds and control drugs are indicated in Tables 1 and 2, respectively. All compounds and control drugs were assayed using twofold serial dilutions with a total of 11 test concentrations. Test organisms were added to assay plates to afford a final volume of 200 μl. Candida spp. and C. neoformans were read at 530 nm using the Biotek Powerwave XS plate reader (Bio-Tek Instruments, VT) prior to and after incubation (Candida spp. at 35°C for 48 h; C. neoformans at 35°C for 72 h). A. fumigatus and T. mentagrophytes were read at 544 nm excitation and 590 nm emission using the Polarstar Galaxy plate reader (BMG LabTechnologies, Germany) prior to and after incubation (Aspergillus spp. at 35°C for 48 h; Trichophyton spp. at 35°C for 5 days). Percent growth was calculated, and IC50s were generated using the XLFit software (dose-response model 201; IDBS, Alameda, CA). The MIC is defined as the lowest test concentration that allows no detectable growth (or no more than 20% growth for the azoles and caspofungin). Minimum fungicidal concentrations (MFCs) were determined by removing 100 μl from each clear (or blue) well, transferring to PD (filamentous fungi) or SD (Candida spp. and Cryptococcus spp.) agar, and incubating as described above. The MFC is defined as the lowest test concentration that allows no growth of the organism on agar.
TABLE 1.
In vitro antifungal activities of acetylenic acids 1 to 7a
| Species and strain or isolate | IC50/MIC/MFC (μM) for compound no.b:
|
||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| C. albicans | |||||||
| ATCC 14053 | 81.8/−/− | >187.7c/−/− | 1.0/2.8/5.6 | 0.8/1.3/2.7 | >162.1/−/− | −/−/− | 8.1/63.3/− |
| ATCC 60193 | 85.9/−/− | 150.0/−/− | 3.0/5.6/− | 1.6/2.7/− | 81.0/−/− | −/−/− | 4.5/63.3/− |
| ATCC 32354 | 50.3/198.1/− | 187.7/−/− | 4.0/27.9/− | 1.6/8.0/− | >162.1/−/− | −/−/− | 4.0/31.7/− |
| ATCC 90028 | 56.8/99.1/− | 95.4/−/− | 2.0/2.8/11.1 | 1.3/2.7/− | 67.3/−/− | −/−/− | 5.0/42.2/− |
| ATCC 200955 | 47.8/198.1/− | 66.7/−/− | 1.9/11.1/− | 0.9/1.3/− | 53.4/−/− | −/−/− | 2.0/5.3/21.1 |
| Isolate 1d | 40.5/99.1/− | 82.2/−/− | 4.2/13.9/− | 1.7/2.7/− | 86.6/−/− | −/−/− | 1.7/42.2/− |
| Isolate 17d | 23.3/99.1/198.1 | 18.8/70.4/− | 3.6/16.7/− | 1.5/8.0/− | 55.0/−/− | −/−/− | 3.5/42.2/− |
| Other Candida spp. | |||||||
| C. glabrata ATCC 90030 | >198.1/−/− | >187.7/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− |
| C. krusei ATCC 6258 | 2.9/6.2/49.5 | 1.9/4.4/− | 3.4/5.6/− | 4.1/10.6/− | 106.1/−/− | >178.7/−/− | >168.9/−/− |
| C. parapsilosis ATCC 22019 | >198.1/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | 5.4/63.3/− |
| C. tropicalis ATCC 750 | 29.5/74.3/99.1 | 49.1/−/− | 6.3/111.4/− | 5.2/−/− | −/−/− | −/−/− | 27.7/−/− |
| C. neoformanse | |||||||
| ATCC 90113 | 52.4/−/− | >187.7/−/− | −/−/− | −/−/− | −/−/− | −/−/− | >168.9/−/− |
| ATCC 66031 | 1.2/49.5/99.1 | 1.2/187.7/− | 1.4/−/− | 1.1/−/− | −/−/− | >178.6/−/− | −/−/− |
| Aspergillus spp. | |||||||
| A. fumigatus ATCC 204305 | 9.4/37.1/198.1 | 36.6/140.8/− | 18.7/178.3/− | 1.1/2.7/− | 32.3/−/− | −/−/− | 10.0/84.5/− |
| A. fumigatus ATCC 90906 | 165.6/−/− | −/−/− | −/−/− | 1.4/6.6/− | 81.0/−/− | −/−/− | 13.3/84.5/− |
| A. flavus ATCC 204304 | 105.9/198.1/198.1 | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | >168.9/−/− |
| A. niger ATCC 16404 | 100.9/−/− | >187.7/−/− | −/−/− | −/−/− | −/−/− | −/−/− | 100.0/−/− |
| Trichophyton spp. | |||||||
| T. mentagrophytes ATCC MYA-4439 | 3.9/9.3/12.4 | 3.0/5.9/5.9 | 1.2/2.8/2.8 | 0.4/0.7/0.7 | >162.1/−/− | >178.6/−/− | 6.7/31.7/− |
| T. mentagrophytes ATCC 9533 | 2.3/4.6/6.2 | 3.7/7.3/11.7 | 1.3/2.1/2.8 | 0.9/2.0/2.7 | −/−/− | −/−/− | 14.2/21.1/− |
| T. rubrum ATCC MYA-4438 | 3.8/31.0/198.1 | 4.1/29.3/− | 1.8/5.6/− | 0.6/3.3/− | −/−/− | 80.2/−/− | 2.9/31.7/− |
| T. rubrum ATCC 10218 | 8.7/24.8/24.8 | 6.5/17.6/23.5 | 2.7/5.6/11.1 | 1.0/2.0/5.3 | −/−/− | >178.6/−/− | 8.4/15.8/− |
Highest test concentrations for compounds 1 to 7 were 198.1, 187.7, 178.3, 169.8, 162.1, 178.6, and 168.9 μM, respectively.
