Abstract
Early diagnosis of invasive pulmonary aspergillosis is problematic in some patient groups due to the lack of rapid, sensitive, specific, and reliable diagnostic tests. Fungal burden and therapeutic efficacy were assessed by survival, quantitative culture (CFU counts), galactomannan enzyme immunoassay (GM-EIA), and quantitative PCR (qPCR) in a new guinea pig model of invasive pulmonary aspergillosis using an aerosol challenge. At 1 day postinfection, qPCR determined that the pulmonary fungal burden was 2 log10 higher than that determined by CFU counting and increased significantly (P < 0.03) over time. In contrast, the tissue burden assessed by CFU counting did not rise over the course of the study. Therapy with the antifungal drug voriconazole produced statistically significant decreases in pulmonary fungal burden, as detected by CFU counting (P < 0.02), qPCR, and GM-EIA (both P < 0.0002). Daily assessment of the progression of fungal infection in serum was performed by qPCR and GM-EIA. GM-EIA demonstrated a statistically significant reduction in the fungal load on days 6 and 7 in voriconazole-treated animals compared to time-matched controls (P < 0.02). Confirmation of fungal tissue burden by two or more methods should provide a more precise account of the burden, allowing improved assessment of diagnostic and therapeutic strategies in invasive pulmonary aspergillosis.
Early diagnosis of invasive pulmonary aspergillosis (IPA), primarily caused by Aspergillus fumigatus, is particularly problematic for immunocompromised patients due to the lack of rapid, sensitive, specific, and reliable diagnostic tests. Despite advances in the development of new antifungal agents, mortality from IPA remains high in this population (11, 16, 19, 21). The Platelia Aspergillus galactomannan enzyme immunoassay (GM-EIA), which detects circulating GM (the major component of the fungal cell wall) in serum, along with standard clinical, radiological, and histological examinations, is currently being used to aid in the diagnosis of IPA. However, the sensitivity and specificity of the GM-EIA for detection of the mold varies and false positives are frequent (8, 18, 24, 31, 32).
Although the GM-EIA is approved for use in serum to quantify circulating antigen in serum, it could be used for quantifying tissue burdens in experimental infections. Other methodologies being used for the assessment of fungal burdens in the laboratory include semiquantitative culture as CFU counting and quantitative PCR (qPCR). Recently, qPCR has been used as a tool for detecting and identifying Aspergillus and other pathogenic fungi in a variety of clinical samples (2, 5, 7, 20). An Aspergillus sp., whether in conidial or filamentous form, will often manifest itself as a single colony on an agar plate. Due to this documented growth characteristic, this may lead to an underestimation of the amount of viable cells as assessed by CFU counting. To date, fungal tissue burden assessment by CFU counting has been highly reproducible and consistent among laboratories (26). However, in comparison to qPCR (2), semiquantitative measurement of Aspergillus cultures may not provide an accurate assessment of tissue burdens, as homogenization of hyphae in infected organ tissues may produce an excessive number of colonies with increased homogenization or an underrepresentation of the true burden with limited homogenization (26). The advantages of molecular methodologies such as qPCR are that they are not culture based, are highly sensitive, and are rapid. However, there is a lack of standardization between laboratories using this method (i.e., there are a variety of DNA extraction procedures, detection platforms, and primers for target DNA), which may prove to be problematic for its approval as a standard diagnostic test such as the GM-EIA.
The performances of these diagnostic tests and their abilities to detect and diagnose IPA have been previously evaluated in vitro or in animal models. These models have been used both to evaluate the treatment of invasive aspergillosis and to investigate the pathogenicity and virulence features of Aspergillus (2, 5, 12, 14, 23, 30). These studies have historically used a variety of host animals, such as the persistently neutropenic rabbit model (3). However, species differences and model design parameters such as the route of infection, as well as the degree and type of immune system suppression, make comparison of in vivo studies difficult (4, 6, 15, 22, 26, 29).
The primary goal of this study was to evaluate and compare three different methodologies for the ability to detect pulmonary fungal burdens in a new animal model of experimental pulmonary invasive aspergillosis at selected time points in the course of infection. For this purpose, an inhalational model of IPA in guinea pigs which closely mimics the route of human infection was developed. Within this framework, our goal was to provide the basis for evaluating genomic and nongenomic targets in order to improve the diagnosis and treatment of IPA, ultimately resulting in improved outcomes for patients with this frequently fatal infection.
MATERIALS AND METHODS
Animals and immunosuppression.
