Abstract
Ceftobiprole (BPR) is an investigational cephalosporin with activity against Staphylococcus aureus, including methicillin-resistant S. aureus (MRSA) strains. The pharmacodynamic (PD) profile of BPR against S. aureus strains with a variety of susceptibility phenotypes in an immunocompromised murine pneumonia model was characterized. The BPR MICs of the test isolates ranged from 0.25 to 2 μg/ml. Pharmacokinetic (PK) studies were conducted with infected neutropenic BALB/c mice; and the BPR concentrations were measured in plasma, epithelial lining fluid (ELF), and lung tissue. PD studies with these mice were undertaken with eight S. aureus isolates (two methicillin-susceptible S. aureus strains, three hospital-acquired MRSA strains, and three community-acquired MRSA strains). Subcutaneous BPR doses of 2 to 125 mg/kg of body weight/day were administered, and the change in the number of log10 CFU/ml in lungs was evaluated after 24 h of therapy. The PD profile was characterized by using the free drug exposures (f) determined from the following parameters: the percentage of time that the concentration was greater than the MIC (T > MIC), the maximum concentration in serum/MIC, and the area under the concentration-time curve/MIC. The BPR PK parameters were linear over the dose range studied in plasma, and the ELF concentrations ranged from 60 to 94% of the free plasma concentration. fT > MIC was the parameter that best correlated with efficacy against a diverse array of S. aureus isolates in this murine pneumonia model. The 80% effective dose (ED80), ED50, and stasis exposures appeared to be similar among the isolates studied. BPR exerted maximal antibacterial effects when fT > MIC ranged from 6 to 22%, regardless of the phenotypic profile of resistance to β-lactam, fluoroquinolone, erythromycin, clindamycin, or tetracycline antibiotics.
Pneumonia has been recognized as a difficult-to-treat infection and is associated with high rates of morbidity and mortality, especially in critically ill and immunocompromised hosts (3). At present, Staphylococcus aureus has been identified as the foremost gram-positive pathogen that causes hospital-acquired (HA) pneumonia and has increasingly been reported as a cause of community-acquired (CA) pneumonia in recent years (12, 16, 18, 23). Methicillin-resistant S. aureus (MRSA) has become a cause for concern in both the hospital and the community settings, as these MRSA infections have been associated with increased rates of mortality, lengths of stay, and costs of care (8, 12, 16, 19, 20).
In the wake of the increasing occurrence of MRSA and, particularly, the increasing rates of occurrence of pneumonia caused by this organism, treatment options are limited. The treatments recommended for health care-associated MRSA pneumonia include vancomycin and linezolid as the preferred agents (23). While vancomycin is considered the “gold standard” treatment, it has been associated with poor clinical outcomes in cases of pneumonia caused by MRSA strains with MICs for susceptibility of 2 μg/ml, presumably due to poor penetration into the lung (24).
Ceftobiprole (BPR) is the first cephalosporin with anti-MRSA activity that has completed phase III clinical trials (5, 6). In vitro, BPR is also active against vancomycin-intermediate S. aureus, vancomycin-resistant S. aureus, and enterococci, as well as some gram-negative pathogens, including Pseudomonas aeruginosa and non-extended-spectrum β-lactamase-producing members of the family Enterobacteriaceae (13, 17). The in vivo efficacy of BPR against MRSA has been confirmed with several animal models (9). Results from phase III studies of complicated skin and skin structure infections confirmed the efficacy of BPR against MRSA (26). Further phase III studies are under way to evaluate the clinical efficacy of BPR for the treatment of other serious infections, such as nosocomial pneumonia (1, 6).
BPR appeared to exert in vivo activity comparable to that of the commercially available expanded-spectrum cephalosporins when it was studied in a model of mouse pneumonia caused by Streptococcus pneumoniae (4) and gram-negative pathogens (28). In a murine thigh infection model, BPR has also demonstrated time-dependent antimicrobial activity against MRSA and Streptococcus pneumoniae strains (2, 4).