Values are means from two independent experiments, each with two replicates; the run-to-run variability was within one- to twofold. −, not active at the highest test concentration.
Some growth inhibition (25 to 49% inhibition) was observed at that concentration.
Patient isolates were obtained during fluconazole therapy; isolate 1 (first isolate) and isolate 17 (last isolate) were azole resistant (35).
Values are means from one experiment using two replicates.
TABLE 2.
In vitro antifungal activities of antifungal drug controlsa
| Species and strain or isolate | IC50/MIC/MFC (μM) of:b
|
|||||
|---|---|---|---|---|---|---|
| AMB | FLU | KTC | CASPO | UDA | TRB | |
| C. albicans | ||||||
| ATCC 14053 | 0.3/1.0/1.4 | 0.5/1.3/− | 0.01/0.02/− | 0.05/0.10/0.26 | >542.7c/−/− | 13.7/−/− |
| ATCC 60193 | 0.2/1.0/1.4 | 0.4/0.6/− | 0.01/0.02/− | 0.03/0.10/0.52 | >542.7/−/− | 5.3/−/− |
| ATCC 32354 | 0.3/1.0/1.4 | 0.8/5.7/− | 0.01/0.03/− | 0.07/0.13/1.03 | >542.7/−/− | 2.6/−/− |
| ATCC 90028 | 0.3/1.0/1.4 | 0.8/6.4/− | <0.01/0.01/− | 0.06/0.19/0.26 | −/−/− | 10.8/−/− |
| ATCC 200955 | 0.6/2.7/2.7 | 0.4/1.3/− | <0.01/0.01/− | 0.05/0.13/0.13 | >542.7/−/− | 3.1/51.5/− |
| Isolate 1d | 0.2/1.0/1.4 | 0.8/5.1/− | <0.01/0.10/− | 0.05/0.10/0.26 | >542.7/−/− | 11.2/−/− |
| Isolate 17d | 0.2/1.0/1.4 | 163.3/−/− | 0.3/−/− | 0.04/0.13/0.26 | >542.7/−/− | >68.3/−/− |
| Other Candida spp. | ||||||
| C. glabrata ATCC 90030 | 0.4/1.4/1.4 | 29.5/81.6/− | 0.7/4.7/- | 0.05/0.13/0.26 | −/−/− | −/−/− |
| C. krusei ATCC 6258 | 0.7/2.0/2.7 | 125.7/163.3/− | 0.4/1.1/− | 0.1/0.4/1.0 | >542.7/−/− | >68.3/−/− |
| C. parapsilosis ATCC 22019 | 0.3/2.0/5.4 | 3.4/10.2/− | 0.04/0.13/− | 0.2/0.5/− | >542.7/−/− | 0.4/42.9/− |
| C. tropicalis ATCC 750 | 0.3/1.0/1.4 | 4.3/15.3/− | 0.04/0.19/− | 0.06/0.10/0.26 | >542.7/−/− | 45.4/−/− |
| C. neoformanse | ||||||
| ATCC 90113 | 0.3/0.7/0.7 | 8.5/20.4/163.3 | 0.1/0.3/1.2 | 0.4/1.0/2.1 | 25.3/271.3/542.7 | 0.5/1.1/1.1 |
| ATCC 66031 | 0.08/0.34/0.34 | 0.7/5.1/20.4 | 0.02/0.15/0.59 | 0.1/0.5/1.0 | 2.7/135.7/271.3 | 0.4/4.3/8.6 |
| Aspergillus spp. | ||||||
| A. fumigatus ATCC 204305 | 1.2/2.0/10.8 | −/−/− | 2.7/4.7/− | ?f/−/− | −/−/− | 1.1/4.3/− |
| A. fumigatus ATCC 90906 | 0.9/1.4/2.7 | −/−/− | 3.2/7.1/− | 0.2/16.5/− | −/−/− | 1.0/4.3/− |
| A. flavus ATCC 204304 | 1.5/2.7/− | −/−/− | 2.8/4.7/− | ?f/−/− | −/−/− | 0.3/2.1/8.6 |
| A. niger ATCC 16404 | 0.9/2.0/− | −/−/− | 6.0/9.4/− | −/−/− | 414.9/−/− | 0.7/2.1/− |
| Trichophyton spp. | ||||||
| T. mentagrophytes ATCC MYA-4439 | 0.9/1.4/1.4 | 24.2/81.6/− | 0.1/0.7/− | 0.2/−/− | 378.9/542.7/− | <0.02/0.17/0.27 |
| T. mentagrophytes ATCC 9533 | 0.6/2.0/2.7 | 29.5/61.2/− | 0.2/0.4/− | 0.3/−/− | 279.0/542.7/− | <0.02/0.05/0.27 |
| T. rubrum ATCC MYA-4438 | 0.6/1.4/5.4 | 2.7/6.4/− | 0.01/0.08/− | 0.06/0.10/− | 111.3/542.7/− | 4.2/21.4/− |
| T. rubrum ATCC 10218 | 0.6/1.4/1.4 | 1.8/1.9/163.3 | 0.02/0.04/− | −/−/− | 270.0/542.7/− | <0.02/0.10/0.13 |
