Abstract
Bacterial alveolar invasion is followed by an inflammatory response. A systemic extension of the compartmentalized immune response has been described in patients with severe pneumonia. The data suggest that some antimicrobials may induce a differential release of cytokines. We conducted a prospective, randomized study in adult patients with severe pneumococcal pneumonia to measure the effects of ceftriaxone and levofloxacin in the systemic cytokine expression over time. Demographic, clinical characteristics, and severity scores were recorded. The serum concentrations of tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-6, IL-8, IL-10, and IL-1 receptor agonist were measured at 0, 24, 72, and 120 h. A total of 32 patients were included in the study. Both groups were homogeneous in terms of age, comorbidities, severity of disease, and corticosteroid or statin use. With the single exception of IL-1β, all cytokines were detected in venous blood. All of the cytokines studied showed a similar pattern of progressive decrease over time. No significant differences in the concentrations of any of the cytokines studied were found, with the exception of TNF-α, for which lower concentrations were obtained at 120 h in the levofloxacin group (P = 0.014). Basal oxygen saturation (P = 0.034) and heart rate (P = 0.029) returned to normal values earlier in the levofloxacin arm. We demonstrated that in patients with severe pneumococcal pneumonia pro- and anti-inflammatory responses could be detected in venous blood, representing a systemic extension of the compartmentalized response. Treatment with a β-lactam agent or a fluoroquinolone has different effects on cytokine production and its systemic expression, impacting the clinical course of the disease.
Streptococcus pneumoniae remains the most common identifiable cause of pneumonia leading to hospitalization. Mortality from pneumococcal infection decreased significantly with the advent of antibiotic therapy. However, despite the advances in diagnostic methods and in intensive care support, mortality among patients with pneumococcal pneumonia remains high, ranging from 5 to 35% (1, 8, 36).
In 1964 Austrian and Gold (3) observed that deaths occurring within the first 5 days of treatment were not due to failure to eradicate the microorganism and suggested that these deaths were due to an inappropriate response of the host. These authors hypothesized that invading pathogens trigger an inflammatory response that can become independent of the bacterial presence and lead to multiple-organ failure and death.
Recent studies have demonstrated that this response is bimodal. In the early stages of infection, the local generation of proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL 8, IL-12, gamma interferon (IFN-γ), and possibly IL-6 are triggered by bacterial products (38).
It is well known that excessive production of these proinflammatory mediators can induce systemic inflammatory response syndrome and that these cytokines play an important role in the development of acute respiratory distress syndrome and multiple-organ dysfunction (15, 46).
However, the anti-inflammatory cytokines act as specific inhibitors of this network (mainly, IL-1 receptor antagonist [IL-1ra], IL-10, IL-4, IL-11, and IL-13) (35). It seems, therefore, that the balance between pro- and counter-inflammatory agents determines the final result and that insufficient or excessive production of any of these molecules may cause deleterious effects.
Adding to the complexity of this process, the inflammatory response could be influenced by many factors not only related to the host and the infective organism but also to the antimicrobial therapy. The immunomodulatory effects of antibiotics include alteration of phagocytosis, chemotaxis, endotoxin release, tumoricidal effects on certain cancer cells, and cytokine production with the potential of either accelerating or downregulating cytokine levels (30, 45).
Some data suggest that different antimicrobials classes may interact with the immune system in a different manner. It is known that many fluoroquinolones have an ability to inhibit the production of TNF-α and other pro/inflammatory interleukins (13). On the other hand, β-lactams cell wall activity causes the release of pneumococcal cell wall components, which act as potent inflammatory inducers.
Many studies have analyzed cytokine concentrations either at a single time point (14, 21, 29, 37) or over time (2, 9, 17, 22, 25, 28, 31-34) in pneumonia. These studies included a heterogeneous population with different etiologies, severity of disease, comorbidities, and treatment. To our knowledge, no studies have been reported on the effects of antimicrobials in the systemic expression of cytokine response in patients with pneumococcal pneumonia. To circumvent some of these difficulties, we performed a prospective study in a well-defined, homogeneous population of patients with documented severe pneumococcal pneumonia.
The aim of our study was to compare the evolution of pro- and anti-inflammatory cytokine systemic levels over time in patients treated either with ceftriaxone or with levofloxacin. We also assessed the relationship between cytokine expression, clinical variables, and the severity of disease.
MATERIALS AND METHODS
Setting and study design.
This prospective, randomized, open-label study was conducted at Hospital Mutua de Terrassa, a 450-bed acute care teaching hospital with ∼25,000 admissions/year for a population of ca. 300,000 inhabitants, between August 2004 and September 2005.
Adults with pneumonia classes III, IV, and V of the pneumonia severity index (PSI) developed by the Pneumonia Outcome Research Team (18) and with a confirmed pneumococcal etiology were included. The subjects were randomized to receive a single daily dose of intravenous ceftriaxone (1 g/day) or intravenous levofloxacin (500 mg/day). The local ethics and research committee approved the study, and informed consent was obtained from all patients.
Inclusion criteria and clinical variables.
