Abstract
The synergy of the activities between chloroquine and various human immunodeficiency virus protease inhibitors was investigated in chloroquine-resistant and -sensitive malaria parasites. In both in vitro and in vivo assay systems, ritonavir was found to be the most potent in potentiating the antimalarial action of chloroquine.
Chloroquine has been commonly used as an antimalarial drug due to its rapid onset of action, easy use, low cost, and toxicity (18). However, the resistance to chloroquine developed by the malaria parasite Plasmodium falciparum has significantly reduced the efficacy of the drug. Many efforts have been made to restore chloroquine's efficacy, and several compounds, such as calcium channel blockers, tricyclic antidepressants, antipsychotic calmodulin antagonists, and histamine H1 receptor antagonists, have been found to be able to enhance the antimalarial action of chloroquine in resistant lines in vitro and in vivo (7). Most of these agents, however, have little intrinsic antimalarial activity and hardly enhance the sensitivity to chloroquine in sensitive lines. In addition, high doses of these agents, which could be toxic to the host and lead to systemic side effects, are required for effective synergistic activity.
Previous studies have suggested that a number of human immunodeficiency virus protease inhibitors (HIV PIs) are active against P. falciparum in vitro (1, 12, 15) and against Plasmodium chabaudi in a murine model (1). Although the mechanism of the antimalarial activities of HIV PIs is not clear, saquinavir and ritonavir behaved synergistically with chloroquine against the chloroquine-resistant line in vitro (14). In this report, the synergistic effects of five HIV PIs, saquinavir, lopinavir, atazanavir, ritonavir, and nelfinavir, with chloroquine on both the chloroquine-sensitive clone 3D7 and the chloroquine-resistant clone Dd2 were investigated in vitro. Potentiation of chloroquine antimalarial action by HIV PIs was further studied in vivo in a rodent model of malaria.
P. falciparum clones were maintained continuously in blood group O+ human erythrocytes and 10% human serum in a gas mixture consisting of 7% CO2, 5%O2, and 88% N2 (16) and synchronized by serial treatments with 5% d-sorbitol (10). Drug interaction studies were performed with a modification of the fixed-ratio method as previously described (6). Parasite growth was determined by light microscopy of Giemsa-stained smears, and the percent inhibition of growth was calculated. Fractional inhibitory concentrations (2, 6) were determined, and isobolograms were constructed to quantitate the interaction between HIV PIs and chloroquine. The degree of interaction was indicated by the parameter I. Positive values of I indicate synergism, and negative values represent antagonism; addition occurs when they equal zero (2). In vivo drug interactions were measured by using a P. chabaudi rodent malaria 4-day suppressive test (3, 5). Experimental groups of six female NIH mice (average body weight, ∼25 g) were inoculated by intraperitoneal injection with 2 × 107 or 5 × 107 parasitized erythrocytes of the chloroquine-sensitive line ASS or the chloroquine-resistant line ASCQ, respectively. At 4, 24, 48, and 72 h postinoculation, the mice were orally administered drugs. On day 4, thin blood films were made, and the parasitemias were determined. All experiments included a drug-free control group, a chloroquine-treated group, and groups treated with different doses of the HIV PIs administered alone or in combination with chloroquine. Statistical analysis was carried out by Student's t test, and P values of less than 0.05 were considered significant.
