Abstract
Candida parapsilosis has emerged as a common cause of invasive fungal infection, especially in Latin America and in the neonatal setting. C. parapsilosis is part of a closely related group of organisms that includes the species Candida orthopsilosis and Candida metapsilosis. All three species show elevated MICs for the new echinocandin class drugs caspofungin, micafungin, and anidulafungin relative to other Candida species. Despite potential impacts on therapy, the mechanism behind this reduced echinocandin susceptibility has not been determined. In this report, we investigated the role of a naturally occurring Pro-to-Ala substitution at amino acid position 660 (P660A), immediately distal to the highly conserved hot spot 1 region of Fks1p, in the reduced-echinocandin-susceptibility phenotype. Kinetic inhibition studies demonstrated that glucan synthase from the C. parapsilosis group was 1 to 2 logs less sensitive to echinocandin drugs than the reference enzyme from C. albicans. Furthermore, clinical isolates of C. albicans and C. glabrata which harbor mutations at this equivalent position also showed comparable 2-log decreases in target enzyme sensitivity, which correlated with increased MICs. These mutations also resulted in 2.4- to 18.8-fold-reduced Vmax values relative to those for the wild-type enzyme, consistent with kinetic parameters obtained for C. parapsilosis group enzymes. Finally, the importance of the P660A substitution for intrinsic resistance was confirmed by engineering an equivalent P647A mutation into Fks1p of Saccharomyces cerevisiae. The mutant glucan synthase displayed characteristic 2-log decreases in sensitivity to the echinocandin drugs. Overall, these data firmly indicate that a naturally occurring P660A substitution in Fks1p from the C. parapsilosis group accounts for the reduced susceptibility phenotype.
Invasive candidiasis is a leading cause of mycosis-associated morbidity and mortality (28). The new echinocandin class drugs caspofungin (CSF), micafungin (MCF), and anidulafungin (ANF) are widely used for primary therapy (8, 18, 36). Recent surveillance studies confirm that after several years of patient exposure, these drugs retain potent in vitro activity against a wide range of Candida spp. (12, 25-27). However, these studies also reveal Candida spp. with reduced echinocandin susceptibility, such as Candida parapsilosis, which have MICs 10- to 100-fold greater than those observed for Candida albicans. Moreover, the recently described C. parapsilosis sibling species, Candida orthopsilosis and Candida metapsilosis (35), also show higher MICs for echinocandin drugs (14, 17). Although the elevated MICs for the C. parapsilosis group do not appear to result in significant clinical failures (3), there is a concern that such strains may be predisposed to higher-level resistance. This is not a minor concern, as C. parapsilosis is one of the most common non-albicans Candida species isolated from blood worldwide, especially in Latin America (1, 4, 6, 7, 24, 25, 29). C. parapsilosis infections are prevalent in low-birth-weight neonates, surgical patients, and those with indwelling invasive devices (31-33).
Echinocandin drugs inhibit the fungal β-1,3-glucan synthase (GS) complex, which is responsible for the biosynthesis of the principal cell wall glucan, by targeting the putative catalytic subunit Fks1p (9, 10). Resistance to these antifungal drugs is associated with mutations in two highly conserved regions of Fks1p, known as hot spot 1 and hot spot 2 (15, 21, 22). A naturally occurring amino acid polymorphism in the highly conserved Fks1p hot spot 1 region from C. parapsilosis relative to other Candida species has been suggested to be responsible for the reduced echinocandin susceptibilities of these species (22).
The objective of this study was to determine if the intrinsic reduced echinocandin susceptibilities showed by C. parapsilosis, C. orthopsilosis, and C. metapsilosis are due to the naturally occurring amino acid change in the hot spot 1 region of Fks1p. To achieve this goal, glucan synthases were isolated from control and clinical strains, and their kinetic inhibition properties were studied. Moreover, clinical strains of C. albicans and C. glabrata containing mutations at the equivalent position in Fks1p, as well as an engineered strain of Saccharomyces cerevisiae, were evaluated. Overall, this study provides evidence that a naturally occurring amino acid change in Fks1p from the C. parapsilosis group accounts for their inherent reduced susceptibilities to echinocandin drugs.
MATERIALS AND METHODS
Strains and compounds.
The 16 strains used throughout this work are listed in Table 1. The echinocandin drugs used were CSF (Merck & Co., Inc., Rahway, NJ), ANF (Pfizer, New York, NY), and MCF (Astellas Pharma USA, Inc., Deerfield, IL). The drugs were obtained as standard powders from their manufacturers. CSF and MCF were dissolved in sterile distilled water, and ANF was dissolved in 100% dimethyl sulfoxide (Sigma-Aldrich). Stock solutions of each drug were kept at −86°C. C. parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were used as control strains for antifungal susceptibility testing.
TABLE 1.
Profiles of in vitro whole-cell susceptibility (MIC) and GS inhibition (IC50) in the strains included in the study
| Organism | Strain | FKS1-HS1 sequence or genotypea | Origin | MIC (μg/ml)b
|
IC50 (ng/ml)c
|
||||
|---|---|---|---|---|---|---|---|---|---|
| ANF | CSF | MCF | ANF | CSF | MCF | ||||
| C. parapsilosis | 22019 | FLTLSLRDA | ATCC | 1.10 | 1.40 | 1.60 | 442.03 ± 38.41 | 21.21 ± 2.27 | 245.27 ± 37.17 |
| C. parapsilosis | H4 | FLTLSLRDA | Clinical | 2.24 | 2.24 | 1.12 | 110.12 ± 16.44 | 79.19 ± 7.69 | 340.83 ± 41.98 |
| C. parapsilosis | H5 | FLTLSLRDA | Clinical | 1.59 | 2.24 | 1.26 | 237.07 ± 19.86 | 12.53 ± 2.30 | 493.63 ± 134.27 |
| C. orthopsilosis | H10 | FLTLSLRDA | Clinical | 0.79 | 1.00 | 0.63 | 163.17 ± 67.33 | 58.16 ± 3.66 | 152.70 ± 17.71 |
| C. orthopsilosis | 981224 | FLTLSLRDA | Clinical | 1.26 | 0.79 | 1.26 | 141.90 ± 13.77 | 75.26 ± 19.53 | 77.52 ± 45.08 |
| C. metapsilosis | am-2006-0113 | FLTLSLRDA | Clinical | 0.63 | 0.79 | 1.59 | 126.40 ± 2.26 | 40.28 ± 5.94 | 70.32 ± 1.10 |
| C. metapsilosis | 960161 | FLTLSLRDA | Clinical | 0.79 | 1.00 | 1.59 | 133.30 ± 7.36 | 25.15 ± 0.43 | 113.73 ± 16.16 |
| S. cerevisiae | BY4742 | FLVLSLRDP | Parental | 0.03 | 0.03 | 0.03 | 107.37 ± 14.90 | 65.54 ± 15.26 | 159.45 ± 7.00 |
| S. cerevisiae | BY4742-P649A | FLVLSLRDA | Laboratory mutant | 0.5 | 0.5 | 0.5 | 1,663.00 ± 18.00 | 1,328.67 ± 10.02 | 5,199.00 ± 132.94 |
| S. cerevisiae | BY4742-FKS1Δ | fks1D453-649::URA3 | Laboratory mutant | 0.015 | 0.02 | 0.015 | ND | ND | ND |
| C. albicans | Sc5314 | FLTLSLRDP | Control strain | 0.08 | 0.40 | 0.05 | 0.89 ± 0.06 | 3.88 ± 0.08 | 58.25 ± 0.32 |
| C. albicans | 90028 | FLTLSLRDP | ATCC | 0.02 | 0.20 | 0.03 | 1.63 ± 0.16 | 0.52 ± 0.04 | 10.20 ± 5.20 |
| C. albicans | 36082 | FLTLSLRDP | ATCC | 0.02 | 0.20 | 0.02 | 1.83 ± 0.10 | 0.60 ± 0.10 | 18.88 ± 2.00 |
| C. albicans | M122 | FLTLSLRDH | Clinical | 0.15 | 4.00 | 0.25 | 530.13 ± 156.38 | 79.48 ± 5.92 | 943.10 ± 82.65 |
| C. glabrata | 90030 | FLILSLRDP | ATCC | 0.05 | 0.10 | 0.06 | 3.77 ± 1.44 | 3.12 ± 0.56 | 0.68 ± 0.27 |
| C. glabrata | T51916 | FLILSLRDT | Clinical | 1.59 | 2.00 | 0.40 | 206.86 ± 15.03 | 157.50 ± 12.32 | 112.35 ± 2.05 |
FKS2-HS1 sequences are shown for C. glabrata strains. Substituted amino acids are in bold.