The pharmacodynamic (PD) characteristics of BPR in staphylococcal pneumonia have not yet been studied; thus, the aim of this current study was to characterize the PD profile of BPR against S. aureus isolates, including methicillin-susceptible S. aureus (MSSA), HA-MRSA, and CA-MRSA isolates with a variety of resistance phenotypes in a murine pneumonia model.
MATERIALS AND METHODS
Antimicrobials.
BPR (BAL9141) and BPR medocaril (BAL5788, prodrug of BPR) were supplied by Johnson & Johnson Pharmaceutical Research & Development (Raritan, NJ) for in vitro and in vivo experiments, respectively. Compound BAL9141 is water insoluble; thus, the water-soluble prodrug BAL5788 (BPR medocaril) was used for the in vivo studies. Vancomycin, erythromycin, doxycycline, clindamycin, and trimethoprim-sulfamethoxazole were obtained from Sigma-Aldrich (St. Louis, MO). Levofloxacin and linezolid were provided by Johnson & Johnson Pharmaceutical Research & Development and Pharmacia & Upjohn (Pharmacia Corp., Kalamazoo, MI), respectively.
Bacteria.
Eight S. aureus isolates (two MSSA, three CA-MRSA, and three HA-MRSA isolates) were used for the PD evaluation of BPR. The two MSSA isolates used were ATCC 29213 and ATCC 25923. The HA-MRSA strains (strains 56, 149, and 152) and the CA-MRSA strains (strains 144, 146 and 147) were clinical isolates that have been phenotypically and genotypically characterized (15, 20, 22, 30). All isolates were maintained in double-strength skim milk medium (BD Biosciences, Sparks, MD) at −80°C. Before they were used in experiments, the isolates were subcultured twice on Trypticase soy agar with 5% sheep blood (BD Biosciences). The MICs of BPR and the other compounds tested against these S. aureus isolates were determined in triplicate by broth microdilution methods, according to the guidelines of the Clinical and Laboratory Standards Institute (10).
Animals.
Pathogen-free inbred female BALB/cAnNCr mice (ages, 7 to 9 weeks; weight range, 15 to 22 g) were obtained from the National Cancer Institute, Frederick, MD. The study protocol was approved by the Hartford Hospital Institutional Animal Care and Use Committee. The animals were acclimated for 7 to 14 days before the experiments were initiated and were adequately supplied with water and chow throughout the studies. Two separate injections of cyclophosphamide (Cytoxan; Bristol-Myers Squibb, Princeton, NJ) were used to create neutropenia in the mice. The first dose of cyclophosphamide was administered intraperitoneally at 250 mg/kg of body weight 4 days before organism inoculation for the establishment of pneumonia, followed by the injection of a second intraperitoneal dose of 100 mg/kg on the day before bacterial challenge.
Induction of experimental pneumonia.
A bacterial inoculum (containing 107 CFU/ml of S. aureus) was prepared in suspension with 3% mucin (from porcine stomach, type II; Sigma Chemical Co.) and normal saline. The neutropenic mice were anesthetized with vaporized isofluorane and then administered an oral inoculum (0.05 ml). The animals' nostrils were then blocked until the fluid was aspirated. After inoculation, the mice recovered in an oxygen-rich environment and were then randomized into the various treatment groups.
Pharmacokinetic studies.
BAL5788 powder was reconstituted with sterile water for injection, and dilutions were prepared such that all doses could be administered in 0.2-ml volumes. Single injection studies were conducted with four dosages of BPR: 1, 2.5, 10, and 25 mg/kg. BPR was administered subcutaneously (s.c.) to the neutropenic, infected mice 6 h after challenge with HA-MRSA 56. Blood samples were obtained by intracardiac puncture and collected into EDTA-containing vials at 0.25, 0.5, 1, 1.5, 2, 2.5, 3, and 4 h following drug administration from a total of six mice per each time point. Plasma was collected after centrifugation, and 3 μl citric acid (2 M) was added to stabilize BAL5788.