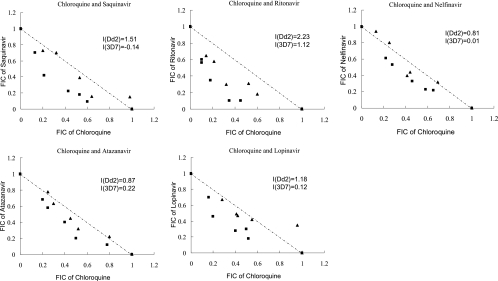
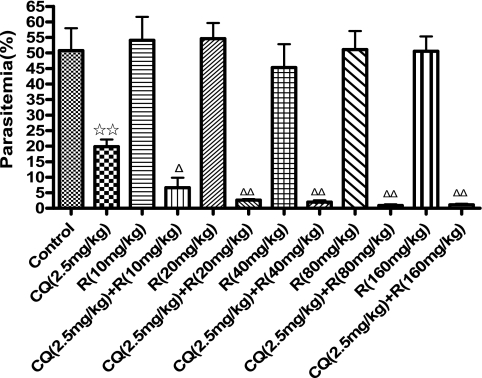
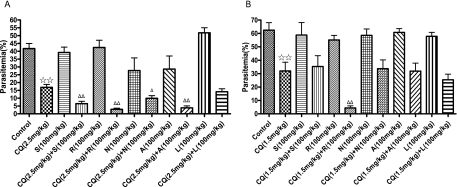
Isobologram analysis showed that all HIV PIs tested were able to enhance the antimalarial action of chloroquine (I > 0), and ritonavir exerted the most synergistic action (I = 2.23) on chloroquine against the chloroquine-resistant clone Dd2 (Fig. 1). However, all curves following roughly the diagonal (I ≈ 0), except ritonavir (I = 1.12), were obtained with chloroquine-sensitive clone 3D7, clearly evidencing simple additive effects of these combinations (Fig. 1). Because ritonavir was strongly synergistic with chloroquine against both chloroquine-sensitive and -resistant clones in vitro, we further examined its synergism activity by in vivo experiments. It was observed that administration of 10 to 160 mg ritonavir per kg body weight alone did not affect the growth of chloroquine-resistant parasites and showed no signs of toxicity in mice. When ritonavir was coadministered with 2.5 mg/kg chloroquine, significant parasite-suppressive effects were observed in the chloroquine-resistant clone of the parasite in comparison with the chloroquine-alone control group, and all doses of ritonavir tested showed similar levels of synergy with chloroquine (Fig. 2). Oral administration of saquinavir, atazanavir, ritonavir, or nelfinavir at 100 mg/kg also potentiated the antimalarial action of chloroquine in resistant parasites, but the same dose of lopinavir did not restore sensitivity to chloroquine (Fig. 3A). The in vivo experiment also revealed that the most efficient compound for potentiating the antimalarial action of chloroquine among these HIV PIs was ritonavir (Fig. 3A). Furthermore, the synergistic effect between ritonavir and chloroquine was pronounced either in the chloroquine-sensitive line P. chabaudi ASS (Fig. 3B) or in the resistant line ASCQ (Fig. 3A). The synergy of chloroquine and ritonavir in ASS and ASCQ parasites is probably partly due to the pharmacokinetic interaction of the two drugs. The potent ability of ritonavir in inhibiting the cytochrome P450 metabolic pathway (9) could be exploited to increase or maintain the level of chloroquine in the blood.
FIG. 1.
Isobolograms of the interaction of chloroquine with HIV PIs against chloroquine-resistant clone Dd2 (▪) and chloroquine-sensitive clone 3D7 (▴) in vitro. The effect of the combination of HIV PIs with chloroquine against malaria parasites was tested by titration of the two drugs at fixed ratios proportional to their 50% effective concentrations. The results were expressed as fractional inhibitory concentrations (FICs), and I values were calculated to quantitate the interaction between HIV PIs and chloroquine. The results are means of three independent experiments.
FIG. 2.
Synergistic suppression of chloroquine resistance of P. chabaudi ASCQ by chloroquine in combination with ritonavir. Mice infected with chloroquine-resistant P. chabaudi ASCQ were treated with the single drug chloroquine (CQ) or ritonavir (R) or a combination of both at specified dosages by oral administration. The results are expressed as means and standard errors (n = 6). ⋆⋆, P < 0.01 versus the untreated group; ▵, P < 0.05, and ▵▵, P < 0.01 versus single CQ- or R-treated groups.
FIG. 3.