Values are geometric means (at least three repetitions on three separate days).
IC50 values were obtained using trapped GS enzyme and are expressed as arithmetic means ± standard deviations (three repetitions on three separate days). ND, not done.
Antifungal susceptibility testing.
Echinocandin drug susceptibility testing was performed in accordance with the guidelines in CLSI document M27-A2 (19) with the modifications previously suggested (20, 25). S. cerevisiae MICs were obtained using the same procedure but using YPD (1% yeast extract, 2% Bacto peptone, 2% dextrose) broth medium.
Identification of FKS in C. parapsilosis, C. metapsilosis, and C. orthopsilosis.
The FKS1 sequence from C. albicans (GenBank accession no. XM_716336) was used to search the C. parapsilosis genome databank at the Sanger institute website (http://www.sanger.ac.uk/sequencing/Candida/parapsilosis/). This sequence showed high homology with three different C. parapsilosis sequences. Annotations were assigned to the putative C. parapsilosis, C. metapsilosis, and C. orthopsilosis open reading frame sequences by BLASTX comparison with S. cerevisiae, C. albicans, and the nonredundant-gene database from GenBank. Moreover, synteny analysis with C. albicans and S. cerevisiae unambiguously identified these genes as FKS1, FKS2, or FKS3 (GenBank accession no. EU221325, EU221326, or EU221327, respectively). Related annotations were obtained from the Saccharomyces Genome Database (SGD) (http://www.yeastgenome.org/) for S. cerevisiae, from the Candida Genome Database (CGD) (http://www.candidagenome.org/) and CandidaDB (ftp://ftp.pasteur.fr/pub/GenomeDB/CandidaDB/FlatFiles/) for C. albicans, and from GenBank (http://www.ncbi.nlm.nih.gov/).
DNA sequence analysis of Candida FKS genes.
Genomic DNA was extracted from yeast cells grown overnight in YPD broth medium with a Q-Biogene (Irvine, CA) FastDNA kit. PCR and sequencing primers were designed based on the C. parapsilosis FKS1, FKS2, and FKS3 gene sequences (GenBank accession no. EU221325, EU221326, and EU221327, respectively); C. albicans FKS1 and FKS2 gene sequences (GenBank accession no. XM_716336 and XM_712867, respectively); and C. glabrata FKS1 and FKS2 gene sequences (GenBank accession no. XM_446406 and XM_448401, respectively). Also, the hot spot 1 and 2 regions of the C. metapsilosis and C. orthopsilosis putative FKS genes were amplified and sequenced using Candida FKS universal primers flanking the FKS1 hot spot regions (1HS1F/1HS1R and 1HS2F/1HS2R primer sets) (Table 2). Based on the information derived from the sequencing of the first PCR fragments, specific C. metapsilosis and C. orthopsilosis primers were designed to amplify and sequence the region in between both hot spots (Table 2). DNA sequencing was performed with a CEQ dye terminator cycle sequencing quick-start kit (Beckman Coulter, Fullerton, CA) according to the manufacturer's recommendations. Sequence analysis was performed with CEQ 8000 genetic analysis system software (Beckman Coulter, Fullerton, CA) and BioEdit sequence alignment editor (Ibis Therapeutics, Carlsbad, CA).
TABLE 2.
