Abstract
The objective of this study was to assess the impact of impaired renal function on the pharmacokinetics of tomopenem (RO4908463/CS-023), a novel carbapenem antibiotic, and its major metabolite in humans. Thirty-two subjects were enrolled in an open-label, two-center study. Subjects were evenly assigned to one of four groups, based on creatinine clearance ranges of ≥80, 50 to 79, 30 to 49, and <30 ml/min. The drug was given as a single 1,500-mg constant-rate intravenous infusion over 60 min. There were no safety concerns with increasing renal dysfunction. Renal impairment had a significant impact on exposure of both tomopenem and its metabolite. Mean (± standard deviation) areas under the curve for tomopenem increased with decreasing renal function, from 191 ± 35.2 to 1,037 ± 238 μg·h/ml. The maximum concentration of drug in plasma (Cmax) increased with a maximum difference of 44% between the severe and normal groups. In contrast, the corresponding increase in Cmax of the metabolite was much higher, at 174%. Total body clearance was linearly correlated with creatinine clearance (R2 = 0.97; P < 0.0001). Renal clearance for tomopenem decreased with increasing severity of disease, with mean values decreasing from 4.63 ± 0.89 to 0.59 ± 0.19 liters/h. The results of this study indicated a strong correlation between the creatinine clearance and total clearance of tomopenem. While renal impairment appeared to have a significant effect on the pharmacokinetics of tomopenem, an even greater effect was seen on the elimination of the inactive metabolite.
Despite the widespread availability of effective antibiotics, the continued emergence and spread of bacteria resistant to existing agents highlight the need for the development of new, safe, and effective antibiotic therapies.
The carbapenem antibiotics, i.e., imipenem, meropenem, and more recently, ertapenem, are currently used for the treatment of moderate to severe infections in hospitalized patients (8, 11, 14). These agents have potent broad-spectrum antibacterial activity, covering a wide range of gram-positive and gram-negative pathogens, including commonly encountered anaerobes (13). While imipenem is associated with central nervous system (CNS) toxicity, meropenem is safely used for the treatment of meningitis in children (1).
The carbapenems are primarily excreted by the kidney, and consequently, their pharmacokinetics are influenced by the extent of renal function (2). The pharmacokinetics of meropenem in the most severely renally impaired individuals (who were not candidates for dialysis) revealed that estimates of exposure (area under the curve [AUC]) to a single 500-mg dose rose approximately 10-fold in comparison with those for healthy volunteers. Dose adjustment is therefore recommended for meropenem and other carbapenems in the case of renal impairment (2, 9).
Tomopenem is a novel 1β-methyl carbapenem (Fig. 1). In common with other carbapenems, tomopenem inhibits the activity of penicillin binding proteins and disrupts bacterial cell wall peptidoglycan biosynthesis. Tomopenem has demonstrated potent broad-spectrum in vitro bactericidal activity against beta-lactam-susceptible and -resistant target species (6). Tomopenem has also demonstrated in vitro activity against significant drug-resistant pathogens, including methicillin-resistant Staphylococcus aureus, ceftazidime-resistant Pseudomonas aeruginosa, and expanded-spectrum cephalosporin-resistant Enterobacteriaceae (10, 19). Similar to other carbapenems, tomopenem demonstrates a very low tendency for the spontaneous emergence of resistance (7). Tomopenem proved efficacious in several infection models in mice, including local, systemic, pneumonia, and urinary tract infection models (12). Together, the data support the potential of tomopenem to treat similar infections in humans.
FIG. 1.
Molecular structures of tomopenem (A) and its metabolite (RO4958463) (B).
Previous studies with other carbapenems have shown that some of the analogs have neurotoxic potential. Effects of tomopenem on the CNS were investigated in a panel of comparative studies with rats and mice (internal reports [not published]). CNS toxicity of tomopenem was less than that of cefazolin and <1/10 that of imipenem. No seizure activity, neurotoxicity, or mortality was noted in mice (or rats) at doses of up to 600 mg/kg of body weight, given intravenously (i.v.). Additionally, no proconvulsant activity was observed with tomopenem in rats at doses resulting in exposures of up to 7,750 μg/ml (internal report [not published]).
