Abstract
All 198 Salmonella isolates (58.6% of isolates were resistant to tetracycline), 92 Vibrio isolates (4.4% of isolates were resistant to tetracycline), and 200 of 201 Aeromonas isolates (39.3% of isolates were resistant to tetracycline; 1 A. caviae isolate had a tigecycline MIC of 4 μg/ml) in our study were susceptible to tigecycline, by U. S. Food and Drug Administration criteria for Enterobacteriaceae.
Salmonella and Vibrio species are commonly associated with food-borne disease, bacteremia, peritonitis, and soft tissue infections (4, 6, 9). Wound infection and bacteremia caused by V. vulnificus are associated with mortality rates as high as 55% (4, 9). Strains of Aeromonas species could also cause fatal soft tissue infection in immunocompromised hosts and especially in cirrhotic patients (10, 11, 14). Traditionally, cefotaxime, ceftriaxone, and fluoroquinolones were active against V. parahaemolyticus and V. vulnificus infection (14). However, emerging resistance among Aeromonas and Salmonella species has been reported recently (15).
Tigecycline, a novel glycylcycline antimicrobial agent, has an expanded spectrum of antimicrobial activities against various clinically important pathogens including most members of the family Enterobacteriaceae (8, 18). Except for tigecycline activity against Salmonella species, the in vitro activities of tigecycline against Aeromonas and Vibrio species have not been previously reported (16).
A total of 491 nonduplicated (one isolate per patient) isolates were included in the current study (Table 1). These isolates were recovered from various clinical specimens, including blood (58%), stool (17%), wound pus (15%), and abscess fluid (10%). These organisms were identified by conventional methods (8, 10, 13). All of these isolates were collected from patients between January 2001 and December 2006 in National Taiwan University Hospital, a 2,200-bed tertiary referral hospital in northern Taiwan.
TABLE 1.
Distribution of MICs of tigecycline for 491 isolates of Aeromonas, Salmonella, and Vibrio speciesa
| Bacteria (no. isolates tested) | No. of isolates susceptible to each indicated MIC (accumulated %)
|
% of susceptibility (FDA/EUCAST)b
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | Susceptible | Intermediate | Resistant | |
| Aeromonas spp. (total, 201) | ||||||||||||
| A. hydrophila (81) | 0 (0) | 2 (1) | 7 (11) | 50 (73) | 14 (90) | 7 (99) | 1 (100) | 0 (100) | 0 (100) | 100/98.8 | 0/1.2 | 0/0 |
| A. caviae (63) | 0 (0) | 0 (0) | 2 (3) | 34 (57) | 22 (92) | 3 (97) | 1 (98) | 1 (100) | 0 (100) | 98.4/96.8 | 1.6/1.6 | 0/1.6 |
| A. sobria (57) | 0 (0) | 0 (0) | 16 (28) | 28 (77) | 11 (96) | 2 (100) | 0 (100) | 0 (100) | 0 (100) | 100/100 | 0/0 | 0/0 |
| Salmonella spp. (total, 198) | ||||||||||||
| S. serotype Choleraesuis (63) | 0 (0) | 0 (0) | 1 (2) | 4 (8) | 39 (70) | 18 (98) | 1 (100) | 0 (100) | 0 (100) | 100/98.4 | 0/1.6 | 0/0 |
| S. serotype Typhimurium (63) | 0 (0) | 0 (0) | 0 (0) | 29 (46) | 30 (94) | 3 (98) | 1 (100) | 0 (100) | 0 (100) | 100/98.4 | 0/1.6 | 0/0 |
| Salmonella serogroup O7 (13) | 0 (0) | 0 (0) | 0 (0) | 5 (38) | 8 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 100/100 | 0/0 | 0/0 |
| Salmonella serogroup O8 (14) | 0 (0) | 0 (0) | 0 (0) | 7 (50) | 7 (60) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 100/100 | 0/0 | 0/0 |
| Salmonella serogroup O9 (45) | 0 (0) | 0 (0) | 1 (2) | 11 (27) | 30 (93) | 3 (100) | 0 (100) | 0 (100) | 0 (100) | 100/100 | 0/0 | 0/0 |
| Vibrio spp. (total, 92) | ||||||||||||
| V. vulnificus (15) | 14 (93) | 0 (93) | 1 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 100/100 | 0/0 | 0/0 |
| V. parahaemolyticus (41) | 0 (0) | 22 (54) | 19 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 100/100 | 0/0 | 0/0 |
| V. cholerae non-O1 (26) | 10 (38) | 15 (96) | 1 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 100/100 | 0/0 | 0/0 |
| Other Vibrio species (10) | 1 (11) | 4 (56) | 5 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | 100 | 0 | 0 |
MICs (μg/ml) of tigecycline for Aeromonas, Salmonella, and Vibrio species were determined by the broth microdilution method.
