Abstract
Recent studies using laboratory clones have demonstrated that several antiretroviral protease inhibitors (PIs) inhibit the growth of Plasmodium falciparum at concentrations that may be of clinical significance, especially during human immunodeficiency virus type 1 (HIV-1) and malaria coinfection. Using clinical isolates, we now demonstrate the in vitro effectiveness of two HIV-1 aspartic PIs, saquinavir (SQV) and ritonavir (RTV), against P. vivax (n = 30) and P. falciparum (n = 20) from populations subjected to high levels of mefloquine and artesunate pressure on the Thailand-Myanmar border. The median 50% inhibitory concentration values of P. vivax to RTV and SQV were 2,233 nM (range, 732 to 7,738 nM) and 4,230 nM (range, 1,326 to 8,452 nM), respectively, both within the therapeutic concentration range commonly found for patients treated with these PIs. RTV was fourfold more effective at inhibiting P. vivax than it was at inhibiting P. falciparum, compared to a twofold difference in SQV sensitivity. An increased P. falciparum mdr1 copy number was present in 33% (3/9) of isolates and that of P. vivax mdr1 was present in 9% of isolates (2/22), but neither was associated with PI sensitivity. The inter-Plasmodium sp. variations in PI sensitivity indicate key differences between P. vivax and P. falciparum. PI-containing antiretroviral regimens may demonstrate prophylactic activity against both vivax and falciparum malaria in HIV-infected patients who reside in areas where multidrug-resistant P. vivax or P. falciparum is found.
Malaria and human immunodeficiency virus type 1 (HIV-1)/AIDS are two important challenges for public health in Southeast Asia. Both diseases are of particular concern in the western border provinces of Thailand, where thousands of displaced people from Myanmar reside. The Thai province of Tak shares a 570-km border with Myanmar, with approximately 40% of the total population lacking formal Thai resident status. As malaria and HIV-1/AIDS are associated with displaced populations, it is not surprising that Tak is the most malarious province in Thailand, accounting for almost a third of malaria cases in 2006 (Communicable Disease Survey, internal report, Department of Disease Control, Royal Thai Ministry of Public Health, Bangkok, Thailand, 2006). The prevalence of HIV-1 infection in pregnant women reporting to one migrant clinic in Tak Province was 2.34% (43/1,838) in 2006 (HIV Prevalence in the Mae Tao Health Clinic, unpublished internal report, Mae Tao Health Clinic, Mae Sod, Thailand, 2006).
The adverse effects of malaria and HIV infection are synergistic and bidirectional (32). Malaria-infected people have a higher viral load, an important cofactor in the pathogenesis and transmission of HIV-1, particularly in pregnant women (7, 17, 37). Although no published data exist on malarial parasite-HIV-1 coinfections in Thailand, data from sub-Saharan Africa suggest that HIV-infected individuals are at higher risk of clinical malaria (34). There are two important differences between the malaria situation in sub-Saharan Africa and the Thailand-Myanmar border; first, 50% of malaria cases in Tak Province are due to Plasmodium vivax, and second, there is a high prevalence of P. falciparum resistance to chloroquine (CQ), antifolates, mefloquine, and amodiaquine (6, 22, 28).
The emergence of multidrug-resistant P. falciparum parasites and the importance of understanding the consequences and interactions of malaria-HIV coinfection and treatment have recently provided an impetus for investigations of the antimalarial activity of antiretroviral agents (2, 8, 20, 21). These studies have shown that aspartic protease inhibitors (PIs) such as saquinavir (SQV) and ritonavir (RTV) can inhibit the in vitro development of P. falciparum at clinically relevant concentrations (20, 27, 33). It has also been demonstrated that these compounds act synergistically in combination with the well-known antimalarials mefloquine and CQ (31). To date, in vitro PI antimalarial studies have used laboratory clones of P. falciparum. The effectiveness of PIs against clinical isolates and against the other major Plasmodium species of human importance, P. vivax, has not been assessed (16, 24).
HIV PIs and antimalarials, such as mefloquine, are substrates for P glycoprotein (Pgp) efflux pumps; consequently, their pharmacodynamics and pharmacokinetics are modulated by Pgp cellular transport (19, 38). Preliminary work on a Pgp in P. falciparum (Pfmdr1) suggests that its amplification is not associated with the synergistic action of PIs with mefloquine (31). As populations of P. falciparum and P. vivax on the Thailand-Myanmar border have an increased mdr1 copy number relative to that of other regions not subjected to mefloquine pressure (25, 35), it is important to better understand the PI sensitivity phenotype in relation to this Pgp polymorphism. As both RTV and SQV demonstrate effective Pgp efflux inhibition (10, 11), we would expect that any association with mdr1 copy number and Plasmodium sp. sensitivity to PIs would be detected by assessing the antimalarial activity of these drugs against field isolates.
