Abstract
The toxicity and antileishmanial effectiveness of a novel liposome formulation of meglumine antimoniate in mongrel dogs with visceral leishmaniasis (VL) obtained from a region where VL is endemic in Brazil have been investigated. Groups of 12 animals received by the intravenous route four doses (with 4-day intervals) of either liposomal meglumine antimoniate (group I [GI], 6.5 mg Sb/kg of body weight/dose), empty liposomes (GII), or isotonic saline (GIII). Evaluation of markers of hematopoietic, hepatic, and renal functions before and just after treatment showed no significant change. On the other hand, transitory adverse reactions, including prostration, defecation, tachypnea, and sialorrhea, were observed during the first 15 min after injections in GI and GII. Parasitological evaluation of sternal bone marrow 4 days after the last dose showed a significant reduction of parasite burden in GI, compared to the other groups. Immunocytochemical evaluations of the skin, bone marrow, cervical lymph nodes, livers, and spleens of dogs for parasites, 150 days after treatment, indicated significant parasite suppression (higher than 95.7%) in the lymph nodes, livers, and spleens of GI, compared to control groups. Feeding of Lutzomyia longipalpis phlebotomines on dogs from GI, 150 days after treatment, resulted in a significant reduction of sand fly infection efficiency, compared to feeding on animals from GII and GIII. This is the first report of both long-term parasite suppression and reduction of infectivity to sand flies in naturally infected dogs following treatment with a liposome-encapsulated drug. Importantly, this was achieved using a 20-fold-lower cumulative dose of Sb than is used for conventional antimonial treatment.
The leishmaniases are a group of diseases produced by invasion of the reticuloendothelial system of a mammalian host by a parasite of the genus Leishmania. This parasite is found as a motile promastigote in the sand fly and transforms into an amastigote when engulfed by host macrophages (1). Visceral leishmaniasis (VL) is the most severe form of the disease, causing death of humans if not treated. The pentavalent organoantimonial complexes meglumine antimoniate and sodium stibogluconate are the first-line drugs for the treatment of all forms of leishmaniasis (9). The achievement of a complete cure in dogs with VL, or at least the blockade of infectivity to sand flies, is currently a great challenge, since dogs are the main reservoir for the transmission of VL to humans and respond poorly to conventional treatment with pentavalent antimonials (2).
In the 1970s, a major advance occurred when it was found that liposome-encapsulated antimonial drugs were hundreds of times more effective than unencapsulated ones for the treatment of experimental VL (5). This spectacular effect of liposome encapsulation was attributed to the ability of liposomes to provide sustained drug release and to their natural tendency to be cleared from the circulation by the fixed macrophages of the mononuclear phagocyte system, mainly the liver, spleen, and bone marrow, which are major sites of parasite infection. Similar results were obtained with other antileishmanial agents (4, 29) and other vesicular systems made from nonionic surfactants (7) instead of phospholipids.
Much effort has also been devoted to the evaluation of liposomal formulations in dogs (12, 13, 30, 33, 36-38, 44, 45); however, very few studies used the clinically relevant naturally infected dogs and none performed important parasitological evaluations, such as determinations of skin parasite load and dog infectivity to sand flies.
Despite the need to improve antimonial chemotherapy and the extremely promising results obtained with liposomes in experimental models of VL, no pharmaceutical composition associating liposomes and antimonials has reached commercialization so far. This fact can be attributed, at least in part, to the technological difficulties usually encountered in the preparation of a stable liposomal composition for water-soluble substances. This is in contrast with the lipophilic fungicidal and leishmanicidal drug amphotericin B, whose liposomal formulation, called AmBisome, has recently been approved by the Food and Drug Administration for treatment of VL (27). On the other hand, the high cost of the latter formulation limits its large-scale use in developing countries. Furthermore, although AmBisome did promote clinical and parasitological improvements in dogs naturally infected with Leishmania infantum, cure was not achieved (33).
Novel liposomal formulations of meglumine antimoniate that are obtained through rehydration of freeze-dried empty liposomes with an aqueous solution of the antimonial compound have recently been described (18-20, 38). A significant technological advantage of this method over conventional ones (3, 35) is that liposomes may be stored as preformed freeze-dried empty vesicles and that rehydration may be performed just before use. Two liposomal formulations differing in their mean vesicle diameters were obtained (38). The large-liposome formulation (mean hydrodynamic vesicle diameter of 1,200 nm) was previously evaluated in dogs with VL (14, 37). Following a multiple-dose regimen, this formulation resulted in a significantly lower number of positive dogs (compared to the group of dogs treated with empty liposomes or that of untreated dogs) but was unable to clear Leishmania parasites from the bone marrow, suggesting that this tissue may be critical for treatment with this liposomal formulation.
In a recent study, the pharmacokinetics of the small-liposome formulation of meglumine antimoniate (mean hydrodynamic vesicle diameter of 410 nm) was evaluated in mongrel dogs naturally infected by Leishmania (Leishmania) chagasi. Importantly, this formulation was found to target antimony to the bone marrow of dogs at a threefold-higher level than the formulation of meglumine antimoniate in large liposomes (38), suggesting that it may more effectively clear parasites from this tissue.
The aim of the present study was to evaluate the small-liposome formulation of meglumine antimoniate in dogs naturally infected by Leishmania (Leishmania) chagasi for its side effects and its antileishmanial efficacy. In addition to the clinical evaluation of dogs, complete parasitological evaluations were performed through determination of the parasitic load in the liver, spleen, bone marrow, lymph nodes, and skin as well as of the infectivity of dogs to sand flies.
MATERIALS AND METHODS
Animals.
Fifty mongrel dogs (weighing 8 to 15 kg) of unknown age naturally infected with L. (Leishmania) chagasi, exhibiting different clinical forms of canine leishmaniasis (26), were identified and captured during an epidemiological survey carried out by the Control Zoonosis Center in Santa Luzia City Hall (Minas Gerais State, southeast Brazil). The animals manifested the classical clinical signs of canine leishmaniasis. Lymphoadenopathy was the most frequent, followed by skin disorders, which included, in decreasing order of frequency, hypotricosis/alopecia, dull fur, exfoliative dermatitis, and skin ulcers.
