Abstract
For treatment of mild to moderate Clostridium difficile-associated disease (CDAD), oral metronidazole has been recommended as the preferred agent, in part due to concern that vancomycin may be more likely to promote colonization by vancomycin-resistant enterococci (VRE). We performed a prospective observational study to examine the effects of oral metronidazole or vancomycin treatment of CDAD on acquisition and concentration of VRE stool colonization. Before, during, and after 90 courses of CDAD therapy, stool samples were cultured for VRE, and the concentrations were quantified. Eighty-seven subjects (97%) had received antibiotics within the past month. For 56 treatment courses in which preexisting VRE colonization was present, metronidazole (n = 37 courses) and vancomycin (n = 19 courses), each promoted persistent VRE overgrowth during therapy, and the concentration decreased significantly in both groups by ∼2 weeks after completion of treatment (P <0.049). For 34 treatment courses in which baseline cultures were negative for VRE, new detection of VRE stool colonization occurred during 3 (14%) of the 22 courses of metronidazole and 1 (8%) of the 12 courses of vancomycin (P = 1.0). These results demonstrate that both oral metronidazole and oral vancomycin promote the overgrowth of VRE during treatment of CDAD. New CDAD treatments are needed that are less likely to disrupt the intestinal microflora and promote overgrowth of healthcare-associated pathogens.
Clostridium difficile is the most common infectious cause of healthcare-associated diarrhea in developed countries (16). Currently, oral vancomycin is the only medication approved by the U.S. Food and Drug Administration for the treatment of C. difficile-associated disease (CDAD) (10). However, oral metronidazole has been recommended as the preferred agent for treatment of mild to moderate CDAD (4, 9, 10). Metronidazole is much less expensive than oral vancomycin, costing a few dollars per treatment course versus hundreds of dollars for oral vancomycin (9). More importantly, studies conducted in the 1980s and 1990s demonstrated that both drugs were equally effective (9, 10, 20, 21). However, several recent reports have raised concerns about the efficacy of metronidazole for treatment of CDAD, especially for severe disease and in the setting of outbreaks associated with an emerging epidemic strain, termed North American pulsed-field electrophoresis type 1 or ribotype 027 (10, 13, 15, 22).
Another rationale for the preferential use of metronidazole for treatment of CDAD is the concern that oral vancomycin may be more likely to promote acquisition and overgrowth of vancomycin-resistant enterococci (VRE) (9, 10). Several studies have demonstrated that oral vancomycin promotes the acquisition and overgrowth of VRE (7, 11); however, metronidazole has also been associated with VRE in case-control studies (8). In addition, subcutaneous metronidazole promoted persistent overgrowth of VRE in stools of colonized mice (5).
Although metronidazole is almost entirely absorbed in the small bowel of healthy individuals (1), in patients with acute CDAD significant concentrations (mean ± the standard deviation [SD], 9.3 ± 7.5 μg/g) are achieved in stool (3), presumably due to decreased absorption associated with diarrhea and due to excretion into the colon in the setting of inflammation (3, 9, 10). The concentration of metronidazole in stool decreases to undetectable levels as diarrhea resolves during treatment (3, 9). In contrast, oral vancomycin is not absorbed and achieves very high concentrations (520 ± 197 μg/g of stool with a 0.5-g/day dose; >1,000 μg/g of stool with a 2-g/day dose) in the gastrointestinal tract that persist during therapy (7, 9). Based on these characteristics of the drugs, we hypothesized that both agents promote VRE but that oral vancomycin might do so to a greater extent because of its persistent high levels in the intestinal tract during therapy. The major objective of the present study was to compare the concentration of VRE overgrowth during and after CDAD therapy with the two agents among patients with preexisting VRE colonization. A secondary goal was to compare the rates of new detection of colonization in patients with negative cultures for VRE at the beginning of therapy.
MATERIALS AND METHODS
Setting and study design.