In a previous clinical trial (15) with healthy volunteers, tomopenem was well tolerated after single i.v. doses of 50 to 2,100 mg. Pharmacokinetic analysis showed that the drug had a half-life (t1/2) of 1.7 h, a clearance (CL) of 8.1 liters/h, and a volume of distribution of 17 liters. It was primarily (59%) eliminated unchanged in the urine. It has a high free fraction in human plasma, with plasma protein binding of 8.9%. Based on its chemical class and significant renal clearance (CLR), it is anticipated that renal function will impact the pharmacokinetics of this drug and its major, inactive metabolite, RO4957463. It is therefore necessary to quantify the magnitude of this effect so that dose adjustments can be made for renally impaired patients, if appropriate.
(Part of this work was presented at the 2007 AAPS Annual Meeting and Exposition, San Diego, CA.)
MATERIALS AND METHODS
Subjects.
A total of 32 eligible male and female subjects were enrolled from two centers. They were assigned, on the basis of renal function, to one of four groups of eight subjects, and all were given active drug. The active drug (tomopenem) was given as a constant-rate 1,500-mg i.v. infusion over 60 min. Twenty-four subjects had various degrees of impaired renal function, as determined by their creatinine clearance rates (CLCR). Renal function was defined as normal or mildly, moderately, or severely impaired, based on CLCR ranges of ≥80 (group A), 50 to 79 (group B), 30 to 49 (group C), and ≤30 ml/min (group D), respectively, based on CHMP guidance (4). Subjects were classified into one of these four groups (A through D) based on the CLCR estimated at screening, using the Cockcroft-Gault equation (3). All enrolled subjects gave informed consent and were willing to comply with study procedures. Subjects were excluded from the study if they were women of childbearing potential and men with partners of childbearing potential who would not use barrier contraception during the study. Subjects who were on hemodialysis or peritoneal dialysis or who had nephrotic syndrome were also excluded. Subjects were allowed to remain on previously stabilized concomitant medication.
Study design.
This study was an open-label, single-dose study of male and female subjects with various degrees of renal function. After written informed consent was obtained, subjects were given a single dose of 1,500 mg of tomopenem as an i.v. infusion. The pharmacokinetics of tomopenem and its major metabolite were assessed for up to 48 h postdosing. A follow-up visit was performed 7 to 9 days after dosing. The study was conducted at the following sites: Roche Clinical Pharmacology Unit, Welwyn, United Kingdom, and the Christchurch Clinical Studies Trust, Christchurch, New Zealand.
The study was approved by the local institutional review board of each site and conducted according to the principles outlined in the Guideline for Good Clinical Practice (5a), which has its basis in the principles of the Declaration of Helsinki (1996) and the principles of good clinical practice, as outlined in the current version of 21 CFR, subchapter D, parts 312, 50, and 56.
Pharmacokinetic sampling.
Blood samples (5 ml) were taken for the assay of tomopenem and its major metabolite (RO4957463). Samples were collected predosing and at 1, 1.5, 2, 3, 4, 6, 9, 12, 15, 24, 28, 34, and 48 h postdosing. Plasma samples were mixed with an equal volume of 0.5 M N-morpholinepropanesulfonic acid (MOPS) buffer (pH 6.0) to stabilize the drug.
Urine was collected at the following intervals: 0 to 6, 6 to 12, and 12 to 24 h postdosing. Urine volume and pH were measured, and approximately 4 ml of urine was transferred to a polypropylene tube containing 4 ml of 0.5 M MOPS buffer.
All samples were kept protected from light during handling and frozen at −70°C to prevent degradation of the drug to its ring-opened metabolite.
Bioanalytical methods.