Category MIC values were derived from U. S. FDA and EUCAST breakpoints.
Susceptibility test results for cefotaxime and ciprofloxacin were obtained from routine laboratory reports generated by the disk diffusion method according to Clinical and Laboratory Standards Institute (CLSI) guidelines (5). MICs of tigecycline and tetracycline were determined by the broth microdilution method (5). The range of antibiotic concentrations tested was 0.03 μg/ml to 128 μg/ml. Interpretation of tigecycline MIC results was determined according to the recommendations of the United States Food and Drug Administration (U. S. FDA) given in the package insert for treating Enterobacteriaceae (susceptible, ≤2 μg/ml; resistant, ≥8 μg/ml) and those recommended by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (susceptible, ≤1 μg/ml; resistant, >2 μg/ml) (7). Escherichia coli ATCC 25922 was used as the control strain in each test.
All isolates of Salmonella species were susceptible to cefotaxime. One isolate of V. cholerae (non-O1 serotype) was intermediate to cefotaxime. The susceptibility rates of A. hydrophila, A. caviae, and A. sobria to cefotaxime were 79%, 73%, and 95%, respectively. All isolates of Vibrio species were susceptible to ciprofloxacin. The susceptibility rates of A. hydrophila, A. caviae, and A. sobria to ciprofloxacin were 90%, 86%, and 93%, respectively. Isolates of the Salmonella species had lower rates of susceptibility to ciprofloxacin (22% for S. enterica serotype Choleraesuis, 75% for S. enterica serotype Typhimurium, 77% for Salmonella serogroup O7, and 57% for Salmonella serogroup O8) than isolates of Aeromonas species, except for Salmonella serogroup O9 (96%).
The tigecycline MICs for the control strain were within the recommended range. All Vibrio and Salmonella isolates tested were susceptible to tigecycline, and one isolate (1.6%) of A. caviae was intermediate (MIC of 4 μg/ml) to tigecycline by U. S. FDA criteria (Table 1). According to EUCAST criteria, 1.2% of A. hydrophila isolates, 3.2% of A. caviae isolates, 1.6% of Salmonella serotype Typhimurium isolates, and 1.6% of Salmonella serotype Choleraesuis isolates were not susceptible to tigecycline (Table 1).
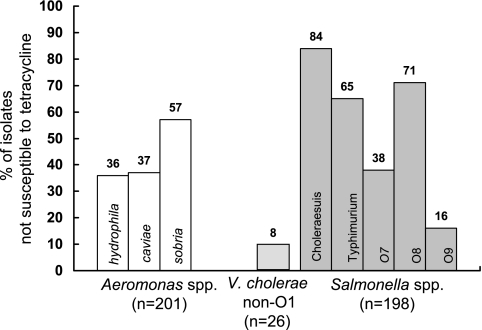
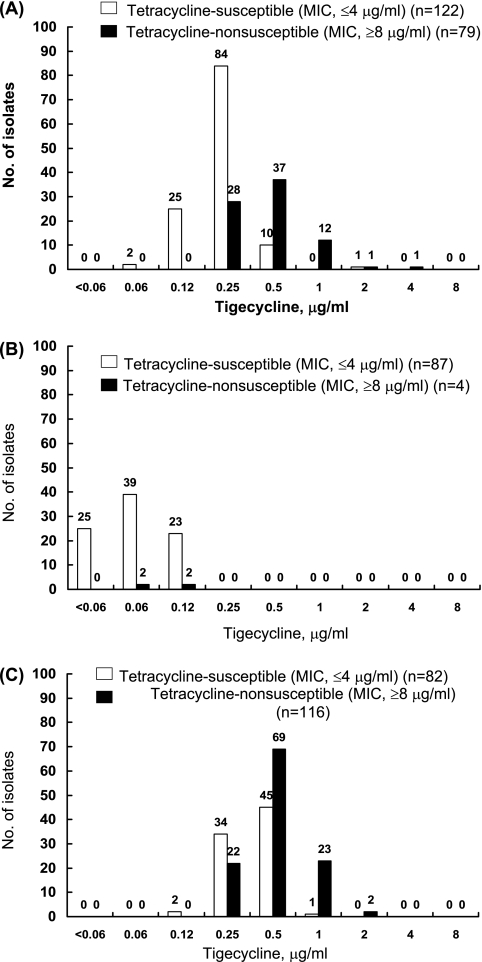
As shown in Fig. 1, four isolates (4.4%) of Vibrio species (tetracycline MIC range, 0.12 to 16 μg/ml; MIC90 of 1 μg/ml), 79 isolates (39.3%) of Aeromonas species (tetracycline MIC range, 0.25 to >128 μg/ml; MIC90 of 16 μg/ml), and 116 isolates (58.6%) of Salmonella species (tetracycline MIC range, 1 to >128 μg/ml; MIC90 of >128 μg/ml) were not susceptible to tetracycline by CLSI criteria (MICs of ≥8 μg/ml) (5). The MIC90s of tigecycline for tetracycline-nonsusceptible isolates of Aeromonas species and Salmonella species (1 μg/ml and 1 μg/ml, respectively) were two- or fourfold higher than those for tetracycline-susceptible isolates (0.25 μg/ml and 0.5 μg/ml, respectively) (Fig. 2). The tigecycline-intermediate A. caviae isolate was also resistant to cefotaxime and ciprofloxacin and exhibited a tetracycline MIC of >128 μg/ml.