The aim of the present study was to investigate the in vitro effectiveness of two antiretroviral PIs, RTV and SQV, against clinical isolates of P. vivax and P. falciparum in an area subjected to high levels of mefloquine and artesunate pressure and to determine if the RTV and SQV sensitivities of P. falciparum and P. vivax are associated with mdr1 copy number.
MATERIALS AND METHODS
Field location and sample collection.
In December 2006, 30 P. vivax and 20 P. falciparum isolates were collected from malaria outpatients attending the Mae Sod Hospital located in the Thai province of Tak on the northwestern Thailand-Myanmar border. Patients with symptomatic malaria presenting to an outpatient facility were recruited into the study if they were singly infected with P. falciparum or P. vivax and had a parasitemia of between 2,000 and 20,000 parasites per microliter. Five milliliters of infected blood was collected by venipuncture into lithium-heparin Vacutainers (Becton Dickinson). After the removal of host white blood cells using a CF-11 column, 2 ml of packed infected red blood cells was divided as follows: 1 ml was cryopreserved in Glycerolyte, 200 μl was spotted onto filter paper, and 800 μl was used for the in vitro drug susceptibility assay.
In vitro drug susceptibility assay.
A modified WHO schizont maturation assay was used to test the antimalarial susceptibility of P. vivax and P. falciparum isolates as described previously (29, 30). A 2% hematocrit blood-medium mixture (BMM) consisting of McCoy's 5A medium and 20% AB-positive human serum was made for P. vivax and P. falciparum isolates. A total of 200 μl of BMM was added to each well of predosed drug plates containing serial dilutions of RTV (34,676 nM), SQV (37,265 nM), CQ (2,992 nM), artesunate (67 nM), and mefloquine (338 nM) (maximum concentrations are shown in parentheses). Predosed drug plates containing the BMM were placed in a gas chamber containing 5% CO2, 5% O2, and 90% N2 at 37.5°C until ≥50% of parasites in the drug-free control had matured to schizonts (24 to 42 h). To minimize the loss of antimalarial action, the predosed plates were stored at 4°C and used within a month of their manufacture. Predosed plates were quality assured by using the K1 clone of P. falciparum before and after field trials.
Thick blood films made from each well were stained with 5% Giemsa stain for 30 min and examined microscopically. Differential counts of 200 asexual parasites in the preincubation and test slides were classified into ring stage (ring-shaped trophozoites), mature trophozoites (one or two chromatin dots and hemazoin pigment visible), and schizonts (three or more chromatin dots visible). Free merozoites and gametocytes were not included in the count. To ensure optimal maturity and ease of parasite identification and to reduce parasite classification error, only schizonts with at least five well-defined chromatin dots were classified as schizonts at the time of harvest. The number of schizonts (five or more chromatin dots visible) per 200 asexual-stage parasites was determined for each drug concentration and normalized to the number in the control well. The dose-response data were analyzed by using nonlinear regression analysis (WinNonlin 4.1; Pharsight), and 50% inhibitory concentration (IC50) values were derived by using an inhibitory sigmoid Emax model.
Determining the mdr1 copy number.
P. vivax mdr1 (Pvmdr1; GenBank accession no. AY618622) and Pfmdr1 (GenBank accession no. M29154) gene copy numbers were determined by quantitative real-time PCR using a Rotor-Gene 6000 (Corbett) as previously described (25, 35). The assessment of the copy number was repeated at least twice for all isolates. The repeatability coefficient was determined to be 0.30 (viz. 95% of repeated estimates of the mdr1 copy number were within 0.15 of the first estimate).
Data analysis.
Plasmodium vivax results were stratified into two groups according to whether parasites were set up in culture with a predominance of rings (a ring-to-trophozoite stage ratio [RT] of >1) or trophozoites (RT < 1). Except for antimalarial stage specificity analysis, only P. vivax isolates with an RT of >1 were prospectively used for analysis, so that all erythrocytic stages of P. vivax were exposed to the therapeutic of interest. Analysis was performed using SPSS for Windows (version 14; SPSS Inc., Chicago, IL). The Mann-Whitney U test or Kruskal-Wallis method was used for nonparametric comparisons. The significance of nonparametric correlation coefficients was examined using a Spearman's rho. The statistical significance level of 0.05 was set prior to analysis. Scatter plots were constructed using GraphPad Prism (version 5; GraphPad Software Inc., San Diego, CA).