The concentrations of tomopenem and its metabolite, RO4957463, in human plasma or urine (prebuffered with 0.5 M MOPS) were determined by using a selective, accurate, reproducible, and validated bioanalytical method (Advion BioServices, Inc., Ithaca, NY). MOPS was added to prevent in vitro degradation of tomopenem. Isotopic internal standards were used for both analytes. The analytes were extracted from plasma or urine by protein precipitation. Reconstituted samples were analyzed by liquid chromatography-tandem mass spectrometry in the positive mode. The lower limits of quantitation for tomopenem and its metabolite were 0.2 μg/ml for plasma and 2 μg/ml for urine, using aliquots of 50 μl and 25 μl, respectively, for analysis.
Pharmacokinetic analysis.
Pharmacokinetic parameters for tomopenem and its major metabolite were estimated by the noncompartmental method, using WinNonlin Enterprise, version 5.0.1. The actual sampling times recorded on the case report form were used to calculate individual pharmacokinetic parameters (maximum concentration of drug in plasma [Cmax], time to Cmax [Tmax], AUC∞, t1/2, volume of distribution at steady state [Vss], and CL). Nominal times were used for graphical presentation and descriptive pharmacokinetic summation. Prior to estimation of pharmacokinetic parameters, plasma concentrations that were below the limit of quantitation for the assay were assigned a value of zero if they occurred prior to the first measurable concentration in the profile and were treated as missing and eliminated from the analysis if they occurred during the terminal phase.
The Cmax and Tmax were taken from the raw data. The t1/2 was calculated as ln 2/λz, calculated by ordinary least-squares linear regression, using at least three points in the terminal part of the log concentration-time curve. The software was allowed to automatically select the terminal elimination phase for estimation of t1/2, with a visual examination of each individual profile to ensure proper selection. CLR was calculated as Ae24/AUC24, where Ae24 is the amount excreted in urine and AUC24 is the plasma AUC over 24 h. The fraction of metabolite exposure to parent was calculated as AUC∞(metabolite)/AUC∞(parent).
CLCR was estimated individually at screening by use of the Cockcroft-Gault equation (3).
Statistical analysis.
The primary parameter for investigation of the effect of renal impairment on the pharmacokinetics of tomopenem and its major metabolite was the total body CL, calculated as dose divided by AUC∞. Evaluation of the relationship between CLCR and drug CL was based on linear regression analysis.
RESULTS
Subjects.
All subjects were aged between 19 and 74 years, with the mean age increasing across the four treatment groups as follows: 39 (group 1; normal), 56 (group 2; mild), 57 (group 3; moderate), and 64 (group 4; severe) years. The majority of subjects were Caucasian (n = 28 [88%]). Heights, weights, and body mass indexes (BMIs) were generally normally distributed. The mean BMI ranged from 26.2 to 31.8 kg/m2. Additional demographic details can be found in Table 1.
TABLE 1.
Summary of demographic data by trial treatment
| Parameter | Value for group
|
|||
|---|---|---|---|---|
| A (CLCR, ≥80 ml/min) | B (CLCR = 50-79 ml/min) | C (CLCR = 30-49 ml/min) | D (CLCR, <30 ml/min) | |
| Total no. of subjects (n) | 8 | 8 | 8 | 8 |
| No. of males | 6 | 5 | 5 | 3 |
| No. of females | 2 | 3 | 3 | 5 |
| Mean (range) age (yr) | 39.1 (19-70) | 55.9 (21-73) | 56.8 (33-74) | 63.6 (49-72) |
| Mean (range) wt (kg) | 77.16 (66.0-90.0) | 72.9 (51.1-96.6) | 83.23 (62.9-107.0) | 89.84 (60.0-120.0) |
| Mean (range) height (cm) | 171.6 (162-179) | 166.6 (152-179) | 168.6 (157-180) | 167.8 (159-176) |
| Mean (range) BMI | 26.18 (22.8-30.8) | 26.06 (21.6-35.2) | 29.24 (24.4-37.0) | 31.75 (22.2-40.9) |
| Mean (range) CLCR (ml/min) | 121 (87.1-153) | 74.3 (65.2-82) | 46.9 (34.3-59) | 20.6 (16.4-25) |
Bioanalytical analysis.