The time line of the studies conducted herein is shown in Fig. 1. Two days prior to infection, 85 male Hartley guinea pigs (0.5 kg; Charles River Laboratories, Wilmington, MA) were made neutropenic with cyclophosphamide (250 mg/kg; Cytoxan; Mead Johnson, Princeton, NJ) given intraperitoneally and further immunosuppressed with cortisone acetate (250 mg/kg; Sigma, St. Louis, MO) given subcutaneously. In addition, ceftazidime (100 mg/kg; Glaxo SmithKline Beecham Pharmaceuticals, Philadelphia, PA) was administered daily throughout the study for prevention of bacterial infections. In order to maintain immunosuppression throughout the study, additional doses of cyclophosphamide (200 mg/kg) and cortisone acetate (250 mg/kg) were administered on day 3 postinfection (28, 29). All animal research procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio.
FIG. 1.
Guinea pig survival after a challenge with nebulized Aspergillus conidia at 108 CFU/ml (day 0). Dashed arrows denote days in the study on which immunosuppressive agents were administered to the animals. Assessment of total white blood cell (WBC) counts (per cubic millimeter) in response to immunosuppression was performed with uninfected controls, and the results are shown below the days indicated by solid arrows.
Preparation of inoculum.
A. fumigatus AF293 was grown on potato dextrose agar (PDA) plates for 10 days at 37°C. Conidia were aseptically dislodged from PDA plates and suspended in sterile 0.9% saline-0.1% Tween 80 (Sigma, St. Louis, MO) as described, filtered for the removal of any hyphal fragments, and concentrated through centrifugation (28). On the day of infection, conidial counts were assessed by hemacytometer and the inoculum was adjusted to obtain a concentration of 108 conidia/ml. Viability testing and confirmation of the inoculum and conidial concentration were performed by plate dilutions. Conidial viability was assessed to be 50 to 70% in these studies.
Inhalational challenge.
As previously described in murine studies of IPA (28, 29), a large-scale aerosol chamber was used to establish our model of IPA through the normal route of infection in the guinea pigs by using an inhalational challenge. A portable, inexpensive acrylic chamber was used to simultaneously infect groups of six to eight guinea pigs per group with a nebulized inoculum of 108 CFU/ml A. fumigatus conidia (the total volume of the inoculum was 12 ml). Animals were exposed to the aerosol mist for 1 h. After an additional hour, two to seven guinea pigs were sacrificed to confirm and assess the average conidial delivery of each run. Guinea pigs were sacrificed by terminal exsanguination after being anesthetized with 44 mg/kg ketamine-HCl and 10 mg/kg xylazine. A cohort of 12 immunosuppressed guinea pigs was left noninfected but underwent the same immunosuppression and placebo therapeutic regimen as the infected cohort. Lungs and serum samples were also obtained from these animals at specified times (1 h and 1, 3, 5, 6, 7, and 8 days) postinfection. Guinea pigs were monitored daily for signs of distress and any obvious signs of illness. Moribund animals were euthanized humanely by approved methods.
Fungal burden assessment.
Fungal burdens in the lungs of 30 guinea pigs were determined by CFU counting, GM-EIA, and qPCR by sacrificing groups of animals at selected time points (1 h and 1, 3, 5, 6, 7, and 8 days) postinfection to monitor the development and spread of infection.
CFU count assessment.
One gram of lung tissue was aseptically removed and homogenized with an overhead RW16 Basic S1 Overhead Stirrer (IKA Works Inc., Wilmington NC) in 9 ml of sterile saline with gentamicin (0.025 g/liter; Gibco BRL, Grand Island, NY) and chloramphenicol (0.4 g/liter; Sigma). Primary homogenate dilutions were quantitatively cultured by serial dilution, plated on PDA plates, and incubated at 37°C for 24 to 36 h, after which A. fumigatus fungal burdens (numbers of CFU per gram of lung tissue) were determined (10).
Assessment of conidial equivalents (CE) by qPCR.
To facilitate and maximize fungal DNA extraction and subsequent qPCR, primary lung homogenates were subjected to a secondary homogenization step with glass beads to aid in the release and extraction of cell nuclei from all of the conidial and hyphal forms present (2). An aliquot of 500 μl of lung homogenate or 300 μl of serum was added to a sterile 2-ml screw-cap centrifuge tube (Sarstedt, Newton, NC) containing 0.5-mm-diameter glass beads (Biospec, Bartlesville, OK), bead beaten (3,200 rpm) with a Biospec Bead Beater homogenizer (Biospec) for 1 min 30 s, and immediately placed on ice. Secondary lung homogenate (100 μl) or serum homogenate (200 μl) was processed for DNA extraction with the QIAamp DNA Mini Kit (Qiagen, Valencia CA) according to the manufacturer's directions. To prevent degradation, extracted fungal DNA was stored at −20°C until use. The amount of fungal cell nuclei in lung tissue (number of CE per gram) was quantified by real-time TaqMan PCR by using the previously reported sequences for the primers AFKS1 (5′-GCCTGGTAGTGAAGCTGAGCGT-3′) and AFKS2 (5′-CGGTGAATGTAGGCATGTTGTCC-3′) and the TaqMan (Applied Biosystems, Foster City, CA) FKS probe (5′-6-FAM-TCACTCTCTACCCCCATGCCCGAGCC-MGB-3′) (7) targeting a 101-bp fragment of the single-copy A. fumigatus FKS gene. For serum samples, fungal cell nuclei were assessed by using the previously reported sequences for the multicopy 18S ribosomal DNA (rDNA) gene (2). The sequences were as follows: forward primer, 5′-GGCCCTTAAATAGCCCGGT-3′; reverse primer, 5′-TGAGCCGATAGTCCCCCTAA-3′; TaqMan (Applied Biosystems, Foster City, CA) 18S rDNA probe, 5′-6-FAM-AGCCAGCGGCCCGCAAATG-MGB-3′. Standards were derived from genomic DNA obtained from A. fumigatus AF293 as previously described (13). All assays were run under the following conditions on an Applied Biosystems 7300 real-time PCR system. The reaction mixture was held at 50°C for 2 min and then at 95°C for 10 min, followed by 40 cycles of 15 s at 95°C and 65°C for 1 min.