Pneumonia was defined as an acute febrile respiratory illness accompanied by a new radiographic infiltrate consistent with this diagnosis. Exclusion criteria were as follows: age <18 years, pneumonia distal to endobronchial obstruction, pulmonary tuberculosis, bronchiectasis, known allergy to β-lactams or fluoroquinolones, underlying systemic autoimmune disease, and immunocompromised states, including patients on maintenance oral corticosteroids, patients with human immunodeficiency virus infection, pregnant subjects, patients that received antimicrobial therapy in the 15 days preceding the actual episode, and patients who had received fluoroquinolones in the last month or who had received nonsteroidal anti-inflammatory therapy in the last 2 weeks. Patients with serum creatinine at >2 mg/dl or patients on hemodialysis, as well as patients with documented pneumococcal pneumonia within the previous 4 weeks, were also excluded.
On admission, data were prospectively collected and included demographic characteristics, smoking and alcohol habits, comorbidities based on the Charlson score (10) and prognosis measured by the PSI (18) and APACHE II scores (27), immunosuppressive conditions, previous or current therapy with statins, use of nonsteroidal or steroidal anti-inflammatory drugs during the process, length of hospital stay, and 30-day mortality. The time between pneumonia onset and inclusion was also recorded.
Vital signs (heart rate, blood pressure, axillary temperature, and respiratory rate) and oxygen saturation while patients were breathing room air were assessed every 8 h during the first 5 days of hospital admission. Intravenous therapy was maintained during the first 5 days of admission and was switched to oral therapy afterward at the discretion of the attending physician.
Collection of blood samples and laboratory processing.
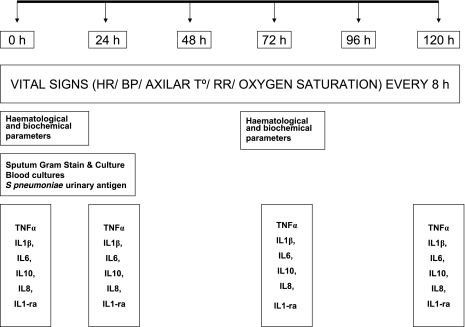
Blood samples were collected at inclusion, immediately prior to the initiation of antibiotic therapy, and at 24, 72, and 120 h (Fig. 1). Samples were centrifuged at 1,500 × g for 15 min at 4°C, distributed into four aliquots of 2 ml, and stored at −80°C. The assays were performed by one of the authors (M.A.), who was blinded to the clinical details of individual patients. The circulating levels of the cytokines TNF-α, IL-1β, IL-6, IL-8, IL-10, and IL-1ra were measured. All determinations were done in duplicate, and averages are reported.
FIG. 1.
Flow chart of cytokine determinations in cases of severe pneumococcal pneumonia. HR, heart rate; BP, blood pressure; AXILAR T°, axillary temperature; RR, respiratory rate.
TNF-α, IL-1β, IL-6, IL-10, and IL-8 concentrations were measured by using commercially available kits (Immulite; Immulite Systems). The procedure consisted of a solid-phase chemiluminescent immunometric assay. The standards defined in the operator's manual were applied. The limits of detection were 1.7 pg/ml for TNF-α, 1.5 pg/ml for IL-1β, 2 pg/ml for IL-6, 1 pg/ml for IL-10, and 2 pg/ml for IL-8.
IL-1ra concentrations were determined by using a commercial quantitative sandwich enzyme immunoassay technique (Quantikine; R&D Systems, Inc.). The minimum detectable dose of IL-1ra was 14 pg/ml.
Microbiological studies.
Two sets of blood cultures were obtained in all patients prior to commencing antibiotic therapy. Blood cultures were processed with the system BacT-Alert (bioMérieux, Durham, NC). When available, sputum samples were processed for Gram stain and culture.
The pneumococcal etiology was also determined by the detection of S. pneumoniae antigen in urine (Binax NOW S. pneumoniae urinary antigen test). To exclude other etiological diagnoses, the detection of Legionella pneumophila serogroup I antigen in urine, and Legionella, Mycoplasma, Coxiella, and Chlamydophila serologies were performed in paired samples obtained at admission and 4 weeks later.
Statistical analysis.
Variables that were not normally distributed were compared using nonparametric tests. The Mann-Whitney U-test was used to compare quantitative variables between treatment groups. The Kruskal-Wallis one-way analysis of variance was used for interleukin concentrations at every time point, and the Wilcoxon signed-rank test was used for two quantitative variables for a single group. For categorical variables, the Fisher exact test was used. Normally distributed data (age, blood pressure, respiratory rate, heart rate, temperature, and oxygen saturation) were compared by using unpaired t tests.
Studies evaluating serum concentrations of cytokines over time (the fall-down pattern) were done by using the general linear model for repeated-measures test, considering both within-subject and between-subject factors (attributable differences to antimicrobial therapy). The select contrasts used were both “difference” and “polynomic” for within subject factors. The analysis was adjusted for potential confounder variables (the use of corticosteroids or the presence of bacteremia). Due to the small sample size and the multiple statistical tests used, the Bonferroni honestly significant difference test was applied. The data analysis was performed with SPSS software version 13. Statistical significance was taken as a P value of <0.05.