Primers used in this study
| Primer | Orientation (5′ → 3′) | Sequence (5′ → 3′) | Purpose |
|---|---|---|---|
| FKS1-453-URA3Fa | Sense | AAAGAGACCCGTACTTGGTTACATTTGGTCACCAACTTCAGAGTGCACCATACCACAGCT | S. cerevisiae mutant generation |
| FKS1-649-URA3Ra | Antisense | GTATTCACCTGTACACCTCATTGCAGTGGTGGACAAAATTGGTATTTCACACCGCATAGG | S. cerevisiae mutant generation |
| FKS1-mut647Rb | Antisense | ATTCACCTGTACACCTCATTGCAGTGGTGGACAAAATTCTAATTGNATCTCTCAAAGATA | S. cerevisiae mutant generation |
| FKS1-375F | Sense | GGTCGTTTTGTCAAGCGTGA | S. cerevisiae mutant generation |
| FKS1-707R | Antisense | GATTTCCCAACAGAGAAAATGG | S. cerevisiae mutant generation |
| URA3iR2 | Antisense | TGCCTTTAGCGGCTTAACTG | S. cerevisiae mutant generation |
| 1HS1F | Sense | AATGGGCTGGTGCTCAACAT | Candida universal FKS1 primers |
| 1HS1R | Antisense | CCTTCAATTTCAGATGGAACTTGATG | Candida universal FKS1 primers |
| 1HS2F | Sense | AAGATTGGTGCTGGTATGGG | Candida universal FKS1 primers |
| 1HS2R | Antisense | TAATGGTGCTTGCCAATGAG | Candida universal FKS1 primers |
| CoinsHS1F | Sense | GGTATGGTGATATTGTCTG | C. orthopsilosis hot spot 1 sequencing |
| CoinsHS2R | Antisense | GGTATGGTGATATTGTCTG | C. orthopsilosis hot spot 2 sequencing |
| CminsHS1F | Sense | CAGAGAACATTTGTTAGCC | C. metapsilosis hot spot 1 sequencing |
| CminsHS2R | Antisense | GTATAACGTCTGATCCAGTC | C. metapsilosis hot spot 2 sequencing |
| CpFKS1expF | Sense | ATCCAAGATCTTCCGGTGCCTCAA | Expression profiling |
| CpFKS1expR | Antisense | ATCAGCTGACCATGCTGGATATGG | Expression profiling |
| CpFKS2expF | Sense | AATGGGCAGAGGTTGAGAAGGTAG | Expression profiling |
| CpFKS2expR | Antisense | GGGTTCCAAGCAGGATATGGATCA | Expression profiling |
| CpFKS3expF | Sense | TCGTAGGTTCGAATCCTGCTGAGA | Expression profiling |
| CpFKS3expR | Antisense | ATGGTGAAGGCGCAACGGTGTAAA | Expression profiling |
| CaFKS1expF | Sense | TGATACTGGTAATCATAGACCAAAAA | Expression profiling |
| CaFKS1expR | Antisense | AACTCTGAATGGATTTGTAGAATAAGG | Expression profiling |
| CaFKS2expF | Sense | ACTTGCTAGCAGTCGCCAAT | Expression profiling |
| CaFKS2expR | Antisense | ACCACCATGAGCGGTTAGAC | Expression profiling |
| CaFKS3expF | Sense | ACCTCAATATTCAGCTTGGTGCCC | Expression profiling |
| CaFKS3expR | Antisense | GGACAACTCATTCGACTTGACCGT | Expression profiling |
| CgFKS1expF | Sense | CAATTGGCAGAACACCGATCCCAA | Expression profiling |
| CgFKS1expR | Antisense | AGTTGGGTTGTCCGTACTCATCGT | Expression profiling |
| CgFKS2expF | Sense | TACCAACCAGAAGACCAACAGAATGG | Expression profiling |
| CgFKS2expR | Antisense | TCACCACCGCTGATGTTTGGGT | Expression profiling |
| CgFKS3expF | Sense | GGGAGAGCACGTAAACGTAACTCAA | Expression profiling |
| CgFKS3expR | Antisense | TTTGCTGCTGTAAGGTTAGTGGCG | Expression profiling |
| But33 | Sense | ATGATAGAGTTGAAAGTAGTTTGGTCAATA | Expression profiling (C. parapsilosis housekeeping gene) |
| But34 | Antisense | ACTACTGCTGAAAGAGAAATTGTTAGAGAC | Expression profiling (C. parapsilosis housekeeping gene) |
| CaURA3F | Sense | CAACACTAAGACCTATAGTGAGAGAGC | Expression profiling (C. albicans housekeeping gene) |
| CaURA3R | Antisense | TGCACATAAATTGGTTTTCTTCA | Expression profiling (C. albicans housekeeping gene) |
| CgURA3F | Sense | CGAGAACACTGTTAAGCCATTG | Expression profiling (C. glabrata housekeeping gene) |
| CgURA3R | Antisense | CACCATGAGCGTTGGTGATA | Expression profiling (C. glabrata housekeeping gene) |
Sequences corresponding to URA3 flanking sequences in pRS416 are underlined.
Codon 647, incorporating N into the first position, is underlined.
Directed mutagenesis of S. cerevisiae FKS1.
A novel two-step replacement method for PCR-based site-directed mutagenesis was employed (S. Katiyar and T. Edlind, unpublished results). In step 1, a partial internal FKS1 deletion (fks1Δ453-649) was constructed with a PCR-generated URA3 cassette. This deletion conferred sensitivity to calcineurin inhibitor FK506, which is required for expression of the “backup” gene FKS2 (9). To generate BY4742 fks1Δ453-649::URA3, primers FKS1-453-URA3F and FKS1-649-URA3R (Table 2) were designed with 20 bases at their 3′ ends to amplify URA3 plus flanking sequences and 40 bases at their 5′ ends homologous to FKS1 sequences upstream of codon 453 and downstream of codon 649, respectively. The template was the URA3-containing plasmid pRS416 (GenBank accession no. U03450). PCR was performed with ExTaq polymerase as recommended by the manufacturer (Takara Bio USA). Products were purified (IsoPure; Denville), and about 1 μg was used to transform BY4742 with selection on SD-URA plates (11). Colonies were screened for sensitivity on YPD plates containing 0.75 μg/ml FK506 (Tecoland, Edison, NJ). PCR with primers FKS1-375F and URA3iR was used to confirm the deletion and replacement with URA3.
In step 2, the deletant was transformed with an FKS1 PCR product that spanned the deleted region but incorporated all 4 bases (N) into the first position of codon 647. Mutagenic reverse primer FKS1-mut647R (Table 2), which incorporated 43 bases at its 5′ end homologous to the FKS1 sequence downstream of codon 647, followed by codon 647 (replaced with N at its first position), followed by an additional 14 upstream bases at its 3′ end, was designed. This primer was used for PCR in conjunction with forward primer FKS1-375F and wild-type BY4742 DNA as a template. The product was purified and about 1 μg transformed into the fks1Δ453-649::URA3 strain described above, with selection on FK506 plates. Colonies were screened on SD-ura medium for loss of URA3, and PCR with primers FKS1-375F and FKS1-707R was used to confirm FKS1 reconstitution. To confirm the FKS1 restoration, FKS1 regions corresponding to codons 453 to 649 were amplified and sequenced employing the same PCR product and primers. Echinocandin susceptibility was determined with YPD medium, as described previously (34).
GS isolation and assay.
All the isolates used in this work were grown with vigorous shaking at 30°C to early stationary phase in YPD broth, and cells were collected by centrifugation. Cell disruption, membrane protein extraction, and partial GS purification by product entrapment were performed, as described previously for wild-type C. albicans, C. glabrata, and S. cerevisiae strains (21). When these procedures were employed to obtain GS enzymes from C. parapsilosis, C. metapsilosis, C. orthopsilosis, and mutant C. albicans, C. glabrata, and S. cerevisiae strains, the enzyme activities were 5- to 10-fold lower than those of the wild-type strains. These enzyme activities were not high enough for kinetics studies to be performed. In these strains, the cell disruption procedure was performed using 10-fold more cells. This protocol change was sufficient to obtain GS enzymes with the requisite activity and quality for further kinetic studies. Sensitivity to echinocandin drugs was measured in a polymerization assay using a 96-well multiscreen high-throughput-screen filtration system (Millipore corporation, Bedford, MA) with a final volume of 100 μl, as previously described (21). Serial dilutions of echinocandin drugs (0.01 to 10,000 ng/ml) were used to determine 50% inhibitory concentration (IC50) values. Control reactions were performed in the presence of 1% dimethyl sulfoxide when ANF was used. The reactions were initiated by addition of GS. Inhibition profiles and IC50 were determined using a sigmoidal response (variable-slope) curve fitting algorithm with GraphPad Prism software (version 4.0; Prism Software, Irvine, CA).
Characterization of glucan product.