GM-EIA assessment.
From the primary lung homogenates, 400-μl samples were aliquoted into a 1.5-ml Eppendorf tube and centrifuged at 2,300 × g for 5 min and the supernatant (approximately 300 μl) was placed into a fresh tube. Lung supernatants and serum were sent to the Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio for GM determination with Platelia GM-EIA kits (Bio-Rad Laboratories, Redmond, WA).
Histological evaluation.
Samples of guinea pig lungs were aseptically removed and fixed in 10% neutral buffered formalin. Paraffin-embedded tissue sections were then sectioned and stained with either periodic acid-Schiff or Grocott-Gomori methenamine-silver stain for microscopic examination of Aspergillus conidia or hyphae. In addition, hematoxylin-eosin stain was also used to evaluate the inflammatory response in the immunosuppressed animals.
Serum samples.
Animals were sedated with ketamine HCl (44 mg/kg; Fort Dodge Laboratories Inc., Fort Dodge, IA), atropine (0.04 mg/kg; Elkin-Sinn, Inc., Cherry Hill, NJ), and xylazine (5 mg/kg; Agriculture Division, Bayer Corporation, Shawnee Mission, KS) (15). Blood was collected on alternate days from the saphenous vein from a total of 20 infected guinea pigs, beginning on day 4 postinfection until termination. Ten of the 20 guinea pigs were made available to be bled each day to decrease the physical stress placed on the animals if they were to be bled on a daily schedule. Approximately 1 ml of blood was collected per animal and placed in serum collection tubes, left overnight at room temperature, and centrifuged at 3,000 × g for 10 min the next day. Determination of the serum fungal load was assessed by either qPCR or GM-EIA.
Antifungal therapy.
Daily antifungal treatment was begun 24 h after infection in 16 guinea pigs with the antifungal azole drug voriconazole (VRC) (Vfend; Pfizer-Roerig, Inc., New York, NY). A stock VRC solution of 10 mg/ml was prepared in sterile water. VRC was administered orally at 10 mg/kg twice a day (BID) for 8 days postinfection. For untreated controls (n = 7), water was used as a placebo and similarly administered.
Statistical analysis.
Statistical analyses were performed with Graph Pad Prism, version 4.0 (Graph Pad Software, Inc., San Diego, CA). A log rank t test for statistical comparisons of survival between animal groups was used to interpret results. A one-way analysis of variance, the Kruskal-Wallis test, and Dunn's multiple-comparison test were also performed for multiple comparisons. Statistical significance was noted at P < 0.05.
RESULTS
Conidia were detected by histopathology in the pulmonary alveolar spaces of the guinea pigs within 1 h postinfection (Fig. 2B). By day 5 postinfection, guinea pig lungs were visibly infected with Aspergillus lesions compared to lungs obtained from uninfected animals. Lung histology showed significant hyphal masses present by day 5 postinfection, when the first death occurred in the model (Fig. 2D to F), and due to the immunosuppressive therapy, no notable infiltration of inflammatory cells in the lungs was observed throughout the study. By day 5 postinfection, the first death occurred, after which all of the animals succumbed to the infection by day 8 (the mean time to death without antifungal treatment was 6.76 ± 0.18 days) (Fig. 1).
FIG. 2.
Histological examination of guinea pig lungs exposed to and infected with aerosolized A. fumigatus 293 at 1 h (A [magnification, ×1,000] and B [magnification, ×2,500], conidia stained with periodic acid-Schiff stain) and 5 days postinfection (D [magnification, ×100] and E [magnification, ×400]), fungal hyphae stained with Grocott-Gomori methenamine-silver stain). To assess inflammatory responses, both samples were also stained with hematoxylin and eosin (C [magnification, ×1,000], F [magnification, ×100]). At 1 h postinfection, Aspergillus conidia were present in the guinea pig alveolar airways, as denoted by black ovals (A and C) and arrows (B).
