Abstract
Telavancin is an investigational, rapidly bactericidal lipoglycopeptide antibiotic that is being developed to treat serious infections caused by gram-positive bacteria. A baseline prospective surveillance study was conducted to assess telavancin activity, in comparison with other agents, against contemporary clinical isolates collected from 2004 to 2005 from across the United States. Nearly 4,000 isolates were collected, including staphylococci, enterococci, and streptococci (pneumococci, beta-hemolytic, and viridans). Telavancin had potent activity against Staphylococcus aureus and coagulase-negative staphylococci (MIC range, 0.03 to 1.0 μg/ml), independent of resistance to methicillin or to multiple agents. Telavancin activity was particularly potent against all streptococcal groups (MIC90s, 0.03 to 0.12 μg/ml). Telavancin had excellent activity against vancomycin-susceptible enterococci (MIC90, 1 μg/ml) and was active against VanB strains of vancomycin-resistant enterococci (MIC90, 2 μg/ml) but less active against VanA strains (MIC90, 8 to 16 μg/ml). Telavancin also demonstrated activity against vancomycin-intermediate S. aureus and vancomycin-resistant S. aureus strains (MICs, 0.5 μg/ml to 1.0 μg/ml and 1.0 μg/ml to 4.0 μg/ml, respectively). These data may support the efficacy of telavancin for treatment of serious infections with a wide range of gram-positive organisms.
Antibiotic resistance in gram-positive bacteria is a continuing health care problem, both in hospitals and in the community. Telavancin is a novel, once-daily, intravenously administered lipoglycopeptide that is being developed to treat serious infections caused by gram-positive bacteria. It has shown promising results in patients with complicated skin and skin structure infections (SSSIs) (i.e., cellulitis, major abscess, infected wound/ulcer, or burn complicated by a requirement for surgical intervention and/or involvement of deeper tissues), including those infected with methicillin-resistant Staphylococcus aureus (MRSA) (19, 20, 21). A U.S. Food and Drug Administration New Drug Application has been filed for telavancin based on two completed phase 3 clinical trials for the treatment of complicated SSSIs (20), and two phase 3 trials for the treatment of hospital-acquired pneumonia have finished patient enrollment.
Like vancomycin and teicoplanin, telavancin inhibits the polymerization of cell wall peptidoglycan precursors by binding to their d-alanyl-d-alanine termini, but telavancin has greater activity than vancomycin in this interaction (50% inhibitory concentration, 0.14 μM versus 2.0 μM) (8). Additionally, the interaction of telavancin with peptidoglycan precursors facilitates the perturbation of bacterial plasma membrane function, which leads to concentration-dependent membrane depolarization and increases in membrane permeability (8). The second mode of action is likely responsible for the more rapid and extensive bactericidal activity of telavancin than vancomycin and teicoplanin.
Telavancin exhibits potent in vitro antibacterial activity against a broad range of clinically important gram-positive bacteria, including MRSA. In several studies, telavancin MICs for MRSA ranged from two to eight times lower than those observed for vancomycin, teicoplanin, and linezolid (9, 12, 15). Telavancin also demonstrated excellent activity against MRSA and coagulase-negative staphylococci (CoNS), with reduced susceptibility to glycopeptides (13) and both vancomycin-susceptible and -resistant enterococci (MIC90s, 1 μg/ml and 4 μg/ml, respectively) (9, 12). The in vitro spectrum of telavancin also includes anaerobic gram-positive bacteria and Corynebacterium spp. (7).
We report the results of the first prospective surveillance study of the in vitro activity of telavancin against gram-positive pathogens collected in the United States.
(Parts of this study have been presented previously in abstract format [4, 5, 22]).
MATERIALS AND METHODS
During 2004 and 2005, 53 hospital laboratories, distributed across all nine U.S. Census Bureau regions, collected gram-positive clinical isolates for testing. The participating institutions included community, teaching, and university hospitals. Each center was asked to submit approximately 80 consecutive clinical isolates from patient specimens, with the aim of obtaining from each center approximately 45 S. aureus, 5 CoNS, 10 Enterococcus faecalis, 10 Enterococcus faecium, and 5 Streptococcus pneumoniae isolates and 5 isolates of other Streptococcus species. Bacterial species were identified using routine microbiological methods and automated systems (VITEK 1; bioMérieux, Durham, NC) as appropriate. Only unique isolates (one per species per patient) from anatomically relevant sites of infection were to be submitted. Additionally, six vancomycin-intermediate S. aureus (VISA) and three vancomycin-resistant S. aureus (VRSA) isolates from the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) repository were tested.