The highest test concentrations of AMB, FLU, KTC, CASPO, UDA, and TRB were 10.8, 163.3, 9.4, 16.5, 542.7, and 68.3 μM, respectively.
Values are means from two independent experiments, each with two replicates; the run-to-run variability was within one- or twofold. −, not active at the highest test concentration.
Some growth inhibition (25 to 49% inhibition) was found at that concentration.
Patient isolates were obtained during fluconazole therapy; isolate 1 (first isolate) and isolate 17 (last isolate) were azole resistant (35).
Values are means from one experiment with two replicates.
XLFit could not calculate an IC50, as growth was present at high and low test concentrations but inhibition was observed at mid-concentrations, possibly due to a concentration effect.
In vitro cytotoxicity assay.
The mammalian cell lines SK-MEL (melanoma), KB (epidermal carcinoma, oral), BT-549 (ductal carcinoma, breast), SK-OV-3 (ovary carcinoma), Vero (African green monkey kidney fibroblast), and LLC-PK-1 (pig kidney epithelial) used in this study were obtained from ATCC (Manassas, VA). Cytotoxicity was determined by the neutral red method (4) up to a highest concentration of 32 μM. The detailed procedure was described in a previous paper (39).
In vivo cytotoxicity assay.
Mice (CD-1; Harlan, Indianapolis, IN) were weighed and randomly distributed into seven groups (n = 3/group). They were kept in filter top cages and housed in environmentally controlled rooms (temperature, 22.2 ± 0.8°C; relative humidity, 30 to 50%) with a 12-h day and night light cycle. Food and water were provided ad libitum. The test compounds (3 and 4) were dissolved in peanut oil to the desired concentration. Six groups of mice were given intraperitoneal injections of 100 μl, affording doses of 3.4, 17, or 34 μmol/kg of body weight of compound 3 or 4. One group of mice was given 100 μl of peanut oil. Mice were observed for any discomfort at 2 h, 5 h, and 8 h postinjection. The body weights were recorded daily. At the end of 3 days, necropsy was performed and gross observations were recorded.
RESULTS AND DISCUSSION
Initial dereplication analysis of the antifungal methanol extract of S. sabiceoides by HPLC-ESI-MS revealed the presence of five acetylenic acid derivatives, including 6-octadecynoic acid (compound 3) and 6-nonadecynoic acid (compound 4), which were previously isolated from the plant P. gigantifolia (25). Although compounds 3 and 4 had been previously demonstrated to be active against C. albicans (25), the remaining three natural products were of great interest from the perspective of structure-activity relationships. Subsequent reverse-phase silica gel column chromatography of the extract using aqueous acetonitrile led to the isolation of five acetylenic acids (compounds 1 to 5), which correspond to the five peaks in the HPLC-ESI-MS (negative mode) analysis with retention times/pseudo-molecular ions of 7.17 min/251, 9.97 min/265, 14.82 min/279, 19.81 min/293, and 26.84 min/307, respectively.