The precision of the assay was measured by the coefficient of variation (CV) from the analysis of quality control samples. In plasma analysis, CV ranged from 3.9% to 9.9% for tomopenem and from 5.1% to 8.1% for the metabolite, RO4957463. In urine analysis, CV ranged from 5.3% to 11.1% for tomopenem and from 4.3% to 7.1% for RO4957463. The accuracy was measured by relative error from the analysis of the quality control samples. In plasma analysis, the accuracy ranged from 0.2% to 5.2% for tomopenem and from −0.6% to 1.8% for RO4957463. In urine analysis, the accuracy ranged from 1.5% to 7.8% for tomopenem and from 0.6 to 4.3% for RO4957463.
Plasma pharmacokinetics.
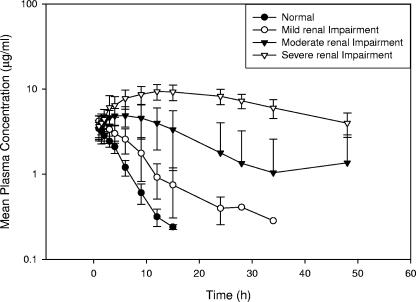
The plasma concentration-time profile of tomopenem and the principal pharmacokinetic parameters after a single dose of tomopenem (1,500 mg) are presented in Table 2 and Fig. 2. The total plasma systemic CL decreased with an increasing degree of renal impairment. CL values were 8.07, 5.08, 3.06, and 1.52 liters/h for the normal, mild, moderate, and severe groups, respectively. AUC∞ increased about fivefold across the groups, as follows: normal, 191 μg·h/ml; mild, 304 μg·h/ml; moderate, 517 μg·h/ml; and severe, 1,037 μg·h/ml. The mean half-life increased with an increasing degree of renal impairment. In subjects with normal renal function, the mean half-life was 2.23 h. This value increased to 3.00, 4.59, and 7.94 h for subjects with mildly, moderately, and severely impaired renal function, respectively. The median Tmax for tomopenem corresponded to the end of the 60-min infusion for all groups, and the volume of distribution remained unchanged. Two subjects (one in group C and one in group D) had Tmax values of greater than the time to the end of infusion. In both cases, no apparent explanation was available, and thus these might reflect inaccuracies in the data. These data are included in the analysis.
TABLE 2.
Pharmacokinetic parameters of tomopenem following single-dose administration of 1,500 mg of tomopenem to subjects with various degrees of renal functiona
| Groupb | Cmax (μg/ml) | Tmax (h) | t1/2 (h) | AUC∞ (μg·h/ml) | Vss (liters) | CL (liters/h) |
|---|---|---|---|---|---|---|
| A (normal) | 81.4 ± 16.9 | 1.00 (0.95-1.03) | 2.23 ± 0.23 | 191 ± 35.2 | 18.8 ± 3.5 | 8.07 ± 1.41 |
| B (mild) | 106 ± 11.2 | 1.00 (0.98-1.00) | 3.00 ± 0.73 | 304 ± 54.5 | 16.2 ± 2.0 | 5.08 ± 0.91 |
| C (moderate) | 100 ± 14.4 | 1.00 (1.00-1.68) | 4.59 ± 1.13 | 517 ± 146 | 17.2 ± 2.7 | 3.06 ± 0.67 |
| D (severe) | 116 ± 38.9 | 1.02 (1.00-3.02) | 7.94 ± 1.23 | 1,037 ± 238 | 16.4 ± 5.0 | 1.52 ± 0.35 |
Data are means ± standard deviations (SD), except for values for Tmax, which are median (range) values.
Each group included eight subjects.
FIG. 2.
Mean plasma concentration-versus-time profiles for tomopenem following a single i.v. dose of 1,500 mg.
As observed with the parent compound, the apparent half-life of the metabolite, RO4957463, increased across the four treatment groups (Fig. 3). Apparent half-life values were 2.98, 5.02, 8.32, and 25.3 h for the normal, mild, moderate, and severe groups, respectively (Table 3). The median Tmax values for RO4957463 were similar for the normal and mild groups and corresponded to the end of infusion. However, the Tmax values for the moderate and severe groups were markedly prolonged. The median values for Tmax were 1.01, 1.00, 5.00, and 13.5 h for the normal, mild, moderate, and severe groups, respectively (Table 3). The ratio of metabolite to parent, estimated as the ratio of AUC∞ values for the two compounds, increased from 10 to 49% over the four groups.