From 1 November 2006 to 31 October 2007 we performed a 1-year prospective observational study of CDAD patients from the acute-care and long-term care facilities of the Louis Stokes Cleveland Veterans Affairs Medical Center, which has a high rate of endemic VRE colonization (6). Patients were diagnosed with CDAD based on symptoms of diarrhea and by positive stool toxin assay (C. difficile Tox A/B II; Wampole Laboratories, Princeton, NJ). The choice of therapy for CDAD was made by the physicians caring for the patients. Information regarding the demographic characteristics, coexisting illnesses, and antibiotic therapy was obtained through standardized chart review. All patients diagnosed with CDAD during the study period were enrolled unless they refused to provide informed consent, were scheduled for discharge within 2 days of the diagnosis, or had received empirical therapy with oral metronidazole or oral vancomycin prior to the diagnosis of CDAD. Patients who had recurrent episodes of CDAD occurring at least 3 months apart were eligible for re-enrollment as a separate case of CDAD. The hospital's institutional review board approved the study protocol.
We monitored the presence and concentration of VRE in stool before, during, and after therapy of CDAD with oral metronidazole or vancomycin. Patients were monitored for the duration of their stay in the acute-care hospital and for up to 3 weeks after completion of CDAD therapy if they were residents of the long-term care facility. Stool samples were refrigerated at 4°C and processed within 1 week. Samples were cultured for the presence of VRE by plating onto Enterococcosel agar (Becton Dickinson, Sparks, MD) containing 20 μg of vancomycin/ml. For a subset of 20 isolates, speciation and broth dilution MICs for vancomycin were performed according to standard methods (14). If VRE were present in stool, the concentration was quantified as previously described (5, 6).
Natural history of VRE colonization in the absence of antibiotic treatment.
All patients diagnosed with CDAD during the study period received treatment for C. difficile. Thus, there was no concurrent group of untreated control patients. Therefore, to provide information on the natural history of VRE colonization in the absence of continued antibiotic therapy, we assessed the rate of clearance of VRE stool colonization in patients enrolled in two previous observational studies of the effect of antibiotic therapy on VRE stool colonization (2, 6). We included patients whose VRE stool concentration was monitored for ≥2 weeks after anti-anaerobic antibiotic regimens were discontinued in the absence of any additional antibiotic therapy. Both of the previous studies demonstrated that VRE-colonized patients receiving anti-anaerobic antibiotic regimens maintained high-concentration colonization during therapy, and the concentration decreased after discontinuing treatment (2, 6). Antibiotics on the hospital formulary were classified as anti-anaerobic if they had been shown to inhibit intestinal anaerobes in humans, including piperacillin-tazobactam, ampicillin/sulbactam, amoxicillin/clavulanate, cefoxitin, cefotetan, imipenem-cilastatin, meropenem, ertapenem, metronidazole, clindamycin, vancomycin, and ceftriaxone (2, 6).
Statistical analysis.
Data were analyzed with the use of SPSS statistical software version 10.0 (SPSS, Inc., Chicago, IL) and STATA 9.1 (StataCorp, College Station, TX). Bivariate analyses were performed to compare the characteristics of patients treated with metronidazole versus vancomycin. For patients with VRE colonization prior to initiation of CDAD therapy, a Student unpaired t test was used to compare the densities of VRE in the two treatment groups at different time points during and after completion of therapy. For patients with negative cultures for VRE prior to initiation of CDAD therapy, chi-square and the Fisher exact test were used to compare the rates of acquisition of VRE.
RESULTS
Patient characteristics.
We studied 82 patients who received 90 courses of CDAD therapy; 8 patients received separate courses of CDAD therapy more than 3 months apart and were analyzed as new cases. The characteristics of the patients are shown in Table 1. The vancomycin-treated patients were more likely to be in the intensive care unit while being treated for CDAD. There was also a nonsignificant trend toward more concurrent use of antibiotics for other indications in the vancomycin group. In 56 of the 90 (62%) courses of CDAD therapy, VRE stool colonization was present at the time treatment was initiated, whereas VRE was undetectable (limit of detection, ∼2.5 log10 CFU/g) at initiation of the remaining 34 courses of therapy. The mean length of follow-up for the metronidazole group was 13.68 days (range, 6 to 25 days) versus 13.29 (range, 7 to 25 days) for the vancomycin group (P = 0.675). A total of 16 of 37 (43.2%) metronidazole-treated patients had stool samples collected at 21 to 25 days versus 8 of 19 (42.1%) vancomycin-treated patients (P = 0.902).
TABLE 1.