The product of the reaction mixtures was characterized as β(1,3)-glucan by using a Glucatell kit (Associates of Cape Cod, Inc., Falmouth, MA), following the manufacturer's instructions. Microcentrifuge tubes were used to perform the product characterization reactions, which were done with a 100-μl final volume. The addition of the GS purified complex was used to initiate the reaction. Reaction mixtures were incubated at 25°C for 60 min and then were stopped by rapid cooling on ice. Using the endpoint assay Glucatell kit and comparing the results obtained with those for the [3H]UDPG incorporation assay, it was established that 2.4 × 10−2 nmol of glucose was incorporated (∼10 pg of glucan/ml).
Kinetic analyses.
All reactions were run in a 96-well multiscreen high-throughput-screen filtration system (Millipore) with a final volume of 100 μl. Each well contained 50 mM HEPES (pH 7.5), 10% (wt/vol) glycerol, 1.5 mg/ml bovine serum albumin, 25 mM KF, 1 mM EDTA, 25 μM GTP-γ-S, 1 μg GS enzyme, [3H]UDPG (7,000 dpm/nmol), and echinocandin drugs, as indicated below. The plates were incubated for 60 min at 25°C. The reactions were initiated by addition of enzyme. [3H]UDPG was used as the substrate in concentrations ranging from 0.015 to 2 mM to determine the different kinetic parameters, which were analyzed by linear regressions to obtain slopes in dpm/min. This value was then converted to nM of glucose incorporated per minute. The maximum velocity (Vmax) and the Michaelis-Menten constant (Km) were determined for trapped GS enzyme by varying the amount of UDPG (between 0.015 and 2 mM), using Lineweaver-Burke plots. Ki values were calculated by varying the echinocandin drug concentration (between 0.01 and 50 ng/ml for wild-type GS and between 10 and 10,000 ng/ml for mutant GS) at different fixed substrate concentrations (0.125 to 0.5 mM UDPG).
RNA isolation and expression profiling.
C. parapsilosis, C. albicans, and C. glabrata strains were grown in YPD and incubated at 37°C with shaking (150 rpm) for 16 h. Total RNA was extracted using an RNeasy mini kit (Qiagen), and gene expression profiles were performed using a one-step Sybr green quantitative reverse transcription-PCR kit (Stratagene, La Jolla, CA) with the Stratagene Mx3005P multiplex quantitative PCR system (Stratagene). Differential expression was analyzed for the three FKS C. parapsilosis, C. albicans, and C. glabrata genes. C. albicans and C. glabrata FKS3 expression profiling primers were designed using GenBank accession no. XM_713421 and XM_449945, respectively. The relative expression levels were evaluated using the Pfaffl method (23). URA3 (for C. albicans, GenBank accession no. XP_721787.1, and for C. glabrata, GenBank accession no. AY771209) and ACT1 (for C. parapsilosis) genes were used for normalization (30). The primers used for gene expression profiling are listed in Table 2.
Statistical analysis.
The kinetic data are the result of experiments performed in triplicate. Arithmetic means and standard deviations were used to statistically analyze all the continuous variables (IC50, Km, Vmax, and Ki). Geometric means were used to statistically compare MIC results. The significance levels of MIC differences and kinetic parameters were determined by Student's t test (unpaired, unequal variance). A P value of <0.05 was considered significant. In order to approximate a normal distribution, the MICs were transformed to log2 values to establish susceptibility differences between strains. Both on-scale and off-scale results were included in the analysis. The off-scale MICs were converted to the next concentration up or down. Statistical analyses were performed with the Statistical Package for the Social Sciences (version 13.0; SPSS, Inc., Chicago, IL).
Nucleotide sequence accession numbers.
The full nucleotide sequences of the C. parapsilosis FKS1, FKS2, and FKS3 genes and the partial C. metapsilosis and C. orthopsilosis FKS1 gene sequences determined in this work appear in the GenBank nucleotide sequence database under accession numbers EU221325, EU221326, EU221327, EU350514, and EU350513, respectively.
RESULTS
Identification and comparative genomics of C. parapsilosis, C. metapsilosis, and C. orthopsilosis FKS genes.
The FKS1 sequence from C. albicans (GenBank accession no. XM_716336) was used to do a BLAST search of the C. parapsilosis genome databank. The submitted sequence showed high percentages of nucleotide identity with three different C. parapsilosis DNA contigs (contig 174.5, 84%; contig 179.6, 61%; and contig 178.1, 69%), revealing three GS homologs. Comparing these contigs by using BLASTX revealed that contig 174.5 was most closely related to the Fks1p orthologs from C. albicans and S. cerevisiae, while contigs 179.6 and 178.1 were more closely related to orthologs of C. albicans and S. cerevisiae Fks2p and Fks3p, respectively. Moreover, synteny with C. albicans and S. cerevisiae unambiguously identified these genes as FKS1, FKS2, and FKS3. C. metapsilosis (2,663 bp) and C. orthopsilosis (2,467 bp) sequences were compared using BLASTX. Both sequences showed high percentages of identity with C. albicans FKS1p and were considered orthologs.
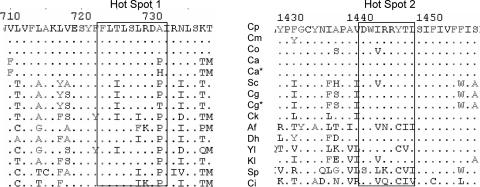
Full-length C. albicans, C. glabrata, C. parapsilosis, and S. cerevisiae Fks1p, Fks2p, and Fks3p homologs and hot spot regions of Fks1p from C. orthopsilosis and C. metapsilosis were aligned with the ClustalW multiple analysis tool (Fig. 1). A naturally occurring Ala substitution for a highly conserved Pro residue is apparent immediately distal to the C-terminal end of FKS1 hot spot 1 from C. parapsilosis, C. orthopsilosis, and C. metapsilosis. An additional amino acid change (Val to Ile) was observed in C. orthopsilosis Fks1p hot spot 2. However, this amino acid variant is found in several other echinocandin-susceptible fungal species, including S. cerevisiae and Aspergillus fumigatus (Fig. 1).
FIG. 1.
Sequence alignments of Fks1p and Fks2p hot spot regions from diverse fungal species. The aligned sequences are as follows: C. parapsilosis (Cp; GenBank accession no. ABX80511); C. metapsilosis (Cm; ABY67254); C. orthopsilosis (Co; ABY67253); C. albicans wild-type strain 5314 (Ca; XP_721429); C. albicans mutant strain 122, FKS1p P649H (Ca*); S. cerevisiae (Sc; AAC48981); C. glabrata Fks2p (Cg; XP_448401); C. glabrata mutant strain 916, Fks2p P633T (Cg*); C. krusei (Ck; AAY40291); Aspergillus fumigatus (Af; AAB58492); Debaryomyces hansenii (Dh; XP_457762); Yarrowia lipolytica (Yl; XP_504213); Kluyveromyces lactis (Kl; CAH02189); Schizosaccharomyces pombe (Sp; NP_588501); and Coccidioides immitis (Ci; EAS36399).