RESULTS
During the study period, 45 adult patients met the inclusion criteria and were randomized to receive levofloxacin (23) or ceftriaxone (22). Thirteen patients were excluded: seven in the levofloxacin group because of final diagnoses other than pneumonia (n = 2), unknown allergy (n = 1), human immunodeficiency virus infection (n = 1), and lack of confirmed pneumococcal etiology (n = 3) and six in the ceftriaxone group due to the lack of confirmed pneumococcal pneumonia (n = 5) and the loss of blood samples (n = 1). Among the excluded patients, one died. Sixteen patients were finally included in each arm and were the subject of this analysis.
No differences were found between the two groups in terms of demographics or clinical characteristics, including time from pneumonia onset to inclusion, previous use of statins, and concomitant use of steroids or nonsteroidal anti-inflammatory drugs. Patients in the ceftriaxone arm had a slightly longer time from pneumonia onset to inclusion and a lower frequency of use and doses of acute corticosteroids, although none of these differences reached statistical significance (Table 1). The presence of comorbidities (Table 2) and length of stay, severity of disease at presentation, and mortality (Table 3) were also similar among the two groups.
TABLE 1.
Demographics and other clinical data for the patients with pneumococcal pneumonia in this study
| Parameter | Patient treatment
|
P | |
|---|---|---|---|
| Levofloxacin (n = 16) | Ceftriaxone (n = 16) | ||
| No. of males (%) | 7 (43.8) | 9 (56.3) | 0.3 |
| Mean age in yr (SD) | 70.3 (14.3) | 64.8 (18.8) | 0.7 |
| Age range (yr) | 34-85 | 19-89 | |
| No. of community-acquired cases (%) | 15 (93.8) | 15 (93.8) | 0.7 |
| Mean time (h) from pneumonia onset to inclusion (SD) | 53.75 (47.5) | 61.6 (49.6) | 0.7 |
| Time (h) from pneumonia onset to inclusion (range) | 4-168 | 3-144 | |
| No. of patients receiving previous statin therapy/total no. of patients (%) | 3/15 (20) | 1/16 (6.3) | 0.3 |
| No. of patients with history of acute use of nonsteroidal anti-inflammatory agents/total no. of patients (%) | 3/16 (18.8) | 3/16 (18.8) | 1 |
| No. of patients with history of acute concomitant corticosteroid therapy (%) | 9 (56.3) | 4 (25) | 0.14 |
| Mean dose of prednisone in mg (SD) | 131 (182) | 56 (123) | 0.1 |
| No. of patients in a long-term care facility (%) | 1 (6.3) | 0 | 0.3 |
TABLE 2.
Comorbidities
| Parameter | Patient treatment
|
P | |
|---|---|---|---|
| Levofloxacin (n = 16) | Ceftriaxone (n = 16) | ||
| Mean Charlson score (SD) | 1.5 (1.5) | 1.4 (1.2) | 0.8 |
| No. of patients with COPDa (%) | 7 (43.8) | 6 (40) | 0.7 |
| No. of patients with a smoking habit (%) | 4 (25) | 9 (56.3) | 0.1 |
| Mean no. of cigarette packs smoked/yr (SD) | 18.75 (38.2) | 23.5 (30.9) | 0.7 |
| No. of patients acknowledging regular alcohol consumption (%) | 4 (25) | 4 (25) | 1 |
| Mean alcohol consumption (g/day) | 72 | 62.6 | |
| No. of patients with diabetes mellitus (%) | 4 (25) | 2 (12.5) | 0.65 |
COPD, chronic obstructive pulmonary disease.
TABLE 3.
Severity of disease and outcome
| Parametera | Patient treatment
|
P | |
|---|---|---|---|
| Levofloxacin (n = 16) | Ceftriaxone (n = 16) | ||
| No. of patients with bilobar Rx involvement (%) | 6 (37.5) | 5 (31.3) | 0.5 |
| No. of patients with pleural effusion/total number of patients (%) | 4/16 (25) | 2/16 (12.5) | 0.3 |
| No. of patients with empyema (%) | 1 (6.3) | 2 (12.5) | 1 |
| No. of patients with PSI class IV (%) | 11 (68.8) | 9 (56.3) | 0.7 |
| No. of patients with PSI class V (%) | 2 (12.5) | 5 (31.3) | 0.39 |
| Mean APACHE score (SD) | 14.5 (3.8) | 14.8 (4.9) | 0.8 |
| No. of patients with shock | 1 (6.3) | 2 (12.5) | 1 |
| No. of patients with ICU admission | 0 | 2 (12.5) | 0.4 |
| No. of patients with mechanical ventilation | 0 | 2 (12.5) | 0.4 |
| Mean length of hospital stay in days (SD) | 8.44 (3.1) | 12.06 (8.9) | 0.1 |
| 30-day mortality (%) | 0 | 1 (6.3) | 1 |
ICU, intensive care unit.
The microbiological diagnoses confirmed the pneumococcal etiology of pneumonia in all patients (Table 4). In fact, most patients had several positive tests. Only two patients in each arm had a pneumococcal etiology sustained exclusively on the sputum results. The four patients with positive sputum cultures had also diplococci in the sputum Gram stain. We recovered 16 isolates (14 from blood cultures and 2 from sputum cultures). All were susceptible to levofloxacin and ceftriaxone.
TABLE 4.