FIG. 1.
Rates of isolates of Aeromonas, Vibrio, and Salmonella species that are not susceptible to tetracycline, as determined by the broth microdilution method.
FIG. 2.
Distribution of MICs of tigecycline among Aeromonas (A), Vibrio (B), and Salmonella (C) isolates based on their susceptibilities to tetracycline.
This study showed that tigecycline had excellent in vitro activity against clinical isolates of Aeromonas, Vibrio, and nontyphoid Salmonella species (NTS). Chuang et al. reported a synergistic effect between cefotaxime and minocycline against V. vulnificus isolates (2, 3). Due to the low MICs of tigecycline among isolates of V. vulnificus and V. parahaemolyticus and the high concentration of tigecycline in treating skin and soft tissue infections, this agent alone or in combination may be a promising option for the treatment of infections due to Vibrio species (1).
Antimicrobial susceptibilities of Aeromonas species varied with different geographic areas and different species. In a study from North America, A. hydrophila was more resistant to cephalosporins and tetracycline than either A. caviae or A. sobria (13, 17). In Taiwan, Ko et al. (12) reported that the resistance rate to tetracycline was 48% for A. hydrophila, 58% for A. sobria, and 41% for A. caviae, rates which are much higher than in other areas. In the present study, 28.4% (46/210) of all Aeromonas isolates tested were not susceptible to cefotaxime, but one cefotaxime- and ciprofloxacin-resistant A. caviae isolate, which was highly resistant to tetracycline, was intermediate to tigecycline.
About 5% of NTS may cause invasive or systemic infections and require antimicrobial therapy (19, 22). Resistance to extended-spectrum cephalosporins among NTS has been reported globally since these isolates were recognized in the 1980s (15). In Taiwan, the ceftriaxone resistance rate among NTS isolates ranged from 0.8% to 2.1% in different serogroups (21, 22). Morosini et al. reported that five isolates of extended-spectrum β-lactamase-producing Salmonella species were susceptible to tigecycline (16). All of the clinical isolates of Salmonella species in the present study were fully susceptible to both cefotaxime and tigecycline.
The limitation of this study is that all the isolates tested were identified by conventional biochemical and serological methods. Molecular methods are more reliable than conventional methods for species identification, particularly for the identification of Aeromonas and Vibrio species.
In conclusion, the promising antimicrobial activities of tigecycline against Aeromonas, Vibrio, and Salmonella isolates suggest the need for further in vivo trials to determine if treatment with this agent could provide a better clinical response than responses to currently available treatment options.
Footnotes
Published ahead of print on 12 May 2008.
REFERENCES
- 1.Breedt, J., J. Teras, J. Gardovskis, F. J. Maritz, T. Vaasna, D. P. Ross, M. Gioud-Paquet, N. Dartois, E. J. Ellis-Grosse, and E. Loh for the Tigecycline 305 cSSSI Study Group. 2005. Safety and efficacy of tigecycline in treatment of skin and skin structure infections: results of a double-blind phase 3 comparison study with vancomycin-aztreonam. Antimicrob. Agents Chemother. 49:4658-4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chuang, Y.-C., W.-C. Ko, S.-T. Wang, J.-W. Liu, C.-F. Kuo, J.-J. Wu, and K.-Y. Huang. 1998. Minocycline and cefotaxime in the treatment of experimental murine Vibrio vulnificus infection. Antimicrob. Agents Chemother. 42:1319-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chuang, Y.-C., J.-W. Liu, W.-C. Ko, K. Y. Lin, J.-J. Wu, and K.-Y. Huang. 1997. In vitro synergism between cefotaxime and minocycline against Vibrio vulnificus. Antimicrob. Agents Chemother. 41:2214-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chuang, Y. C., C. Y. Yuan, C. Y. Liu, C. K. Lan, and A. H. Huang. 1992. Vibrio vulnificus infection in Taiwan: report of 28 cases and review of clinical manifestations and treatment. Clin. Infect. Dis. 15:271-276. [DOI] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing; 16th informational supplement. CLSI document M100-S16. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Cohen, J. I., J. A. Bartlett, and G. R. Corey. 1987. Extra-intestinal manifestations of salmonella infections. Medicine (Baltimore) 66:349-388. [DOI] [PubMed] [Google Scholar]