The serological diagnosis was established by the Serology Laboratory of the Institute of Biological Sciences, Federal University of Minas Gerais (UFMG) by indirect immunofluorescence assay and enzyme-linked immunosorbent assay. All animals were found to be positive by indirect immunofluorescence assay (≥1:40 dilutions) and enzyme-linked immunosorbent assay (optical density > 0.100; ≥1:400 dilutions). In addition, parasitological diagnosis was performed by observation of parasite forms in both cytological examinations and cultures of bone marrow aspirates in Novy-Nicolle-McNeal medium enriched with minimum essential medium. Prior to treatment, the animals were maintained in quarantine in kennels and were treated for intestinal helminthic infections (Canex Composto; Vetbrands Health Animal) and ectoparasite infestations (Front Line; Merial) and immunized against viral infections (Defensor and Vanguard HTLP 5/CV-L vaccine; Pfizer, Brazil). During the whole experimental period, the dogs were housed in a screened kennel to avoid reinfection and received drinking water and a balanced feed ad libitum (Pedigree Champ; Effem). The present research adhered to the Principles of Laboratory Animal Care (28a) and received approval from the Ethical Committee for the use of Experimental Animals of the UFMG (Brazil), protocol 123/05.
Materials.
Cholesterol and dicetylphosphate were purchased from Sigma Co. (St. Louis, MO). Distearoylphosphatidylcholine was obtained from Avanti Polar Lipids Inc. (Alabaster, AL). N-Methyl-d-glucamine and antimony pentachloride (SbCl5; 99%) were obtained from Aldrich Chemical Co. (Milwaukee, WI).
Preparation of meglumine antimoniate.
Meglumine antimoniate was synthesized as previously described (16, 17) from an equimolar mixture of N-methyl-d-glucamine and pentavalent antimony oxyhydrated in water. The resulting product contained approximately 29% antimony by weight, as determined by inductively coupled plasma optical emission spectroscopy using a Perkin-Elmer Optima 3000 plasma emission spectrometer.
Preparation and characterization of meglumine antimoniate-containing liposomes.
Meglumine antimoniate-containing liposomes with reduced size were prepared as described previously (38). Briefly, small unilamellar vesicles (SUVs) were obtained by ultrasonication of a suspension of multilamellar vesicles in deionized water, made from distearoylphosphatidylcholine, cholesterol, and dicetylphosphate (molar ratio of 5:4:1) at the final lipid concentration of 55 g/liter. After filtration through a sterile 0.22-μm membrane, the SUV suspension was mixed with sucrose at a sugar/lipid mass ratio of 3:1 and a final sugar concentration of 0.3 M. The resulting mixture was immediately frozen in liquid nitrogen and subsequently dried (freeze dryer; 4.5 liters; Labconco, United Kingdom). Rehydration of the dried powder was performed with a meglumine antimoniate aqueous solution (antimony concentration of 0.65 M) and phosphate-buffered saline (PBS; 0.15 M NaCl, 0.01 M phosphate, pH 7.2) as follows. Forty percent of the original SUV volume of meglumine antimoniate solution was added to the lyophilized powder, and the mixture was vortexed and incubated for 30 min at 55°C. The same volume of PBS was then added, and the mixture was vortexed and incubated for 30 min at 55°C. Drug-containing liposomes were separated from nonencapsulated drug by centrifugation (14,000 × g, 30 min). The liposome pellet was then washed twice and finally resuspended in isotonic saline at a final antimony concentration of about 10 g/liter. The concentration of encapsulated antimony and the phospholipid concentration in the resulting liposome suspension were determined using previously described colorimetric assays (34, 38). The drug encapsulation efficiency and the final Sb/lipid ratio were equal to 40% ± 4% and 0.25:1 (wt/wt), respectively. The final vesicular suspension was sized by photon correlation spectroscopy at 25°C with a 90° scattering angle and using a channel correlator (ZEN3500; Malvern Instruments, United Kingdom) in conjunction with a laser of wavelength 532 nm. The mean hydrodynamic diameter of the vesicles was 400 nm, with a mean polydispersity factor of 0.3. Empty liposomes with a comparable mean hydrodynamic diameter were obtained using the same method as that described above, except that the meglumine antimoniate solution was replaced by a 0.65 M N-methyl-d-glucamine aqueous solution at pH 7.2.
Treatment protocol.
Thirty-six dogs were stratified by weight, sex, and clinical forms and randomly distributed into three groups, each group containing initially four asymptomatic, four oligosymptomatic, and four symptomatic dogs (26). Group I was treated with four doses of liposomal meglumine antimoniate at 6.5 mg Sb/kg of body weight/dose given with 4-day intervals. Group II received four doses of antimony-free liposomes given at the same lipid dose as that for group I. Group III received four doses of isotonic saline given at the same volume as that for group I. The animals were clinically monitored during the 5 months of the experiment and were euthanized 150 days after treatment with a lethal dose of intravenous (i.v.) thiopental. The mean value of the clinical state in each animal group was calculated by assuming values of 1, 2, 3, and 4 for asymptomatic, oligosymptomatic, and symptomatic dogs and dogs subjected to euthanasia due to disease evolution, respectively.
Toxicity studies.
The animals from groups I, II, and III were evaluated for clinical and behavioral changes as well as for changes in some hematological and biochemical markers of hematopoietic, hepatic, and renal functions before and 4 days after treatment. Temperature and food and water intake were registered, and heart and respiratory frequencies were measured. Blood samples were taken from the cephalic vein contralateral to that used for infusion for analysis of hemogram (red blood cells, packed cell volume, hemoglobin, white blood cells, neutrophils, lymphocytes, monocytes, eosinophils, and platelets), renal profile (urea and creatinine), and hepatic profile (aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and total bilirubins).
In another independent experiment, toxicity was evaluated in mongrel dogs with VL following a single dose of liposomal meglumine antimoniate. These animals received an i.v. bolus injection of the liposome formulation at 4.2 mg Sb/kg of body weight and were sacrificed 24 h (three animals), 48 h (three animals), 72 h (three animals), and 96 h (five animals) after administration. The dogs were monitored continuously for clinical alterations. Laboratory parameters were evaluated at the time intervals of 0, 24, 48, 72, and 96 h.
Parasitological evaluations.
Immunocytochemical evaluation for Leishmania tissue burden was carried out after euthanasia of animals as described previously (25). Briefly, samples of ear skin, bone marrow, cervical lymph node, spleen, and liver were collected and fixed in 10% neutral buffered formalin solution. All tissue samples were dehydrated, cleared, embedded in paraffin, and cut into 4- to 5-μm-thick sections for immunocytochemical studies. Deparaffinated slides were hydrated and incubated with hydrogen peroxide (4% [vol/vol]) in 0.01 M PBS, pH 7.2, followed by incubation with normal goat serum (diluted 1:50). Heterologous immune serum from dogs naturally infected with L. chagasi (diluted 1:100 in 0.01 M PBS) was used as the primary antibody. The slides were incubated for 18 to 22 h at 4°C in a humid chamber, washed in PBS, incubated with biotinylated goat anti-mouse and anti-rabbit antibodies (LSAB2 kit; Link-DAKO, CA), washed once more with PBS, and incubated with streptavidin-peroxidase complex (LSAB2 kit; Link-DAKO, CA) for 20 min at room temperature. The reaction was developed with 0.024% diaminobenzidine (Sigma, St. Louis, MO) and hydrogen peroxide (0.16% [vol/vol]). The slides were counterstained with Harris's hematoxylin, dehydrated, cleared, and mounted with coverslips. The Leishmania tissue burden was determined by counting the immunolabeled amastigotes in 20 microscopic fields randomly chosen.