The mass data from the HPLC-ESI-MS analysis indicated that compounds 1 to 5 are analogs with an increase of a methylene group (14 mass units) starting from 1 to 5. The molecular formulae of compounds 1 to 5 were further confirmed by high-resolution ESI-MS as C16H28O2, C17H30O2, C18H32O2, C19H34O2, and C20H36O2, respectively. The structural identification of the three acetylenic acid analogs (compounds 1, 2, and 5) were readily achieved by careful comparison of their NMR spectra with those of compounds 3 and 4, whose structures were established by detailed chemical and spectroscopic methods (25). As indicated in the 13C-NMR spectra for compounds 1 to 5, the chemical shifts of these compounds are very similar, particularly for C-1 to C-8 and the last three carbons starting from the terminal methyl group. The only difference is the number of the carbon signals attributable to the multimethylenes in the center of their straight carbon chains that resonate in the range of δ 29 to 31 ppm, showing the presence of 6, 7, 8, 9, and 10 carbon signals for compounds 1 to 5, respectively. This finding is also supported by the 1H-NMR spectra, which showed close resemblance, with the exception of the integral protons in the range of δ 1.2 to 1.4 ppm from 12H, 14H, 16H, 18H, and 20H for compounds 1 to 5, respectively. Therefore, the structures of compounds 1, 2, and 5 are 6-hexadecynoic acid, 6-heptadecynoic acid, and 6-icosynoic acid, respectively. The last two represent new additions to the class of acetylenic acids. It is worthwhile to note that the aqueous acetonitrile system is an excellent mobile phase for column chromatography on reverse-phase silica gel (RP-18) to separate these fatty acids with minor structural modifications.
Compounds 1 to 5 and two commercially available acetylenic acid analogs, 9-octadecynoic acid (compound 6, a DNA binding agent [2]) and 5,8,11,14-eicosatetraynoic acid (compound 7, an inhibitor of arachidonic acid metabolism [31]), were tested for in vitro antifungal activity against 21 fungal pathogens: seven strains of Candida albicans, including a fluconazole-resistant isolate (35); four species of non-albicans Candida, including C. glabrata, C. krusei, C. parapsilosis, and C. tropicalis; two strains of Cryptococcus neoformans; four strains of Aspergillus, including A. fumigatus, A. flavus, and A. niger; two strains of Trichophyton mentagrophytes; and two strains of T. rubrum. Six antifungal drugs with different mechanisms of action were used as positive controls: the polyene AMB, which interacts with fungal ergosterol, thereby disrupting the cytoplasmic membrane (15); the azoles FLU and KTC, which inhibit 14α-lanosterol demethylase in the ergosterol biosynthesis pathway (15); the lipopeptide CASPO, which inhibits the synthesis of 1,3-β-glucan (15); the fatty acid UDA, inhibiting morphogenesis of C. albicans (27); and the allyamine TRB, which inhibits squalene epoxidase in the ergosterol synthesis pathway (15). The results are shown in Tables 1 and 2.