- 7.European Committee on Antimicrobial Susceptibility Testing (EUCAST) Steering Committee. 2006. EUCAST technical note on tigecycline. Clin. Microbiol. Infect. 12:1147-1149. [DOI] [PubMed] [Google Scholar]
- 8.Fritsche, T. R., R. A. Strabala, H. S. Sader, M. J. Dowzicky, and R. N. Jones. 2005. Activity of tigecycline tested against a global collection of Enterobacteriaceae, including tetracycline-resistant isolates. Diagn. Microbiol. Infect. Dis. 52:209-213. [DOI] [PubMed] [Google Scholar]
- 9.Hsueh, P. R., C. Y. Lin, H. J. Tang, H. C. Lee, J. W. Liu, Y. C. Liu, and Y. C. Chuang. 2004. Vibrio vulnificus in Taiwan. Emerg. Infect. Dis. 10:1363-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko, W. C., and Y. C. Chuang. 1995. Aeromonas bacteremia: review of 59 episodes. Clin. Infect. Dis. 20:1298-1304. [DOI] [PubMed] [Google Scholar]
- 11.Ko, W. C., H. C. Lee, Y. C. Chuang, C. C. Liu, and J. J. Wu. 2000. Clinical features and therapeutic implications of 104 episodes of monomicrobial Aeromonas bacteraemia. J. Infect. 40:267-273. [DOI] [PubMed] [Google Scholar]
- 12.Ko, W.-C., K. W. Yu, C. Y. Liu, C. T. Huang, H. S. Leu, and Y.-C. Chuang. 1996. Increasing antibiotic resistance in clinical isolates of Aeromonas strains in Taiwan. Antimicrob. Agents Chemother. 40:1260-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koehler, J. M., and L. R. Ashdown. 1993. In vitro susceptibilities of tropical strains of Aeromonas species from Queensland, Australia, to 22 antimicrobial agents. Antimicrob. Agents Chemother. 37:905-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu, J. W., I. K. Lee, H. J. Tang, W. C. Ko, H. C. Lee, Y. C. Liu, P. R. Hsueh, and Y. C. Chuang. 2006. Prognostic factors and antibiotics in Vibrio vulnificus septicemia. Arch. Intern. Med. 166:2117-2123. [DOI] [PubMed] [Google Scholar]
- 15.Miriagou, V., P. T. Tassios, N. J. Legakis, and L. S. Tzouvelekis. 2004. Expanded-spectrum cephalosporin resistance in non-typhoid Salmonella. Int. J. Antimicrob. Agents 23:547-555. [DOI] [PubMed] [Google Scholar]
- 16.Morosini, M. I., M. García-Castillo, T. M. Coque, A. Valverde, A. Novais, E. Loza, F. Baquero, and R. Cantón. 2006. Antibiotic coresistance in extended-spectrum-β-lactamase-producing Enterobacteriaceae and in vitro activity of tigecycline. Antimicrob. Agents Chemother. 50:2695-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motyl, M. R., G. McKinley, and J. M. Janda. 1985. In vitro susceptibilities of Aeromonas hydrophila, Aeromonas sobria, and Aeromonas caviae to 22 antimicrobial agents. Antimicrob. Agents Chemother. 28:151-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sacchidanand, S., R. L. Penn, J. M. Embil, M. E. Campos, D. Curcio, E. Ellis-Grosse, E. Loh, and G. Rose. 2005. Efficacy and safety of tigecycline monotherapy compared with vancomycin plus aztreonam in patients with complicated skin and skin structure infections: results from a phase 3, randomized, double-blind trial. Int. J. Infect. Dis. 9:251-261. [DOI] [PubMed] [Google Scholar]
- 19.Stein, G. E., and W. A. Craig. 2006. Tigecycline: a critical analysis. Clin. Infect. Dis. 43:518-524. [DOI] [PubMed] [Google Scholar]
- 20.Su, H. P., S. I. Chiu, J. L. Tsai, C. L. Lee, and T. M. Pan. 2005. Bacterial food-borne illness outbreaks in northern Taiwan, 1995-2001. J. Infect. Chemother. 11:146-151. [DOI] [PubMed] [Google Scholar]
- 21.Su, L. H., C. H. Chiu, C. Chu, and J. T. Ou. 2004. Antimicrobial resistance in nontyphoid Salmonella serotypes: a global challenge. Clin. Infect. Dis. 39:546-551. [DOI] [PubMed] [Google Scholar]
- 22.Su, L. H., T. L. Wu, J. H. Chia, C. Chu, A. J. Kuo, and C. H. Chiu. 2005. Increasing ceftriaxone resistance in Salmonella isolates from a university hospital in Taiwan. J. Antimicrob. Chemother. 55:846-852. [DOI] [PubMed] [Google Scholar]