Giemsa-stained sternal bone marrow aspirates were also obtained from animals in each group 4 days after treatment and used for the determination of the number of amastigotes/1,000 cell nuclei. Giemsa-stained impression smears of spleen were obtained from all animals after euthanasia, and the Leishman-Donovan unit value was calculated as the number of amastigotes per 1,000 host cell nuclei × organ weight in grams.
Xenodiagnosis.
The Lutzomyia longipalpis phlebotomines used in the present study belonged to generation F1 and were kindly provided by Edelberto Santos Dias, Institute Research Renée Rachou, Fundation Oswaldo Cruz, Belo Horizonte, Minas Gerais, Brazil. The xenodiagnosis was realized as previous described (15). In brief, sand flies were fed on dogs by putting the insects in a specially designed receptacle (FleboContainer). Approximately 40 females and about 20 males were used in each experimental replicate. During 40 min in a darkened room, the FleboContainer netting was placed in direct contact with the medial skin of the dog's right ear (43), previously sedated with 0.8 or 1.2 ml/kg of acepromazine (Univet). After feeding, the insects were returned to the insectary for 5 days and provided with a solution of fructose in distilled water (50:50, vol/vol) and kept at 28°C. Five days after feeding, live male and female sand flies were counted and the engorged females were dissected in a drop of PBS. The guts were examined under interference microscopy (24), and the proportions of infected females and appearance of flagellates within the digestive tract of each sand fly were recorded. The approximate numbers of promastigotes and their distribution in different parts of the gut were estimated based on observations using the 40× objective of a microscope. The intensity of infection was classified as “low” when few promastigotes were seen in the sand fly midgut and they had little or no motility. It was classified as “medium” when many motile promastigotes where seen in the fore- and midgut and as “high” when large numbers of promastigotes were seen throughout the gut and rosettes of parasites were visible (15).
Statistical analysis.
Comparisons of tissue parasitic loads between two groups were performed with the Mann-Whitney test or Student's t test, while those between three groups were carried out using analysis of variance or Kruskal-Wallis tests, followed by Tukey′s or Dunn's multiple comparison test. Fisher's exact, chi-square, and Kruskal-Wallis tests were used to compare the clinical states of animals between the different groups. The analysis of correlations between variables (i.e., between clinical condition, parasite load, and infectivity of dogs to sand flies) was performed by the method of Spearman. The chi-square test was used to compare the efficiencies of infected sand flies between different groups. All statistical analyses were performed using Prism 3.0 and Minitab 14 software (95% significance level).
RESULTS
Impact of treatment with liposomal meglumine antimoniate on clinical parameters.
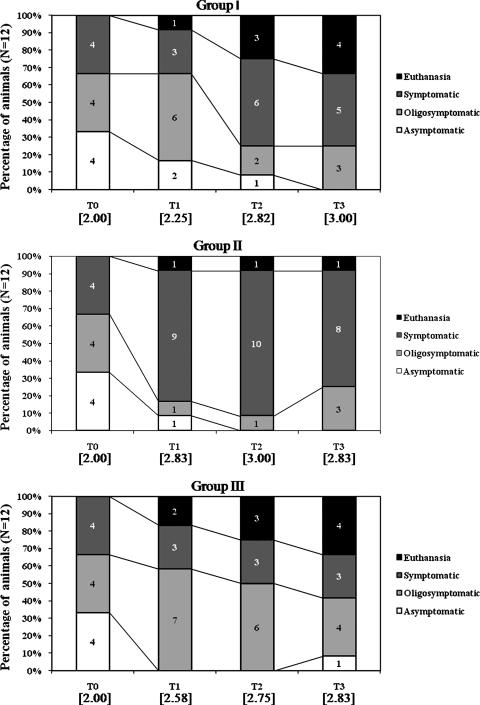
Treatment of mongrel dogs naturally infected with Leishmania chagasi was performed with either liposomal meglumine antimoniate (group I, n = 12, 4 doses of 6.5 mg Sb/kg, with an interval of 4 days between each dose), antimony-free liposomes (group II, n = 12, liposomes given at the same lipid dose as for group I), or saline (group III, n = 12). In the present study, after inclusion of animals according to laboratory, serological, and parasitological criteria, animals were classified according the clinical exams as asymptomatic, oligosymptomatic, or symptomatic, as described previously (26). Since the clinical condition of the dogs is expected to influence the response to treatment (6, 26), the different groups were designed to have initially the same proportion of dogs in each category (four animals of each category), as shown in Fig. 1.
FIG. 1.
Clinical status of dogs naturally infected with Leishmania (Leishmania) chagasi, just before treatment (T0) and 30 (T1), 90 (T2), and 150 (T3) days after treatment with different formulations. Group I received liposomal meglumine antimoniate as four doses by the i.v. route of 6.5 mg Sb/kg/dose, with 4-day intervals; group II received antimony-free liposomes as four doses by the i.v. route of liposomes at the same lipid dose as in group I, with 4-day intervals; group III received saline as four doses by the i.v. route at the same volume as group I, with 4-day intervals. Numbers within brackets represent the mean values of the clinical state for each of the three groups at each time period, assuming values of 1, 2, 3, and 4 for asymptomatic, oligosymptomatic, and symptomatic dogs and dogs subjected to euthanasia (due to disease evolution), respectively.
Before treatment, the main clinical signs were lymphadenopathy, alopecia, dull fur, exfoliative dermatitis, and skin ulcers, in decreasing order of occurrence. In all animals exhibiting clinical signs of the disease, the lymph nodes showed also size increase, with a higher frequency for cervical ones, followed by the popliteal and submandibular nodes.
As illustrated in Fig. 1, during the experiment, nine animals were subjected to euthanasia either because of the disease evolution (eight dogs) or because of lesions resulting from fights (one dog).