At 1 h postinfection, the mean pulmonary fungal burden was assessed by semiquantitative culture (log10 5.59 ± 0.04 CFU/g); there was no significant change in the fungal burden 24 h later (Table 1). Although an extensive fungal burden was detected by CFU counting throughout the study, a statistically significant decrease (compared to the 1-h time point) in the fungal burden was demonstrated from day 3 through day 7.
TABLE 1.
Comparison of diagnostic markers at selected time points
| Tissue or fluid and time postinfection (no. of animals) | Fungal burden
|
||
|---|---|---|---|
| No. of CFU/ga | GM indexb | No. of CE/ga | |
| Lung tissue | |||
| 1 h (4) | 5.59 ± 0.04 | BLDc | 5.92 ± 0.13 |
| 1 day (4) | 5.51 ± 0.25 | 7.32 ± 1.09 | 7.70 ± 0.13d |
| 3 days (4) | 5.04 ± 0.16d | 7.32 ± 1.14 | 7.68 ± 0.10d |
| 5 days (4) | 4.62 ± 0.10d | 9.87 ± 1.05 | 8.55 ± 0.23d |
| 6 days (4) | 4.29 ± 0.02d | 10.59 ± 1.13 | 8.73 ± 0.08d |
| 7 days (6) | 4.06 ± 0.06d | 11.25 ± 1.06 | 9.20 ± 0.11d |
| 8 days (4) | 4.33 ± 0.23d | 10.18 ± 1.08 | 9.21 ± 0.48d |
| Serum | |||
| 1 h (7) | NDe | BLD | 1.37 ± 0.11 |
| 1 day (7) | ND | BLD | 2.17 ± 0.17 |
| 2 days (8) | ND | BLD | 2.06 ± 0.20 |
| 3 days (9) | ND | 1.03 ± 1.03 | 1.75 ± 0.18 |
| 4 days (8) | ND | 1.05 ± 1.05 | 1.83 ± 0.13 |
| 5 days (6) | ND | 1.35 ± 1.20 | 1.29 ± 0.26 |
| 6 days (4) | ND | 2.69 ± 1.51 | ND |
| 8 days (5) | ND | 3.06 ± 1.48 | ND |
Mean log10 ± standard error.
Mean index ± standard error.
BLD, below level of detection.
Statistically significant difference (P < 0.03) compared to diagnostic marker assessment at 1 h.
ND, not done.
In comparison to CFU counts, pulmonary GM levels were undetectable at 1 h postinfection (Table 1). The earliest detection of GM in the lungs occurred at day 1 postinfection, and the level appeared to stabilize at subsequent time points, with a total increase of 0.4 log10.
Using an AF293 single-copy gene, FKS-1, in order to obtain a 1:1 ratio of cell nuclei to copy number, we assessed the number of CE present in the lungs. The initial qPCR-based fungal burden counts were within half a log10 of those obtained by semiquantitative culture (at 1 h, 5.92 ± 0.13 versus 5.59 ± 0.04 CFU/g) (Table 1). However, qPCR at day 1 detected CE numbers over 2 logs higher in comparison to CFU counting, and the number of CE increased significantly throughout the duration of the study (P < 0.03). By day 8, qPCR assessed the pulmonary fungal burden as log10 9.21 ± 0.48 CE/g in comparison to the log10 4.33 ± 0.23 CFU/g assessed by semiquantitative culture.
qPCR and GM-EIA were used to detect and further assess serum fungal burdens in the model. As shown in Table 1, the GM-EIA did not detect any significant amount of GM in the sera until day 3 postinfection (0.03 ± 0.03) and the amount increased more than a log throughout the same time period (day 8 = 1.12 ± 0.39). In contrast, qPCR was able to detect Aspergillus DNA in serum as early as 1 h postinfection (Table 1) and throughout the study, with levels gradually leveling off at log10 1.29 ± 0.26 CE/g by day 5. There was no significant increase or decrease in the number of CE detected in the serum compared to the pulmonary data. Finally, disseminated infection in this model did not occur, as evidenced by the lack of a detectable fungal burden in the kidneys, as assessed by semiquantitative culture, GM-EIA, and qPCR. In comparison, disseminated infection, as assessed by the previous assays, did occur in the intravenous aspergillosis model previously used in this laboratory (data not shown).