FIG. 3.
Mean plasma concentration-versus-time profiles for RO4957463 following a single i.v. dose of 1,500 mg of tomopenem.
TABLE 3.
Plasma pharmacokinetic parameters of the metabolite, RO4957463, and AUC ratios of metabolite to parent following single-dose administration of 1,500 mg of tomopenem to subjects with various degrees of renal functiona
| Groupb | Cmax (μg/ml) | Tmax (h) | t1/2 (h) | AUC∞(μg·h/ml) | AUCM/P |
|---|---|---|---|---|---|
| A | 3.51 ± 0.81 | 1.01 (1.00-1.50) | 2.98 ± 0.36 | 18.5 ± 3.44 | 0.10 ± 0.01 |
| B | 4.31 ± 0.77 | 1.00 (0.98-1.50) | 5.02 ± 1.68 | 37.3 ± 12.9 | 0.12 ± 0.02 |
| C | 5.24 ± 1.70 | 5.00 (3.00-9.10) | 8.32 ± 3.45 | 115 ± 94.2 | 0.21 ± 0.09 |
| D | 9.63 ± 1.87 | 13.5 (12.0-24.0) | 25.3 ± 12.6 | 490 ± 139 | 0.49 ± 0.16 |
Data are means ± SD, except for Tmax values, which are median (range) values.
Each group included eight subjects.
Urine pharmacokinetics.
Table 4 shows the mean urine pharmacokinetic parameters for tomopenem and its metabolite. The CLR decreased with an increasing degree of renal impairment. Mean values were as follows: 4.63 (normal), 3.68 (mild), 1.98 (moderate), and 0.59 (severe) liters/h. This decrease in CLR was mimicked for the metabolite, RO4957463 (Table 4), with values decreasing from 13.2 to 8.27, 3.91, and 1.01 liters/h, respectively. The percent decrease in CLR was 86% and 92% for the parent compound and the metabolite, respectively. There were no marked differences among the treatment groups in terms of amount and percentage of tomopenem excreted, except in the severe group, where the percentage of drug as a function of dose was 41.2%, relative to 57.4, 72.6, and 62.6% for the normal, mild, and moderate groups, respectively. A similar lack of trend was noted for the metabolite levels in urine, perhaps with the exception of the severely impaired group, where the percentage of RO4957463 excreted decreased to 13.0% from the 16.0 to 18.7% observed for the other groups.
TABLE 4.
Urine pharmacokinetic parameters of tomopenem and its major metabolite following single-dose administration of 1,500 mg of tomopenem to subjects with various degrees of renal functiona
| Parameter | Value for group
|
|||
|---|---|---|---|---|
| A | B | C | D | |
| Tomopenem parameters | ||||
| CLR (liters/h) | 4.63 ± 0.89 | 3.68 ± 0.94 | 1.98 ± 0.77 | 0.59 ± 0.19 |
| Ae (mg) | 862 ± 79.0 | 1,090 ± 220 | 939 ± 265 | 618 ± 270 |
| Ae (% dose) | 57.4 ± 5.3 | 72.6 ± 14.7 | 62.6 ± 17.7 | 41.2 ± 18.0 |
| RO4957463 parameters | ||||
| CLR (liters/h) | 13.2 ± 5.6 | 8.27 ± 2.9 | 3.91 ± 1.8 | 1.01 ± 0.5 |
| Ae (mg) | 240 ± 99.3 | 279 ± 107 | 281 ± 59.7 | 194 ± 120 |
| Ae (% dose) | 16.0 ± 6.62 | 18.6 ± 7.13 | 18.7 ± 3.98 | 13.1 ± 8.01 |
Data are means ± SD (n = 8 for each group).