Microbiological diagnoses
| Finding | Patient treatment
|
P | |
|---|---|---|---|
| Levofloxacin (n = 16) | Ceftriaxone (n = 16) | ||
| S. pneumoniae antigen in urine (%) | 12 (75) | 14 (87.5) | 0.2 |
| S. pneumoniae bacteremia (%) | 6 (37.5) | 8 (50) | 0.3 |
| Presence of diplococci in sputum as determined by Gram stain (%) | 7 (43.8) | 7 (43.5) | 1 |
| S. pneumoniae isolated in sputum culture (%) | 4 (25) | 4 (25) | 1 |
Concentrations of all studied cytokines, with the exception of IL-1β, could be detected in peripheral venous blood samples in all patients. A total of 19% of the patients at 0 h, 47% at 24 h, 65% at 72 h, and 59% at 120 h had IL-1β levels under the limit of detection. The median concentrations of all cytokines tested were compared between both groups at 0, 24, 72, and 120 h. No significant differences in the systemic concentrations of each of the cytokines studied were found at baseline and at 24, 72, and 120 h with the single exception of TNF-α: a higher concentration of this cytokine was documented at 120 h in the group treated with ceftriaxone (P = 0.014).
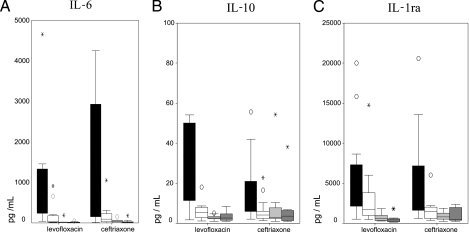
Figures 2 and 3 show the evolution of pro- and anti-inflammatory cytokine systemic concentrations. All cytokines concentrations had a significant decreasing pattern over time. The impact of antimicrobial therapy in the reduction of the cytokines measured became apparent at 120 h in the ceftriaxone arm, and this difference remained significant after adjustment for the use of corticosteroids and the presence of bacteremia.
FIG. 2.
Sequential proinflammatory cytokine levels in patients with severe pneumococcal pneumonia. A general linear model for a repeated-measures test was used. Analysis was adjusted for potential confounder variables (i.e., the use of corticosteroids or the presence of bacteremia). (A) TNF-α; (B) IL-1β; (C) IL-8. Determinations are shown for each set of four shaded boxes from left to right as follows: at entry, at 24 h, at 72 h, and at 120 h. Circles and asterisks represent outliers. The boxplots show the medians, interquartile ranges, and outliers. Outliers are cases with values that are between 1.5 and 3 box lengths from either end of the box.
FIG. 3.
Sequential anti-inflammatory cytokines levels in patients with severe pneumococcal pneumonia. A general linear model for a repeated-measures test was used. Analysis was adjusted for potential confounder variables (i.e., the use of corticosteroids or the presence of bacteremia). (A) IL-6; (B) IL-10; (C) IL-1ra. Determinations are shown for each set of four shaded boxes from left to right as follows: at entry, at 24 h, at 72 h, and at 120 h. Circles and asterisks represent outliers. The boxplots show the medians, interquartile ranges, and outliers. Outliers are cases with values that are between 1.5 and 3 box lengths from either end of the box.
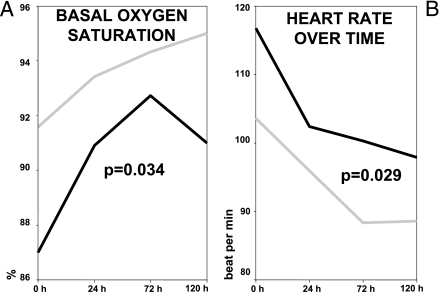
No differences were found between the two groups when blood pressure, temperature, or respiratory rate were analyzed (data not shown). However, basal oxygen saturation (P = 0.034) and heart rate (P = 0.029) went back to normal values earlier in the levofloxacin group (Fig. 4).
FIG. 4.
Vital signs over time in patients with severe pneumococcal pneumonia. (A) Oxygen saturation. Top line, levofloxacin; bottom line, ceftriaxone. (B) Heart rate. Top line, ceftriaxone; bottom line, levofloxacin.
DISCUSSION
Our study shows that the production and release of a number of pro- and anti-inflammatory cytokines, with the single exception of IL-1β, can be detected in the systemic circulation in all patients with severe pneumococcal pneumonia. It is well known that the initial inflammatory response is a local phenomenon. In pneumonia, a compartmentalized cytokine production has been described using bronchoalveolar lavage fluid studies (7, 14, 33). Our findings confirm the hypothesis of a systemic extension of the compartmentalized immune response in severe pneumonia, as previous studies have shown (22, 31, 34).
Many of these studies have analyzed cytokine concentrations at a single time point. Since the temporal changes in cytokine concentrations may better reflect the dynamics of the immune response, we measured the progression of cytokine release over time. In our study, sequential determinations showed a pattern of decline, with progressive normalization of all pro- and anti-inflammatory cytokines detected, which was accompanied by clinical recovery. In the mouse model of pneumococcal pneumonia (5, 12), this response is a process involving in a sequential manner the recruitment of polymorphonuclear monocytes, lymphocytes, and the pulmonary and/or systemic release of inflammatory mediators. In these models, survival was associated with rapid bacterial clearance and low inflammation profiles. Thus, the progressive normalization of cytokines that our study shows is part of the homeostatic response to the infection. In fact, the only patient who died (72 h after inclusion in the study) showed persistent high levels of TNF-α and IL-6 and a significant increase over time of IL-10 and IL-1ra (data not shown). Persistent elevation of TNF-α and IL-6 have been markers of poor prognosis in sepsis (41), and high levels of both anti-inflammatory cytokines have been correlated with severity (21) and less-favorable outcome (2).