Echinocandin drug susceptibilities.
The in vitro activities of ANF, CSF, and MCF against all the strains used in this work are summarized in Table 1. The in vitro drug susceptibilities of C. parapsilosis, C. metapsilosis, and C. orthopsilosis clinical and control isolates to ANF and MCF were 20- to 50-fold higher than those of the C. albicans and C. glabrata control strains. CSF showed a lower but statistically significant increase in MIC (3.6- to 7.8-fold; P = 2 × 10−6). C. albicans FKS1 and C. glabrata FKS2 clinical strains harboring the amino acid changes P649H and P633T (equivalent in position to A660 of C. parapsilosis Fks1p), respectively, showed echinocandin drug MICs that were 4- to 21-fold higher than the wild-type strains. Moreover, comparing the MICs obtained for the mutant clinical strains with those obtained for C. parapsilosis and its sibling species revealed no significant difference (P > 0.05). These data are consistent with an increase in MIC when an amino acid change occurs in the distal amino acid of the hot spot 1 C terminus.
Inhibition of glucan synthase.
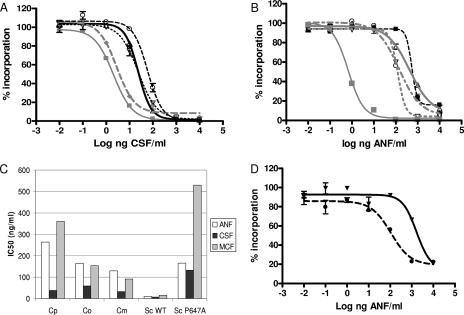
To evaluate the in vitro inhibition of product-entrapped enzymes from C. parapsilosis, C. metapsilosis, and C. orthopsilosis, the IC50 was determined (Table 1). It should be noted that the GS complexes obtained from C. parapsilosis and it sibling species have several Fks isoenzymes, but only one with the P660A amino acid substitution. However, the kinetic profiles did not reveal the presence of mixed enzyme species, as was expected. Only the mutant enzyme was dominant in the kinetic analyses. As a group, these enzymes show statistically higher IC50 values than enzymes from wild-type C. albicans and C. glabrata strains for all three echinocandin drugs tested (P < 0.001) (Fig. 2). The echinocandin IC50 values obtained for enzymes from the clinical FKS1 mutant isolates of C. albicans P649H and C. glabrata P633T increased 50- to 290-fold and 50- to 164-fold, respectively, relative to those for wild-type reference strains (Table 1). These higher IC50 values were consistent with elevated MICs for these strains and mimicked the elevated IC50 values obtained for the C. parapsilosis group (Table 1).
FIG. 2.
Kinetic properties of GS inhibition by echinocandin drugs. (A) CSF inhibition profiles of trapped GS complexes from wild-type C. albicans SC5314 (gray squares), C. glabrata ATCC 90030 (gray diamonds), C. parapsilosis ATCC 22019 (triangles), C. metapsilosis 960161 (inverted triangles), and C. orthopsilosis H10 (circles). (B) ANF titration of the GS complexes isolated from C. albicans Sc5314 (gray squares), C. albicans mutant strain 122 (black squares), C. parapsilosis ATCC 22019 (triangles), C. metapsilosis 960161 (inverted triangles), and C. orthopsilosis H10 (circles). (C) Average IC50 values comparison between the following groups: C. parapsilosis (Cp), C. metapsilosis (Cm), C. orthopsilosis (Co), and S. cerevisiae wild type (Sc WT) and P647A mutant (Sc P647A). (D) ANF inhibition profiles of partially purified GS complexes from S. cerevisiae wild type (circles) and FKS1 P647A (inverted triangles).
To better understand the behavior of echinocandin drugs on the enzymes obtained from C. parapsilosis and its sibling species, a more detailed kinetic study was performed to assess the inhibition constant (Ki) (Table 3). Overall, the average Ki values (n = 3) for the C. parapsilosis enzyme for ANF, CSF, and MCF were 479.9, 19.3, and 407.7 ng/ml, respectively, which were significantly greater than the values of 1.1, 1.5, and 22.2 ng/ml for enzymes from C. albicans (Table 3). The average Ki values for ANF and MCF for C. parapsilosis were more than 21-fold higher than those for CSF (Fig. 2). A similar pattern was observed with C. orthopsilosis but not for C. metapsilosis, which showed comparable levels for ANF and CSF. The Ki values for the P649H mutant C. albicans enzyme for ANF, CSF, and MCF were increased 571-, 204-, and 117-fold, respectively, relative to those for the C. albicans enzyme (Table 3). In the same way, the P633T mutant strain of C. glabrata showed a 10- to 451-fold increase in Ki for each of the echinocandin drugs (Table 3).
TABLE 3.
Summary of kinetic properties and Ki values of the strains included in the study
| Organism | Strain | FKS1-HS1 sequencea | Vmax (nmol/min)b | Km (mM)b |
Ki (ng/ml)b
|
||
|---|---|---|---|---|---|---|---|
| ANF | CSF | MCF | |||||
| C. parapsilosis | 22019 | FLTLSLRDA | 0.52 ± 0.14 | 0.60 ± 0.16 | 639.50 ± 10.61 | 47.99 ± 0.72 | 476.00 ± 2.83 |
| C. parapsilosis | H4 | FLTLSLRDA | 0.44 ± 0.16 | 0.45 ± 0.07 | 368.50 ± 13.44 | 3.93 ± 2.22 | 486.00 ± 7.07 |
| C. parapsilosis | H5 | FLTLSLRDA | 0.46 ± 0.04 | 0.59 ± 0.05 | 431.67 ± 15.89 | 6.01 ± 0.71 | 261.00 ± 11.31 |
| C. orthopsilosis | H10 | FLTLSLRDA | 0.33 ± 0.20 | 0.51 ± 0.07 | 111.00 ± 5.66 | 4.29 ± 1.11 | 177.50 ± 2.12 |
| C. orthopsilosis | 981224 | FLTLSLRDA | 0.40 ± 0.04 | 0.93 ± 0.11 | 46.50 ± 12.02 | 7.40 ± 2.69 | 248.00 ± 2.83 |
| C. metapsilosis | am-2006-0113 | FLTLSLRDA | 0.18 ± 0.07 | 0.22 ± 0.13 | 41.40 ± 5.09 | 76.90 ± 0.85 | 266.00 ± 8.49 |
| C. metapsilosis | 960161 | FLTLSLRDA | 0.25 ± 0.20 | 0.20 ± 0.02 | 129.00 ± 11.31 | 80.45 ± 0.49 | 382.50 ± 2.12 |
| S. cerevisiae | BY4742 | FLVLSLRDP | 2.65 ± 0.16 | 0.10 ± 0.02 | 68.95 ± 12.51 | 183.94 ± 35.27 | 179.01 ± 8.08 |
| S. cerevisiae | BY4742-P649A | FLVLSLRDA | 1.58 ± 0.13 | 0.13 ± 0.08 | 2374.0 ± 9.90 | 1215.00 ± 12.73 | 5706.50 ± 20.51 |
| C. albicans | Sc5314 | FLTLSLRDP | 6.72 ± 0.25 | 0.12 ± 0.01 | 1.33 ± 0.56 | 2.97 ± 1.58 | 27.47 ± 1.82 |
| C. albicans | 90028 | FLTLSLRDP | 5.87 ± 0.20 | 0.09 ± 0.01 | 1.43 ± 0.14 | 0.95 ± 0.05 | 23.53 ± 2.37 |
| C. albicans | 36082 | FLTLSLRDP | 4.71 ± 0.48 | 0.07 ± 0.01 | 0.63 ± 0.18 | 0.46 ± 0.04 | 15.47 ± 2.05 |
| C. albicans | M122 | FLTLSLRDH | 1.95 ± 0.29 | 0.15 ± 0.01 | 646.07 ± 26.72 | 291.67 ± 50.16 | 2588.53 ± 32.25 |
| C. glabrata | 90030 | FLILSLRDP | 6.97 ± 0.57 | 0.13 ± 0.01 | 16.15 ± 1.06 | 18.95 ± 3.04 | 1.13 ± 0.18 |
| C. glabrata | T51916 | FLILSLRDT | 0.37 ± 0.06 | 0.05 ± 0.01 | 712.00 ± 9.90 | 194.50 ± 23.33 | 510.00 ± 67.88 |
FKS2-HS1 sequences are shown for C. glabrata strains. Substituted amino acids are in bold.