Synergistic suppression of P. chabaudi ASCQ and ASS by chloroquine in combination with HIV PIs. Mice infected with either the chloroquine-resistant line ASCQ (A) or the chloroquine-sensitive line ASS (B) of P. chabaudi were treated with a single drug (CQ or HIV PIs) or a combination of two drugs at specified dosages by oral administration. The results shown are means and standard errors (n = 6). ⋆⋆, P < 0.01 versus the untreated group; ▵, P < 0.05, and ▵▵, P < 0.01 versus the single-drug CQ-treated group. CQ, chloroquine; S, saquinavir; L, lopinavir; A, atazanavir; R, ritonavir; N, nelfinavir.
Mutations in pfcrt (P. falciparum chloroquine resistance transporter) play an important role in producing the chloroquine resistance phenotype in P. falciparum. The mutations of PfCRT may alter the substrate specificity of the transporter and facilitate the diffusion of the diprotonated chloroquine from the digestive vacuole (17). Nitrogen atoms of the HIV PIs, which can be protonated in acidic environments, and the hydrophobic heterocyclic ring may play certain roles in their reversal activities in vitro. Protonated HIV PIs may bind to the mutated PfCRT and block the pore because of its hydrophobicity. Consequently, chloroquine is not effluxed from the digestive vacuole and may efficiently reach therapeutic concentration to kill the parasites. Although P. chabaudi has a pfcrt-like gene (pccg10), it is unlikely to be linked to chloroquine resistance in that species (8). Our observation that treatment of mice with the HIV PIs increased chloroquine susceptibilities in both human and rodent malaria parasite species suggests that other mechanisms may exist. It has been shown that the increased glutathione levels in resistant malaria parasite species may enable the detoxification of chloroquine in resistant parasites (4, 11). HIV PIs may interfere with glutathione metabolism to increase the chloroquine sensitivity in both P. falciparum and P. chabaudi malaria parasite species. In addition, ritonavir may reduce glutathione levels in chloroquine-resistant and -sensitive parasites to enhance the antimalarial action of chloroquine. Further experiments are needed to determine whether and how HIV PIs influence the intracellular levels of glutathione to restore sensitivity to chloroquine.
HIV PIs can directly inhibit the growth of P. falciparum in vitro at or below the concentrations found in human plasma after oral drug administration (1, 12, 15). Because HIV PIs have relatively short half-lives, it might be beneficial to use these PIs for malaria patients who are being treated with the long half-life chloroquine to prevent recrudescence (15). Previous studies have demonstrated that chloroquine has anti-HIV effect and showed synergism with HIV PIs (13). The potential ability of chloroquine to overcome resistance to HIV PIs could be important in the treatment of drug-experienced HIV-positive subjects. Therefore, coadministration of HIV PIs and chloroquine to HIV/malaria parasite-coinfected or singly infected individuals may enhance the efficacies of the two groups of drugs in patients.
Acknowledgments
We thank Zhong Su at our institute for his critical review of the manuscript. All parasite strains used in this study were obtained from the U.S. Malaria Research and Reference Reagent Center.
This work was supported in part by a grant from Guangzhou Science and Technology Programs (2007Z3-E4131).
Footnotes
Published ahead of print on 28 April 2008.