Baseline characteristics of 90 patients treated with oral metronidazole or oral vancomycin
| Characteristica | No. and % of patients
|
P | |||||
|---|---|---|---|---|---|---|---|
| Overall (n = 90)
|
Metronidazole (n = 57)
|
Vancomycin (n = 33)
|
|||||
| No. | % | No. | % | No. | % | ||
| Male sex | 89 | 98.9 | 56 | 98.3 | 33 | 100.0 | |
| Preexisting VRE colonization | 56 | 62.2 | 39 | 68.4 | 17 | 51.5 | 0.111 |
| ICU admission during treatment | 19 | 21.1 | 7 | 12.3 | 12 | 36.4 | 0.014 |
| Proton pump inhibitor | 61 | 67.8 | 40 | 70.2 | 21 | 63.6 | 0.522 |
| Antibiotics within past mo | 87 | 96.7 | 56 | 98.3 | 31 | 93.9 | 0.552 |
| Concurrent antibioticsb | 9 | 10.0 | 4 | 7.0 | 5 | 15.2 | 0.279 |
| Fecal incontinence | 48 | 53.3 | 31 | 54.4 | 17 | 51.5 | 0.792 |
| Feeding tube | 16 | 17.8 | 10 | 17.5 | 6 | 18.2 | 1.000 |
| Diabetes mellitus | 34 | 37.8 | 22 | 38.6 | 12 | 36.4 | 0.833 |
| Cancer | 23 | 25.6 | 15 | 26.3 | 8 | 24.2 | 1.000 |
| End-stage renal disease | 8 | 8.9 | 7 | 12.3 | 1 | 3.0 | 0.250 |
| Cirrhosis | 7 | 7.8 | 5 | 8.8 | 2 | 6.1 | 1.000 |
| Cerebrovascular accident | 21 | 23.3 | 14 | 24.6 | 7 | 21.2 | 0.800 |
| Surgery within 3 mo | 20 | 22.2 | 14 | 24.6 | 6 | 18.2 | 0.602 |
| Nursing home resident | 46 | 51.1 | 29 | 50.9 | 17 | 51.5 | 0.953 |
| Previous CDAD | 14 | 15.5 | 2 | 3.5 | 12 | 36.4 | 0.004 |
The median interquartile ages in years (range) for all patients, patients receiving metronidazole, and patients receiving vancomycin were 73.5 (60 to 79), 75 (63 to 80), and 68 (59 to 77), respectively (P = 0.099). ICU, intensive care unit.
Concurrent antibiotics refers to systemic antibiotics administered for another indication that were continued for more than 2 days after the diagnosis of CDAD. Specific concurrent antibiotics included piperacillin-tazobactam, ciprofloxacin, moxifloxacin, vancomycin (intravenous), and cefepime.
Effect of treatment on concentration of VRE in patients with preexisting colonization.
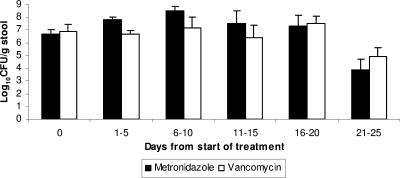
Of the 56 CDAD treatment courses in which prior VRE colonization was present, 37 (66%) were treated with metronidazole, and 19 (34%) were treated with oral vancomycin. The mean duration of therapy was not significantly different for the metronidazole and vancomycin groups (mean ± the SD, 11.2 ± 1.9 and 12.1 ± 2.0, respectively; P = 0.088). There were no significant differences in the concentrations of VRE for the metronidazole and vancomycin groups prior to beginning CDAD therapy, during therapy, or up to ∼2 weeks after completion of therapy (P >0.350) (Fig. 1). For both groups, the density of VRE decreased significantly by days 21 to 25 (i.e., ∼2 weeks after completion of treatment) (P <0.049) (Fig. 1). It should be noted that only 16 of 37 (43%) metronidazole-treated subjects and 8 of 19 (42%) vancomycin-treated subjects had stool samples available for analysis at days 21 to 25. However, on days 6 to 10, there was no difference in the mean concentrations of VRE among subjects who were subsequently monitored to 21 to 25 days versus those who were lost to follow-up prior to days 21 to 25 (mean ± the SD, 7.6 ± 0.9 versus 7.2 ± 0.4 log10 CFU/g; P = 0.914). One subject in each treatment group developed a VRE bloodstream infection during the study.