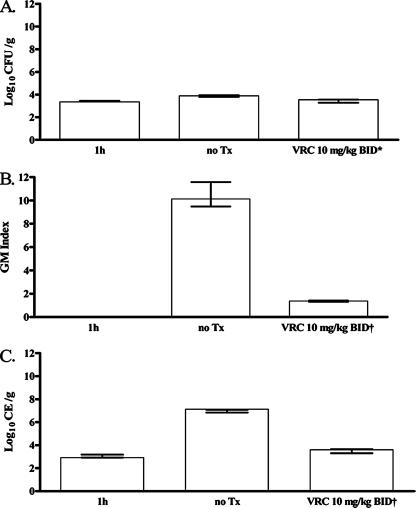
In order to determine the effect of VRC on tissue burden quantification, we evaluated the efficacy of VRC in this model. However, in order to prolong the mean time to death in this particular study, so as to assess antifungal drug effects on survival, 16 guinea pigs were exposed to a lower concentration of nebulized fungal conidia (107 CFU/ml). After infection, guinea pigs were randomly placed into the following three groups: first group, sacrificed 1 h after infection for confirmation of inoculum delivery; second group, untreated controls; third group, animals receiving VRC at 10 mg/kg BID for 8 days. At this lower inoculum, the mean time to death for untreated infected control animals shifted to 8.0 ± 0.50 days, with 12.5% survival overall in the study. In comparison, the daily treatment regimen in infected animals given VRC at 10 mg/kg BID led to a mean time to death of 9.13 ± 0.85 days or 62.5% survival of all treated animals. As shown in Fig. 3, at the time of death, semiquantitative culture was able to detect Aspergillus conidia (log10 3.4 ± 0.04 CFU/g) as early as 1 h postinfection. In addition, there was a statistically significant difference between the fungal burdens of the treated and nontreated groups (P < 0.02, log rank t test).
FIG. 3.
Comparison of pulmonary fungal burdens at 1 h postinfection (n = 4) and at the time of death in guinea pigs undergoing no treatment (n = 5) or treatment (n = 5) with 10-mg/kg BID doses of VRC, as assessed by three different methodologies, i.e., semiquantitative culture (A), GM-EIA (B), and qPCR (C) An asterisk denotes a statistically significant difference compared to untreated controls at P < 0.02; a dagger denotes a statistically significant difference compared to untreated controls at P < 0.0002. Tx, treatment.
qPCR also detected a pulmonary fungal burden at 1 h postinfection, and the amount of fungus closely correlated with that determined by semiquantitative culture (log10 3.05 ± 0.13 compared to log10 3.41 ± 0.03 CFU/g) (Fig. 3C). A statistically significant decrease in the Aspergillus burden in lung tissue at the time of death (day 8) was seen (P < 0.0002) in the VRC group. This decrease in the pulmonary fungal burden was also demonstrated by GM-EIA (Fig. 3B). Although GM-EIA was unable to detect Aspergillus at the 1-h time point, there was a statistically significant drop in measurable GM in lung tissue in the VRC group from day 4 to day 6 (P < 0.0002) (Fig. 3B).
Serial serum samples were also tested for the presence of Aspergillus by GM-EIA and qPCR. In the untreated group, a statistically significant (P < 0.01) increase in GM levels was seen from day 5 to day 7 (mean, log10 0.71 ± 0.08 versus log10 1.90 ± 0.17), in contrast to the VRC treatment group, which maintained low GM levels (index of <0.61) with no significant change over time. Although the average serum fungal burden assessed by qPCR was half a log higher in the untreated group compared to the VRC-treated group from day 4 through day 7 of the study (mean, log10 3.45 ± 0.36 versus log10 2.98 ± 0.22), this was not statistically significant.
DISCUSSION
We examined the utility of a large-scale low-cost acrylic inhalational chamber previously used in a murine model of IPA (26) to establish a reproducible model of IPA in guinea pigs. Previous studies in this laboratory and elsewhere have used guinea pigs in their assessment of a variety of mycoses and therapies, including invasive aspergillosis (1, 4, 5, 6, 15). In addition to rabbits, which have also been used extensively in the field (3, 8, 23), this larger-animal model also provides a greater amount of solid organ tissues and other biological samples per animal compared to mice to investigate various aspects of IPA at a relatively lower cost than rabbits. Serial blood sampling from a single animal is an additional key feature of the model that enhances the monitoring of fungal surrogate markers throughout the course of infection (for example, GM and the 18S rRNA gene), as opposed to the murine model.
Difficulties in the early diagnosis and therapy of IPA led us to compare three different methods of fungal detection in order to assess their abilities to detect pulmonary fungal burdens in our guinea pig model. Other investigators have previously evaluated these diagnostic markers in other animal models (2, 9, 23, 27) or retrospectively in human clinical samples (7, 17, 18, 20). We show, as Sheppard et al. (27) demonstrated, that fungal burdens assessed by qPCR and GM-EIA increased significantly during the progression of infection and that those assessed by semiquantitative culture, after an initial drop, remained relatively stable. The tissue fungal burden assessed by qPCR consistently increased throughout infection, even after the other two diagnostic markers (CFU counts and GM-EIA results) appeared to reach a plateau by day 5. Although semiquantitative culture of lung tissue provides an important measurement of tissue burden, it may underestimate the absolute fungal burden in an established infection compared to qPCR and GM-EIA. Cultures are useful in the demonstration of the viability and characteristics of the organism and to show the extent of infection and/or eradication of the same. However, variations in tissue homogenization could result in CFU counts that may over- or underestimate therapeutic effectiveness. In intralaboratory studies conducted by our group, we demonstrated that the method of tissue disruption used herein resulted in consistently higher CFU counts (26).