At 0.25, 1, 2, and 4 h, a bronchoalveolar lavage (BAL) was also performed on each animal, as described elsewhere (14), to obtain epithelial lining fluid (ELF). Briefly, a catheter was inserted into the trachea and an aliquot (0.4 ml) of normal saline was instilled, followed by immediate removal of the dispellate. Three additional saline aliquots were instilled and removed, and subsequently, the total volume recovered was combined. Immediately following the BAL, all lung tissue (five lobes) was collected from each animal. Supernatant aliquots were separated from the plasma and BAL fluid samples after centrifugation. Citric acid (2 M, 10 μl) was added to the BAL fluid supernatant samples to stabilize BAL5788.
The BPR concentrations in plasma, BAL fluid, and lung tissues were analyzed by Johnson & Johnson Pharmaceutical Research & Development by a validated high-performance liquid chromatography assay. The limit of quantification for the drug concentration assay was 0.01 μg/ml in all matrices. Standard curves were run for each analysis, and the coefficients of variation (CVs) were <12% for all matrices.
Portions of plasma and BAL fluid samples were tested for the urea concentration by a commercially available urea assay (Teco Diagnostics, Anaheim, CA). The urea assay was run within a 0.1- to 2.0-mg/dl standard curve range. The interday precision of the urea assay ranged from −4.67 to +9.00% (average CVs, 2.8 to 3.2%), and the intraday precision ranged from −2.53 to +5.33% (average CVs, 0.97 to 1.64%).
The drug concentrations in ELF were calculated from the following formula (31): ELF drug concentration = BAL fluid drug concentration × (plasma urea concentration/BAL fluid urea concentration). Prior to analysis by high-performance liquid chromatography, the lungs were weighed and then homogenized to extract the BPR. The calculation of the drug concentrations in lung tissue was based on an 80% lung water content (21). The pharmacokinetic parameters calculated included the elimination half-life, the area under the curve (AUC), the volume of distribution, and the elimination clearance and were derived by one-compartment analysis (WinNonLin Pro; Pharsight Corp.). The AUCs from 0 to 4 h (AUC0-4) of BPR in ELF and lung tissue were compared with the AUC of free drug in plasma to estimate the penetration ratio.
PD studies.
Multiple dosing regimens were administered s.c. to immunocompromised, infected mice to provide different exposures, with particular regard given to the percentage of the time that the concentration remained above the MIC (T > MIC) for the eight S. aureus isolates. Five mice were used in each dose-exposure group. At approximately 6 h after inoculation (0 h), lungs were collected from a group of untreated controls to provide a baseline measurement of the lung bacterial density. BPR or sham treatment (sterile water for injection) for all groups was initiated 6 h after inoculation and continued for 24 h. In order to provide a wide range of BPR exposures, dosages of 1 to 25 mg/kg were administered once to five times daily.
Twenty-four hours after drug administration began, lungs were aseptically harvested and then homogenized in 1.0 ml of normal saline, as illustrated previously (29). Dilutions of homogenates from 100 to 105 in saline were plated onto 5% sheep blood agar and Columbia nutrient agar (for the prevention of contamination with gram-negative bacteria; Remel Inc., Lenexa, KS) and incubated at 35°C up to 48 h. The limit of detection for the lung tissue culture was 2 × 102 CFU/ml. The mean bacterial density (log10 CFU/ml) in the lungs from untreated control mice and all BPR-treated mice at 24 h were calculated and compared with the starting (0-h) bacterial density in the untreated controls. Data on the level of plasma protein binding of BPR in mice (19%) were provided by the study sponsor, Johnson & Johnson Pharmaceutical Research & Development. Graphs of the log10 change in the numbers of CFU at 24 h versus the PD parameters T > MIC, maximum concentration of drug in plasma (Cmax)/MIC, and AUC/MIC, constructed by using the level of free drug exposure (f), were plotted using the sigmoid maximum-effect model. The 80% effective dose (ED80), ED50, and stasis exposure values were calculated from the individual curve for each S. aureus isolate as well as from a composite curve for all eight isolates.
RESULTS
Table 1 displays the phenotypic resistance profiles to antimicrobial compounds of the eight S. aureus isolates. The BPR MICs for the isolates ranged from 0.25 to 2 μg/ml. The MICs of BPR were lower than those of vancomycin for the majority of the isolates.
TABLE 1.