Notably, few patients had detectable concentrations of IL-1β. Previous studies have inconsistently documented the presence of IL-1β in the systemic circulation in patients with CAP (2, 9, 14, 17, 22, 28, 32, 33). In general, the differing results reported would support the short half-life or the rapid local clearance (16) as the causes of the absence of detectable levels of IL-1β in the systemic circulation.
Surprisingly, concentrations of TNF-α in serum and concentration changes over time have not been detected in previous studies of cytokine release in pneumonia (17, 25, 31, 32, 33). A common explanation offered to explain this fact has been that its production is predominantly local and that its half-life is short. The number and timing of the sequential determinations, the degree of lung injury (4), and a great improvement assay sensitivity, as well as the pneumococcal etiology of our cases, are critical in explaining our results. It has been suggested that the magnitude of cytokine secretion depends on the bacterial species (17, 29, 37, 47). TNF-α activity has been shown to be a critical factor in the protective response to pneumococcal pneumonia. Increased susceptibility to infection and higher bacterial loads have been found in mouse strains with a reduced capacity to produce TNF-α or after systemic neutralization of TNF-α during pneumococcal pneumonia (26). TNF-α has already been detected in rabbits with experimental pneumococcal meningitis (19) or pneumonia (40) and in the cerebrospinal fluid (20) or sera of patients with pneumococcal meningitis or pneumonia (2, 9, 22, 34).
No differences were found in the progressive pattern of decrease over time among all of the studied cytokines between both treatment groups with the exception of TNF-α. In this sense, the most remarkable finding of our study was the lower concentrations of TNF-α in the last determination, at 120 h, in parallel with an earlier clinical recovery phase (expressed as an earlier recovery of oxygen saturation and slower cardiac rate) in patients treated with levofloxacin, a fluoroquinolone, compared to the group treated with a β-lactam.
Two possible reasons could explain these findings. First, the ability of many fluoroquinolones to inhibit the production of TNF-α (13) is well documented. A dose-dependent effect of levofloxacin in inhibiting the production of cytokines by lipopolysaccharide-stimulated human monocytes has been demonstrated (48). Many mechanisms have been described to explain this inhibition. With ciprofloxacin, it occurs in very early stages of TNF-α synthesis, probably due to the quinolone effect as a phosphodiesterase inhibitor, leading to cyclic AMP accumulation in the cells, resulting in enhanced cyclic AMP-protein kinase A activity, which in turn is known to inhibit TNF-α production (6). Others have described the ability of fluoroquinolones, such as moxifloxacin, to interfere with NF-κB activation by inhibiting the degradation of IκBα, thus reducing the levels of production of proinflammatory cytokines (11).
On the other hand, β-lactam cell wall activity causes the release of cell wall components, namely, C polysaccharide and lipoteichoic acid (23, 42, 43, 44), as well as cytoplasmic proteins, such as pneumolysin (24, 39), which act as potent inflammatory inducers. In this scenario, the continuous release of degradation bacterial products to the systemic circulation stimulating the monocytes could explain the persistent higher levels of TNF-α in the ceftriaxone group.
The time from the onset of symptoms to cytokine determination is also critical in order to properly interpret our results. In a pneumococcal pneumonia mouse model (12), bacterial growth reached a plateau of 107 CFU in lung tissue by 36 h postinoculation. However, the inflammatory response further amplified after 36 h, and peak levels of most mediators were observed at 84 h postinfection. This late burst of inflammation is most likely due to the lysis of dying pneumococci and the release of large amounts of toxins rather than by living pneumococci in a well-established infection. The median time from the onset of symptoms to study entry was >50 h in both arms. This fact offers a plausible explanation for the high levels of TNF-α found in both arms ab initio and the higher persistent concentrations in the ceftriaxone treated patients, where lysis was prominent with the initiation of the antimicrobial therapy.
An early and significant recovery of basal oxygen saturation and normalization of heart rate were observed in the levofloxacin-treated patients. We could not correlate these findings to evolving cytokine concentrations, other than the more intense decay of TNF-α in this group. Other contributing factors to the earlier clinical stabilization of the levofloxacin treated group remain to be determined.
Some differences at inclusion among groups, although not significant when considered individually, would justify a possible higher proinflammatory status at the baseline in the β-lactam arm, as measured by the higher initial TNF-α levels in this group. Previous use of statins and acute use of corticosteroids were more frequent in the levofloxacin group. In addition, patients in the ceftriaxone group were included later after onset of symptoms and had a higher proportion of cases of bacteremia, and a higher proportion of these patients were smokers. All of these factors coincided with the fact that baseline levels of TNF-α, IL-1β, and IL-8 (all proinflammatory cytokines) were higher in the ceftriaxone group, whereas baseline IL-10 concentrations were higher in the levofloxacin-treated patients. In the animal model, the presence of bacteremia (12), as well as TNF-α production (40), has been correlated with bacterial growth in the lung; since the bacterial burden increases over time following the onset of infection, a late inclusion could represent a higher inflammatory state. Therefore, the sustained proinflammatory response in the ceftriaxone group that we found could be explained at least in part by these differences. Still, after adjustment for steroid use and bacteremia, a more intense decay of TNF-α concentrations and a shorter time to reach clinical stability remained significantly associated with levofloxacin treatment.