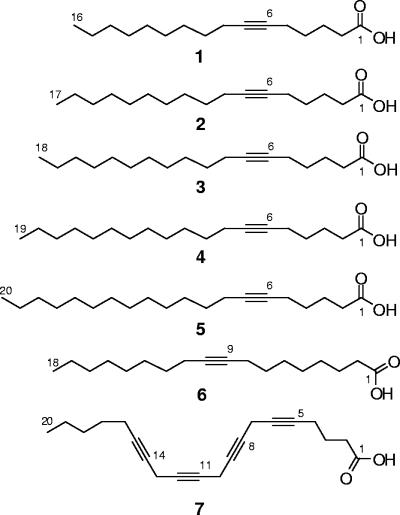
The seven acetylenic acids tested (Fig. 1), which differ only in their chain lengths or the position or number of triple bonds, showed various degrees of antifungal activity (Table 1). Compounds 3 and 4, which have a C-6 triple bond and carbon chain lengths of 18 and 19, respectively, demonstrated consistently good activity against the C. albicans strains. The remaining three 6-acetylenic acids, compounds 1, 2, and 5, which have chain lengths of 16, 17, and 20, respectively, were essentially inactive or marginally active, as was compound 6, which has a carbon chain length of 18 but a triple bond located at C-9 instead of C-6. These susceptibility patterns for compounds 1 to 6 seem to be similar to those against the Trichophyton spp. For example, compounds 3 and 4 showed potent fungicidal activities against T. mentagrophytes ATCC MYA-4439, T. mentagrophytes ATCC 9533, and T. rubrum ATCC 10218, with MFCs ranging from 0.7 to 11.1 μM, while compounds 1 and 2 exhibited decreased activities and compounds 5 and 6 were essentially inactive. These data suggest that both the chain length and the position of the triple bond are important for antifungal activity. Additionally, it appears that multiple triple bonds diminish or eliminate activity, as with compound 7, which has four triple bonds on a C20 carbon backbone. Interestingly, in the 6-acetylenic acid series compounds 1 to 4 are also active against C. krusei (MIC, 4.4 to 10.6 μM), which is inherently resistant to FLU (MIC, 163.3 μM). None of the other non-albicans Candida spp. nor the C. neoformans, A. flavus, or A. niger strains was significantly inhibited by any of the seven compounds tested. These data suggest that the antifungal activities of these acetylenic acids against respective fungal pathogens are strongly associated with their chain lengths and positional triple bonds.
FIG. 1.
Structures of acetylenic acids 1 to 7.
Within the series of acetylenic acids tested, compound 4 showed the most consistent and potent activity across various strains within each species of fungal pathogen, with activity comparable to positive control drugs against C. albicans (MICs, 1.3 to 8.0 μM, compared to FLU MICs of 0.6 to >163.3 μM), A. fumigatus (MICs, 2.7 to 6.6 μM, compared to AMB MICs of 1.4 to 2.0 μM), and all four Trichophyton strains (MICs, 0.7 to 3.3 μM, compared with AMB MICs of 1.4 to 2.0 μM, KTZ MICs of 0.04 to 0.7 μM, and TRB MICs of 0.05 to 21.4 μM). Additionally, compound 4 has potent activity against the FLU-resistant C. albicans clinical isolate 17 (MIC, 8.0 μM; FLU MIC, >163.3 μM).
Our work reported here confirms the observations reported by others that the in vitro antifungal activity of an agent is largely dependent upon the susceptibility testing methods, and considerable variations for intra- and interlaboratory results may occur (19, 36), depending on the methods employed. For example, in a previous study, we isolated compound 4 from the plant P. gigantifolia and reported its activity against C. albicans ATCC 90028 (MIC, 1.8 μM [0.52 μg/ml]), as well as several FLU-resistant C. albicans strains (25). In that previous work, we used a slightly modified CLSI method, in which RPMI 1640 supplemented with 2% dextrose was used as the incubation medium, a larger inoculum size was employed, and plates were incubated at 37°C (25). In contrast, Carballeira et al. recently reported the synthesis and in vitro antifungal evaluation of compound 4 and reported it to be inactive against two strains of C. albicans (ATCC 14053, MIC of 6,672 μM; ATCC 60193, MIC of 8,896 μM) (6, 7) but active against Cryptococcus neoformans ATCC 66031 (MIC, <4.3 μM). Our results in the present study show compound 4 to be significantly active against a variety of Candida spp. strains (>800× more active than reported by Carballeira et al.) and inactive against C. neoformans (no MICs were observed). One explanation for these differences is that Carballeira et al. evaluated the antifungal activity of compound 4 by a modified CLSI method using SD broth (rather than RPMI medium) and possibly a shorter incubation time (24 to 48 h, versus 48 h in our present study). In the present study, we followed the CLSI methods (29, 30), using the recommended inoculum size, temperature, incubation time, and medium conditions for all the Candida strains. Due to poor growth in RPMI, SD broth was substituted for analysis of activity against C. neoformans in order to afford susceptibility data. Our results indicate that compound 4, isolated from S. sabiceoides, showed potent antifungal activities against C. albicans ATCC 14053 (MIC, 1.3 μM), C. albicans ATCC 60193 (MIC, 2.7 μM), and C. albicans ATCC 90028 (MIC, 2.7 μM) (Table 1). In addition, this compound did not show strong activity against C. neoformans ATCC 66031, even though the same SD broth was used in both studies for this strain. While the reason for this discrepancy is unclear at this time, it is possible that other experimental conditions (such as inoculum size, incubation time, etc.) could account for these differences (6, 7).