In vitro susceptibilities of Staphylococcus aureus strains to BPR and other compounds
| Druga | MIC (μg/ml)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| MSSA ATCC 29213 | MSSA ATCC 25923 | CA-MRSA 144 | CA-MRSA 146 | CA-MRSA 147 | HA-MRSA 56 | HA-MRSA 149 | HA-MRSA 152 | |
| BPRb | 0.25b | 0.25 | 1 | 2 | 1 | 1 | 1 | 0.5 |
| LZD | 2 | 4 | 2 | 2 | 2 | 8 | 2 | 2 |
| VAN | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| ERY | 1 | 0.5 | >32 | >32 | >32 | 32 | >32 | 1 |
| CLI | 0.125 | 0.125 | >16 | 0.25 | 0.125 | 0.25 | 0.125 | 0.125 |
| LVX | 0.25 | 0.25 | 0.5 | 8 | 8 | 0.25 | 8 | 0.25 |
| TMP-SXT | 0.125 | 0.06 | 0.25 | 0.125 | 0.125 | 0.06 | 0.25 | 0.06 |
| DOX | 0.5 | 0.25 | 0.5 | 2 | 2 | 8 | 0.5 | 0.5 |
LZD, linezolid; VAN, vancomycin; ERY, erythromycin; CLI, clindamycin; LVX, levofloxacin; TMP-SXT, trimethoprim-sulfamethoxazole; DOX, doxycycline.
MIC range, 0.25 to 1 μg/ml according to CLSI guidelines (10).
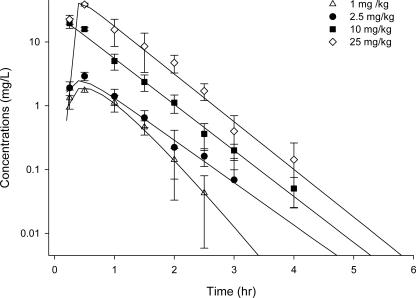
In plasma, a linear pharmacokinetic profile was noted for BPR over the range of doses studied. The half-life of BPR in mice was estimated to be 0.25 to 0.45 h. The values for other pharmacokinetic parameters are summarized in Table 2. The total plasma concentration for each single dose is displayed in Fig. 1. In plasma, fT > MIC ranged from 3 to 58%, while fCmax/MIC and fAUC/MIC ranged from 1 to 63 and 2 to 262, respectively, with the dosage regimens used.
TABLE 2.
Pharmacokinetic results for BPR after a single s.c. dose in immunocompromised, infected BALB/c micea
| Dosing regimen (mg/kg) | Cmax (mg/liter) | Tmax (h) | AUC0-∝ (mg·h/liter) | VF (liter/kg) | Half-life (h) | CL (ml/h/kg) |
|---|---|---|---|---|---|---|
| 1 | 1.74 | 0.48 | 1.84 | 0.19 | 0.25 | 0.54 |
| 2.5 | 2.91 | 0.41 | 2.57 | 0.63 | 0.45 | 0.97 |
| 10 | 19.75 | 0.15 | 15.79 | 0.38 | 0.42 | 0.63 |
| 25 | 38.59 | 0.47 | 35.25 | 0.41 | 0.40 | 0.71 |
Tmax, time required to achieve Cmax; AUC0-∝, AUC from time zero to infinity; VF, volume of distribution; CL, clearance.
FIG. 1.
Total plasma drug concentration of BPR after a single s.c. dose.
Overall, the concentrations of BPR in ELF and lung tissue increased with escalating dosages, and the concentrations in ELF exceeded those observed in whole lung tissue for all dosages studied (Fig. 2). The AUC0-4 values of BPR in ELF were estimated by use of the trapezoidal rule and ranged from 60 to 94% of the free drug concentration in plasma. The level of lung tissue penetration, as estimated by the ratio of the AUC0-4 for free BPR in the lung to the AUC0-4 for free BPR in plasma, was approximately 25% (range, 17 to 40%).
FIG. 2.
BPR concentrations in ELF (μg/ml) and lung tissue (μg/g) after a single s.c. dose (1, 2.5, 10, and 25 mg/kg).