FIG. 1.
Concentration of VRE in stools of patients with preexisting VRE colonization who were treated with oral metronidazole versus oral vancomycin for C. difficile-associated disease. Day 0, before treatment on day of CDAD diagnosis. For the metronidazole group (n = 37), the number of subjects monitored to 1 to 5 days, 6 to 10 days, 11 to 15 days, 16 to 20 days, and 21 to 25 days were 37, 28 (76%), 22 (59%), 19 (51%), and 16 (43%), respectively. For the vancomycin group (n = 19), the number of subjects monitored to 1 to 5 days, 6 to 10 days, 11 to 15 days, 16 to 20 days, and 21 to 25 days were 19, 14 (74%), 11 (58%), 11 (58%), and 8 (42%), respectively. The durations of treatment (mean ± the SD) with metronidazole and vancomycin were 11.2 ± 1.9 and 12.1 ± 2.0, respectively. Error bars indicate the standard error.
Natural history of VRE colonization in the absence of antibiotic treatment.
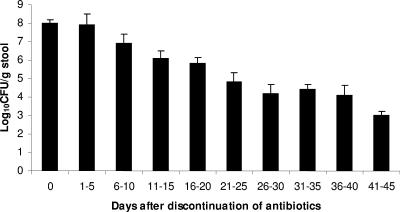
The natural history of VRE colonization in the control patients whose stool concentration was monitored after discontinuation of anti-anaerobic antibiotics is shown in Fig. 2. The concentration decreased significantly by days 6 to 10 after discontinuation of antibiotic therapy (mean ± the SD, 8.0 ± 1.1 to 6.9 ± 0.5 log10 CFU/g; P = 0.015). Further gradual decreases in VRE concentration occurred throughout the follow-up period in the absence of additional antibiotic treatment.
FIG. 2.
Decrease in concentration of VRE in stools of 33 VRE-colonized control patients after discontinuation of anti-anaerobic antibiotic therapy regimens on day 0. None of the patients received antibiotic therapy after day 0, and none had received therapy for CDAD. The control patients were enrolled in two previous observational studies examining the effect on antibiotic therapy on concentration of VRE colonization (2, 6). Error bars indicate the standard error.
New detection of VRE colonization during CDAD treatment.
Of the 34 treatment courses in which baseline cultures were negative for VRE, new detection of VRE stool colonization occurred during 3 (14%) of the 22 courses of metronidazole and 1 (8%) of the 12 courses of vancomycin (P = 1.0). No VRE infections occurred among the subjects who had new detection of stool colonization. Of the 20 VRE isolates subjected to speciation and susceptibility testing, all were Enterococcus faecium, and 19 (95%) had vancomycin MICs of >256 μg/ml; the remaining isolate had a vancomycin MIC of 128 μg/ml.
DISCUSSION
To our knowledge, no previous published studies have directly compared VRE overgrowth and acquisition during treatment of CDAD with metronidazole versus vancomycin. Among patients with preexisting VRE colonization, we found that both drugs promoted similar high-concentration colonization that persisted during the course of therapy and decreased after discontinuation of treatment. Among patients with negative cultures prior to initiation of therapy, new detection of VRE colonization was uncommon in both therapy groups. These findings do not support our hypothesis that oral vancomycin may promote VRE to a greater degree than oral metronidazole and suggest that factors other than promotion of VRE should be the primary considerations in choosing oral vancomycin or metronidazole for treatment of CDAD.
Because multiple genes are necessary to generate vancomycin resistance in enterococci, acquisition of VRE colonization does not occur through mutations in susceptible enterococci in the intestinal tract (4, 11, 18, 19). Rather, selective pressure exerted by oral vancomycin may facilitate the exogenous acquisition of VRE or the transfer of vancomycin resistance genes from other organisms to E. faecium in the intestinal tract (12, 18). Although we evaluated a limited number of patients for new detection of VRE colonization, our data provide some reassurance that this occurrence may be relatively uncommon during oral vancomycin therapy, even in centers with a high prevalence of VRE. Salgado et al. (19) previously reported that no new acquisition of VRE colonization was detected among 20 patients who received 23 courses of oral vancomycin for CDAD in a medical center with a low prevalence of VRE. In addition, we have demonstrated no de novo emergence of VRE in more than 100 mice treated with oral vancomycin in drinking water (5; unpublished data). The previous studies that did find high rates of acquisition of VRE in humans during oral vancomycin treatment were conducted in Europe and may have been confounded that the fact that the food supply was contaminated with VRE of animal origin (7, 12, 18). Finally, it should be noted that the transfer of vancomycin resistance genes in the intestinal tract may occur in the absence of vancomycin selective pressure (12, 18).