The isolates were sent to a central laboratory (Eurofins Medinet, Inc., Herndon, VA) for confirmation of identity and susceptibility testing. The MICs of telavancin and appropriate comparators were determined by the Clinical Laboratory Standards Institute (CLSI) (formerly known as the National Committee for Clinical Laboratory Standards) broth microdilution method (1). Microtiter trays containing serial dilutions of the test agents were prepared by TREK Diagnostic Systems (Cleveland, OH) using standard laboratory powders and shipped frozen. Telavancin was supplied by Theravance, Inc. (South San Francisco, CA). The quality control strains E. faecalis ATCC 29212, E. faecalis ATCC 51299, S. aureus ATCC 29213, and S. pneumoniae ATCC 49619 were tested in parallel (1). Susceptibility to comparators was determined using CLSI breakpoints (2). Methicillin resistance in staphylococci was determined by the oxacillin MIC (2).
RESULTS
In total, 3,988 isolates, comprising 2,299 S. aureus, 372 CoNS, 458 E. faecalis, 337 E. faecium, and 276 S. pneumoniae isolates and 246 other streptococci, were collected from hospitalized (including intensive care) and nonhospitalized adult and pediatric patients. Participating institutions across all nine U.S. Census Bureau Regions each provided between 1 and 131 isolates (mean ± standard deviation, 75.2 ± 20.5) (by census region, there were 395 from five hospitals in the South Atlantic region, 684 from eight hospitals in the Mid-Atlantic region, 312 from four hospitals in New England, 596 from seven hospitals in the East North Central region, 347 from five hospitals in the East South Central region, 314 from five hospitals in the Mountain region, 589 from eight hospitals in the Pacific region, 312 from five hospitals in the West South Central region, and 439 from six hospitals in the West North Central region). Most isolates were from SSSIs (1,724 isolates, including more than two-thirds of the S. aureus isolates) and the bloodstream (1,469 isolates). Enterococci were mainly from the bloodstream and SSSIs. Most S. pneumoniae isolates were from the blood or the lower respiratory tract.
Nearly half of the S. aureus isolates (47.1%) were methicillin resistant, as were 73% of the CoNS (Table 1). Resistance to clindamycin and ciprofloxacin was frequently detected among both MRSA (44% and 76%, respectively) and methicillin-resistant CoNS (46% and 72%, respectively). Overall, erythromycin resistance rates were high among staphylococci (65% and 72% of S. aureus isolates and CoNS, respectively), while resistance to telithromycin mirrored the clindamycin resistance rates (data not shown). Resistance to gentamicin and cotrimoxazole was common among CoNS (19% and 44% of all strains, respectively) but less so among S. aureus isolates (4% and 3%, respectively). No glycopeptide-nonsusceptible S. aureus isolates were identified, but 5% of the CoNS were resistant to or had intermediate susceptibility to teicoplanin. A small number of staphylococcal isolates were nonsusceptible to daptomycin, linezolid, or quinupristin-dalfopristin.
TABLE 1.