Ethical approval.
All research was conducted in accordance with the national and institutional guidelines for human experimentation. Samples were taken only upon written consent after the study was explained in Karen, Burmese, or Thai. Consent and information forms were translated into Karen, Burmese, and Thai. Ethical approval for this study was obtained from the Ethical Review Committee for Research in Human Subjects, Ministry of Public Health, Thailand (reference no. 4/2549, 6 February 2006).
RESULTS
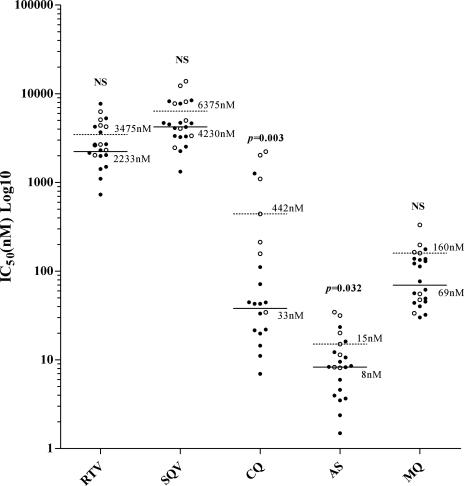
Of the 30 P. vivax and 20 P. falciparum clinical isolates collected, 80% (24/30) and 95% (19/20), respectively, were successfully cultured in the in vitro assay. Whereas all isolates of P. falciparum were synchronous, with all parasites at the ring stage prior to culture, the P. vivax isolates were mostly asynchronous, with a median percentage of rings of 60% (interquartile range [IQR], 28 to 85%). The median duration of the assay was 36 h (IQR, 24 to 43 h), with no difference between species. Sixty-seven percent of P. vivax isolates (16/24) had an RT of ≥1 prior to culture. The median percentage of rings in P. vivax isolates with an RT of ≥1 was 75% (IQR, 60 to 91%), significantly higher than in isolates with an RT of <1 (26%) (IQR, 10 to 29%; P, <0.001). Plasmodium vivax isolates with an RT of <1 had a significantly higher median CQ IC50 of 442 nM (IQR, 157 to 2,034 nM) versus 38 nM (IQR, 19 to 51 nM) in isolates with an RT of >1 (Fig. 1). In view of the significant stage specificity of CQ and a similar trend with the other antimalarials, further analysis of P. vivax was restricted to isolates with an RT of >1 (29). The median IC50s of P. falciparum and P. vivax (RT > 1) for each of the PIs and control antimalarial agents are presented in Table 1. Plasmodium vivax was significantly more sensitive to RTV, SQV, CQ, and mefloquine (Table 1). The in vitro sensitivities of both species to RTV, SQV, and artesunate were significantly correlated; i.e., parasites with increased sensitivity to other PIs also had increased sensitivity to these PIs (Table 2).
FIG. 1.
Effect of the initial development stage of the P. vivax isolate, either predominantly ring stage (RT > 1) (filled circles) or predominantly trophozoite stage (RT < 1) (open circles) on RTV, SQV, CQ, artesunate (AS), and mefloquine (MQ) sensitivity. Lines indicate median IC50s (nM) for an RT of >1 (solid) or an RT of <1 (dashed). NS, no significant difference. Subsequent analysis of P. vivax was restricted to isolates with an RT of >1.
TABLE 1.
Comparison of the in vitro antimalarial susceptibilities of P. vivax and P. falciparum isolates from Mae Sod, Thailand
| Therapeutic |
P. vivaxa
|
P. falciparum
|
Pb | ||
|---|---|---|---|---|---|
| No. of isolates | Median IC50 (range) (nM) | No. of isolates | Median IC50 (range) (nM) | ||
| Ritonavir | 14 | 2,233 (732-7,738) | 19 | 9,664 (909-21,103) | <0.001 |
| Saquinavir | 15 | 4,230 (1,326-8,452) | 19 | 8,305 (1,105-26,269) | 0.018 |
| Chloroquine | 14 | 38 (7-1,264) | 18 | 152 (32-380) | <0.001 |
| Artesunate | 15 | 8 (1-23) | 19 | 8 (1-27) | 0.682 |
| Mefloquine | 16 | 69 (30-177) | 17 | 137 (42-341) | 0.028 |
Analysis of Plasmodium vivax was restricted to isolates with an RT of >1.
Underlined values indicate that the correlation is significant at least at the 0.05 level.
TABLE 2.