To assess potential toxicity, the compounds were evaluated for in vitro cytotoxicity (39) in a panel of two noncancerous mammalian kidney cell lines (Vero and LLC-PK-1) and four human cancer cell lines (SK-MEL, KB, BT-549, and SK-OV-3). The results indicate that compounds 1 to 7 are not toxic up to the concentration of 32 μM. Follow-up evaluations of compounds 3 and 4 for in vivo toxicity in mice showed no obvious toxic effects at intraperitoneal doses up to 34 μmol/kg/day for 3 days.
Naturally occurring acetylenic acids are widely distributed in nature and are found in terrestrial plants, fungi, microorganisms, and marine organisms (3, 11). Biosynthetically, they are produced by further desaturation of olefinic systems in the fatty acid-derived molecules. The acetylenic acids containing multiple triple and/or double bonds are generally unstable, particularly for those with conjugated systems. For example, mycomycin (3,5,7,8-tridecatetraene-10,12-diynoic acid), isolated in 1950 and shown to be active against Mycobacterium tuberculosis, is highly unstable (8, 21). Compound 7 used in this study is also unstable. However, compounds 1 to 5, with a single triple bond, are stable enough for potential pharmaceutical development.
Extensive studies have been conducted regarding the chemistry and biological activities of acetylenic acids. A recent comprehensive review on acetylenic lipids, which also covered acetylenic acids, highlighted their anticancer activities (12). The antibacterial, anti-human immunodeficiency virus, and pesticidal activities of some acetylenic acids have also been reported (5, 9, 13, 17, 18, 22). Several studies have also shown that natural and synthetic acetylenic acids have activity against human and agricultural fungal pathogens (5, 9, 14, 16, 17, 24). As with the antibacterial activity, their antifungal activity is generally associated with their chain length and positional triple bond (6, 16, 22, 28). For example, 4-, 5-, and 6-octynoic acids and 9-undecynoic acid are antifungal against T. mentagrophytes, with the last being the most active compound and slightly less potent than undecylenic acid (9). Our findings are consistent with those reported in the literature.
UDA is the only compound within the fatty acid class that is used as a topical antifungal drug for the treatment of dermatomycosis as well as oral thrush and denture stomatitis (20, 27). T. mentagrophytes and T. rubrum are two major causative fungal pathogens for dermatomycosis, while C. albicans, generally considered the most pathogenic Candida species, has been identified as the most prevalent yeast encountered in oral candidiasis (23). The antifungal mechanism of fatty acids may involve interference with fungal fatty acid biosynthesis (32, 33, 38). The advantage of UDA is its low toxicity and favorable safety profile; its shortcoming is its low cure rate due to its weak antifungal potency, as shown by the weak in vitro activity against the tested pathogens in Table 2, especially when compared to TRB, a powerful fungicidal drug for the treatment of dermatomycosis (with adverse events in 10.5% of the recipients) (10, 37). It is interesting that McLain et al. demonstrated that UDA inhibits morphogenesis of C. albicans at a 10 μM concentration; however, no inhibition of growth was observed up to 100 μM in vitro (27). Additionally, in that report, UDA was present at a very high concentration of approximately 70 mM in the denture liners used to study in vivo denture stomatitis. The discovery of these naturally occurring acetylenic acids with potent antifungal activities against T. mentagrophytes, T. rubrum, and C. albicans may represent an opportunity for the development of new topical antifungal agents derived from the fatty acid class.
In conclusion, the current study has demonstrated that acetylenic acids, e.g., compound 4, with low in vitro and in vivo toxicity profiles, are highly superior to UDA in terms of their in vitro antifungal potencies against T. mentagrophytes, T. rubrum, and C. albicans (Tables 1 and 2). Their strong fungicidal activities against T. mentagrophytes and T. rubrum indicates that they may quickly eradicate these pathogens and thus may reduce the chance of developing drug resistance in the treatment of topical infections. In addition, the synthetic methodologies for this class of compounds are available in the literature (6, 7, 26). Taking into account their antifungal potencies, low toxicities, synthetic accessibilities, and good pharmaceutical properties (similar to those of UDA), these 6-acetylenic acids may be excellent leads for further preclinical studies on their use as topical antifungal drug candidates to manage oral candidiasis and dermatomycosis.
Acknowledgments
We thank Marsha Wright and John Trott for biological testing; D. Chuck Dunbar, Bharthi Avula, and John Coyne for MS data; and Troy A. Smillie for database management.
This work was supported by the NIH, NIAID, Division of AIDS, grant no. AI 27094 and USDA Agricultural Research Service Specific Cooperative Agreement no. 58-6408-2-0009.
Footnotes
Published ahead of print on 5 May 2008.