The starting (0-h) bacterial density in the lungs of the controls was consistently 105 to 106 CFU/ml (5.80 ± 0.22, mean ± standard deviation) between each experiment for all of the S. aureus isolates. At 24 h after inoculation, the bacterial density had increased 1.3 to 1.9 log units in the untreated control mice. The maximal change in bacterial density in the lungs at 24 h after BPR treatment was approximately a 2.5-log decrease compared to the initial numbers of CFU.
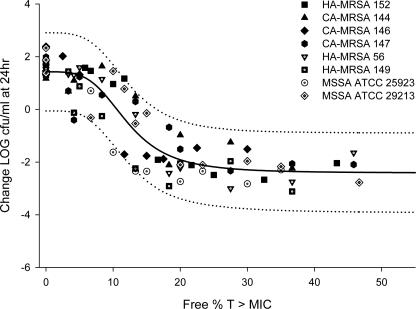
The PD profiles of BPR appeared to be similar against the eight S. aureus isolates. The relationship between the antimicrobial activity of BPR and each PD parameter was assessed for each individual S. aureus isolate separately, as well as for a composite of all eight isolates (Fig. 3). The correlations (R2) of the composite curves for the eight isolates tested between the change in the log10 numbers of CFU and the three PD parameters fT > MIC, fCmax/MIC, and fAUC/MIC were 0.831, 0.771, and 0.807, respectively. Overall, in comparison to fCmax/MIC and fAUC/MIC, fT > MIC was the parameter that best correlated with efficacy by the determination of R2 and the distribution of the data along the fitted curve. As demonstrated in Table 3, the individually generated ED80, ED50, and stasis exposure values appeared to be similar among the eight S. aureus isolates studied. The maximum changes in the numbers of CFU were determined by measurement of the reductions in the numbers of CFU in the treatment groups in relation to the numbers of CFU in the control animals at 24 h. For all test isolates, the maximum lung bacterial titer reduction occurred at fT > MIC values of 6 to 22%, with an average fT > MIC for an ED80 of 15% ± 5%.
FIG. 3.
Antimicrobial activity of BPR versus fT > MIC against eight S. aureus isolates (solid line, maximum-effect model curve; dotted lines, 95% population confidence intervals).
TABLE 3.
fT > MICs for corresponding EDs of BPR against all S. aureus isolates in an immunocompromised murine pneumonia model
| S. aureus strain | % fT > MIC for:
|
Maximum log10 CFU reduction | ||
|---|---|---|---|---|
| ED80 | ED50 | Stasis | ||
| MSSA 25923 | 10.2 | 8.1 | 7.6 | 4.2 |
| MSSA 29213 | 19.5 | 14.8 | 13.3 | 3.9 |
| HA-MRSA 56 | 15.5 | 13.7 | 13.1 | 3.9 |
| HA-MRSA 149 | 13.8 | 8.6 | 6.6 | 4.1 |
| HA-MRSA 152 | 15.9 | 14.0 | 13.4 | 3.8 |
| CA-MRSA 144 | 14.3 | 13.8 | 13.6 | 2.9 |
| CA-MRSA 146 | 6.0 | 5.5 | 5.6 | 3.6 |
| CA-MRSA 147 | 21.6 | 17.1 | 14.9 | 3.2 |
| Mean (SD) | 14.6 (4.9) | 11.9 (4.0) | 11.0 (3.7) | 3.7 (0.5) |
Likewise, BPR displayed similar effects against this consortium of S. aureus isolates when the data were taken together. When they were analyzed as one data set, the fT > MICs required to achieve ED80, ED50, and stasis, as determined from the composite curve for the eight isolates (Fig. 3), were 17%, 12%, and 11%, respectively. The fCmax/MICs required to achieve ED80 and ED50 for the eight isolates ranged from 2 to 21 (15 ± 7) and 2 to 87 (19 ± 28), respectively. The fAUC/MICs required to achieve ED80 and ED50 for the eight isolates ranged from 3 to 34 (21 ± 12) and 3 to 420 (72 ± 154), respectively.
DISCUSSION
While the S. aureus isolates used in the current study displayed diverse phenotypic profiles, the BPR MICs ranged from 0.25 to 2 μg/ml and were consistent with the values previously reported for the compound (17).