In vitro susceptibility correlation coefficients (Spearman's rho) of P. vivax and P. falciparum to RTV, SQV, and three commonly used antimalarial therapeuticsb
| Therapeutic combinations |
P. vivaxa
|
P. falciparum
|
||||
|---|---|---|---|---|---|---|
| Correlation | P | No. of isolates | Correlation | P | No. of isolates | |
| Ritonavir plus: | ||||||
| Chloroquine | 0.294 | 0.354 | 12 | 0.304 | 0.219 | 18 |
| Artesunate | 0.533 | 0.061 | 13 | 0.489 | 0.033 | 19 |
| Mefloquine | 0.349 | 0.221 | 14 | 0.282 | 0.257 | 18 |
| Saquinavir | 0.753 | 0.003 | 13 | 0.772 | <0.001 | 19 |
| Saquinavir plus: | ||||||
| Chloroquine | −0.291 | 0.334 | 13 | 0.353 | 0.151 | 18 |
| Artesunate | 0.697 | 0.006 | 14 | 0.626 | 0.004 | 19 |
| Mefloquine | 0.346 | 0.206 | 15 | 0.396 | 0.103 | 18 |
Analysis of Plasmodium vivax was restricted to isolates with an RT of ≥1.
Underlined P values indicate that the correlation is significant at least at the 0.05 level.
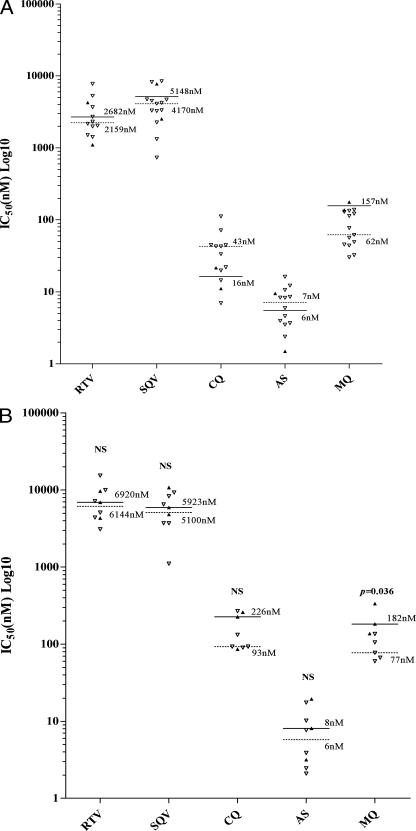
The Pvmdr1 copy number could be reliably determined in 92% (22/24) of the P. vivax isolates tested, with amplification present in two (9%) isolates, one containing a double amplification and the other containing a triple amplification. Neither of these isolates demonstrated RTV or SQV IC50 values outside of the range determined for the remainder of the isolates (Fig. 2A). The median IC50 to mefloquine in the 13 isolates with a single Pvmdr1 copy number was 43 nM (range, 7.0 to 112 nM), compared to IC50s in the two isolates with Pvmdr1 amplification levels of 137 nM and 176 nM (Fig. 2A).
FIG. 2.
Effect of Pvmdr1 (A) and Pfmdr1 (B) copy amplification on the sensitivity of P. vivax and P. falciparum to RTV, SQV, CQ, artesunate (AS), and mefloquine (MQ). Filled triangles represent isolates with mdr1 amplification (solid lines represent median IC50s [nM]). Open triangles represent isolates with a single copy of mdr1 (dashed lines represent median IC50s [nM]).
The Pfmdr1 copy number was successfully assayed in 77% (10/13) of the P. falciparum isolates in which DNA samples were available. Forty percent (3/9) of the P. falciparum isolates had Pfmdr1 amplification (two double mutants and one quadruple mutant). Isolates with an increased Pfmdr1 copy number had significantly higher IC50s to mefloquine (median, 182 nM; range, 137 to 335 nM) compared to those with a single copy number (median, 77 nM; range, 60 to 136 nM; P, 0.036). Although there were only three P. falciparum isolates with increased copy numbers, there was no significant difference or trend for reduced sensitivity in these isolates in comparison to the sensitivity of the isolates with a single copy number for either RTV or SQV (Fig. 2B).
DISCUSSION
The spread of drug-resistant P. vivax isolates throughout areas of Asia already affected by a high prevalence of HIV-1 infection is a cause for great concern. Little is known about the clinical or public health implications of HIV-1 and P. vivax coinfections. Compared to those in Indonesia, P. vivax populations in Thailand are generally considered to be more sensitive to antimalarials (3, 26, 35). Indeed, our data show that Thai P. vivax isolates are generally more sensitive to CQ than Indonesian isolates (except for 14% [2/14] of isolates with an RT of >1 and with a CQ IC50 greater than 100 nM) (35) (Fig. 1). However, our data also indicate that Thai P. vivax isolates have a significantly lower sensitivity to mefloquine and artesunate (Table 1) (29). These findings highlight the emergence of multidrug-resistant strains of Plasmodium spp., a likely reflection of local drug pressure.