Figure 4 shows the individual values of total CL and CLCR displayed in a scattergram, using different symbols for the four groups. A fitted line through the origin is shown. The line of the proportionality model that was fitted to the data describes the mean relationship between total CL and CLCR reasonably well over the whole range of the observed values. The estimate of the slope was 1.11 ± 0.034, with a regression value of 0.97 and a P value of <0.0001, showing a strong linear relationship between the total CL and CLCR. A comparison of the change in CLR versus total CL relative to renal function is shown in Fig. 5. A difference in the rates of decline for the two CLs is evident. In addition, the graph shows a very low intercept (close to zero) on the y axis for both regression lines.
FIG. 4.
Relationship between total CL of tomopenem and CLCR following a single i.v. dose of 1,500 mg of tomopenem.
FIG. 5.
Total plasma CL and CLR of tomopenem as a function of CLCR.
Safety results.
Eighteen subjects (56%) experienced a total of 31 adverse events (AEs). There were no serious AEs, and no subjects were withdrawn prematurely due to AEs. There were no severe AEs. The majority of AEs (dizziness, dyspepsia, and flatulence) were of mild intensity. Moderate AEs (inflammation and headache) resolved without sequelae. The most commonly related AE was headache. There were no clinically relevant changes from baseline in any of the laboratory parameters, vital signs, or physical examinations. There were no apparent trends in the number and severity of AEs relative to the degree of renal impairment.
DISCUSSION
This study was an open-label, single-dose study including subjects with various degrees of renal impairment. The pharmacokinetics of tomopenem and its open-ring metabolite, RO4957463, were studied in four groups of eight subjects each to evaluate the effect of impaired renal function on the pharmacokinetics of both compounds. A single dose of 1,500 mg of tomopenem was administered as an i.v. infusion over 60 min. The drug was well tolerated in all subjects. Data analysis revealed that the pharmacokinetics for the healthy subjects were in good agreement with the results of a previous study (15). In the healthy subject group, the compound was largely renally excreted in its unchanged form (57%). The CLR was approximately 60% of the total systemic CL.
The t1/2 of the unchanged tomopenem increased as a function of decreasing renal function. The t1/2 increased from 2.23 h in healthy subjects to 7.94 h in the severely renally impaired group. Total body CL was linearly correlated with CLCR. Both AUC and Cmax increased with decreasing renal function. Since the drug was administered i.v., the effect on AUC was higher than that on Cmax. The AUC∞ increased approximately fivefold, from 191 to 1,037 μg·h/ml, whereas the Cmax increased only 44%, from 81.4 to 116 μg/ml. The volume of distribution was constant across the four groups.
The Tmax was consistent across the dose groups for the parent compound but increased with degree of renal impairment for the metabolite. It is likely that reduced CL of the metabolite contributes to its delayed Tmax.
There was a 44% difference in Cmax values for tomopenem between the severe and normal groups, whereas the increase in Cmax for the metabolite was much higher, at 174%, between the severe and normal groups. The AUC∞ ratio of the metabolite to the parent compound increased with decreasing renal function. The increase in ratio was due primarily to a greater increase in levels of metabolite versus an increase in parent levels, and this was due to renal impairment having an apparently greater effect on the elimination of the metabolite.
In healthy subjects, the unbound CLR (CLR × unbound fraction = 70.3 ml/min) of tomopenem was lower than CLCR (121 ml/min) (Table 1), indicating a high probability that in addition to glomerular filtration, the drug undergoes tubular reabsorption and/or metabolism in the kidney. While tomopenem has much greater stability against dihydropeptidase-1 than other carbapenems do, some substrate affinity does exist. As such, it is possible that hydrolysis of this drug occurs in the brush border membrane of the proximal tubule. It is likely that the lower unbound CLR of tomopenem is a result of metabolism after filtration in the kidney. The CLR of metabolite in healthy subjects was apparently greater than the glomerular filtration rate. This could be a result of metabolism of tomopenem to the metabolite at the brush border membrane. Since hydrolysis could result in a smaller amount of tomopenem in urine, CLR of tomopenem may be underestimated. On the other hand, since the hydrolysis of tomopenem at the brush border membrane in the kidney would result in a larger amount of the metabolite in urine, CLR of the metabolite may be overestimated.