In pneumonia, ELF is believed to be the primary site of infection for extracellular organisms like S. aureus (27). The pharmacokinetic data from this study showed that BPR sufficiently penetrated the ELF and achieved concentrations in excess of the MICs for the isolates. As whole lung tissue contains both extracellular and intracellular fluid that may dilute the antibiotic concentration, lower concentrations in lung tissue compared to those in ELF are not unexpected. The concentrations of BPR in the lung tissue in our study with infected, neutropenic mice were lower than those obtained with uninfected, nonneutropenic mice reported by Azoulay-Dupuis et al. (4). The use of different strains of mice, especially a strain with a functional immune system, may have heightened the dissimilarity in the values of the pharmacokinetic parameters obtained between the studies. The concentrations of BPR in ELF and lung tissue obtained from four sampling time points in our study provided an estimation of BPR's rate and extent of penetration into the target sites of infection.
In the current study, we found that fT > MIC was the PD parameter which best defined the efficacy of BPR against a diverse array of S. aureus isolates in a murine pneumonia model. BPR exerted maximal antibacterial effects when fT > MIC was approximately 20%, regardless of the phenotype of resistance to other antimicrobial compounds, after 24 h of drug exposure. Our results bear similarity to those previously determined with a S. aureus murine thigh infection model (2) and S. pneumoniae lung infection model (4). Against two MRSA isolates in a neutropenic thigh infection model, BPR displayed time-dependent killing, as an exposure with a T > MIC equal to 23 to 33% resulted in a static effect; however, the authors did not specify whether these results were calculated with total or free drug exposures (2). In the study with the S. pneumoniae pneumonia model, T > MICs between 9 and 18% were required for efficacy (4).
While other cephalosporin antibiotics require fT > MICs of approximately 30 to 40% for stasis or 60 to 70% for bactericidal effects (11), our study demonstrated that BPR exerted consistent killing activity against diversely resistant S. aureus isolates at a lower level of free drug exposure in neutropenic hosts. PD studies conducted with neutropenic models, such as that used in the current study, are challenging trials for BPR since the animals lack a functioning immune system. Several studies have supported the differences in antibiotic treatment outcomes between neutropenic and nonneutropenic hosts (7, 25). The antimicrobial effects of BPR were predictable for both MSSA and MRSA isolates and were not affected by resistance to other classes of antibiotics. The low level of drug exposure in plasma required for BPR may be related in part to the low percentage of protein binding, which improved penetration into target tissues. The good penetration of BPR into ELF and lung tissue potentially accounts for its reliable efficacy in this pneumonia model; thus, this agent should offer an attractive option for the treatment of serious MRSA infections, including pneumonia, in critically ill or immunocompromised patients.
S. aureus has been identified as a common causative organism in HA pneumonia and, more recently, as a pathogen in CA pneumonia (16, 19, 23). Moreover, Kollef et al. identified S. aureus as the leading pathogen in pneumonia and as the only pathogen independently associated with mortality (19). For the treatment of either CA pneumonia or HA pneumonia, a compound must not only display microbiological activity but must also achieve sufficient antimicrobial exposures at the site of infection (27). Our study has shown the penetration of BPR into target tissues and its resultant efficacy against S. aureus. Moreover, this agent displayed consistent activity not only against MSSA isolates but also against MRSA isolates, including both HA-MRSA and CA-MRSA genotype isolates. BPR appears to have several important characteristics such as potent in vitro activity, low levels of protein binding, and good penetration into the lungs; thus, it should prove to be a valuable tool in the armamentarium for the management of bronchopulmonary infections due to S. aureus strains, including MRSA strains, possessing diverse phenotypic profiles.
Acknowledgments
This study was funded by a grant from Johnson & Johnson Pharmaceutical Research & Development.
We thank Darren Abbanat at Johnson & Johnson Pharmaceutical Research & Development for assistance with the determination of BPR concentrations in biological samples and providing protein binding data.
Footnotes
Published ahead of print on 14 April 2008.