As shown in Fig. 1, the initial condition of animals was found to be altered 30 days after treatment in all three groups. The disease showed an absence of evolution or an evolution toward higher intensity in the animals of groups II and III. On the other hand, animals from group I showed a variable response. Among initially symptomatic animals in group I, three showed a considerable remission of the clinical signs after 30 days, which permitted their reclassification as oligosymptomatic. Among initially oligosymptomatic animals in group I, one showed a positive response to treatment and did not show clinical signs of the disease in any subsequent evaluations and two dogs showed a negative evolution of the disease (one subsequently subjected to euthanasia and the other reclassified as symptomatic). Among initially asymptomatic animals in group I, one remained asymptomatic 30 days after treatment, two changed to oligosymptomatic, and one changed to symptomatic. The positive impact of the treatment with liposomal Sb was also revealed, after 30 days, by the lower mean value of the clinical state of group I, compared to those of groups II and III (Fig. 1). Although the mean values of dog clinical state did not differ significantly between animal groups, group I showed a significantly higher number of animals with clinical state lower than 3, compared to group II (P < 0.05, Fisher's exact test). Ninety days after treatment, the clinical status was maintained in groups II and III, whereas the animals of group I showed a worsening of the clinical status. At the end of the experiment, all groups were found to be equivalent with respect to their clinical status.
Toxicity evaluations.
The group that received multiple doses of liposomal meglumine antimoniate was compared to those that received antimony-free liposomes and saline. Side effects in each animal were monitored clinically during the initial hour after each of the four doses given at 4-day intervals (total of 48 recordings per group). Although in the control group (saline) no adverse effect was observed, undesired effects were observed in 90% and 77% of recordings in the groups receiving liposomal meglumine antimoniate and empty liposomes, respectively. These adverse reactions were observed only during the first 15 min after dosing with the liposomal formulations and varied from discrete tachycardia and/or tachypnea to clinical manifestation of tachycardia, tachypnea, sialorrhea, and mydriasis, followed by miosis, prostration, urination, muscle tremor, vomiting, and defecation.
Levels of serum markers of the hepatic (aspartate aminotransferase, alkaline phosphatase, alanine aminotransferase, total bilirubins) and renal functions (urea, creatinine) in all groups, as determined 4 days after treatment, showed no significant changes from levels measured before treatment. In addition, evaluation of the hemogram parameters for treated groups showed no suppression of the hematopoietic function (data not shown).
In another experiment performed with 14 additional mongrel dogs with VL evaluated for short-time side effects of a single dose of liposomal meglumine antimoniate (4.2 mg Sb/kg by the i.v. route), the hemogram and the levels of the markers of hepatic and renal functions showed no significant changes 24, 48, 72, and 96 h after administration (data not shown).
Impact of treatment on tissue parasite burden.
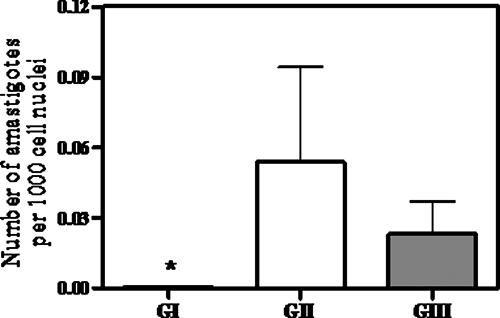
Figure 2 displays the mean number of amastigotes/1,000 host cell nuclei determined from aspirates of the sternal bone marrow of dogs in each group, 4 days after the end of treatment. In group I, the presence of the parasite was observed in only 1 animal out of 12 (8.3%). In groups II and III, parasites were encountered in 60% (n = 10) and 66% (n = 12) of animals, respectively. Statistical analysis indicated a significantly lower parasite burden in group I than in groups II and III (P < 0.05, Kruskal-Wallis).
FIG. 2.
Parasite burdens in the sternal bone marrow of dogs naturally infected with Leishmania (Leishmania) chagasi, 4 days after treatment with different formulations. Group I received liposomal meglumine antimoniate as four doses by the i.v. route of 6.5 mg Sb/kg/dose, with 4-day intervals; group II received antimony-free liposomes as four doses by the i.v. route of liposomes at the same lipid dose as for group I, with 4-day intervals; group III received saline as four doses by the i.v. route at the same volume as groups II and III, with 4-day intervals. Parasite burden is the amastigote number/1,000 host mononuclear cell nuclei. Data are given as means ± standard errors (n = 10 to 12). *, P < 0.05 (Kruskal-Wallis, followed by Dunn's multiple comparison test, for comparison between groups I [GI] and II and I and III.
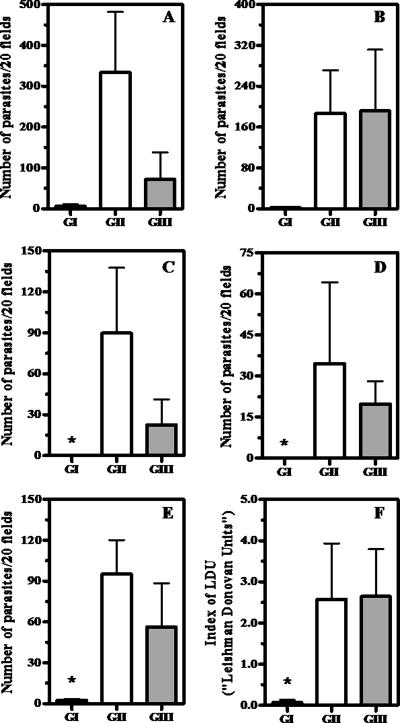
Figure 3 shows the results obtained from immunocytochemical evaluations for Leishmania burden in the skin, cervical lymph nodes, bone marrow, liver, and spleen, carried out 150 days after treatment. Statistical analyses indicated significantly lower parasite burdens in the lymph nodes, livers, and spleens of group I than in those of groups II and III (P < 0.05, Kruskal-Wallis). Strikingly, no parasite was detected in the livers and lymph nodes in animals of group I, whereas at least 60% of animals of groups I and III were found to be positive for Leishmania amastigotes in the same tissues (with at least one parasite in 20 microscopic fields). According to these data, more than a 95.7% reduction of the parasite burden was achieved in the liver, spleen, and cervical lymph nodes following treatment with liposome-encapsulated drug, compared to treatment with either saline or empty liposomes. It is also noteworthy that only group I animals (two out of nine) were parasitologically negative in all analyzed tissues. One of these animals was oligosymptomatic and the other one symptomatic.
FIG. 3.
Parasite burdens in the skin (A), bone marrow (B), cervical lymph nodes (C), livers (D), and spleens (E and F) of dogs naturally infected with Leishmania (Leishmania) chagasi, 150 days after treatment with different formulations. Group I received liposomal meglumine antimoniate as four doses by the i.v. route of 6.5 mg Sb/kg/dose, with 4-day intervals; group II received antimony-free liposomes as four doses by the i.v. route of liposomes at the same lipid dose as for group I, with 4-day intervals; Group III received saline as four doses by the i.v. route at the same volume as groups II and III, with 4-day intervals. (A to E) Parasites were determined immunocytochemically as described in Materials and Methods, and parasite burden is the number of Leishmania parasites per 20 microscopic fields. (F) Parasites were determined through Giemsa staining, and the LDU index is the number of amastigotes per 1,000 host cell nuclei × organ weight in grams. Data are given as means ± standard errors (n = 9 to 11). *, P < 0.05 (Kruskal-Wallis, followed by Dunn's multiple comparison test, for comparison between groups I [GI] and II and I and III).