The effect of impaired renal function appeared to be larger for the metabolite than for the parent drug. In this study, the data indicate that the estimated decrease in total CL of the metabolite (18-fold, from 12.9 to 0.7 liters/h; calculated using the assumption that the metabolite is cleared exclusively in the urine [5]) was more significant than that of the parent drug (5-fold). It is possible that the metabolite has an extrarenal component responsible for its clearance which is also affected by the disease state. This might be a reason for the overall increase in metabolite-to-parent ratio. Depending on the dosing regimen, accumulation of the metabolite is possible in patients with reduced renal function. Fortunately, the toxicity profile, in particular the neurotoxic potential of this compound, appears to be less adverse than that for other analogs in this class.
It is known that t1/2 values for imipenem and meropenem in humans are similar, at approximately 1 h. The t1/2 of tomopenem in healthy subjects was 2.23 h in this study. A possible explanation is that filtration is largely responsible for the renal excretion of this drug, in contrast to renal filtration and excretion for imipenem and meropenem. Recent in vivo work has shown that tubular secretion in rabbits is not involved in the clearance of tomopenem, in contrast to meropenem (16). In vitro work using cells expressing human organic transporters also supported the idea by showing that tomopenem was not a substrate of these transporters, again in contrast to meropenem (17).
The CLR of metabolite in healthy subjects was greater than the glomerular filtration rate. This could be due to filtration, secretion, and metabolism in the kidney playing a role in the elimination of the metabolite in the urine. Since renal impairment would be expected to influence both filtration and secretion (18), and possibly metabolism, this would explain why renal impairment had a greater effect on the metabolite. In addition, the lack of parallel decline in the total CL and CLR curves (Fig. 5) provides evidence that renal impairment, besides reducing the filtration component of total CL, also results in a decreased capacity of the kidneys to metabolize the drug.
While the CLR of both tomopenem and its metabolite decreased as a function of renal impairment, the total amount of each compound excreted in urine remained relatively constant. The lower recovery of both compounds in the severely renally impaired group could reflect incomplete collection of the drug excreted in the urine over the period of urine collection (24 h).
In conclusion, tomopenem appears to be cleared largely by renal elimination with glomerular filtration. Renal impairment appeared to have a greater effect on the elimination of the metabolite, RO4957463. The mechanism for this increased effect on the metabolite needs to be elucidated further. Based on the significant differences in pharmacokinetics, it is likely that in subjects with moderate and severe renal impairment, some dosing adjustment will be necessary to ensure safe and adequate exposure. A reduction in dose and/or dosing frequency would correspondingly mitigate the possibility of accumulation of the metabolite.
Acknowledgments
Funding for this study was provided by Hoffmann-La Roche, Inc.
We gratefully acknowledge the contributions of Brian Dannemann and Rosemary Petric. We are also grateful to the following colleagues in Daiichi-Sankyo for a critical review of the manuscript: Kazutaka Yoshihara, Naotoshi Yamamura, and Tetsufumi Koga.
Footnotes
Published ahead of print on 28 April 2008.
REFERENCES
- 1.Abi-Hanna, P., and J. Quinn. 1998. Meropenem: a comprehensive review. P&T 23:632-640. [Google Scholar]
- 2.Chimata, M., M. Nagase, Y. Sukuki, M. Shimomura, and S. Kakuta. 1993. Pharmacokinetics of meropenem in patients with various degrees of renal function, including patients with end-stage renal disease. Antimicrob. Agents Chemother. 37:229-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 4.Committee for Medicinal Products for Human Use. June 2004. Note for guidance on the evaluation of the pharmacokinetics of medicinal products in patients with impaired renal function. Committee for Medicinal Products for Human Use, London, United Kingdom.
- 5.Gibaldi, M. 1991. Metabolite kinetics, p. 369-371. In Biopharmaceutics and clinical pharmacokinetics, 4th ed. Lea and Febiger, Malvern, PA.