Many recent trials have analyzed the relationship between the intensity of the systemic cytokine production over time and the severity of the community-acquired pneumonia (2, 17, 25, 28). A direct relationship between the intensity of the inflammatory response and the severity of the episode has been well established. Only patients with severe pneumonia were included in our study. This fact represents a voluntary selection bias and probably keeps us from finding differences in terms of severity among groups.
The main limitation of our study was the small sample size. This caused a limited power and the consequent risk of false-positive results. Another limitation was the fact that patients were admitted at different stages of their disease and, although we recorded the time from onset of symptoms to inclusion in order to control this factor, it is clear that there is no real-time zero. Finally, too few deaths occurred during the study to make any firm statement about the clinical relevance of the changes we observed.
In summary, in severe pneumococcal pneumonia, with the single exception of IL-1β, all of the cytokines were detected in venous blood, representing a systemic extension of the compartmentalized response. All of the cytokines studied showed a similar pattern of progressive decrease over time. Our results must be viewed with prudence, due to the small size sample and the initial differences between both treatments at inclusion. However, it seems that the levofloxacin group showed a shorter time to clinical stability (expressed as an earlier recovery of O2 saturation and a slower cardiac rate), in parallel with lower TNF-α levels in the last determination. Fluoroquinolone ability to inhibit cytokine production, coupled with the β-lactam cell wall activity (that might result in a second wave of TNF-α production), could explain these findings.
Acknowledgments
This study was supported by the Fondo Investigaciones Sanitarias and grant G03/103 from the Ministerio de Sanidad y Consumo of Spain.
Footnotes
Published ahead of print on 21 April 2008.
REFERENCES
- 1.Afessa, B., W. L. Greaves, and W. R. Frederick. 1995. Pneumococcal bacteraemia in adults: a 14-year experience in an inner-city university hospital. Clin. Infect. Dis. 21:345-351. [DOI] [PubMed] [Google Scholar]
- 2.Antunes, G., S. A. Evans, J. L. Lordan, and A. J. Frew. 2002. Systemic cytokine levels in community-acquired pneumonia and their association with disease severity. Eur. Respir. J. 20:990-995. [DOI] [PubMed] [Google Scholar]
- 3.Austrian, R., and J. Gold. 1964. Pneumococcal bacteraemia with especial reference to bacteraemic pneumococcal pneumonia. Ann. Intern. Med. 60:759-776. [DOI] [PubMed] [Google Scholar]
- 4.Bauer, T. T., C. Monton, A. Torres, H. Cabello, X. Fillela, A. Maldonado, J. N. Nicolas, and E. Zavala. 2000. Comparison of systemic cytokine levels in patients with acute respiratory distress syndrome, severe pneumonia, and controls. Thorax 55:46-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergeron, Y., N. Ouellet, A. M. Deslauriers, M. Simard, M. Olivier, and M. G. Bergeron. 1998. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect. Immun. 66:912-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaine, T. A., P. F. Pollice, R. N. Rosier, P. R. Reynolds, J. E. Puzas, and R. J. O'Keefe. 1997. Modulation of the production of cytokines in titanium-stimulated human peripheral blood monocytes by pharmacological agents.: the role of camp-mediated signaling mechanism. J. Bone Joint Surg. 79:1519-1528. [DOI] [PubMed] [Google Scholar]
- 7.Boutten, A., M. S. Dehoux, N. Seta, J. Ostinelli, P. Venembre, B. Crestani, M. C. Dombret, G. Durand, and M. Aubier. 1996. Compartmentalized IL-8 and elastase release within the human lung in unilateral pneumonia. Am. J. Respir. Crit. Care Med. 153:336-342. [DOI] [PubMed] [Google Scholar]
- 8.Brandenburg, J. A., T. J. Marrie, C. M. Coley, D. E. Singer, D. S. Obrosky, W. N. Kapoor, and M. J. Fine. 2000. Clinical presentation, processes and outcomes of care for patients with pneumococcal pneumonia. J. Gen. Intern. Med. 15:638-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruunsgaard, H., P. Skinhoj, J. Qvist, and B. K. Pedersen. 1999. Elderly humans show prolonged in vivo inflammatory activity during pneumococcal infections. J. Infect. Dis. 180:551-554. [DOI] [PubMed] [Google Scholar]
- 10.Charlson, M. E., P. Pompei, K. L. Ales, and C. R. MacKenzie. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40:373-383. [DOI] [PubMed] [Google Scholar]