REFERENCES
- 1.Adis International Data Information. 2006. Ceftobiprole medocaril: BAL5788, JNJ 30982081, JNJ30982081, RO 65-5788, RO 655788. Drugs R. D. 7:305-311. [DOI] [PubMed] [Google Scholar]
- 2.Andes, D. R., and W. A. Craig. 2000. In-vivo pharmacodynamics of RO 63-9141 against multiple bacterial pathogens, abstr. 1079. Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 3.Anonymous. 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171:388-416. [DOI] [PubMed] [Google Scholar]
- 4.Azoulay-Dupuis, E., J. P. Bedos, J. Mohler, A. Schmitt-Hoffmann, M. Schleimer, and S. Shapiro. 2004. Efficacy of BAL5788, a prodrug of cephalosporin BAL9141, in a mouse model of acute pneumococcal pneumonia. Antimicrob. Agents Chemother. 48:1105-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogdanovich, T., L. M. Ednie, S. Shapiro, and P. C. Appelbaum. 2005. Antistaphylococcal activity of ceftobiprole, a new broad-spectrum cephalosporin. Antimicrob. Agents Chemother. 49:4210-4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bush, K., M. Heep, M. J. Macielag, and G. J. Noel. 2007. Anti-MRSA beta-lactams in development, with a focus on ceftobiprole: the first anti-MRSA beta-lactam to demonstrate clinical efficacy. Expert Opin. Investig. Drugs 16:419-429. [DOI] [PubMed] [Google Scholar]
- 7.Capitano, B., D. Maglio, M. A. Banevicius, C. H. Nightingale, and D. P. Nicolau. 2003. Bactericidal effect of cethromycin (ABT-773) in an immunocompetent murine pneumococcal pneumonia model. Int. J. Antimicrob. Agents 22:588-593. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2007. Severe methicillin-resistant Staphylococcus aureus community-acquired pneumonia associated with influenza—Louisiana and Georgia, December 2006-January 2007. MMWR Morb. Mortal. Wkly. Rep. 56:325-329. [PubMed] [Google Scholar]
- 9.Chambers, H. F. 2006. Ceftobiprole: in-vivo profile of a bactericidal cephalosporin. Clin. Microbiol. Infect. 12(Suppl. 2):17-22. [DOI] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed. CLSI publication M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 11.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed] [Google Scholar]
- 12.Deresinski, S. 2005. Methicillin-resistant Staphylococcus aureus: an evolutionary, epidemiologic, and therapeutic odyssey. Clin. Infect. Dis. 40:562-573. [DOI] [PubMed] [Google Scholar]
- 13.Deshpande, L., P. R. Rhomberg, T. R. Fritsche, H. S. Sader, and R. N. Jones. 2004. Bactericidal activity of BAL9141, a novel parenteral cephalosporin against contemporary gram-positive and gram-negative isolates. Diagn. Microbiol. Infect. Dis. 50:73-75. [DOI] [PubMed] [Google Scholar]
- 14.Du, X., C. Li, H. K. Sun, C. H. Nightingale, and D. P. Nicolau. 2005. A sensitive assay of amoxicillin in mouse serum and broncho-alveolar lavage fluid by liquid-liquid extraction and reversed-phase HPLC. J. Pharm. Biomed. Anal. 39:648-652. [DOI] [PubMed] [Google Scholar]
- 15.Francis, J. S., M. C. Doherty, U. Lopatin, C. P. Johnston, G. Sinha, T. Ross, M. Cai, N. N. Hansel, T. Perl, J. R. Ticehurst, K. Carroll, D. L. Thomas, E. Nuermberger, and J. G. Bartlett. 2005. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin. Infect. Dis. 40:100-107. [DOI] [PubMed] [Google Scholar]
- 16.Fridkin, S. K., J. C. Hageman, M. Morrison, L. T. Sanza, K. Como-Sabetti, J. A. Jernigan, K. Harriman, L. H. Harrison, R. Lynfield, and M. M. Farley. 2005. Methicillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 352:1436-1444. [DOI] [PubMed] [Google Scholar]