Activities of telavancin and selected comparators against gram-positive clinical isolates from centers across the United States
| Organism (no. tested) | Agent | MIC (μg/ml)
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| S. aureus | ||||
| Methicillin susceptible (1,217) | Telavancin | 0.03-1 | 0.25 | 0.5 |
| Vancomycin | ≤0.25-2 | 1 | 1 | |
| Teicoplanin | ≤0.12-8 | 1 | 2 | |
| Linezolid | ≤0.5->4 | 2 | 2 | |
| Daptomycin | 0.12-1 | 0.5 | 0.5 | |
| Quinupristin-dalfopristin | ≤0.12-4 | 0.25 | 0.5 | |
| Gentamicin | ≤0.06->16 | 0.25 | 0.5 | |
| Cotrimoxazolea | ≤0.5->4 | ≤0.5 | ≤0.5 | |
| Methicillin resistant (1,082) | Telavancin | 0.06-1 | 0.25 | 0.25 |
| Vancomycin | 0.5-2 | 1 | 1 | |
| Teicoplanin | ≤0.12-8 | 0.5 | 1 | |
| Linezolid | ≤0.5->4 | 2 | 2 | |
| Daptomycin | 0.12->1 | 0.5 | 0.5 | |
| Quinupristin-dalfopristin | ≤0.12-4 | 0.25 | 0.5 | |
| Gentamicin | ≤0.06->16 | 0.5 | 2 | |
| Cotrimoxazolea | ≤0.5->4 | ≤0.5 | ≤0.5 | |
| Coagulase-negative staphylococcib | ||||
| Methicillin susceptible (100) | Telavancin | 0.06-1 | 0.25 | 0.5 |
| Vancomycin | ≤0.25-4 | 1 | 2 | |
| Teicoplanin | ≤0.12-32 | 2 | 8 | |
| Linezolid | ≤0.5-2 | 1 | 1 | |
| Daptomycin | 0.12->1 | 0.5 | 1 | |
| Quinupristin-dalfopristin | ≤0.12-1 | 0.25 | 0.5 | |
| Methicillin resistant (272) | Telavancin | 0.12-1 | 0.25 | 0.5 |
| Vancomycin | 0.5-4 | 2 | 2 | |
| Teicoplanin | 0.25-32 | 4 | 8 | |
| Linezolid | ≤0.5->4 | 1 | 1 | |
| Daptomycin | 0.12->1 | 0.5 | 1 | |
| Quinupristin-dalfopristin | ≤0.12-4 | 0.25 | 0.25 | |
| E. faecalisc | ||||
| Vancomycin susceptible (429) | Telavancin | 0.12-1 | 0.5 | 1 |
| Vancomycin | ≤0.5-4 | 1 | 2 | |
| Teicoplanin | ≤0.03-0.5 | 0.25 | 0.25 | |
| Linezolid | 0.25-32 | 1 | 2 | |
| Daptomycin | 0.12-2 | 1 | 1 | |
| Ampicillin | ≤0.25-8 | 1 | 1 | |
| VanA (22) | Telavancin | 4-16 | 8 | 16 |
| Vancomycin | 256->512 | 512 | >512 | |
| Teicoplanin | 32->128 | 64 | 128 | |
| Linezolid | 0.5-2 | 1 | 2 | |
| Daptomycin | 0.5-2 | 0.5 | 1 | |
| Ampicillin | 0.5-128 | 1 | 2 | |
| VanB (4) | Telavancin | 0.25-1 | ||
| Vancomycin | 32-512 | |||
| Teicoplanin | 0.25 | |||
| Linezolid | 1 | |||
| Daptomycin | 0.5-2 | |||
| Ampicillin | 0.5-1 | |||
| E. faeciumd | ||||
| Vancomycin susceptible (92) | Telavancin | ≤0.015-0.5 | 0.12 | 0.25 |
| Vancomycin | ≤0.5-2 | ≤0.5 | 1 | |
| Teicoplanin | ≤0.03-2 | 0.5 | 0.5 | |
| Linezolid | ≤0.015-4 | 2 | 2 | |
| Daptomycin | ≤0.015-8 | 2 | 4 | |
| Quinupristin-dalfopristin | ≤0.06-4 | 0.5 | 2 | |
| Ampicillin | ≤0.25->128 | 128 | >128 | |
| VanA (223) | Telavancin | ≤0.015-16 | 4 | 8 |
| Vancomycin | 32->512 | 512 | 512 | |
| Teicoplanin | 16->128 | 64 | >128 | |
| Linezolid | 1-16 | 2 | 2 | |
| Daptomycin | 0.25-8 | 2 | 4 | |
| Quinupristin-dalfopristin | 0.25-4 | 0.5 | 1 | |
| Ampicillin | 1->128 | 128 | >128 | |
| VanB (17) | Telavancin | 0.12-4 | 0.25 | 2 |
| Vancomycin | 32-512 | 128 | 512 | |
| Teicoplanin | 0.5-8 | 0.5 | 8 | |
| Linezolid | 1-8 | 2 | 2 | |
| Daptomycin | 0.5-4 | 2 | 4 | |
| Quinupristin-dalfopristin | 0.25-4 | 1 | 4 | |
| Ampicillin | 64->128 | 128 | >128 | |
| S. pyogenes (68) | Telavancin | 0.015-0.12 | 0.03 | 0.06 |