Our study has some limitations. The study was observational; however, the characteristics of the groups were similar. Because vancomycin is often prescribed for more severe CDAD cases, any bias due to patient selection should have favored the metronidazole group. The number of patients with negative cultures for VRE at initiation of CDAD therapy was low, and therefore additional studies are needed to further assess the risk for new detection of VRE during CDAD treatment, which was a secondary aim of our study. It is possible that a difference between the study groups would have been detected if there had been a longer follow-up period. However, the mean concentration of VRE decreased significantly by days 21 to 25 (i.e., approximately 2 weeks after completion of CDAD therapy) in both treatment groups. In addition, patients were monitored for the duration of their stay in the acute care facility, and therefore the absence of long-term follow-up reflects the fact that most subjects were discharged during or within 1 to 2 weeks of completing CDAD treatment. Because all of the patients were treated for CDAD, it is possible that some of the persistent VRE overgrowth may have been attributable to the previous antibiotics that caused the CDAD. However, our findings from two previous observational studies demonstrate that the concentrations of VRE in the stools of colonized patients decreases significantly by 1 to 2 weeks after discontinuation of treatment with a wide range of different anti-anaerobic antibiotic regimens (2, 6). Finally, the effects of the agents on the indigenous microflora were not assessed. However, previous studies have confirmed that oral metronidazole and vancomycin cause significant disruption of the intestinal microflora, including Bacteroides species (5, 7, 17).
In summary, we found that both oral vancomycin and oral metronidazole treatment of CDAD was associated with persistent overgrowth of preexisting VRE stool colonization. In contrast, the concentration of VRE in stool of colonized patients decreased significantly by 1 to 2 weeks after discontinuation of treatment with anti-anaerobic antibiotics (2, 6). Among patients with negative cultures prior to initiation of therapy, new detection of VRE colonization was uncommon in both therapy groups. These findings suggest that factors other than promotion of VRE should be the primary considerations in choosing oral vancomycin or metronidazole for treatment of CDAD. In addition, our findings highlight the importance of developing new treatments for CDAD that are less likely to disrupt the intestinal microflora and promote overgrowth of healthcare-associated pathogens.
Acknowledgments
This study was supported by an Advanced Research Career Development Award from the Department of Veterans Affairs to C.J.D. and in part by a grant from ViroPharma, Inc.
Footnotes
Published ahead of print on 28 April 2008.
REFERENCES
- 1.Arabi, Y., F. Dimock, D. W. Burdon, J. Alexander-Williams, and M. R. Keighley. 1979. Influence of neomycin and metronidazole on colonic microflora of volunteers. J. Antimicrob. Chemother. 5:531-537. [DOI] [PubMed] [Google Scholar]
- 2.Bhalla, A., N. J. Pultz, A. J. Ray, C. K. Hoyen, E. C. Eckstein, and C. J. Donskey. 2003. Anti-anaerobic antibiotic therapy promotes overgrowth of antibiotic-resistant, gram-negative bacilli and vancomycin-resistant enterococci in stool of colonized patients. Infect. Control Hosp. Epidemiol. 47:3610-3612. [DOI] [PubMed] [Google Scholar]
- 3.Bolton, R. P., and M. A. Culshaw. 1986. Faecal metronidazole concentrations during oral and intravenous therapy for antibiotic associated colitis due to Clostridium difficile. Gut 27:1169-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1995. Recommendations for preventing the spread of vancomycin resistance: recommendations of the Hospital Infection Control Practices Advisory Committee (HIC-PAC). Am. J. Infect. Control 23:87-94. [DOI] [PubMed] [Google Scholar]