- 17.Hebeisen, P., I. Heinze-Krauss, P. Angehrn, P. Hohl, M. G. Page, and R. L. Then. 2001. In vitro and in vivo properties of Ro 63-9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 45:825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kollef, M. H., and S. T. Micek. 2006. Methicillin-resistant Staphylococcus aureus: a new community-acquired pathogen? Curr. Opin. Infect. Dis. 19:161-168. [DOI] [PubMed] [Google Scholar]
- 19.Kollef, M. H., A. Shorr, Y. P. Tabak, V. Gupta, L. Z. Liu, and R. S. Johannes. 2005. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest 128:3854-3862. [DOI] [PubMed] [Google Scholar]
- 20.Kowalski, T. J., E. F. Berbari, and D. R. Osmon. 2005. Epidemiology, treatment, and prevention of community-acquired methicillin-resistant Staphylococcus aureus infections. Mayo Clin. Proc. 80:1201-1207. [DOI] [PubMed] [Google Scholar]
- 21.Lange, N. R., and D. P. Schuster. 1999. The measurement of lung water. Crit. Care (London) 3:R19-R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaPlante, K. L., M. J. Rybak, M. Amjad, and G. W. Kaatz. 2007. Antimicrobial susceptibility and staphylococcal chromosomal cassette mec type in community- and hospital-associated methicillin-resistant Staphylococcus aureus. Pharmacotherapy 27:3-10. [DOI] [PubMed] [Google Scholar]
- 23.Mandell, L. A., R. G. Wunderink, A. Anzueto, J. G. Bartlett, G. D. Campbell, N. C. Dean, S. F. Dowell, T. M. File, Jr., D. M. Musher, M. S. Niederman, A. Torres, and C. G. Whitney. 2007. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 44(Suppl. 2):S27-S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohr, J. F., and B. E. Murray. 2007. Point: vancomycin is not obsolete for the treatment of infection caused by methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 44:1536-1542. [DOI] [PubMed] [Google Scholar]
- 25.Nicolau, D. P., H. M. Mattoes, M. Banevicius, D. Xuan, and C. H. Nightingale. 2003. Pharmacodynamics of a novel des-F(6)-quinolone, BMS-284756, against Streptococcus pneumoniae in the thigh infection model. Antimicrob. Agents Chemother. 47:1630-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noel, G. J., R. S. Strauss, R. Pypstra, and the 00154 Study Group. 2006. Successful treatment of complicated skin infections (cSSSI) due to staphylococci, including methicillin-resistant Staphylococcus aureus (MRSA) with ceftobiprole, abstr. L-1212. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 27.Pea, F., and P. Viale. 2006. The antimicrobial therapy puzzle: could pharmacokinetic-pharmacodynamic relationships be helpful in addressing the issue of appropriate pneumonia treatment in critically ill patients? Clin. Infect. Dis. 42:1764-1771. [DOI] [PubMed] [Google Scholar]
- 28.Rouse, M. S., M. M. Hein, P. Anguita-Alonso, J. M. Steckelberg, and R. Patel. 2006. Ceftobiprole medocaril (BAL5788) treatment of experimental Haemophilus influenzae, Enterobacter cloacae, and Klebsiella pneumoniae murine pneumonia. Diagn. Microbiol. Infect. Dis. 55:333-336. [DOI] [PubMed] [Google Scholar]
- 29.Tessier, P. R., M. K. Kim, W. Zhou, D. Xuan, C. Li, M. Ye, C. H. Nightingale, and D. P. Nicolau. 2002. Pharmacodynamic assessment of clarithromycin in a murine model of pneumococcal pneumonia. Antimicrob. Agents Chemother. 46:1425-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zetola, N., J. S. Francis, E. L. Nuermberger, and W. R. Bishai. 2005. Community-acquired methicillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect. Dis. 5:275-286. [DOI] [PubMed] [Google Scholar]
- 31.Ziglam, H. M., D. R. Baldwin, I. Daniels, J. M. Andrew, and R. G. Finch. 2002. Rifampicin concentrations in bronchial mucosa, epithelial lining fluid, alveolar macrophages and serum following a single 600 mg oral dose in patients undergoing fibre-optic bronchoscopy. J. Antimicrob. Chemother. 50:1011-1015. [DOI] [PubMed] [Google Scholar]