GM-EIA assessment of lung tissue burdens showed increased burdens after day 5, which correlated with the extent of infection, as demonstrated by histological examination, as we saw in this study and as others have described (8, 9). However, the delayed (day 3) detection by the GM-EIA in serum could prove problematic for the initiation of effective therapy in the clinical setting. In this study, qPCR, when used for tissue burden measurement, was positive early, correlating with the fungal aerosol delivery, and the burden continued to increase throughout the course of the study, as was expected on the basis of histopathological examination and the rise in levels determined by GM-EIA. Although qPCR and GM-EIA appeared to most closely correlate with the rise in the fungal burden and the extent of infection, there is still debate as to whether the elevated fungal burden as assessed by these two assays in comparison to semiquantitative culture is a result of the presence of cell nuclei or cell wall components from viable and nonviable organisms.
Serial blood sampling in this model allowed us to evaluate the kinetics of serum markers in infection. Although undetectable early in our study, circulating GM antigen rose threefold within 6 days (Table 1.) In contrast, qPCR was able to detect low levels of fungal cell nuclei as early as day 1. However, levels rapidly reached a plateau before becoming undetectable by day 5. Due to the nature of the kinetics of the markers (GM antigen consistently being released by viable and nonviable cells versus cell nuclei, which are cell bound in viable cells), GM assessment in serum remains the most practical and valuable method in the clinical setting. In addition, the lack of externally validated methods remains a limiting factor for the standardization of qPCR for the early diagnosis of IPA. Due to the experimental limitations and cost involved in each methodology, the use of one or more of these methods to provide a more reliable indication of the true fungal burden in IPA must be carefully determined so as to increase therapeutic success rates.
The relevance of the guinea pig model to the clinical setting is demonstrated by our assessment of the antifungal effect of treatment with VRC, which is metabolized similarly in both guinea pigs and humans (1, 25). We have demonstrated in this study that this model has the capability of delivering Aspergillus conidia directly to the lungs, emulating the normal route of infection in IPA. We were able to manipulate the length of survival by using high conidial doses, which led to shorter survival times of infected animals, or by lowering conidial doses, which led to prolonged survival, for our assessment of drug efficacy. Furthermore, we were able to take advantage of serial blood sampling in this model in our evaluation of the effect of therapy on the levels of diagnostic markers detected by both GM-EIA and qPCR in serum.
Previously, in an intravenous aspergillosis guinea pig model used in this laboratory, intravenous challenge led to a disseminated infection producing high fungal burdens in the livers and kidneys of the animals compared to their lungs (15). The intravenous model demonstrated a significantly greater difference between the fungal tissue burdens of untreated control animals and animals treated with the same doses of VRC compared to the present inhalational model due to different organ targets in the models. However, due to the precise targeting of the lungs in the present model, the qPCR and GM response may closely mimic what is seen in human IPA. Therefore, therapeutic data obtained from both models of infection may be of more use in the evaluation of the preclinical antifungal dose response.
These studies demonstrate that this new animal model is amenable to studies that would include the evaluation of novel diagnostics, host response to infection, and organism virulence factors through molecular manipulations; in vivo expression analysis; and the evaluation of therapeutic compounds.
Acknowledgments
This work was supported with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under contract N01-AI-30041. J.R.G. received research support from Pfizer Inc., Schering-Plough Corporation, Merck & Co., and Fujisawa. T.F.P. received research support from Merck & Co., Pfizer Inc., Schering-Plough Corporation, and Nektar Therapeutics.
J.R.G. serves on the speaker's bureaus of Merck & Co. and Schering-Plough Corporation and is a consultant for Merck & Co., Schering-Plough Corporation, Indevus, Vicuron, and Nektar. T.F.P. serves on the speaker's bureaus of Merck & Co. and Pfizer Inc. and is a consultant for Basilea, Merck & Co., Nektar Therapeutics, Pfizer Inc., and Toyoma Pharmaceuticals. A.C.V., W.R.K., L.K.N., R.B., M.C.K., A.W.F., M.L.H., and B.L.W. have no conflicts of interest to declare.
We thank D. I. McCarthy, M. Olivo, Jr., and D. D. Molina for their technical contributions to the completion of this project.
Footnotes
Published ahead of print on 12 May 2008.