As shown in Fig. 3, the spleens of dogs were evaluated both immunocytochemically and by Giemsa staining. In both cases, a significant reduction of spleen parasite burden was observed in group I, compared to groups II and III (P < 0.05, Kruskal-Wallis). The dog from group I that was positive according to both assays was found to be oligosymptomatic.
Importantly, none of the treated animals showed complete elimination of Leishmania parasites, as evidenced by in vitro culture of bone marrow aspirates 150 days after treatment (data not shown).
Impact of treatment on the infectivity of dogs to sand flies.
Table 1 shows the intensity and percentage of infection of Lutzomyia longipalpis females fed on the dogs of the different groups, 150 days after treatment. At the end of the experiment, it was possible to visualize the promastigotes in the digestive tracts of the fed phlebotomines in 10 out of 29 animals (34.5%) subjected to the xenodiagnosis. Among the 10 dogs that were infective, 1 belonged to group I (n = 9, 11%), 5 to group II (n = 11, 45%), and 4 to group III (n = 9; 44%). The infection efficiencies of phlebotomines, given by the ratio of infected phlebotomines to fed phlebotomines, were 0.65%, 14.3%, and 21.5%, for groups I, II, and III, respectively. Strikingly, the infection efficiency of phlebotomines fed on group I dogs was found to be significantly lower than those for the other groups (P < 0.01, χ2 test).
TABLE 1.
Intensity and efficiency of infection of Lutzomyia (Lutzomyia) longipalpis fed on dogs naturally infected with Leishmania (Leishmania) chagasi, 150 days after treatment, in different experimental groupsa
| Group | Code no. of dog | Sand fly infection
|
||
|---|---|---|---|---|
| No. of positive sand flies/no. fed | % Infection | Intensity of infection | ||
| I | 2 | 0/16 | 0 | − |
| 3 | 0/18 | 0 | − | |
| 4 | 1/17 | 5.88 | + | |
| 6 | 0/26 | 0 | − | |
| 8 | 0/26 | 0 | − | |
| 9 | 0/10 | 0 | − | |
| 10 | 0/8 | 0 | − | |
| 33 | 0/18 | 0 | − | |
| 34 | 0/10 | 0 | − | |
| II | 11 | 5/18 | 27.8 | + |
| 12 | 0/17 | 0 | − | |
| 13 | 0/7 | 0 | − | |
| 14 | 0/24 | 0 | − | |
| 15 | 0/15 | 0 | − | |
| 16 | 3/9 | 33.3 | + | |
| 18 | 0/14 | 0 | − | |
| 19 | 9/25 | 36.0 | ++ | |
| 20 | 3/9 | 33.3 | + | |
| 21 | 0/29 | 0 | − | |
| 23 | 8/30 | 26.7 | + | |
| III | 17 | 0/8 | 0 | − |
| 25 | 0/26 | 0 | − | |
| 27 | 4/18 | 22.2 | + | |
| 29 | 0/5 | 0 | − | |
| 30 | 14/22 | 63.6 | + | |
| 32 | 6/8 | 75 | +++ | |
| 35 | 0/30 | 0 | − | |
| 36 | 4/12 | 33.3 | + | |
| 38 | 0/7 | 0 | − | |
The data show the percentages of infection to Lutzomyia longipalpis 5 days after feeding on dogs and the intensity of the infection by the dog, according to the number, the activity, and the localization of the promastigote forms in the digestive tract of the phlebotomines. −, no infection detected; +, low infection intensity (few promastigotes were seen in the sand fly midgut with little or no motility); ++, medium infection intensity (many motile promastigotes where seen in the fore- and midgut); +++, high infection intensity (large numbers of promastigotes were seen throughout the gut and rosettes of parasites were visible). Group 1 treatment was liposomal meglumine antimoniate, given as four doses at 4-day intervals at 6.5 mg Sb/kg; group II treatment was empty liposomes, given as four doses at 4-day intervals; group III treatment was isotonic saline at 4-day intervals.
DISCUSSION
The present study reports the toxicity and antileishmanial efficacy of a novel liposomal formulation of meglumine antimoniate in mongrel dogs naturally infected with Leishmania (Leishmania) chagasi. This represents a continuation of our previous work which led to the design and the pharmacokinetic characterization of a novel liposomal formulation (20, 37, 38; F. Frézard, C. Demicheli, D. A. Schettini, R. R. Ribeiro, M. N. Melo, and M. S. M. Michalick, November 2004, Brazilian patent pending). As main advantages, this formulation can be stored in an intermediate lyophilized state and consists of vesicles with a mean diameter in the nanometer range, allowing for safer i.v. administration and improved targeting to the bone marrow (38). The dosage and interval between the doses used in the present study were chosen from pharmacokinetic data determined previously for dogs with VL (38), indicating that high levels of Sb were maintained in the liver and spleen from 24 to 96 h following a single i.v. administration of the liposomal formulation (at 4.2 mg Sb/kg).
Based on hematological parameters and serum biochemical markers, no significant alteration of the hematopoietic, renal, and hepatic functions of dogs could be detected, either within the 96 h following a single dose of the liposome formulation or after the administration of the four consecutive doses. On the other hand, transitory acute side effects, including prostration, defecation, tachypnea, and sialorrhea, were observed during the 15 min following every administration of the liposomal formulation. Since similar side effects were also observed after the administration of empty liposomes, those could be attributed to a reaction to the vesicles but not the metal. Indeed, lipid vesicle-induced acute adverse reactions have been reported previously and were attributed to the activation of the complement system (40). Those were named complement-mediated pseudoallergic reactions. The cardiopulmonary alterations usually initiated just after the infusion of liposomes most probably arise from the action of complement products, presumably the anaphylatoxins C3a and C5a, which cause an increase of the pulmonary arterial pressure and a reduction of the cardiac debit (40, 41), as well as an increase of pulmonary and peripheral vascular resistance.