| Vancomycin | 0.25-1 | 0.25 | 0.5 | |
| Linezolid | 0.5-1 | 1 | 1 | |
| Daptomycin | ≤0.03-0.25 | 0.06 | 0.06 | |
| Levofloxacin | 0.25-2 | 0.5 | 1 | |
| Penicillin | ≤0.06 | ≤0.06 | ≤0.06 | |
| Cotrimoxazolea | ≤0.06->4 | 0.12 | 0.5 | |
| S. agalactiae (45) | Telavancin | 0.03-0.12 | 0.06 | 0.06 |
| Vancomycin | 0.25-0.5 | 0.5 | 0.5 | |
| Linezolid | 0.5-1 | 1 | 1 | |
| Daptomycin | 0.12-0.5 | 0.25 | 0.25 | |
| Levofloxacin | 0.5->8 | 0.5 | 1 | |
| Penicillin | ≤0.06 | ≤0.06 | ≤0.06 | |
| Cotrimoxazolea | ≤0.06-0.25 | ≤0.06 | 0.12 | |
| Streptococcus spp. | Telavancin | 0.03-0.25 | 0.03 | 0.06 |
| Groups C, F, G (31) | Vancomycin | 0.25-1 | 0.25 | 0.5 |
| Linezolid | 1-2 | 1 | 1 | |
| Daptomycin | ≤0.03-0.5 | 0.06 | 0.25 | |
| Levofloxacin | ≤0.12-4 | 0.5 | 0.5 | |
| Penicillin | ≤0.06 | ≤0.06 | ≤0.06 | |
| Cotrimoxazolea | ≤0.06-0.5 | ≤0.06 | 0.25 | |
| Viridans group streptococci (102)e | Telavancin | ≤0.001-1 | 0.03 | 0.12 |
| Vancomycin | 0.12-1 | 0.5 | 0.5 | |
| Linezolid | ≤0.12-2 | 1 | 1 | |
| Daptomycin | ≤0.03->1 | 0.25 | 1 | |
| Levofloxacin | ≤0.12->8 | 0.5 | 1 | |
| Penicillin | ≤0.06->2 | ≤0.06 | 1 | |
| Cotrimoxazolea | ≤0.06->4 | 0.12 | 2 | |
| S. pneumoniae | ||||
| Penicillin susceptible (204) | Telavancin | 0.002-0.06 | 0.015 | 0.03 |
| Vancomycin | ≤0.06-1 | 0.25 | 0.5 | |
| Linezolid | 0.25-1 | 1 | 1 | |
| Levofloxacin | 0.25->8 | 1 | 1 | |
| Penicillin | ≤0.06 | ≤0.06 | ≤0.06 | |
| Cotrimoxazolea | ≤0.06->4 | 0.12 | 0.5 | |
| Penicillin nonsusceptiblef (72) | Telavancin | 0.008-0.03 | 0.015 | 0.015 |
| Vancomycin | 0.25-0.5 | 0.25 | 0.5 | |
| Linezolid | 0.25-1 | 1 | 1 | |
| Levofloxacin | 0.5-8 | 0.5 | 1 | |
| Cotrimoxazolea | 0.12->4 | 4 | >4 | |
MICs expressed as the concentration of trimethoprim.
Includes Streptococcus capitis (8), Streptococcus cohnii (2), Streptococcus epidermidis (214), Streptococcus haemolyticus (14), Streptococcus hominis (13), Streptococcus saprophyticus (1), Streptococcus sciuri (2), Streptococcus simulans (16), Streptococcus warneri (6), Streptococcus xylosus (2), and unspeciated CoNS (94).
Excludes vancomycin-intermediate enterococci (3).
Excludes vancomycin-intermediate enterococci (5).
Includes Streptococcus bovis (1), Streptococcus constellatus (5), Streptococcus intermedius (16), Streptococcus mitis (1), Streptococcus oralis (1), Streptococcus sanguinis (1), and Streptococcus spp. (99).
Includes penicillin-intermediate and -resistant isolates.
Telavancin had potent activity against S. aureus and CoNS isolates (MIC90s, 0.5 μg/ml for both), similar to those of daptomycin and quinupristin-dalfopristin, independent of methicillin resistance (Table 1). Relative to telavancin MIC90 values, linezolid was 4- to 8-fold less active against S. aureus, vancomycin was 4-fold less active against MRSA and CoNS, and teicoplanin was 4- to 16-fold less active against staphylococci. Cotrimoxazole had potent activity against the majority of S. aureus isolates (MIC90, ≤0.5 μg/ml). Six daptomycin-nonsusceptible staphylococci had telavancin MICs that ranged from 0.25 μg/ml to 1.0 μg/ml (Table 2).