The rise in the prevalence of HIV infection in many areas where malaria is endemic also increases the need for novel ways to effectively treat HIV-malaria parasite coinfections. For example, the recent adverse events observed in HIV-infected children following treatment with artesunate-amodiaquine for uncomplicated malaria demonstrate that more attention to this issue is required (13). In this study, we demonstrate the antimalarial activity of an important class of antiretroviral drugs, the PIs, at concentrations likely to be clinically relevant. The median IC50s for RTV and SQV in Thai P. falciparum isolates were higher than for the three standard antimalarials tested (Table 1) and higher than those previously reported for lab strains of P. falciparum (20, 31, 33). In contrast, the median IC50s for RTV and SQV against P. vivax (Table 1) were within the in vivo therapeutic range commonly seen in patients treated with these PIs (maximum concentration of RTV and SQV in serum, 15,500 nM and 3,700 to 5,500 nM, respectively) (20). Interestingly, P. vivax isolates were almost twice as sensitive to RTV as to SQV. RTV was also fourfold more effective at inhibiting P. vivax than P. falciparum, compared to an only twofold difference in effectiveness for SQV (Table 1).
Although the antimalarial actions of PIs are still not fully understood, it was initially thought that PIs inhibited the aspartic proteases associated with the food vacuole (12). The P. falciparum food vacuole-associated plasmepsins (PMs) I, II, and IV and histoaspartic protease are thought to be involved in the initial steps of hemoglobin degradation (4). However, more-recent data from isobologram and transgenic-parasite studies suggest that these food vacuole PMs are unlikely to be the primary targets of these drugs (5, 15, 18, 21, 31). Interestingly, PMIV (GenBank accession no. AAC15792) is the only digestive vacuole PM ortholog found in P. vivax (9, 14). However, it is unlikely that PMIV in P. vivax is associated with the vacuole, as it is transcribed only in the early schizont stage (Z. Bozdech and P. R. Preiser, personal communications). The effective absence of vacuolar PMs in P. vivax and the significant antimalarial efficacy of RTV and SQV against all stages of P. vivax erythrocytic development (including the early ring stage where PMIV is not yet present) (Fig. 1) support the current view that Plasmodium sp. vacuolar PMs are not the targets of PIs. Nonvacuolar aspartic proteases also exist in malaria parasites, and it may be that one or more of these enzymes are the targets for PIs.
An important molecular marker associated with drug resistance in P. falciparum is Pfmdr1. Multiple copy numbers of Pfmdr1 are associated with reduced sensitivity to mefloquine, lumefantrine, and artesunate in P. falciparum (1, 23, 36). An earlier study using laboratory clones with a known mdr1 amplification genotype suggested that mdr1 amplification does not affect PI sensitivity. In this study of clinical isolates, we provide additional evidence to support this assertion, demonstrating that an increase in the Pfmdr1 or Pvmdr1 copy number is not associated with changes in the in vitro sensitivity of P. vivax or P. falciparum to RTV or SQV. In keeping with previous observations, P. falciparum isolates with Pfmdr1 amplification were less sensitive to mefloquine. Although a similar trend appeared to be present for P. vivax, only two isolates with an increased Pvmdr1 copy number could be tested; hence, further studies will be required to confirm these findings. The absence of any cross-resistance between mefloquine and PIs suggests that PIs may be beneficial for patients coinfected with malaria and HIV in areas of mefloquine resistance, particularly given the possible synergistic interactions which may occur as a result of PI and mefloquine coadministration (31). PIs are not used as single-agent therapy for HIV but rather as components of highly active antiretroviral therapy (HAART). While PI-based HAART is not generally considered frontline therapy in the developing world, the enhanced potency of some PI-based HAART regimens, such as RTV-boosted lopinavir, now available in a lower-cost thermostable formulation for marketing in developing countries (Aluvia; Abbott), indicates that usage will expand. RTV, which is generally used as a “low-dose” component of PI-based HAART, is used to boost the pharmacokinetic profile of its partner PI through its potent inhibition of cytochrome P450 enzymes. Our data showing the cross-Plasmodium sp. effectiveness of PIs suggest that the treatment of HIV-infected patients with PI-containing antiretroviral regimens might help prevent malarial parasite coinfection in regions where both P. falciparum and P. vivax are endemic. Furthermore, the differential sensitivities of P. vivax and P. falciparum isolates and the modulatory effect of RTV on antimalarial activity provide some further information on the likely mechanism of the antimalarial action of this class of drugs.