- 5a.ICH. January 1997. Guideline for good clinical practice. ICH, Geneva, Switzerland.
- 6.Jones, M. E., N. P. Brown, B. Dannemann, and D. F. Sahm. 2006. Bactericidal activity of RO4908463 against beta-lactam-susceptible and resistant target species, abstr. E-0227. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother.
- 7.Jones, M. E., N. P. Brown, B. Dannemann, and D. F. Sahm. 2006. RO4908463 demonstrates a low potential for in vitro selection of resistance in S. aureus, S. pneumoniae, E. coli, E. cloacae, K. pneumoniae, and P. aeruginosa, abstr. C1-0035. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother.
- 8.Kawamoto, I., and S. Ohya. 1998. New aspects in carbapenem antibiotics. Annu. Rev. Sankyo Res. Lab. 50:1-14. [Google Scholar]
- 9.Mouton, J. W., D. J. Touw, A. M. Horrevorts, and A. A. T. M. M. Vinks. 2000. Comparative pharmacokinetics of the carbapenems clinical implications. Clin. Pharmacokinet. 39:185-201. [DOI] [PubMed] [Google Scholar]
- 10.Noel, A. R., K. E. Bowker, and A. P. MacGowan. 2006. The RO4908463 (CS-023) T>MIC antibacterial effect relationship for MRSA established in an in vitro pharmacokinetic model of infection, abstr. A-638. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother.
- 11.Norrby, S. R. 2000. Carbapenems in serious infections: a risk-benefit assessment. Drug Saf. 22:191-194. [DOI] [PubMed] [Google Scholar]
- 12.Ohya, S., T. Fukuoka, H. Kawada, M. Kubota, A. Kitayama, T. Abe, H. Yasuda, and S. Kuwahara. 2000. R-115685, a novel parenteral carbapenem: in vivo antibacterial activity, abstr. F-1232. Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother.
- 13.Pfaller, M. A., and R. N. Jones. 1997. A review of the in vitro activity of meropenem and comparative antimicrobial agents tested against 30,254 aerobic and anaerobic pathogens isolated world wide. Diagn. Microbiol. Infect. Dis. 26:157-163. [DOI] [PubMed] [Google Scholar]
- 14.Shah, P. M., and R. D. Isaacs. 2003. Ertapenem, the first of a new group of carbapenems. J. Antimicrob. Chemother. 52:538-542. [DOI] [PubMed] [Google Scholar]
- 15.Shibayama, T., Y. Matsushita, T. Hirota, T. Ikeda, and S. Kuwahara. 2006. Pharmacokinetics of CS-023 (RO4908463), a novel parenteral carbapenem, in healthy male Caucasian volunteers. Antimicrob. Agents Chemother. 50:4186-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibayama, T., N. Yamamura, Y. Matsushita, T. Tokui, T. Hirota, and T. Ikeda. 2006. Renal handling of CS-023 (RO4908463), a novel parenteral carbapenem antibiotic, in rabbits in comparison with meropenem. Xenobiotica 36:1273-1287. [DOI] [PubMed] [Google Scholar]
- 17.Shibayama, T., D. Sugiyama, E. Kamiyama, T. Tokui, T. Hirota, and T. Ikeda. 2007. Characterization of CS-023 (RO4908463), a novel parenteral carbapenem antibiotic, and meropenem as substrates of human renal transporters. Drug Metab. Pharmacokinet. 22:41-47. [DOI] [PubMed] [Google Scholar]
- 18.Tett, S., C. Kirkpatrick, A. Gross, and A. McLachlan. 2003. Principles and clinical application of assessing alterations in renal elimination pathways. Clin. Pharmacokinet. 42:1193-1211. [DOI] [PubMed] [Google Scholar]
- 19.Thomson, K. S., and E. S. Moland. 2004. CS-023 (R-115685), a novel carbapenem with enhanced in vitro activity against oxacillin-resistant staphylococci and Pseudomonas aeruginosa. J. Antimicrob. Chemother. 54:557-562. [DOI] [PubMed] [Google Scholar]