Arithmetic means ± standard deviations (three repetitions).
Kinetic properties of GS.
Little is known about the kinetic properties of GS from different Candida species as well as from drug-resistant mutants, and a detailed kinetic study was undertaken to explore these properties (Table 3). The relative affinities for UDPG for GS from C. parapsilosis, C. orthopsilosis, and C. metapsilosis, as reflected in Km values, were on average two- to threefold lower than those for enzymes from C. albicans and C. glabrata (P < 0.0001) (Table 3). The C. metapsilosis enzyme displayed the lowest average Km value relative to C. parapsilosis and C. orthopsilosis enzymes (P < 0.001) (0.21 ± 0.01 mM versus 0.55 ± 0.08 mM and 0.72 ± 0.30 mM, respectively) (Table 3). GS enzymes from C. parapsilosis displayed average Vmax values (0.47 ± 0.34 nmol/min) that were >12-fold lower than those from the susceptible C. albicans and C. glabrata strains (P < 0.001). Significant decreases in Vmax were observed for C. albicans Fks1p P649H and C. glabrata Fks2p P633T mutants relative to those for fully drug-susceptible GS, suggesting that amino acid substitutions in hot spot 1, resulting in reduced drug susceptibility, may have a kinetic cost to the enzyme and a potential fitness cost to the cell.
Validation of Pro-to-Ala substitution (P647A) in FKS1 from Saccharomyces cerevisiae.
To confirm that the Pro-to-Ala variant is responsible for reduced echinocandin susceptibility in the C. parapsilosis group, a P647A mutation was engineered into Fks1p of S. cerevisiae. As in most fungi, S. cerevisiae FKS1 encodes Pro at the position (residue 647) corresponding to C. parapsilosis A660. A novel two-step replacement method for PCR-based site-directed mutagenesis was employed (see Materials and Methods). Two clones encoding wild-type P647 and two encoding A647 were identified. The wild-type clones and parent BY4742 exhibited identical echinocandin MICs, while the P647A mutants exhibited 16-fold-elevated MICs for all echinocandin drugs (Table 1). GS from the Fks1p P647A mutant showed comparable increases in echinocandin IC50 values of 15.49-, 20.27-, and 33.20-fold (Table 1) and Ki values of 34.4-, 6.6-, and 31.9-fold (Table 3) for ANF, CSF, and MCF, respectively. The data strongly support the hypothesis that the P647A mutation alters the echinocandin susceptibility of GS from S. cerevisiae, which is consistent with the phenotypic and biochemical properties of the C. parapsilosis group enzymes.
FKS gene expression differs between Candida species wild-type and mutant strains.
Real-time PCR was used to evaluate transcription levels for the three different FKS genes. Table 4 indicates that both FKS2 and FKS3 were expressed at greater levels than FKS1 at 16 h of growing. Moreover, it was confirmed that all three C. parapsilosis FKS genes were expressed at levels comparable to those for the housekeeping gene used (data not shown). FKS2 was expressed at a higher level than FKS1 in the C. glabrata wild-type strain. In contrast, FKS1 was the most dominantly expressed FKS gene in C. albicans. The C. glabrata Fks2p P633T mutant showed a greater FKS1 expression level than FKS2, while the C. albicans mutant showed no significant change compared with the wild-type strain (P > 0.05). FKS3 gene expression was not detectable in C. albicans and C. glabrata wild-type and mutant strains.
TABLE 4.
Relative expression ratios between FKS genes
| Organism | Strain | FKS1-HS1 sequencea | Expression ratiob
|
|
|---|---|---|---|---|
| FKS2/FKS1 | FKS3/FKS1 | |||
| C. parapsilosis | 22019 | FLTLSLRDA | 6.94 | 2.88 |
| C. parapsilosis | H4 | FLTLSLRDA | 4.04 | 3.23 |
| C. albicans | 90028 | FLTLSLRDP | 0.21 | 0.03 |
| C. albicans | 36082 | FLTLSLRDP | 0.39 | 0.02 |
| C. albicans | M122 | FLTLSLRDH | 0.28 | 0.04 |
| C. glabrata | 90030 | FLILSLRDP | 1.53 | 0.02 |
| C. glabrata | T51916 | FLILSLRDT | 0.81 | 0.005 |
FKS2-HS1 sequences are shown for C. glabrata strains. Substituted amino acids are in bold.
Differences (n-fold) relative to FKS1 levels.
DISCUSSION
The reduced in vitro susceptibilities of C. parapsilosis and its sibling species, C. metapsilosis and C. orthopsilosis, to echinocandin class drugs are notable. Most infections due to C. parapsilosis respond to primary echinocandin therapy (3, 32), although resistance to CSF resulting in clinical failure has been reported (5). In this context, there is a concern that as echinocandin therapy expands to countries in Latin America, where C. parapsilosis is a common cause of fungal bloodstream infection (24, 29), the elevated baseline drug susceptibility could represent an initial step toward clinical resistance or a new factor contributing to an even higher prevalence. The latter notion is supported by a recent report correlating CSF usage and an increased incidence of C. parapsilosis candidemia (13). Acquisition of resistance to echinocandin drugs in several Candida species and S. cerevisiae is associated with amino acid substitutions in two highly conserved regions of Fks1p or its paralog Fks2p (22). The highest frequency of resistance mutations is found within hot spot 1 (2). In C. parapsilosis, an Ala at position 660 in Fks1p replaces the Pro found in other fungal species, and this amino acid variation is also observed in Fks1p from sibling species C. metapsilosis and C. orthopsilosis (Fig. 1). This naturally occurring substitution has not been observed in any other species, although other hot spot 1 substitutions have been identified in species such as Candida guilliermondii, which also show reduced echinocandin susceptibility (15). Given its location in hot spot 1, it has been suggested that this naturally occurring amino acid change is linked to reduced echinocandin susceptibility (22).