REFERENCES
- 1.Andes, D., K. Marchillo, T. Stanstad, and R. Conklin. 2003. In vivo pharmacokinetics and pharmacodynamics of a new triazole, voriconazole, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:3165-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowman, J. C., G. K. Abruzzo, J. W. Anderson, A. M. Flattery, C. J. Gill, V. B. Pikounis, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2001. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob. Agents Chemother. 45:3474-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capilla, J., K. V. Clemons, and D. A. Stevens. 2007. Animal models: an important tool in mycology. Med. Mycol. 45:657-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandrasekar, P. H., J. L. Cutright, and E. K. Manavathu. 2004. Efficacy of voriconazole plus amphotericin B or micafungin in a guinea-pig model of invasive pulmonary aspergillosis. Clin. Microbiol. Infect. 10:925-928. [DOI] [PubMed] [Google Scholar]
- 5.Clemons, K. V., and D. A. Stevens. 2006. Efficacy of micafungin alone or in combination against experimental pulmonary aspergillosis. Med. Mycol. 44:69-73. [DOI] [PubMed] [Google Scholar]
- 6.Clemons, K. V., and D. A. Stevens. 2005. The contribution of animal models of aspergillosis to understanding pathogenesis, therapy and virulence. Med. Mycol. 43(Suppl. 1):S101-S110. [DOI] [PubMed] [Google Scholar]
- 7.Costa, C., D. Vidaud, M. Olivi, E. Bart-Delabesse, M. Vidaud, and S. Bretagne. 2001. Development of two real-time quantitative TaqMan PCR assays to detect circulating Aspergillus fumigatus DNA in serum. J. Microbiol. Methods 44(3):263-269. [DOI] [PubMed] [Google Scholar]
- 8.Francesconi, A., M. Kasai, R. Petraitiene, V. Petraitis, A. M. Kelaher, R. Schaufele, W. W. Hope, Y. R. Shea, J. Bacher, and T. J. Walsh. 2006. Characterization and comparison of galactomannan enzyme immunoassay and quantitative real-time PCR assay for detection of Aspergillus fumigatus in bronchoalveolar lavage fluid from experimental invasive pulmonary aspergillosis. J. Clin. Microbiol. 44:2475-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez-Lopez, A., M. Martin Gomez, P. Martin-Davila, P. Lopez-Onrubia, J. Gavalda, J. Fortun, A. Pahissa, J. Rodriguez-Tudela, and M. Cuenca-Estrella. 2006. Detection of fungal DNA by real time polymerase chain reaction: evaluation of 2 methodologies in experimental pulmonary aspergillosis. Diagn. Microbiol. Infect. Dis. 56:387-393. [DOI] [PubMed] [Google Scholar]
- 10.Graybill, J. R., and S. R. Kaster. 1984. Experimental murine aspergillosis. Comparison of amphotericin B and a new polyene antifungal drug, SCH 28191. Am. Rev. Respir. Dis. 129(2):292-295. [PubMed] [Google Scholar]
- 11.Groll, A. H., M. Kurz, W. Schneider, V. Witt, H. Schmidt, M. Schneider, and D. Schwabe. 1999. Five-year-survey of invasive aspergillosis in a pediatric cancer centre: epidemiology, management and long-term survival. Mycoses 42:431-442. [DOI] [PubMed] [Google Scholar]
- 12.Hachem, R., P. Bahna, H. Hanna, L. C. Stephens, and I. Raad. 2006. EDTA as an adjunct antifungal agent for invasive pulmonary aspergillosis in a rodent model. Antimicrob. Agents Chemother. 50:1823-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin, J., Y. K. Lee, and B. L. Wickes. 2004. Simple chemical extraction method for DNA isolation from Aspergillus fumigatus and other Aspergillus species. J. Clin. Microbiol. 42:4293-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkpatrick, W. R., B. J. Coco, and T. F. Patterson. 2006. Sequential or combination antifungal therapy with voriconazole and liposomal amphotericin B in a guinea pig model of invasive aspergillosis. Antimicrob. Agents Chemother. 50:1567-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirkpatrick, W. R., R. K. McAtee, A. W. Fothergill, M. G. Rinaldi, and T. F. Patterson. 2000. Efficacy of voriconazole in a guinea pig model of disseminated invasive aspergillosis. Antimicrob. Agents Chemother. 44:2865-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin, S., J. Schranz, and S. M. Teutsch. 2001. Aspergillosis case-fatality rate: systematic review of the literature. Clin. Infect. Dis. 32:358-366. [DOI] [PubMed] [Google Scholar]