- 11.Choi, J. H., M. J. Song, S. H. Kim, S. M. Choi, D. G. Lee, J. H. Yoo, and W. S. Shin. 2003. Effect of moxifloxacin on production of proinflammatory cytokines from human peripheral blood mononuclear cells. Antimicrob. Agents Chemother. 47:3704-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dallaire, F., N. Oullet, Y. Bergeron, V. Turmel, M. C. Gauthier, M. Simard, and M. G. Bergeron. 2001. Microbiological and inflammatory factors associated with the development of pneumococcal pneumonia. J. Infect. Dis. 184:292-300. [DOI] [PubMed] [Google Scholar]
- 13.Dalhoff, A., and I. Shalit. 2003. Immunomodulatory effect of quinolones. Lancet Infect. Dis. 3:359-371. [DOI] [PubMed] [Google Scholar]
- 14.Dehoux, M. S., A. Boutten, J. Ostinelli, N. Seta, M. C. Dombret, B. Crestani, M. Deschenes, J. L. Trouillet, and M. Aubier. 1994. Compartmentalized cytokine production within the human lung in unilateral pneumonia. Am. J. Respir. Crit. Care Med. 150:710-716. [DOI] [PubMed] [Google Scholar]
- 15.Dinarello, C. A. 1997. Proinflammatory and anti-inflammatory cytokines as mediators in the pathogenesis of septic shock. Chest 112:321S-329S. [DOI] [PubMed] [Google Scholar]
- 16.Dinarello, C. A., and J. G. Cannon. 1993. Cytokine measurements in septic shock. Ann. Intern. Med. 119:853-854. [DOI] [PubMed] [Google Scholar]
- 17.Fernández-Serrano, S., J. Dorca, M. Coromines, Carratalà, J. F. Gudiol, and F. Manresa. 2003. Molecular inflammatory responses measured in blood of patients with severe community acquired pneumonia. Clin. Diagn. Lab. Immunol. 10:813-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fine, M. J., T. E. Auble, D. M. Yealy, B. H. Hanusa, L. A. Weissfeld, D. E. Singer, C. M. Coley, T. J. Marrie, and W. N. Kapoor. 1997. A prediction rule to identify low-risk patients with community-acquired pneumonia. N. Engl. J. Med. 336:243-250. [DOI] [PubMed] [Google Scholar]
- 19.Friedland, I. R., M. M. Paris, S. Hickey, S. Shelton, K. Olsen, J. C. Paton, and G. H. McCracken. 1995. The limited role of pneumolysin in the pathogenesis of pneumococcal meningitis. J. Infect. Dis. 172:805-809. [DOI] [PubMed] [Google Scholar]
- 20.Glimaker, M., P. Kragsbjerg, M. Forsgren, and P. Olcen. 1993. Tumor necrosis factor alpha in cerebrospinal fluid from patients with meningitis of different etiologies: high levels of TNF indicate bacterial meningitis. J. Infect. Dis. 167:882-889. [DOI] [PubMed] [Google Scholar]
- 21.Glynn, P., R. Coakley, I. Kilgallen, N. Murphy, and S. O'Neill. 1999. Circulating interleukin 6 and interleukin 10 in community-acquired pneumonia. Thorax 54:51-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gon, Y., S. Hashimoto, S. Hayashi, T. Koura, K. Matsumoto, and T. Horie. 1996. Lower serum concentrations of cytokine in elderly patients with pneumonia and the impaired production of cytokines by peripheral blood monocytes in the elderly. Clin. Exp. Immunol. 106:120-126. [PubMed] [Google Scholar]
- 23.Heumann, D., C. Barras, A. Severin, M. P. Glauser, and A. Tomasz. 1994. Gram-positive cell wall stimulates synthesis of tumor necrosis factor alpha and interleukin 6 by human monocytes. Infect. Immun. 62:2715-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houldsworth, S. P., P. W. Andrew, and T. J. Mitchell. 1994. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin 1 beta by human mononuclear phagocytes. Infect. Immun. 62:7501-7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Igonin, A. A., V. W. Armstrong, M. Shipkova, N. B. Lazareva, V. G. Kukes, and M. Oellerich. 2004. Circulation cytokines as markers of systemic inflammatory response in severe community-acquired pneumonia. Clin. Biochem. 37:204-209. [DOI] [PubMed] [Google Scholar]
- 26.Kerr, A. R., J. J. Irvine, J. J. Search, N. A. Gingles, A. Kadioglu, P. W. Andrew, W. L. McPheat, C. G. Booth, and T. J. Mitchell. 2002. Role of inflammatory mediators in resistance and susceptibility to pneumococcal infection. Infect. Immun. 70:1547-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knaus, W. A., E. A. Draper, D. P. Wagner, and J. E. Zimmerman. 1985. APACHE II: a severity of disease classification system. Crit. Care Med. 13:818-829. [PubMed] [Google Scholar]
- 28.Kosmas, E. N., C. N. Baxevanis, M. Papamichail, and T. Kordossis. 1997. Daily variation in circulating cytokines and acute-phase proteins correlates with the clinical and laboratory indices in community-acquired pneumonia. Eur. J. Clin. Investig. 27:308-315. [DOI] [PubMed] [Google Scholar]