As mutations within the hot spot regions of Fks1p confer growth resistance to echinocandin drugs, they also alter the in vitro sensitivities of the mutant GS enzymes to drug by as much as 1,000-fold (21). If the P660A change in C. parapsilosis were responsible for the reduced echinocandin susceptibility, then GS from this organism should also show a relative decrease in sensitivity. As shown in Tables 1 and 3, both the IC50 and the Ki values for GS from the C. parapsilosis group showed 40- to 500-fold less sensitivity to all three echinocandin drugs than those for GS from C. albicans. ANF displayed the largest difference in Ki with GS from C. parapsilosis (Table 3), which may account for the slightly elevated MICs relative to those for CSF and MCF (Table 1).
The importance of the C. parapsilosis Fks1p P660A amino acid change was affirmed in an analysis of C. albicans and C. glabrata clinical isolates with highly elevated MICs which harbored amino acid substitutions P649H and P633T, respectively, at this position. Mutant GS from both organisms showed several-hundred-fold increases in Ki values for all drugs relative to enzymes from susceptible reference strains (Table 3). The data strongly implicate changes in the conserved distal Pro in Fks1p hot spot 1 as playing an important role in phenotypic sensitivity to echinocandin drugs. Final confirmation of the importance of the Pro change was obtained by engineering a P649A substitution into Fks1p from S. cerevisiae. The FKS1 mutations resulted in 16-fold increases in MIC for all echinocandin drugs (Table 1) in growth assays and 7- to 34-fold decreases in GS sensitivity in vitro (Table 3). Only cells harboring the FKS1 mutant genotype conferred the reduced-echinocandin-susceptibility phenotype, indicating that it is necessary and sufficient. Overall, the data confirm the hypothesis that a variant occurring at the highly conserved distal Pro in FKS1 hot spot 1 accounts for the reduced susceptibilities of C. parapsilosis and its related species to echinocandin drugs.
In the course of this work, we observed that mutations in the hot spot 1 region of Fks1p had little effect on the Km of GS, but they decreased Vmax in enzymes from C. albicans and C. glabrata. This kinetic effect was mimicked in the engineered Fks1p mutant of S. cerevisiae (Table 3), and it was reflected in the relatively low Vmax values obtained for GS from the C. parapsilosis group (Table 3). These data suggest that mutations in Fks1p can alter the catalytic capacity of GS, which may have a fitness cost to the cell. This reduced catalytic capacity may help account for the expression profiling data (Table 4) in which a hypothetical biological cost in C. parapsilosis and C. glabrata can be compensated for by increases in the expression levels of the nonmutated FKS genes. Interestingly, the level of expression was not reflected in our kinetic assays, as smooth curves, which appeared as a single kinetic species, were obtained. This contrasts with previous studies from our group, where mixed kinetic species (FKS1p/fks1p) were readily apparent (21). The majority of the kinetic response for C. parapsilosis group GS appears to be dominated by a single enzyme species (>80%). This phenomenon could have several possible explanations. One is that expression of FKS2 and FKS3 does not translate into an assembled enzyme, due to posttranscriptional control (e.g., RNA or enzyme turnover), or the enzyme extraction was more selective for Fks1p. The latter is consistent with some of our observations with the S. cerevisiae BY4742-FKS1Δ strain, where the GS complex (Fks2p and/or Fks3p) was difficult to purify. Experiments are under way in our laboratory to confirm that Candida Fks proteins have overlapping and compensatory functions, as was demonstrated with S. cerevisiae (16).
Acknowledgments
This work was supported by NIH grants 1R01AI069397-01 to D.S.P. and R03 AI054289 to S.K.K. and T.D.E.
We thank Frank Odds for kindly providing us with some of the strains used in this work.
Footnotes
Published ahead of print on 28 April 2008.
REFERENCES
- 1.Almirante, B., D. Rodriguez, M. Cuenca-Estrella, M. Almela, F. Sanchez, J. Ayats, C. Alonso-Tarres, J. L. Rodriguez-Tudela, and A. Pahissa. 2006. Epidemiology, risk factors, and prognosis of Candida parapsilosis bloodstream infections: case-control population-based surveillance study of patients in Barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 44:1681-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balashov, S. V., S. Park, and D. S. Perlin. 2006. Assessing resistance to the echinocandin antifungal drug caspofungin in Candida albicans by profiling mutations in FKS1. Antimicrob. Agents Chemother. 50:2058-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett, J. E. 2006. Echinocandins for candidemia in adults without neutropenia. N. Engl. J. Med. 355:1154-1159. [DOI] [PubMed] [Google Scholar]
- 4.Brito, L. R., T. Guimaraes, M. Nucci, R. C. Rosas, L. Paula Almeida, D. A. Da Matta, and A. L. Colombo. 2006. Clinical and microbiological aspects of candidemia due to Candida parapsilosis in Brazilian tertiary care hospitals. Med. Mycol. 44:261-266. [DOI] [PubMed] [Google Scholar]
- 5.Cheung, C., Y. Guo, P. Gialanella, and M. Feldmesser. 2006. Development of candidemia on caspofungin therapy: a case report. Infection 34:345-348. [DOI] [PubMed] [Google Scholar]
- 6.Colombo, A. L., T. Guimaraes, L. R. Silva, L. P. de Almeida Monfardini, A. K. Cunha, P. Rady, T. Alves, and R. C. Rosas. 2007. Prospective observational study of candidemia in Sao Paulo, Brazil: incidence rate, epidemiology, and predictors of mortality. Infect. Control Hosp. Epidemiol. 28:570-576. [DOI] [PubMed] [Google Scholar]
- 7.Colombo, A. L., M. Nucci, B. J. Park, S. A. Nouer, B. Arthington-Skaggs, D. A. da Matta, D. Warnock, and J. Morgan. 2006. Epidemiology of candidemia in Brazil: a nationwide sentinel surveillance of candidemia in eleven medical centers. J. Clin. Microbiol. 44:2816-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denning, D. W. 2003. Echinocandin antifungal drugs. Lancet 362:1142-1151. [DOI] [PubMed] [Google Scholar]
- 9.Douglas, C. M., F. Foor, J. A. Marrinan, N. Morin, J. B. Nielsen, A. M. Dahl, P. Mazur, W. Baginsky, W. Li, and M. el Sherbeini. 1994. The Saccharomyces cerevisiae FKS1 (ETG1) gene encodes an integral membrane protein which is a subunit of 1,3-beta-D-glucan synthase. Proc. Natl. Acad. Sci. USA 91:12907-12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douglas, C. M., J. A. D'Ippolito, G. J. Shei, M. Meinz, J. Onishi, J. A. Marrinan, W. Li, G. K. Abruzzo, A. Flattery, K. Bartizal, A. Mitchell, and M. B. Kurtz. 1997. Identification of the FKS1 gene of Candida albicans as the essential target of 1,3-β-d-glucan synthase inhibitors. Antimicrob. Agents Chemother. 41:2471-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edlind, T. D., K. W. Henry, J. P. Vermitsky, M. P. Edlind, S. Raj, and S. K. Katiyar. 2005. Promoter-dependent disruption of genes: simple, rapid, and specific PCR-based method with application to three different yeast. Curr. Genet. 48:117-125. [DOI] [PubMed] [Google Scholar]