REFERENCES
- 1.Andrews, K. T., D. P. Fairlie, P. K. Madala, J. Ray, D. M. Wyatt, P. M. Hilton, L. A. Melville, L. Beattie, D. L. Gardiner, R. C. Reid, M. J. Stoermer, T. Skinner-Adams, C. Berry, and J. S. McCarthy. 2006. Potencies of human immunodeficiency virus protease inhibitors in vitro against Plasmodium falciparum and in vivo against murine malaria. Antimicrob. Agents Chemother. 50:639-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canfield, C. J., M. Pudney, and W. E. Gutteridge. 1995. Interactions of atovaquone with other antimalarial drugs against Plasmodium falciparum in vitro. Exp. Parasitol. 80:373-381. [DOI] [PubMed] [Google Scholar]
- 3.Deloron, P., L. K. Basco, B. Dubois, C. Gaudin, F. Clavier, J. Le Bras, and F. Verdier. 1991. In vitro and in vivo potentiation of chloroquine against malaria parasites by an enantiomer of amlodipine. Antimicrob. Agents Chemother. 35:1338-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubois, V. L., D. F. Platel, G. Pauly, and J. Tribouley-Duret. 1995. Plasmodium berghei: implication of intracellular glutathione and its related enzyme in chloroquine resistance in vivo. Exp. Parasitol. 81:117-124. [DOI] [PubMed] [Google Scholar]
- 5.Fidock, D. A., P. J. Rosenthal, S. L. Croft, R. Brun, and S. Nwaka. 2004. Antimalarial drug discovery: efficacy models for compound screening. Nat. Rev. Drug. Discov. 3:509-520. [DOI] [PubMed] [Google Scholar]
- 6.Fivelman, Q. L., I. S. Adagu, and D. C. Warhurst. 2004. Modified fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisinin against drug-resistant strains of Plasmodium falciparum. Antimicrob. Agents Chemother. 48:4097-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henry, M., S. Alibert, E. Orlandi-Pradines, H. Bogreau, T. Fusai, C. Rogier, J. Barbe, and B. Pradines. 2006. Chloroquine resistance reversal agents as promising antimalarial drugs. Curr. Drug Targets 7:935-948. [DOI] [PubMed] [Google Scholar]
- 8.Hunt, P., P. V. Cravo, P. Donleavy, J. M. Carlton, and D. Walliker. 2004. Chloroquine resistance in Plasmodium chabaudi: are chloroquine-resistance transporter (crt) and multi-drug resistance (mdr1) orthologues involved? Mol. Biochem. Parasitol. 133:27-35. [DOI] [PubMed] [Google Scholar]
- 9.Ikezoe, T., Y. Hisatake, T. Takeuchi, Y. Ohtsuki, Y. Yang, J. W. Said, H. Taguchi, and H. P. Koeffler. 2004. HIV-1 protease inhibitor, ritonavir, a potent inhibitor of CYP3A4, enhanced the anticancer effects of docetaxel in androgen-independent prostate cancer cells in vitro and in vivo. Cancer Res. 64:7426-7431. [DOI] [PubMed] [Google Scholar]
- 10.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 11.Meierjohann, S., R. D. Walter, and S. Müller. 2002. Regulation of intracellular glutathione levels in erythrocytes infected with chloroquine-sensitive and chloroquine-resistant Plasmodium falciparum. Biochem. J. 368:761-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parikh, S., J. Gut, E. Istvan, D. E. Goldberg, D. V. Havlir, and P. J. Rosenthal. 2005. Antimalarial activity of human immunodeficiency virus type 1 protease inhibitors. Antimicrob. Agents Chemother. 49:2983-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savarino, A., M. B. Lucia, E. Rastrelli, S. Rutella, C. Golotta, E. Morra, E. Tamburrini, C. F. Perno, J. R. Boelaert, K. Sperber, and R. Cauda. 2004. Anti-HIV effects of chloroquine: inhibition of viral particle glycosylation and synergism with protease inhibitors. J. Acquir. Immune Defic. Syndr. 35:223-232. [DOI] [PubMed] [Google Scholar]
- 14.Skinner-Adams, T. S., K. T. Andrews, L. Melville, J. McCarthy, and D. L. Gardiner. 2007. Synergistic interactions of the antiretroviral protease inhibitors saquinavir and ritonavir with chloroquine and mefloquine against Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 51:759-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skinner-Adams, T. S., J. S. McCarthy, D. L. Gardiner, P. M. Hilton, and K. T. Andrews. 2004. Antiretrovirals as antimalarial agents. J. Infect. Dis. 190:1998-2000. [DOI] [PubMed] [Google Scholar]
- 16.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 17.Valderramos, S. G., and D. A. Fidock. 2006. Transporters involved in resistance to antimalarial drugs. Trends. Pharmacol. Sci. 27:594-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward, S. A., and P. G. Bray. 2001. Is reversal of chloroquine resistance ready for the clinic? Lancet 357:904. [DOI] [PubMed] [Google Scholar]