- 29.Kragsbjerg, P., I. Jones, T. Vikerfors, and H. Holmberg. 1995. Diagnostic value of blood cytokine concentrations in acute pneumonia. Thorax 50:1253-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labro, M. T. 2000. Interference of antibacterial agents with phagocyte functions: immunomodulation or “immuno-fairy” tales? Clin. Microbiol. Rev. 3:615-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marik, P., M. Med, P. Kraus, J. Sribante, I. Havlik, J. Lipman, and D. W. Johnson. 1993. Hydrocortisone and tumor necrosis factor in severe community-acquired pneumonia. Chest 104:389-392. [DOI] [PubMed] [Google Scholar]
- 32.Maskin, B., P. A. Fontan, E. G. Spinedi, D. Gammella, and A. Badolati. 2002. Evaluation of endotoxin release and cytokine production induced by antibiotics in patients with gram-negative nosocomial pneumonia. Crit. Care Med. 30:349-354. [DOI] [PubMed] [Google Scholar]
- 33.Monton, C., A. Torres, M. El-Ebiary, X. Filella, A. Xaubet, and J. Puig de la Bellacsa. 1999. Cytokine expression in severe pneumonia: a bronchoalveolar lavage study. Crit. Care Med. 27:1745-1753. [DOI] [PubMed] [Google Scholar]
- 34.Moussa, K., H. J. Michie, I. A. Cree, A. C. McCafferty, J. H. Winter, D. P. Dhillon, S. Stephens, and R. A. Brown. 1994. Phagocyte function and cytokine production in community acquired pneumonia. Thorax 49:107-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Opal, S. M., and V. A. DePalo. 2000. Anti-inflammatory cytokines. Chest 117:1162-1172. [DOI] [PubMed] [Google Scholar]
- 36.Ortqvist, A., A. Grepe, I. Julander, and M. Kalin. 1988. Bacteraemic pneumococcal pneumonia in Sweden: clinical course and outcome and comparison with non-bacteraemic pneumococcal and mycoplasma pneumonias. Scand. J. Infect. Dis. 20:163-171. [DOI] [PubMed] [Google Scholar]
- 37.Ortqvist, A., J. Hedlund, B. Wretlind, A. Carlstom, and M. Kalin. 1995. Diagnostic and prognostic value of interleukin 6 and C reactive protein in community-acquired pneumonia. Scand. J. Infect. Dis. 27:457-462. [DOI] [PubMed] [Google Scholar]
- 38.Pinsky, M. R. 2001. Sepsis: a pro- and anti-inflammatory disequilibrium syndrome. Contrib. Nephrol. 132:354-366. [DOI] [PubMed] [Google Scholar]
- 39.Spreer, A., H. Kerstan, T. Bottcher, J. Gerber, A. Siemer, G. Zysk, T. J. Mitchell, H. Eiffert, and R. Nau. 2003. Reduced release of pneumolysin by Streptococcus pneumoniae in vitro and in vivo after treatment with nonbacteriolytic antibiotics in comparison to ceftriaxone. Antimicrob. Agents Chemother. 47:2649-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takashima, K., K. Tateda, T. Matsumoto, Y. Iizawa, M. Nakao, and K. Yamaguchi. 1997. Role of TNF alpha in pathogenesis of pneumococcal pneumonia in mice. Infect. Immun. 65:257-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taniguchi, T., Y. Koido, J. Aiboshi, T. Yamashita, S. Suzaki, and A. Kurokawa. 1999. Change in the ratio of interleukin-6 to interleukin-10 predicts a poor outcome in patients with systemic inflammatory response syndrome. Crit. Care Med. 27:1262-1264. [DOI] [PubMed] [Google Scholar]
- 42.Tomasz, A., and D. Saukkonen. 1989. The nature of cell wall derived inflammatory components of pneumococci. Pediatr. Infect. Dis. J. 8:902-903. [DOI] [PubMed] [Google Scholar]
- 43.Tuomanen, E., R. Rich, and O. Zak. 1987. Induction of pulmonary inflammation by components of the pneumococcal cell surface. Am. Rev. Respir. Dis. 135:869-874. [DOI] [PubMed] [Google Scholar]
- 44.Tuomanen, E., H. Liu, B. Hengstler, O. Zak, and A. Tomasz. 1985. The induction of meningeal inflammation by components of the pneumococcal cell wall. J. Infect. Dis. 151:859-868. [DOI] [PubMed] [Google Scholar]
- 45.Van Vlem, B., R. Vanholder, P. De Paepe, D. Vogelaers, and S. Ringoir. 1996. Immunomodulating effects of antibiotics: literature review. Infection. 24:275-291. [DOI] [PubMed] [Google Scholar]
- 46.Wheeler, A. P., and G. R. Bernard. 1999. Treating patients with severe sepsis. N. Engl. J. Med. 340:207-214. [DOI] [PubMed] [Google Scholar]
- 47.Wu, C. L., M. C. Chan, G. C. Chang, G. C. Lee, C. S. Chin, K. M. Chang, and J. Y. Hsu. 2006. Aetiology and cytokine expression in patients requiring mechanical ventilation due to severe community-acquired pneumonia. J. Formos. Med. Assoc. 105:49-55. [DOI] [PubMed] [Google Scholar]
- 48.Yoshimura, T., C. Kurita, E. Usami, T. Nakao, S. Watanabe, J. Kobayashi, F. Yamazaki, and H. Nagai. 1996. Immunomodulatory action of levofloxacin on cytokine production by human peripheral blood mononuclear cells. Chemotherapy 42:459-464. [DOI] [PubMed] [Google Scholar]