TABLE 2.
Activities of telavancin against staphylococci not susceptible to daptomycin
| Organism | Isolate identification no. | MIC (μg/ml)
|
||
|---|---|---|---|---|
| Telavancin | Vancomycin | Daptomycin | ||
| S. aureus | 1171055 | 0.5 | 1 | >1 |
| S. aureus | 1171063 | 0.5 | 1 | >1 |
| S. aureus | 1176127 | 0.5 | 2 | >1 |
| S. aureus | 1285404 | 0.25 | 1 | >1 |
| CoNSa | 1285835 | 0.5 | 2 | >1 |
| S. capitis | 1285355 | 1 | 2 | >1 |
This particular isolate could not be identified to the species level with ≥85% confidence.
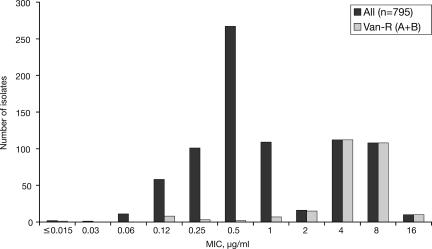
Vancomycin nonsusceptibility was detected in 6% of the E. faecalis and 71% of the E. faecium isolates tested (Table 1). More than 30% of the enterococci expressed high-level resistance to gentamicin and/or streptomycin (data not shown), and 89% of the E. faecium isolates were resistant to ampicillin. Telavancin had potent activity against vancomycin-susceptible enterococci (MIC90s, 1 μg/ml and 0.25 μg/ml for E. faecalis and E. faecium, respectively), as well as against VanB (vancomycin-resistant, teicoplanin-susceptible) strains (E. faecium MIC90, 2 μg/ml) (Table 1). Telavancin was less active against VanA (vancomycin- and teicoplanin-nonsusceptible) strains, with MIC90 values of 16 μg/ml and 8 μg/ml against VanA E. faecalis and VanA E. faecium, respectively. The bimodal nature of the telavancin MIC distribution for enterococci is illustrated in Fig. 1. The MIC90 values of teicoplanin were somewhat lower than those of telavancin against vancomycin-susceptible isolates but were ≥128 μg/ml against VanA strains. The MIC90 values of linezolid and daptomycin against VanA and VanB E. faecium were similar to those observed for vancomycin-susceptible isolates and were lower than those observed with telavancin (Table 1), though a few enterococci nonsusceptible to either daptomycin or linezolid were detected in this study. Nine enterococcal isolates were nonsusceptible to either linezolid or daptomycin. Against these isolates, telavancin displayed MICs ranging from 0.06 μg/ml to 0.5 μg/ml for vancomycin-susceptible E. faecium, MICs ranging from 2 μg/ml to 16 μg/ml for vancomycin-resistant E. faecium, and an MIC of 1 μg/ml against a single strain of vancomycin-susceptible E. faecalis (data not shown). Against VanA E. faecium, telavancin had a MIC90 higher than that of quinupristin-dalfopristin; however, against vancomycin-susceptible and VanB E. faecium, telavancin had a lower MIC90 than quinupristin-dalfopristin (Table 1). Resistance to quinupristin-dalfopristin was detected in 4% of E. faecium isolates tested.
FIG. 1.
Telavancin MIC distribution patterns against enterococci. “All” includes vancomycin-susceptible (n = 521), -resistant (n = 266), and -intermediate (n = 8) isolates. “Van-R (A + B)” includes vancomycin-resistant enterococci of both VanA and VanB phenotypes.