REFERENCES
- 1.Babu, K. S., X.-C. Li, M. R. Jacob, Q.-F. Zhang, S. I. Khan, D. Ferreira, and A. M. Clark. 2006. Synthesis, antifungal activity, and structure activity relationships of coruscanone A analogs. J. Med. Chem. 49:7877-7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry, D. E., J. A. Chan, L. MacKenzie, and S. M. Hecht. 1991. 9-Octadecynoic acid: a novel DNA binding agent. Chem. Res. Toxicol. 4:195-198. [DOI] [PubMed] [Google Scholar]
- 3.Bohlmann, F., T. Burkhardt, and C. Zdero. 1973. Naturally occurring acetylenes. Academic Press, New York, NY.
- 4.Borenfreund, E., and J. Puerner. 1985. Toxicity determined in vitro morphological alterations and neutral red absorption. Toxicol. Lett. 24:119-124. [DOI] [PubMed] [Google Scholar]
- 5.Butler, J. M., and L. A. Miller. November 1964. Method of inhibiting growth of fungi and bacteria with esters of acetylenic acid and polyol. U.S. patent 3,156,612.
- 6.Carballeira, N. M., D. Sanabria, C. Cruz, K. Parang, B. Wan, and S. Franzblau. 2006. 2,6-Hexadecadiynoic acid and 2,6-nonadecadiynoic acid: novel synthesized acetylenic fatty acids as potent antifungal agents. Lipids 41:507-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carballeira, N. M., D. Sanabria, and K. Parang. 2005. Total synthesis and further scrutiny of the in vitro antifungal activity of 6-nonadecynoic acid. Arch. Pharm. 338:441-443. [DOI] [PubMed] [Google Scholar]
- 8.Celmer, W. D., and I. A. Solomons. 1952. Mycomycin. I. Isolation, crystallization, and chemical characterization. J. Am. Chem. Soc. 74:2245-2248. [Google Scholar]
- 9.Cox, C. D., J. C. Forbes, and J. H. Wotiz. 1950. Fungistatic and bacteriostatic studies on some alkynoic acids. J. Am. Pharm. Assoc. 39:55-57. [Google Scholar]
- 10.Darkes, M. J. M., L. J. Scott, and K. L. Goa. 2003. Terbinafine: a review of its use in onychomycosis in adults. Am. J. Clin. Dermatol. 4:39-65. [DOI] [PubMed] [Google Scholar]
- 11.Dembitsky, V. M., and T. Rezanka. 1995. Distribution of acetylenic acids and polar lipids in some acuatic bryophytes. Phytochemistry 40:93-97. [Google Scholar]
- 12.Dembitsky, V. M. 2006. Anticancer activity of natural and synthetic acetylenic lipids. Lipids 41:883-924. [DOI] [PubMed] [Google Scholar]
- 13.Fatope, M. O., O. A. Adoum, and Y. Takeda. 2000. C18 acetylenic fatty acids of Ximenia americana with potential pesticidal activity. J. Agric. Food Chem. 48:1872-1874. [DOI] [PubMed] [Google Scholar]
- 14.Fusetani, N., H.-Y. Li, K. Tamura, and S. Matsunaga. 1993. Bioactive marine metabolites. 46. Antifungal brominated C18 acetylenic acids from the marine sponge Petrosia volcano Hoshino. Tetrahedron 49:1203-1210. [Google Scholar]
- 15.Georgopapadakou, N. H., and T. J. Walsh. 1996. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob. Agents Chemother. 40:279-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gershon, H., and L. Shanks. 1978. Antifungal properties of 2-alkynoic acids and their methyl esters. Can. J. Microbiol. 24:593-597. [DOI] [PubMed] [Google Scholar]
- 17.Hoppe, U., U. Eigener, F. Wolf, and J. Jacob. 1997. Antibacterial, antifungal, and antiviral cosmetic and pharmaceutical preparations containing alkyne-, alkadiyne-, and/or alkatriynecarboxylic acids. DE patent 19535948.