- 17.Loeffler, J., H. Hebart, U. Brauchle, U. Schumacher, and H. Einsle. 2000. Comparison between plasma and whole blood specimens for detection of Aspergillus DNA by PCR. J. Clin. Microbiol. 38:3830-3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maertens J., J. Verhaegen, K. Lagrou, J. van Eldere, and M. Boogaerts. 2001. Screening for circulating galactomannan as a noninvasive diagnostic tool for invasive aspergillosis in prolonged neutropenic patients and stem cell transplantation recipients: a prospective validation. Blood 97:1604-1610. [DOI] [PubMed] [Google Scholar]
- 19.Marr, K. A., T. F. Patterson, and D. Denning. 2002. Aspergillosis: pathogenesis, clinical manifestations, and therapy. Infect. Dis. Clin. N. Am. 16:875-894. [DOI] [PubMed] [Google Scholar]
- 20.Musher, B., D. Fredricks, W. Leisenring, S. A. Balajee, C. Smith, and K. A. Marr. 2004. Aspergillus galactomannan enzyme immunoassay and quantitative PCR for diagnosis of invasive aspergillosis with bronchoalveolar lavage fluid. J. Clin. Microbiol. 42:5517-5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patterson, T. F., W. R. Kirkpatrick, M. White, J. W. Himenez, J. R. Wingard, B. Dupont, M. G. Rinaldi, D. A. Stevens, and J. R. Graybill. 2000. Invasive aspergillosis: disease spectrum, treatment practices, and outcomes. Medicine 79:250-260. [DOI] [PubMed] [Google Scholar]
- 22.Patterson, T. F. 2005. The future of animal models of invasive aspergillosis. Med. Mycol. 43(Suppl. 1):S115-S119. [DOI] [PubMed] [Google Scholar]
- 23.Petraitiene, R., V. Petraitis, A. H. Groll, T. Sein, R. L. Schaufele, A. Francesconi, J. Bacher, N. A. Avila, and T. J. Walsh. 2002. Antifungal efficacy of caspofungin (MK-0991) in experimental pulmonary aspergillosis in persistently neutropenic rabbits: pharmacokinetics, drug disposition, and relationship to galactomannan antigenemia. Antimicrob. Agents Chemother. 46:12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinel, C., H. Fricker-Hidalgo, B. Lebeau, F. Garban, R. Hamidfar, P. Ambroise-Thomas, and R. Grillot. 2003. Detection of circulating Aspergillus fumigatus galactomannan: value and limits of the Platelia test for diagnosing invasive aspergillosis. J. Clin. Microbiol. 41:2184-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roffey, S. J., S. Cole, P. Comby, D. Gibson, S. G. Jezequel, A. N. R. Nedderman, D. A. Smith, D. K. Walker, and N. Wood. 2003. The disposition of voriconazole in mouse, rat, rabbit, guinea pig, dog and human. Drug Metab. Dispos. 31:731-741. [DOI] [PubMed] [Google Scholar]
- 26.Sheppard, D. C., J. R. Graybill, L. K. Najvar, L. Y. Chiang, T. Doedt, W. R. Kirkpatrick, R. Bocanegra, A. C. Vallor, T. F. Patterson, and S. G. Filler. 2006. Standardization of an experimental murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 50:3501-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheppard, D. C., K. A. Marr, D. N. Fredricks, L. Y. Chiang, T. Doedt, and S. G. Filler. 2006. Comparison of three methodologies for the determination of pulmonary fungal burden in experimental murine aspergillosis. Clin. Microbiol. Infect. 12:376-380. [DOI] [PubMed] [Google Scholar]
- 28.Sheppard, D. C., G. Rieg, L. Y. Chiang, S. G. Filler, J. E. Edwards, Jr., and A. S. Ibrahim. 2004. Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 48:1908-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinbach, W. J., D. K. Benjamin, Jr., S. A. Trasi, J. L. Miller, W. A. Schell, A. K. Zaas, W. M. Foster, and J. R. Perfect. 2004. Value of an inhalational model of invasive aspergillosis. Med. Mycol. 42(5):417-425. [DOI] [PubMed] [Google Scholar]
- 30.Steinbach, W. J., R. A. Cramer, Jr., B. Z. Perfect, Y. G. Asfaw, T. C. Sauer, L. K. Najvar, W. R. Kirkpatrick, T. F. Patterson, D. K. Benjamin, Jr., J. Heitman, and J. R. Perfect. 2006. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 5:1091-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sulahian, A., F. Boutboul, P. Ribaud, T. Leblanc, C. Lacroix, and F. Derouin. 2001. Value of antigen detection using an enzyme immunoassay in the diagnosis and prediction of invasive aspergillosis in two adult and pediatric hematology units during a 4-year prospective study. Cancer 91:311-318. [DOI] [PubMed] [Google Scholar]
- 32.Upton, A., A. Gugel, W. Leisenring, A. Limaye, B. Alexander, R. Hayden, and K. A. Marr. 2005. Reproducibility of low galactomannan enzyme immunoassay index values tested in multiple laboratories. J. Clin. Microbiol. 43:4796-4800. [DOI] [PMC free article] [PubMed] [Google Scholar]