Telavancin had potent activity against all groups of streptococci (MIC90, 0.03 μg/ml to 0.12 μg/ml), independent of susceptibility to penicillin or erythromycin (Table 1). All of the comparators had higher MIC90 values against the streptococci, with the exception of penicillin (excluding penicillin-nonsusceptible S. pneumoniae). Among the penicillin-resistant pneumococci (which were 9% of the total; 24/276), 4% were resistant to ceftriaxone, 38% to clindamycin, 83% to erythromycin, 54% to tetracycline, and 79% to cotrimoxazole (data not shown). Among all S. pneumoniae isolates, resistance to levofloxacin was infrequent (<2%). Erythromycin resistance was very common in the viridans streptococci (54%) and Streptococcus agalactiae (36%) but less frequent among Streptococcus pyogenes isolates (6%) and other beta-hemolytic species (13%). Resistance to clindamycin was frequent in the erythromycin-nonsusceptible population of viridans streptococci and S. agalactiae (18% and 41%, respectively), and tetracycline resistance was highest among S. agalactiae, viridans streptococci, and the combined group C, G, and F beta-hemolytic strains (89%, 29%, and 19%, respectively). Levofloxacin resistance was 4% among viridans streptococci and rare among the beta-hemolytic species.
As there were no VISA or VRSA isolates identified in the surveillance study, we separately tested six VISA and three VRSA strains from the NARSA repository (Table 3). Telavancin MICs for the VISA strains were 0.5 μg/ml to 1 μg/ml, which were within the MIC range of telavancin for the surveillance isolates tested in this study. One isolate of VISA (NRS12) tested as vancomycin susceptible in this study, with a vancomycin MIC of 2 μg/ml, potentially indicating the presence of heterogeneous subpopulations of VISA in this strain. Against the VRSA strains, the telavancin MICs ranged from 1 μg/ml to 4 μg/ml versus ≥32 μg/ml for vancomycin. The three VRSA strains and all but one of the VISA strains were also resistant to both methicillin and ciprofloxacin. Seven of the nine tested VISA and VRSA strains were resistant to clindamycin and gentamicin, and four of the VISA strains were nonsusceptible to daptomycin.
TABLE 3.
Activities of telavancin, vancomycin, and teicoplanin against VISA and VRSA isolates from the NARSA repository
| Phenotype | NARSA ID no. | Country of origin | MIC (μg/ml)
|
||
|---|---|---|---|---|---|
| Telavancin | Vancomycin | Teicoplanin | |||
| VISA | NRS17 | United States | 1 | 8 | 4 |
| VISA | NRS74 | United States | 1 | 4 | 4 |
| VISA | NRS118 | United States | 0.5 | 4 | 8 |
| VISA | NRS1 | Japan | 0.5 | 4 | 4 |
| VISAa | NRS12 | France | 0.5 | 2 | 8 |
| VISAb | NRS56 | Brazil | 1 | 4 | 2 |
| VRSA | VRS1 | United States | 4 | >32 | 32 |
| VRSA | VRS2 | United States | 1 | 32 | 8 |
| VRSA | VRS3 | United States | 4 | >32 | 8 |
Isolate identified as vancomycin intermediate based on its antibiotic profile reported within NARSA.
Methicillin susceptible. All other isolates are methicillin resistant.
DISCUSSION
This was the first prospective surveillance study of the activity of telavancin, an investigational lipoglycopeptide, against gram-positive clinical isolates from the United States. The nearly 4,000 isolates collected represent a diverse geographic and patient population and were obtained from clinically relevant infections. The high rates of resistance to comparators and the prevalence of organisms with multiple-drug resistance are consistent with data reported in other recent surveillance studies conducted in the United States and internationally (3, 6, 10, 11, 14, 16-18, 23). Telavancin demonstrated activity similar to that previously reported in smaller retrospective studies (7, 9, 12, 15).
In the present study, telavancin demonstrated potent in vitro activity against prospectively collected isolates of staphylococci and streptococci, including those resistant to other antimicrobial agents. Against more than 1,000 MRSA isolates, most of them resistant to multiple classes of agents, the MIC90 of telavancin was lower than those of all comparators, with the exception of cotrimoxazole (to which, however, 5% of the MRSA isolates were resistant). Interestingly, telavancin also had excellent activity against VISA and VRSA strains from the NARSA repository, and its activity was not altered against the few daptomycin-nonsusceptible isolates encountered in this study. Overall, telavancin was more active against CoNS than all comparators except quinupristin-dalfopristin. Telavancin MICs were lower than those of most comparators against pneumococci and viridans and beta-hemolytic streptococci, independent of their susceptibilities to other agents. It also had excellent in vitro activity against vancomycin-susceptible and VanB vancomycin-resistant enterococci. Although telavancin was somewhat less active against VanA enterococci, the vast majority of strains were inhibited by concentrations of ≤8 μg/ml, a level of activity not associated with the currently available glycopeptides.