Acknowledgments
We are grateful to the Shoklo Malaria Research Unit for providing us with laboratory space and for the expert assistance provided by Anchalee Jaidee, Varakorn Kosaisavee, and Kanlaya Sriprawat.
This study was supported by the Department of Parasitology, Faculty of Public Health, Mahidol University, and the Wellcome Trust-NHRMC (Wellcome Trust ICRG GR071614MA-NHMRC ICRG ID 283321). B.R. is supported by an NHMRC Howard Florey fellowship; N.M.A. and J.S.M. are supported by NHMRC Practitioner Fellowships; D.L.G. is supported by NHMRC program grant 290208 and donations from Mark Nicholson, Alice Hill, and the Tudor Foundation; and R.N.P. is supported by a Wellcome Trust Career Development Award, affiliated to the Wellcome Trust-Mahidol University-Oxford Tropical Medicine Research Programme (074637).
We declare no conflict of interest.
Footnotes
Published ahead of print on 28 April 2008.
REFERENCES
- 1.Alker, A. P., P. Lim, R. Sem, N. K. Shah, P. Yi, D. M. Bouth, R. Tsuyuoka, J. D. Maguire, T. Fandeur, F. Ariey, C. Wongsrichanalai, and S. R. Meshnick. 2007. Pfmdr1 and in vivo resistance to artesunate-mefloquine in falciparum malaria on the Cambodian-Thai border. Am. J. Trop. Med. Hyg. 76:641-647. [PubMed] [Google Scholar]
- 2.Andrews, K. T., M. L. Gatton, T. S. Skinner-Adams, J. S. McCarthy, and D. L. Gardiner. 2007. HIV-malaria interactions: don't forget the drugs. Science 315:1791. [DOI] [PubMed] [Google Scholar]
- 3.Baird, J. K. 2004. Chloroquine resistance in Plasmodium vivax. Antimicrob. Agents Chemother. 48:4075-4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee, R., J. Liu, W. Beatty, L. Pelosof, M. Klemba, and D. E. Goldberg. 2002. Four plasmepsins are active in the Plasmodium falciparum food vacuole, including a protease with an active-site histidine. Proc. Natl. Acad. Sci. USA 99:990-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonilla, J. A., T. D. Bonilla, C. A. Yowell, H. Fujioka, and J. B. Dame. 2007. Critical roles for the digestive vacuole plasmepsins of Plasmodium falciparum in vacuolar function. Mol. Microbiol. 65:64-75. [DOI] [PubMed] [Google Scholar]
- 6.Brockman, A., R. N. Price, M. van Vugt, D. G. Heppner, D. Walsh, P. Sookto, T. Wimonwattrawatee, S. Looareesuwan, N. J. White, and F. Nosten. 2000. Plasmodium falciparum antimalarial drug susceptibility on the north-western border of Thailand during five years of extensive use of artesunate-mefloquine. Trans. R. Soc. Trop. Med. Hyg. 94:537-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brouwer, K. C., L. B. Mirel, C. Yang, R. B. Lal, M. S. Kolczak, A. M. Van Eijk, J. Ayisi, J. A. Otieno, B. L. Nahlen, R. Steketee, Y. P. Shi, and A. A. Lal. 2007. Subclinical Plasmodium falciparum infection and HIV-1 viral load. Emerg. Infect. Dis. 13:351-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coombs, G. H., D. E. Goldberg, M. Klemba, C. Berry, J. Kay, and J. C. Mottram. 2001. Aspartic proteases of Plasmodium falciparum and other parasitic protozoa as drug targets. Trends Parasitol. 17:532-537. [DOI] [PubMed] [Google Scholar]
- 9.Dame, J. B., C. A. Yowell, L. Omara-Opyene, J. M. Carlton, R. A. Cooper, and T. Li. 2003. Plasmepsin 4, the food vacuole aspartic proteinase found in all Plasmodium spp. infecting man. Mol. Biochem. Parasitol. 130:1-12. [DOI] [PubMed] [Google Scholar]
- 10.Dupuis, M. L., M. Flego, A. Molinari, and M. Cianfriglia. 2003. Saquinavir induces stable and functional expression of the multidrug transporter P-glycoprotein in human CD4 T-lymphoblastoid CEMrev cells. HIV Med. 4:338-345. [DOI] [PubMed] [Google Scholar]
- 11.Dupuis, M. L., M. Tombesi, M. Sabatini, and M. Cianfriglia. 2003. Differential effect of HIV-1 protease inhibitors on P-glycoprotein function in multidrug-resistant variants of the human CD4+ T lymphoblastoid CEM cell line. Chemotherapy 49:8-16. [DOI] [PubMed] [Google Scholar]