According to the present work, treatment of infected dogs with four doses of liposomal meglumine antimoniate led to a partial clinical recovery, which was not observed in the dogs treated with saline or empty liposomes. A significant reduction of bone marrow parasite burden was observed 4 days after the end of treatment. Importantly, 5 months after the treatment, significantly lower parasite loads were found in cervical lymph nodes, livers, and spleens of animals treated with the liposomal drug formulation, indicating a long-term reduction of the parasite load of these tissues. The immunocytochemical evaluation of the proportion of positive dogs 5 months after treatment showed significant differences between group I and the other groups, when considering the lymph nodes, livers, and spleens, but not the bone marrow and skin. The apparently lower efficacy of liposomal meglumine antimoniate for parasite suppression in the bone marrow and skin may be explained by the fact that these tissues are less accessible to the liposomes. Our previous observation that Sb levels in the bone marrow of dogs with VL were about 10-fold lower than those in the liver and spleen (38) following administration of the same liposomal formulation in dogs naturally infected with Leishmania chagasi is consistent with this interpretation. Another interesting observation of our previous pharmacokinetic study was that equivalent Sb levels were found in the livers and spleens of infected dogs (38) during the 4 days following administration of the liposome formulation. On the other hand, significantly different levels of parasite suppression were achieved in the liver and spleen 150 days after treatment (100% and 95.7%, respectively; P < 0.05, Kruskal-Wallis test). These data taken altogether suggest a lack of correlation between tissue parasitic load and Sb concentration. This observation is not surprising, since a previous study with a mouse model of Leishmania donovani infection has indicated that sodium stibogluconate, either free or encapsulated in liposomes, was effective in reducing parasite burden in the liver but not in the spleen (11), indicating that the drug antileishmanial activity is organ dependent. Different intraorgan drug distributions and/or levels of activation of tissue Leishmania-infected macrophages may account for this effect. In the case of liposomal drug formulations, another factor that may contribute to the poor correlation between parasite load and tissue drug concentration is the fact that a large proportion of the drug in the organ may still be encapsulated in liposomes and, therefore, may not be bioavailable.
To our knowledge, parasitological evaluations of dogs with VL subjected to treatment with liposome-encapsulated antimonial drug have been reported in only two previous studies (12, 14, 37). In the first study (12), liposome-encapsulated meglumine antimoniate was administered to mongrel dogs experimentally infected with L. donovani (12 days after infection) at doses varying from 0.01 to 0.61 mg Sb/kg/day for 1, 4, and 10 days. The spleen was evaluated parasitologically 4 days after the end of treatment. It was verified that the formulation, when administered at low dose, provided little reduction in the number of parasites in spleen, either during 1 day (31.8% suppression) or 10 days (64.7% suppression) of treatment. On the other hand, the highest dose investigated during 4 days of treatment showed a high (97.4%) parasitic suppression. Although the protocol used in the present study seems, at first sight, no more effective than that used by these investigators, the use of naturally infected dogs and the parasitological evaluation 5 months after treatment make our data much more relevant to the clinical setting. In a previous study performed by our group (14, 37), meglumine antimoniate encapsulated in larger liposomes (mean diameter of 1,200 nm) has been evaluated for its efficacy in naturally infected mongrel dogs using a similar protocol (four doses of 6.5 mg Sb/kg given with 4-day intervals). Determination of the parasite load in the bone marrow aspirates of dogs 30 days after the end of treatment showed a significantly lower number of positive dogs (with at least one amastigote per 1,000 host cells) in the group treated with liposomal drug, compared to the control groups (treated with saline and empty liposomes) (37). On the other hand, the immunocytochemical evaluations of the spleen, 4 months after the end of treatment, and of the skin of the ear at various times (0, 30, 60, 90, and 120 days after the treatment) showed no significant difference between the groups (14). It is also noteworthy that this previous study also included, as a control, a group of animals receiving the free drug at the same dosage as the encapsulated one. As expected from the very low cumulative dose of Sb, the long time interval between each dose (compared to conventional antimony therapy), and the low Sb accumulation in the infection sites (38), no side effect was registered and no difference between the group treated with the free drug and that treated with saline was observed, from both the clinical and parasitological points of view. These data taken altogether indicate that the liposome formulation used in the present study, which consists of vesicles in the nanometer range, promoted a significant long-term parasite suppression, in contrast to the drug encapsulated in larger vesicles and to the free drug.
As evidenced from the culture of bone marrow aspirates, Leishmania parasites were still detected in all treated dogs 5 months after the end of treatment. Considering how rare it is to verify complete elimination of parasites by chemotherapy (31), it was important to evaluate the epidemiological risk of these animals by means of xenodiagnosis (2). Strikingly, the liposome formulation of meglumine antimoniate was found to reduce significantly the infectivity of dogs to Lutzomyia longipalpis 5 months after the end of the treatment. These data indicate that, although the reduction of parasite load in the skin promoted by the liposomal drug was not statistically significant, it was sufficient to reduce significantly the infectivity of the dogs to sand flies.
It is noteworthy that the clinical condition of the animals did not correlate either with the parasite load of tissues or with the infectivity of dogs to sand flies (P > 0.05, Spearman). These observations are in accordance with previous ones from other authors (22, 28, 39, 42), demonstrating the epidemiological importance of the diversity of clinical status in the transmission of the parasite.
Conventional treatments of dogs with VL using free pentavalent antimonial drugs showed high antileishmanial efficacy, promoting both suppression of tissue parasites (32) and reduction of infectivity of animals to sand flies (2, 21). Nevertheless, complete parasite elimination was rarely observed (31). It is noteworthy that the cumulative dose of antimony used in conventional antimonial treatment of infected dogs usually exceeds 500 mg of Sb/kg/treatment. This dose is at least 20-fold higher than that used in the present study. Indeed, the use of a much lower dose of antimony together with a reduced frequency of administration represents a major advantage of the liposome formulation, resulting ultimately in less-pronounced metal-induced side effects. Whether complete parasite elimination can be achieved by using a liposomal drug formulation remains an open question. In addition to the drug targeting to infection sites, liposomes may exert an additional synergistic effect related to their immunoadjuvant properties, for instance, by facilitating Leishmania antigen presentation to immunocompetent cells (8, 10). To improve the efficacy of treatment with our liposome formulation, increasing each dose of Sb is probably not appropriate, since this will result in a lipid dose higher than 25 mg of lipid/kg of body weight (used in our study), which may increase the saturation of the liver and impair its physiological function. Indeed, the saturation of the liver by liposomes in mice and rats from lipid doses ranging from 3 to 10 mg of lipid/kg of body weight was described previously (23). Therefore, increasing the number of doses, and thus the duration of the treatment, should be a more effective strategy.
Acknowledgments
This research was supported by the Brazilian agencies CNPq/MCT (550040/01-3, 472032/2004-6, 307726/2006-1, 477003/2004-4), FAPEMIG (EDT1806/02, REDE 2825/05, CEX549/04, CBB1014/05, CBB 165/07), and FAPEBIO/UFMG.
Footnotes
Published ahead of print on 5 May 2008.