- 5.Donskey, C. J., J. A. Hanrahan, R. A. Hutton, et al. 1999. Effect of parenteral antibiotic administration on persistence of vancomycin-resistant Enterococcus faecium in the mouse gastrointestinal tract. J. Infect. Dis. 180:384-390. [DOI] [PubMed] [Google Scholar]
- 6.Donskey, C. J., T. K. Chowdhry, M. T. Hecker, et al. 2000. Effect of antibiotic therapy on the concentration of vancomycin-resistant enterococci in the stool of colonized patients. N. Engl. J. Med. 343:1925-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edlund, C., L. Barkholt, B. Olsson-Liljequist, and C. E. Nord. 1997. Effect of vancomycin on intestinal flora of patients who previously received antimicrobial therapy. Clin. Infect. Dis. 25:729-732. [DOI] [PubMed] [Google Scholar]
- 8.Edmond, M. B., J. F. Ober, D. L. Weinbaum, et al. 1995. Vancomycin-resistant Enterococcus faecium bacteremia: risk factors for infection. Clin. Infect. Dis. 20:1126-1133. [DOI] [PubMed] [Google Scholar]
- 9.Gerding, D. N., S. Johnson, L. R. Peterson, M. E. Mulligan, and J. Silva. 1995. Clostridium difficile-associated diarrhea and colitis. Infect. Control Hosp. Epidemiol. 16:459-477. [DOI] [PubMed] [Google Scholar]
- 10.Gerding, D. N. 2005. Metronidazole for Clostridium difficile-associated disease: is it okay for Mom? Clin. Infect. Dis. 40:1598-1600. [DOI] [PubMed] [Google Scholar]
- 11.Harbarth, S., S. Cosgrove, and Y. Carmeli. 2002. Effects of antibiotics on nosocomial epidemiology of vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 46:1619-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lester, C. H., N. Frimodt-Moller, T. Lund Sorensen, D. L. Monnet, and A. M. Hammerum. 2006. In vivo transfer of the vanA resistance gene from an Enterococcus faecium isolate of animal origin to an E. faecium isolate of human origin in the intestines of human volunteers. Antimicrob. Agents Chemother. 50:596-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musher, D. M., S. Aslam, N. Logan, I. Bhaila, and F. Borchert. 2005. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin. Infect. Dis. 40:1586-1590. [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 2005. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard M7. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 15.Pepin, J., M. E. Alary, L. Valiquette, et al. 2005. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin. Infect. Dis. 40:1591-1597. [DOI] [PubMed] [Google Scholar]
- 16.Poutanen, S. M., and A. E. Simor. 2004. Clostridium difficile-associated diarrhea in adults. CMAJ 171:51-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pultz, N. J., U. Stiefel, and C. J. Donskey. 2005. Effects of daptomycin, linezolid, and vancomycin on establishment of intestinal colonization with vancomycin-resistant enterococci and extended-spectrum-β-lactamase-producing Klebsiella pneumoniae in mice. Antimicrob. Agents Chemother. 49:3513-3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robredo, B., K. V. Singh, F. Baquero, B. E. Murray, and C. Torres. 1999. From vanA Enterococcus hirae to vanA Enterococcus faecium: a study of feed supplementation with avoparcin and tylosin in young chickens. Antimicrob. Agents Chemother. 43:1137-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salgado, C. D., E. T. Giannetta, and B. M. Farr. 2004. Failure to develop vancomycin-resistant Enterococcus with oral vancomycin treatment of Clostridium difficile. Infect. Control Hosp. Epidemiol. 25:413-417. [DOI] [PubMed] [Google Scholar]
- 20.Teasley, D. G., D. N. Gerding, M. M. Olson, et al. 1983. Prospective randomised trial of metronidazole versus vancomycin for Clostridium difficile-associated diarrhoea and colitis. Lancet ii:1043-1046. [DOI] [PubMed] [Google Scholar]
- 21.Weinisch, C., B. Parschalk, M. Hasenhundl, et al. 1996. Comparison of vancomycin, teicoplanin, metronidazole and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin. Infect. Dis. 22:813-818. [DOI] [PubMed] [Google Scholar]
- 22.Zar, F. A., S. R. Bakkanagari, K. M. Moorthi, and M. B. Davis. 2007. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin. Infect. Dis. 45:302-307. [DOI] [PubMed] [Google Scholar]