- 18.Ishiyama, H., M. Ishibashi, A. Ogawa, S. Yoshida, and J. Kobayashi. 1997. Taurospongin A, a novel acetylenic fatty acid derivative inhibiting DNA polymerase β and HIV reverse transcriptase from sponge Hippospongia sp. J. Org. Chem. 62:3831-3836. [Google Scholar]
- 19.Jonson, E. M. 2008. Issues in antifungal susceptibility testing. J. Antimicrob. Chemother. 61(Suppl. 1):i13-i18. [DOI] [PubMed] [Google Scholar]
- 20.Jurenka, J. S. 1 February 2002. Undecylenic acid—monograph. Altern. Med. Rev. http://www.highbeam.com/Alternative+Medicine+Review/publications.aspx?date=200202. [PubMed]
- 21.King, D. S. 1950. Tuberculosis. N. Engl. J. Med. 243:530-536. [DOI] [PubMed] [Google Scholar]
- 22.Konthikamee, W., J. R. Gilbertson, H. Langkamp, and H. Gershon. 1982. Effect of 2-alkynoic acids on in vitro growth of bacterial and mammalian cells. Antimicrob. Agents Chemother. 22:805-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuriyama, T., D. W. Williams, J. Bagg, W. A. Coulter, D. Ready, and M. A. O. Lewis. 2005. In vitro susceptibility of oral Candida to seven antifungal agents. Oral Microbiol. Immunol. 20:349-353. [DOI] [PubMed] [Google Scholar]
- 24.Li, H.-Y., S. Matsunaga, and N. Fusteani. 1994. Bioactive marine metabolites. Corticatic acids A-C, antifungal acetylenic acids from the marine sponge, Petrosia corticata. J. Nat. Prod. 57:1464-1467. [DOI] [PubMed] [Google Scholar]
- 25.Li, X.-C., M. R. Jacob, H. N. ElSohly, D. G. Nagle, T. J. Smillie, L. A. Walker, and A. M. Clark. 2003. Acetylenic acids inhibiting azole-resistant Candida albicans from Pentagonia gigantifolia. J. Nat. Prod. 66:1132-1135. [DOI] [PubMed] [Google Scholar]
- 26.Lumb, P. B., and J. C. Smith. 1952. Higher aliphatic compounds. Part X. A synthesis of tariric and petroselinic acids. J. Chem. Soc. 1952:5032-5035. [Google Scholar]
- 27.McLain, N., R. Ascanio, C. Baker, R. Strohaver, and J. W. Dolan. 2000. Undecylenic acid inhibits morphogenesis of Candida albicans. Antimicrob. Agents Chemother. 44:2873-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakatani, M., Y. Fukunaga, H. Haraguchi, M. Taniguchi, and T. Hase. 1986. Synthesis and biological activities of acetylenic fatty acids and their esters. Bull. Chem. Soc. Jpn. 59:3535-3539. [Google Scholar]
- 29.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard M27-A2. National Committee on Clinical Laboratory Standards, Wayne, PA.
- 30.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard M38-A. National Committee on Clinical Laboratory Standards, Wayne, PA.
- 31.Ondrey, F., J. E. Harris, and K. M. Anderson. 1989. Inhibition of U937 eicosanoid and DNA synthesis by 5,8,11,14-eicosatetraynoic acid, an inhibitor of arachidonic acid metabolism, and its partial reversal by leukotriene C4. Cancer Res. 49:1138-1142. [PubMed] [Google Scholar]
- 32.Tobias, L. D., and J. G. Hamilton. 1979. The effect of 5,8,11,14-eicosatetraynoic acid on lipid metabolism. Lipids 14:181-193. [DOI] [PubMed] [Google Scholar]
- 33.Upreti, G. C., M. Matocha, and R. Wood. 1981. Effect of 2-hexadecynoic acid on cultured 7288C hepatoma cells. Lipids 16:315-322. [DOI] [PubMed] [Google Scholar]
- 34.Van de Ven, M., H. Van Lengen, W. Verwer, and Y. K. Levine. 1984. Raman spectra and conformations of α-hexadecynoic acids and their potassium salts. Chem. Phys. Lipids 34:185-200. [Google Scholar]
- 35.White, T. C. 1997. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 41:1482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White, T. C., K. A. Marr, and R. A. Bowden. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wingfield, A. B., A. C. Fernandez-Obregon, F. S. Wignall, and D. L. Greer. 2004. Treatment of tinea imbricata: a randomized clinical trial using griseofulvin, terbinafine, itraconazole and fluconazole. Br. J. Dermatol. 150:119-126. [DOI] [PubMed] [Google Scholar]
- 38.Wood, R., and T. Lee. 1981. Metabolism of 2-hexadecynoate and inhibition of fatty acids elongation. J. Biol. Chem. 256:12379-12386. [PubMed] [Google Scholar]
- 39.Yang, C.-R., Y. Zhang, M. R. Jacob, S. I. Khan, Y.-J. Zhang, and X.-C. Li. 2006. Antifungal activity of C-27 steroidal saponins. Antimicrob. Agents Chemother. 50:1710-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]