REFERENCES
- 1.Alexander, A., and D. G. Russel. 1992. The interaction of Leishmania species with macrophages. Adv. Parasitol. 31:175-254. [DOI] [PubMed] [Google Scholar]
- 2.Alvar, J., R. Molina, M. San Andrés, M. Tesouro, J. Nieto, M. Vitutia, F. Gonzalez, M. D. San Andrés, J. Boggio, F. Rodriguez, A. Sainz, and C. Escacena. 1994. Canine leishmaniasis: clinical, parasitological and entomological follow-up after chemotherapy. Ann. Trop. Med. Parasitol. 88:371-378. [DOI] [PubMed] [Google Scholar]
- 3.Alving, C. R., and E. A. Steck. January 1980. Liposome carriers in chemotherapy of leishmaniasis. U.S. patent 4,186,183.
- 4.Alving, C. R., E. A. Steck, W. L. Chapman, Jr., V. B. Waits, L. D. Hendricks, G. M. Swartz, and W. L. Hanson. 1980. Liposomes in leishmaniasis: therapeutic effects of antimonial drugs, 8-aminoquinolines, and tetracyclines. Life Sci. 26:2231-2238. [DOI] [PubMed] [Google Scholar]
- 5.Alving, C. R. 1986. Liposomes as drug carriers in leishmaniasis and malaria. Parasitol. Today 2:101-107. [DOI] [PubMed] [Google Scholar]
- 6.Amusategui, I. 1998. Tratamiento de la leishmaniosis canina: valoración, caracterización y comparación de la respuesta a distintos protocolos a base de Antimoniato de meglumina asociado o no al Alopurinol. Ph.D. thesis. Universidad Complutense de Madrid, Madrid, Spain.
- 7.Baillie, A. J., G. H. Coombs, T. F. Dolan, and J. Laurie. 1986. Nonionic surfactant vesicles, niosomes, as a delivery system for the antileishmanial drug sodium stibogluconate. J. Pharm. Pharmacol. 38:502-505. [DOI] [PubMed] [Google Scholar]
- 8.Banduwardene, R., A. B. Mullen, and K. C. Carter. 1997. Immune responses of Leishmania donovani infected BALB/c mice following treatment with free and vesicular sodium stibogluconate formulations. Int. J. Immunopharmacol. 19:195-203. [DOI] [PubMed] [Google Scholar]
- 9.Berman, J. D. 1997. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin. Infect. Dis. 24:684-703. [DOI] [PubMed] [Google Scholar]
- 10.Bhowmick, S., R. Ravindran, and N. Ali. 2008. Gp63 in stable cationic liposomes confers sustained vaccine immunity to susceptible BALB/c mice infected with Leishmania donovani. Infect. Immun. 76:1003-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter, K. C., A. J. Baillie, J. Alexander, and T. F. Dolan. 1988. The therapeutic effect of sodium stibogluconate in BALB/c mice infected with Leishmania donovani is organ-dependent. J. Pharm. Pharmacol. 40:370-373. [DOI] [PubMed] [Google Scholar]
- 12.Chapman, W. L., W. L. Hanson, C. R. Alving, and L. D. Hendricks. 1984. Antileishmanial activity of liposome-encapsulated meglumine antimonate in the dog. Am. J. Vet. Res. 45:1028-1030. [PubMed] [Google Scholar]
- 13.Collins, M., K. C. Carter, A. J. Baillie, and J. O′Grady. 1993. The distribution of free and non-ionic vesicular sodium stibogluconate in the dog. J. Drug Targeting 1:133-142. [DOI] [PubMed] [Google Scholar]
- 14.Costa-Val, A. P. 2004. Tratamento da leishmaniose visceral canina com antimonial pentavalente encapsulado em lipossomas. Ph.D. thesis. Universidade Federal de Minas Gerais, Belo Horizonte, Brazil.
- 15.da Costa-Val, A. P., R. R. Cavalcanti, N. F. Gontijo, M. S. M. Michalick, B. Alexander, P. Williams, and M. N. Melo. 2007. Canine visceral leishmaniasis: relationships between clinical status, humoral immune response, haematology and Lutzomyia (Lutzomyia) longipalpis infectivity. Vet. J. 174:636-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demicheli, C., T. L. de Figueiredo, S. Carvalho, R. D. Sinisterra, J. C. D. Lopes, and F. Frézard. 1999. Physico-chemical characterization of meglumine antimoniate. Biometals 12:63-66. [DOI] [PubMed] [Google Scholar]
- 17.Demicheli, C., R. Ochoa, I. S. Lula, F. C. Gozzo, M. Eberlin, and F. Frézard. 2003. Pentavalent organoantimonial derivatives: two simple and efficient synthetic methods for meglumine antimonate. Appl. Organomet. Chem. 17:226-231. [Google Scholar]
- 18.Demicheli, C., and F. Frézard. 2005. Pentavalent antimonials: from chemistry to the design of new drugs. Drug Design Rev. 2:243-249. http://www.bentham.org/ddro/. [Google Scholar]
- 19.Frézard, F., M. S. M. Michalick, C. F. Soares, and C. Demicheli. 2000. Novel methods for the encapsulation of meglumine antimoniate in liposomes. Braz. J. Med. Biol. Res. 33:841-846. [DOI] [PubMed] [Google Scholar]
- 20.Frézard, F., D. Schettini, O. G. F. Rocha, and C. Demicheli. 2005. Liposomes: physicochemical and pharmacological properties, applications in antimony-based chemotherapy. Quim. Nova 28:511-518. [Google Scholar]
- 21.Gradoni, L., M. Maroli, M. Grammicia, and F. Mancianti. 1987. Leishmania infantum infection rates in Phlebotomus perniciosus fed on naturally infected dogs under antimonial treatment. Med. Vet. Entomol. 1:339-342. [DOI] [PubMed] [Google Scholar]
- 22.Guarga, J. L., J. Moreno, J. Lucientes, M. J. Gracia, M. A. Peribanez, J. Alvar, and J. A. Castillo. 2000. Canine leishmaniasis transmission: higher infectivity amongst naturally infected dogs to sand flies is associated with lower proportions of T helper cells. Res. Vet. Sci. 69:249-253. [DOI] [PubMed] [Google Scholar]
- 23.Hwang, K. J. 1987. Liposome pharmacokinetics, p. 109-156. In M. J. Ostro (ed.), Liposomes: from biophysics to therapeutics, Marcel Dekker, Inc., New York, NY.