- 12.Ersmark, K., B. Samuelsson, and A. Hallberg. 2006. Plasmepsins as potential targets for new antimalarial therapy. Med. Res. Rev. 26:626-666. [DOI] [PubMed] [Google Scholar]
- 13.Gasasira, A. F., M. R. Kamya, J. Achan, T. Mebrahtu, and J. N. Kalyango. 2008. High risk of neutropenia in HIV-infected children following treatment with artesunate plus amodiaquine for uncomplicated malaria in Uganda. Clin. Infect. Dis. 46:985-991. [DOI] [PubMed] [Google Scholar]
- 14.Li, T., C. A. Yowell, B. B. Beyer, S. H. Hung, J. Westling, M. T. Lam, B. M. Dunn, and J. B. Dame. 2004. Recombinant expression and enzymatic subsite characterization of plasmepsin 4 from the four Plasmodium species infecting man. Mol. Biochem. Parasitol. 135:101-109. [DOI] [PubMed] [Google Scholar]
- 15.Liu, J., I. Y. Gluzman, M. E. Drew, and D. E. Goldberg. 2005. The role of Plasmodium falciparum food vacuole plasmepsins. J. Biol. Chem. 280:1432-1437. [DOI] [PubMed] [Google Scholar]
- 16.Mendis, K., B. J. Sina, P. Marchesini, and R. Carter. 2001. The neglected burden of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 64:97-106. [DOI] [PubMed] [Google Scholar]
- 17.Okereke, C. S. 1999. Management of HIV-infected pregnant patients in malaria-endemic areas: therapeutic and safety considerations in concomitant use of antiretroviral and antimalarial agents. Clin. Ther. 21:1456-1496, 1427-1428. [DOI] [PubMed] [Google Scholar]
- 18.Omara-Opyene, A. L., P. A. Moura, C. R. Sulsona, J. A. Bonilla, C. A. Yowell, H. Fujioka, D. A. Fidock, and J. B. Dame. 2004. Genetic disruption of the Plasmodium falciparum digestive vacuole plasmepsins demonstrates their functional redundancy. J. Biol. Chem. 279:54088-54096. [DOI] [PubMed] [Google Scholar]
- 19.Owen, A., O. Janneh, R. C. Hartkoorn, B. Chandler, P. G. Bray, P. Martin, S. A. Ward, C. A. Hart, S. H. Khoo, and D. J. Back. 2005. In vitro synergy and enhanced murine brain penetration of saquinavir coadministered with mefloquine. J. Pharmacol. Exp. Ther. 314:1202-1209. [DOI] [PubMed] [Google Scholar]
- 20.Parikh, S., J. Gut, E. Istvan, D. E. Goldberg, D. V. Havlir, and P. J. Rosenthal. 2005. Antimalarial activity of human immunodeficiency virus type 1 protease inhibitors. Antimicrob. Agents Chemother. 49:2983-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parikh, S., J. Liu, P. Sijwali, J. Gut, D. E. Goldberg, and P. J. Rosenthal. 2006. Antimalarial effects of human immunodeficiency virus type 1 protease inhibitors differ from those of the aspartic protease inhibitor pepstatin. Antimicrob. Agents Chemother. 50:2207-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price, R., C. Luxemburger, M. van Vugt, F. Nosten, A. Kham, J. Simpson, S. Looareesuwan, T. Chongsuphajaisiddhi, and N. J. White. 1998. Artesunate and mefloquine in the treatment of uncomplicated multidrug-resistant hyperparasitaemic falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 92:207-211. [DOI] [PubMed] [Google Scholar]
- 23.Price, R. N., C. Cassar, A. Brockman, M. Duraisingh, M. van Vugt, N. J. White, F. Nosten, and S. Krishna. 1999. The pfmdr1 gene is associated with a multidrug-resistant phenotype in Plasmodium falciparum from the western border of Thailand. Antimicrob. Agents Chemother. 43:2943-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price, R. N., E. Tjitra, C. A. Guerra, S. Yeung, N. J. White, and N. M. Anstey. 2007. Vivax malaria: neglected and not benign. Am. J. Trop. Med. Hyg. 77:79-87. [PMC free article] [PubMed] [Google Scholar]