In conclusion, telavancin demonstrated potent in vitro activity against contemporary gram-positive clinical isolates from across the United States. Telavancin activity was not affected by resistance to other classes of antimicrobial agents, and it demonstrated potentially useful activity against staphylococcal and some enterococcal isolates expressing high-level acquired glycopeptide resistance. As the clinical development of telavancin progresses, the results of this initial prospective surveillance study can serve as a benchmark for monitoring the in vitro activity of this new agent.
Acknowledgments
This study was sponsored by Theravance, Inc. VISA and VRSA isolates were contributed by the NARSA repository.
Editorial support was provided by Paul MacCallum and was funded by Astellas Pharma, Inc.
Footnotes
Published ahead of print on 28 April 2008.
REFERENCES
- 1.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed. Approved standard M7-A7. Clinical Laboratory Standards Institute, Wayne, PA.
- 2.Clinical and Laboratory Standards Institute. 2007. Performance standards for antimicrobial susceptibility testing, 17th informational supplement. M100-S17. Clinical Laboratory Standards Institute, Wayne, PA.
- 3.Diekema, D. J., B. J. Bootsmiller, T. E. Vaughn, R. F. Woolson, J. W. Yankey, E. J. Ernst, S. D. Flach, M. M. Ward, C. L. Franciscus, M. A. Pfaller, and B. N. Doebbeling. 2004. Antimicrobial resistance trends and outbreak frequency in United States hospitals. Clin. Infect. Dis. 38:78-85. [DOI] [PubMed] [Google Scholar]
- 4.Draghi, D. C., B. M. Benton, M. E. Jones, K. M. Krause, C. Thornsberry, and D. F. Sahm. 2006. In vitro activity of telavancin against enterococci: results from the 2004-2005 U.S. Surveillance Initiative, abstr. E-0717. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 5.Draghi, D. C., B. M. Benton, M. E. Jones, K. M. Krause, C. Thornsberry, and D. F. Sahm. 2006. Baseline antistaphylococcal profile of telavancin: results of the 2004-2005 U.S. Surveillance Initiative, abstr. E-0715. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 6.Draghi, D. C., D. J. Sheehan, P. Hogan, and D. F. Sahm. 2005. In vitro activity of linezolid against key gram-positive organisms isolated in the United States: results of the LEADER 2004 surveillance program. Antimicrob. Agents Chemother. 49:5024-5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein, E. J., D. M. Citron, C. V. Merriam, Y. A. Warren, K. L. Tyrrell, and H. T. Fernandez. 2004. In vitro activities of the new semisynthetic glycopeptide telavancin (TD-6424), vancomycin, daptomycin, linezolid, and four comparator agents against anaerobic gram-positive species and Corynebacterium spp. Antimicrob. Agents Chemother. 48:2149-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins, D. L., R. Chang, D. V. Debabov, J. Leung, T. Wu, K. M. Krause, E. Sandvik, J. M. Hubbard, K. Kaniga, D. E. Schmidt, Jr., Q. Gao, R. T. Cass, D. E. Karr, B. M. Benton, and P. P. Humphrey. 2005. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jansen, W. T., A. Verel, J. Verhoef, and D. Milatovic. 2007. In vitro activity of telavancin against gram-positive clinical isolates recently obtained in Europe. Antimicrob. Agents Chemother. 51:3420-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones, M. E., J. A. Karlowsky, D. C. Draghi, C. Thornsberry, D. F. Sahm, and D. Nathwani. 2003. Epidemiology and antibiotic susceptibility of bacteria causing skin and soft tissue infections in the USA and Europe: a guide to appropriate antimicrobial therapy. Int. J. Antimicrob. Agents 22:406-419. [DOI] [PubMed] [Google Scholar]
- 11.Karlowsky, J. A., C. Thornsberry, M. E. Jones, A. T. Evangelista, I. A. Critchley, and D. F. Sahm. 2003. Factors associated with relative rates of antimicrobial resistance among Streptococcus pneumoniae in the United States: results from the TRUST Surveillance Program (1998-2002). Clin. Infect. Dis. 36:963-970. [DOI] [PubMed] [Google Scholar]