- 24.Johnson, P. T., E. McConnell, and M. Hertig. 1963. Natural infection of leptomonad flagellates in Panamanian Phlebotomus sandflies. Exp. Parasitol. 14:107-122. [DOI] [PubMed] [Google Scholar]
- 25.Lima, W. G., P. S. Oliveira, M. V. Caliari, R. Goncalves, M. S. M. Michalick, M. N. Melo, W. L. Tafuri, and W. L. Tafuri. 2007. Histopathological and immunohistochemical study of type 3 complement receptors (CD11b/CD18) in livers and spleens of asymptomatic and symptomatic dogs naturally infected with Leishmania (Leishmania) chagasi. Vet. Immunol. Immunopathol. 117:129-136. [DOI] [PubMed] [Google Scholar]
- 26.Mancianti, F., M. Gramiccia, I. Gradoni, and S. Pieri. 1988. Studies on canine leishmaniais control. I. Evolution of infection of different clinical forms of canine leishmaniasis following antimonial treatment. Trans. R. Soc. Trop. Med. Hyg. 82:566-567. [DOI] [PubMed] [Google Scholar]
- 27.Meyerhoff, A. 1999. U.S. Food and Drug Administration approval of AmBisome (liposomal amphotericin B) for treatment of visceral leishmaniasis. Clin. Infect. Dis. 28:42-51. [DOI] [PubMed] [Google Scholar]
- 28.Molina, R., C. Amela, J. Nieto, M. San Andrés, F. González, J. A. Castillo, J. Lucientes, and J. Alvar. 1994. Infectivity of dogs naturally infected with Leishmania infantum to colonized Phlebotomus perniciosus. Trans. R. Soc. Trop. Med. Hyg. 88:491-493. [DOI] [PubMed] [Google Scholar]
- 28a.National Institutes of Health. 1985. Principles of laboratory animal care. Publication 85-23. National Institutes of Health, Bethesda, MD.
- 29.New, R. R. C., M. L. Chance, and S. Heath. 1981. Antileishmanial activity of amphotericin and other antifungal agents entrapped in liposomes. J. Antimicrob. Chemother. 8:371-381. [DOI] [PubMed] [Google Scholar]
- 30.Nieto, J., J. Alvar, A. B. Mullen, K. C. Carter, C. Rodríguez, M. I. San Andrés, M. D. San Andrés, A. J. Baillie, and F. González. 2003. Pharmacokinetics, toxicities, and efficacies of sodium stibogluconate formulations after intravenous administration in animals. Antimicrob. Agents Chemother. 47:2781-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noli, C. 1999. Canine leishmaniasis. Waltham Focus 9:16-24. [Google Scholar]
- 32.Noli, C., and S. T. Auxilia. 2005. Treatment of canine Old World visceral leishmaniasis: a systematic review. Vet. Dermatol. 16:213-232. [DOI] [PubMed] [Google Scholar]
- 33.Oliva, G., L. Gradoni, P. Ciaramella, R. De Luna, L. Cortese, S. Orsini, R. N. Davidson, and A. Persechino. 1995. Activity of liposomal amphotericin B (AmBisome) in dogs naturally infected with Leishmania infantum J. Antimicrob. Chemother. 36:1013-1019. [DOI] [PubMed] [Google Scholar]
- 34.Oliveira, F. B., D. A. Schettini, C. S. Ferreira, B. Rates, O. G. F. Rocha, F. Frézard, and C. Demicheli. 2006. Kinetics of antimony (V) reduction by L-cysteine. Pharmacological implications and application to the determination of antimony in pentavalente antimonial drugs. J. Braz. Chem. Soc. 17:1642-1650. [Google Scholar]
- 35.Rao, L. S. June 1986. Anti-leishmanial pharmaceutical formulation. U.S. patent 4,594,241.
- 36.Schettini, D. A., A. P. Costa-Val, L. F. Souza, C. Demicheli, O. G. F. Rocha, M. N. Melo, M. S. M. Michalick, and F. Frézard. 2003. Distribution of liposome-encapsulated antimony in dogs. Braz. J. Med. Biol. Res. 36:269-272. [DOI] [PubMed] [Google Scholar]
- 37.Schettini, D. A., A. P. Costa-Val, L. F. Souza, C. Demicheli, O. G. F. Rocha, M. N. Melo, M. S. M. Michalick, and F. Frézard. 2005. Pharmacokinetic and parasitological evaluation of the bone marrow of dogs with visceral leishmaniasis submitted to multiple dose treatment with liposome-encapsulated meglumine antimoniate. Braz. J. Med. Biol. Res. 38:1879-1883. [DOI] [PubMed] [Google Scholar]
- 38.Schettini, D. A., R. R. Ribeiro, C. Demicheli, O. G. F. Rocha, M. N. Melo, M. S. M. Michalick, and F. Frézard. 2006. Improved targeting of antimony to the bone marrow of dogs using liposomes of reduced size. Int. J. Pharm. 315:140-147. [DOI] [PubMed] [Google Scholar]
- 39.Slappendel, R. J. 1988. Canine leishmaniasis. A review based on 95 cases in the Netherlands. Vet. Q. 10:1-16. [DOI] [PubMed] [Google Scholar]
- 40.Szebeni, J. 1998. The interaction of liposomes with the complement system. Crit. Rev. Ther. Drug Carrier Syst. 15:57-88. [PubMed] [Google Scholar]
- 41.Szebeni, J., L. Baranyi, S. Savay, M. Bodo, D. S. Morse, M. Basta, G. L. Stahl, R. Bunger, and C. R. Alving. 2000. Liposome-induced pulmonary hypertension: properties and mechanism of a complement-mediated pseudoallergic reaction. Am. J. Physiol. Heart Circ. Physiol. 279:1319-1328. [DOI] [PubMed] [Google Scholar]
- 42.Tafuri, W. L., R. L. Santos, R. M. E. Arantes, R. Gonçalves, M. N. Melo, M. S. M. Michalick, and W. L. Tafuri. 2004. Na alternative immunohistochemical method for detecting Leishmania amastigotes in paraffin-embedded canine tissues. J. Immunol. Methods 292:17-23. [DOI] [PubMed] [Google Scholar]
- 43.Travi, B. L., C. J. Tabares, H. Cadena, C. Ferro, and Y. Osorio. 2001. Canine visceral leishmaniasis in Colombia: relationship between clinical and parasitologic status and infectivity for sand flies. Am. J. Trop. Med. Hyg. 64:119-124. [DOI] [PubMed] [Google Scholar]
- 44.Valladares, J. E., J. Freixas, J. Alberola, C. Franquelo, C. Cristofol, and M. Arboix. 1997. Pharmacokinetics of liposome-encapsulated meglumine antimoniate after intramuscular and subcutaneous administration in dogs. Am. J. Med. Hyg. 57:403-406. [DOI] [PubMed] [Google Scholar]
- 45.Valladares, J. E., C. Riera, P. González-Ensenyat, A. Díez-Cascón, G. Ramos, L. Solano-Gallego, M. Gállego, M. Gállego, N. Portús, M. Arboix, and J. Alberola. 2001. Long term improvement in the treatment of canine leishmaniosis using antimony liposomal formulation. Vet. Parasitol. 97:15-21. [DOI] [PubMed] [Google Scholar]