- 25.Price, R. N., A. C. Uhlemann, A. Brockman, R. McGready, E. Ashley, L. Phaipun, R. Patel, K. Laing, S. Looareesuwan, N. J. White, F. Nosten, and S. Krishna. 2004. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet 364:438-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pukrittayakamee, S., A. Chantra, J. A. Simpson, S. Vanijanonta, R. Clemens, S. Looareesuwan, and N. J. White. 2000. Therapeutic responses to different antimalarial drugs in vivax malaria. Antimicrob. Agents Chemother. 44:1680-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redmond, A. M., T. Skinner-Adams, K. T. Andrews, D. L. Gardiner, J. Ray, M. Kelly, and J. S. McCarthy. 2007. Antimalarial activity of sera from subjects taking HIV protease inhibitors. AIDS 21:763-765. [DOI] [PubMed] [Google Scholar]
- 28.Rojanawatsirivet, C., K. Congpuong, S. Vijaykadga, S. Thongphua, K. Thongsri, K. N. Bangchang, P. Wilairatana, and W. H. Wernsdorfer. 2004. Declining mefloquine sensitivity of Plasmodium falciparum along the Thai-Myanmar border. Southeast Asian J. Trop. Med. Public Health 35:560-565. [PubMed] [Google Scholar]
- 29.Russell, B., F. Chalfein, B. Prasetyorini, E. Kenangalem, K. Piera, R. Suwanarusk, A. Brockman, P. Prayoga, P. Sugiarto, Q. Cheng, E. Tjitra, N. M. Anstey, and R. N. Price. 2008. Determinants of in vitro drug susceptibility testing of Plasmodium vivax. Antimicrob. Agents Chemother. 52:1040-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell, B. M., R. Udomsangpetch, K. H. Rieckmann, B. M. Kotecka, R. E. Coleman, and J. Sattabongkot. 2003. Simple in vitro assay for determining the sensitivity of Plasmodium vivax isolates from fresh human blood to antimalarials in areas where P. vivax is endemic. Antimicrob. Agents Chemother. 47:170-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skinner-Adams, T. S., K. T. Andrews, L. Melville, J. McCarthy, and D. L. Gardiner. 2007. Synergistic interactions of the antiretroviral protease inhibitors saquinavir and ritonavir with chloroquine and mefloquine against Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 51:759-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skinner-Adams, T. S., J. S. McCarthy, D. L. Gardiner, and K. T. Andrews. 2 May 2008, posting date. HIV and malaria co-infection: interactions and consequences of chemotherapy. Trends Parasitol. doi: 10.1016/j.pt.2008.03.008. [DOI] [PubMed]
- 33.Skinner-Adams, T. S., J. S. McCarthy, D. L. Gardiner, P. M. Hilton, and K. T. Andrews. 2004. Antiretrovirals as antimalarial agents. J. Infect. Dis. 190:1998-2000. [DOI] [PubMed] [Google Scholar]
- 34.Slutsker, L., and B. J. Marston. 2007. HIV and malaria: interactions and implications. Curr. Opin. Infect. Dis. 20:3-10. [DOI] [PubMed] [Google Scholar]
- 35.Suwanarusk, R., B. Russell, M. Chavchich, F. Chalfein, E. Kenangalem, V. Kosaisavee, B. Prasetyorini, K. A. Piera, M. Barends, A. Brockman, U. Lek-Uthai, N. M. Anstey, E. Tjitra, F. Nosten, Q. Cheng, and R. N. Price. 2007. Chloroquine resistant Plasmodium vivax: in vitro characterisation and association with molecular polymorphisms. PLoS ONE 2:e1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uhlemann, A. C., R. McGready, E. A. Ashley, A. Brockman, P. Singhasivanon, S. Krishna, N. J. White, F. Nosten, and R. N. Price. 2007. Intrahost selection of Plasmodium falciparum pfmdr1 alleles after antimalarial treatment on the northwestern border of Thailand. J. Infect. Dis. 195:134-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verhoeff, F. H., B. J. Brabin, C. A. Hart, L. Chimsuku, P. Kazembe, and R. L. Broadhead. 1999. Increased prevalence of malaria in HIV-infected pregnant women and its implications for malaria control. Trop. Med. Int. Health 4:5-12. [DOI] [PubMed] [Google Scholar]
- 38.Williams, G. C., A. Liu, G. Knipp, and P. J. Sinko. 2002. Direct evidence that saquinavir is transported by multidrug resistance-associated protein (MRP1) and canalicular multispecific organic anion transporter (MRP2). Antimicrob. Agents Chemother. 46:3456-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]