- 12.Espinel-Ingroff, A. 2003. In vitro antifungal activities of anidulafungin and micafungin, licensed agents and the investigational triazole posaconazole as determined by NCCLS methods for 12,052 fungal isolates: review of the literature. Rev. Iberoam. Micol. 20:121-136. [PubMed] [Google Scholar]
- 13.Forrest, G. N., E. Weekes, and J. K. Johnson. 2008. Increasing incidence of Candida parapsilosis candidemia with caspofungin usage. J. Infect. 56:126-129. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Lopez, A., A. Alastruey-Izquierdo, D. Rodriguez, B. Almirante, A. Pahissa, J. Rodriguez-Tudela, and M. Cuenca-Estrella. 2008. Prevalence and susceptibility profile of Candida metapsilosis and Candida orthopsilosis: results from population-based surveillance of candidemia in Spain. Antimicrob. Agents Chemother. 52:1506-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katiyar, S., M. Pfaller, and T. Edlind. 2006. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 50:2892-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazur, P., N. Morin, W. Baginsky, M. el Sherbeini, J. A. Clemas, J. B. Nielsen, and F. Foor. 1995. Differential expression and function of two homologous subunits of yeast 1,3-β-d-glucan synthase. Mol. Cell. Biol. 15:5671-5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melo, A. S., A. L. Colombo, and B. A. Arthington-Skaggs. 2007. Paradoxical growth effect of caspofungin observed on biofilms and planktonic cells of five different Candida species. Antimicrob. Agents Chemother. 51:3081-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison, V. A. 2006. Echinocandin antifungals: review and update. Expert Rev. Anti Infect. Ther. 4:325-342. [DOI] [PubMed] [Google Scholar]
- 19.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard, 2nd ed. NCCLS document M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 20.Odds, F. C., M. Motyl, R. Andrade, J. Bille, E. Canton, M. Cuenca-Estrella, A. Davidson, C. Durussel, D. Ellis, E. Foraker, A. W. Fothergill, M. A. Ghannoum, R. A. Giacobbe, M. Gobernado, R. Handke, M. Laverdiere, W. Lee-Yang, W. G. Merz, L. Ostrosky-Zeichner, J. Peman, S. Perea, J. R. Perfect, M. A. Pfaller, L. Proia, J. H. Rex, M. G. Rinaldi, J. L. Rodriguez-Tudela, W. A. Schell, C. Shields, D. A. Sutton, P. E. Verweij, and D. W. Warnock. 2004. Interlaboratory comparison of results of susceptibility testing with caspofungin against Candida and Aspergillus species. J. Clin. Microbiol. 42:3475-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park, S., R. Kelly, J. N. Kahn, J. Robles, M. J. Hsu, E. Register, W. Li, V. Vyas, H. Fan, G. Abruzzo, A. Flattery, C. Gill, G. Chrebet, S. A. Parent, M. Kurtz, H. Teppler, C. M. Douglas, and D. S. Perlin. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 49:3264-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perlin, D. S. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat. 10:121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfaller, M. A., L. Boyken, R. J. Hollis, J. Kroeger, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2008. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J. Clin. Microbiol. 46:150-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfaller, M. A., L. Boyken, R. J. Hollis, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2006. Global surveillance of in vitro activity of micafungin against Candida: a comparison with caspofungin by CLSI-recommended methods. J. Clin. Microbiol. 44:3533-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaller, M. A., L. Boyken, R. J. Hollis, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2005. In vitro activities of anidulafungin against more than 2,500 clinical isolates of Candida spp., including 315 isolates resistant to fluconazole. J. Clin. Microbiol. 43:5425-5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfaller, M. A., L. Boyken, R. J. Hollis, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2006. In vitro susceptibilities of Candida spp. to caspofungin: four years of global surveillance. J. Clin. Microbiol. 44:760-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfaller, M. A., and D. J. Diekema. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfaller, M. A., D. J. Diekema, D. L. Gibbs, V. A. Newell, K. P. Ng, A. Colombo, J. Finquelievich, R. Barnes, and J. Wadula. 2008. Geographic and temporal trends in isolation and antifungal susceptibility of Candida parapsilosis: a global assessment from the ARTEMIS DISK antifungal surveillance program. 2001 to 2005. J. Clin. Microbiol. 46:842-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossignol, T., M. E. Logue, K. Reynolds, M. Grenon, N. F. Lowndes, and G. Butler. 2007. Transcriptional response of Candida parapsilosis following exposure to farnesol. Antimicrob. Agents Chemother. 51:2304-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Safdar, A., T. W. Bannister, and Z. Safdar. 2004. The predictors of outcome in immunocompetent patients with hematogenous candidiasis. Int. J. Infect. Dis. 8:180-186. [DOI] [PubMed] [Google Scholar]
- 32.Safdar, A., D. Perlin, and D. Armstrong. 2002. Hematogenous infections due to Candida parapsilosis: changing trends in fungemic patients at a comprehensive cancer center during the last four decades. Diagn. Microbiol. Infect. Dis. 44:11. [DOI] [PubMed] [Google Scholar]
- 33.Saiman, L., E. Ludington, J. D. Dawson, J. E. Patterson, S. Rangel-Frausto, R. T. Wiblin, H. M. Blumberg, M. Pfaller, M. Rinaldi, J. E. Edwards, R. P. Wenzel, and W. Jarvis. 2001. Risk factors for Candida species colonization of neonatal intensive care unit patients. Pediatr. Infect. Dis. J. 20:1119-1124. [DOI] [PubMed] [Google Scholar]
- 34.Smith, W. L., and T. D. Edlind. 2002. Histone deacetylase inhibitors enhance Candida albicans sensitivity to azoles and related antifungals: correlation with reduction in CDR and ERG upregulation. Antimicrob. Agents Chemother. 46:3532-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tavanti, A., A. D. Davidson, N. A. Gow, M. C. Maiden, and F. C. Odds. 2005. Candida orthopsilosis and Candida metapsilosis spp. nov. to replace Candida parapsilosis groups II and III. J. Clin. Microbiol. 43:284-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiederhold, N. P., and R. E. Lewis. 2003. The echinocandin antifungals: an overview of the pharmacology, spectrum and clinical efficacy. Expert Opin. Investig. Drugs 12:1313-1333. [DOI] [PubMed] [Google Scholar]