- 12.King, A., I. Phillips, and K. Kaniga. 2004. Comparative in vitro activity of telavancin (TD-6424), a rapidly bactericidal, concentration-dependent anti-infective with multiple mechanisms of action against Gram-positive bacteria. J. Antimicrob. Chemother. 53:797-803. [DOI] [PubMed] [Google Scholar]
- 13.Leuthner, K. D., C. M. Cheung, and M. J. Rybak. 2006. Comparative activity of the new lipoglycopeptide telavancin in the presence and absence of serum against 50 glycopeptide non-susceptible staphylococci and three vancomycin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 58:338-343. [DOI] [PubMed] [Google Scholar]
- 14.National Nosocomial Infections Surveillance System. 2003. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1992 through June 2003, issued August 2003. Am. J. Infect. Control 31:481-498. [DOI] [PubMed] [Google Scholar]
- 15.Pace, J. L., K. Krause, D. Johnston, D. Debabov, T. Wu, L. Farrington, C. Lane, D. L. Higgins, B. Christensen, J. K. Judice, and K. Kaniga. 2003. In vitro activity of TD-6424 against Staphylococcus aureus. Antimicrob. Agents Chemother. 47:3602-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross, J. E., T. R. Fritsche, H. S. Sader, and R. N. Jones. 2007. Oxazolidinone susceptibility patterns for 2005: international report from the Zyvox annual appraisal of potency and spectrum study. Int. J. Antimicrob. Agents 29:295-301. [DOI] [PubMed] [Google Scholar]
- 17.Sader, H. S., J. M. Streit, T. R. Fritsche, and R. N. Jones. 2006. Antimicrobial susceptibility of gram-positive bacteria isolated from European medical centres: results of the Daptomycin Surveillance Programme (2002-2004). Clin. Microbiol. Infect. 12:844-852. [DOI] [PubMed] [Google Scholar]
- 18.Sahm, D. F., M. K. Marsilio, and G. Piazza. 1999. Antimicrobial resistance in key bloodstream bacterial isolates: electronic surveillance with the Surveillance Network Database-USA. Clin. Infect. Dis. 29:259-263. [DOI] [PubMed] [Google Scholar]
- 19.Stryjewski, M. E., V. H. Chu, W. D. O'Riordan, B. L. Warren, L. M. Dunbar, D. M. Young, M. Vallee, V. G. Fowler, Jr., J. Morganroth, S. L. Barriere, M. M. Kitt, and G. R. Corey for the FAST 2 Investigator Group. 2006. Telavancin versus standard therapy for treatment of complicated skin and skin structure infections caused by gram-positive bacteria: FAST 2 study. Antimicrob. Agents Chemother. 50:862-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stryjewski, M. E., D. R. Graham, S. E. Wilson, W. O'Riordan, D. Young, A. Lentnek, D. P. Ross, V. G. Fowler, A. Hopkins, H. D. Friedland, S. L. Barriere, M. M. Kitt, and G. R. Corey on behalf of the Assessment of Telavancin in Complicated Skin and Skin-Structure Infections Study. 2008. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by gram-positive organisms. Clin. Infect. Dis. 46:1683-1693. [DOI] [PubMed] [Google Scholar]
- 21.Stryjewski, M. E., W. D. O'Riordan, W. K. Lau, F. D. Pien, L. M. Dunbar, M. Vallee, V. G. Fowler, Jr., V. H. Chu, E. Spencer, S. L. Barriere, M. M. Kitt, C. H. Cabell, and G. R. Corey. 2005. Telavancin versus standard therapy for treatment of complicated skin and soft-tissue infections due to gram-positive bacteria. Clin. Infect. Dis. 40:1601-1607. [DOI] [PubMed] [Google Scholar]
- 22.Thornsberry, C., D. C. Draghi, B. M. Benton, M. E. Jones, K. M. Krause, and D. F. Sahm. 2006. Baseline profile of telavancin activity against streptococci: results of the. 2004-2005 U.S. Surveillance Initiative, abstr. E-0719. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 23.Watters, A. A., R. N. Jones, J. A. Leeds, G. Denys, H. S. Sader, and T. R. Fritsche. 2006. Antimicrobial activity of a novel peptide deformylase inhibitor, LBM415, tested against respiratory tract and cutaneous infection pathogens: a global surveillance report (2003-2004). J. Antimicrob. Chemother. 57:914-923. [DOI] [PubMed] [Google Scholar]