Abstract
Male mammals of many species exhibit reflexive testosterone release in mating situations. In house mice (Mus musculus), the dramatic robustness of such release, occurring primarily in response to a novel female, suggests some function. The resulting testosterone elevations typically peak during copulatory behavior and may serve to activate transitory motivational and physiological responses that facilitate reproduction. However, such a function requires that testosterone be working through either nongenomic, or very quick genomic, mechanisms. The first part of the review describes reflexive sex hormone release in house mice. The second part summarizes research implicating testosterone’s fast actions in affecting anxiety, reward, learning, analgesia, and penile reflexes in rodents, all of which could optimize male mating success. The review concludes with a speculative model of how spontaneous and reflexive hormone release might interact to regulate reproductive behavior and why mice appear to be an ideal species for examining testosterone’s quick effects.
Keywords: courtship, copulation, environment, genotype, genomic, GnRH, LH, mouse, nongenomic, reflexive release, reproduction, sexual arousal, spontaneous release, testosterone, ultrasonic vocalization
Introduction
The nongenomic effects of various steroids have been extensively demonstrated at molecular and cellular levels and a number of different receptor systems implicated [1; 2; 3]. However, much less work has examined possible nongenomic steroid effects upon organismal physiology and behavior. Although other species likely will prove to be good model systems, I make the case here that male mouse (Mus musculus) responses occurring during reflexive testosterone release represent an excellent system in which to study the quick-acting effects of testosterone upon male behavior/physiology. Whether these quick effects are nongenomic or genomic ultimately will require identification of the receptor mechanisms mediating responsiveness.
In male mammals, testosterone is released in a pulsatile fashion in which high “pulsatile” levels are periodically superimposed upon low “basal” levels. Two different types of pulsatile release occur: spontaneous and reflexive. Spontaneous release typically occurs multiple times per day in response to endogenous homeostatic conditions, accounts for much of a male's circulating testosterone, and is centrally important in organizing and maintaining many aspects of male reproductive physiology and behavior.
In addition, male mammals also reflexively release testosterone less frequently as a result of mating interactions. However, the role of reflexive release is not clear. One hypothesis is that reflexive release simply augments spontaneously released testosterone to better prepare the male (or perhaps the female that stimulated release) for FUTURE reproductive situations. Another hypothesis (that is not mutually exclusive, but more germane to this review) is that reflexive release has IMMEDIATE effects in helping the male cope with the situation that stimulated release.
Spontaneous Sex Hormone Release In Mice
The pulsatile nature of mouse sex hormone release may not be fully appreciated because both baseline levels and pulse amplitudes are often obscured by the manner in which many investigators report their results. Because of large inter-individual variability in testosterone (or LH) levels, the group means typically reported both overestimate basal levels and underestimate peak levels. To truly appreciate its pulsatile nature, hormone levels must be monitored over short intervals in single individuals. Given the difficulty of such research in small animals, it is not surprising that this approach is seldom utilized.
The pulsatile nature of spontaneous sex hormone release in the male house mouse can be seen clearly in Figure 1. In a heroic study by Coquelin and Desjardins [4], blood was withdrawn from individually housed male mice from indwelling atrial catheters and measured for both LH and testosterone levels every 5 min for 8–10 hr. LH was secreted at basal levels (15–25 ng/ml) much of the time interspersed with spontaneous pulses 10 to 15 times higher (150–200 ng/ml). The pulses lasted about 20–30 min until returning to baseline. However, the interval between LH pulses was highly variable both within and between mice with some mice showing as many as 6 pulses in the 9-hr sampling period and some mice showing none. Coquelin and Desjardins [4] estimate that a young sexually mature male mouse will usually show around 6 or 8 spontaneous LH pulses per day or a pulse approximately every 3 to 4 hrs. If data from rats [5] are representative, these cycles likely proceed with similar regularity both during wakefulness and sleep.
Figure 1.
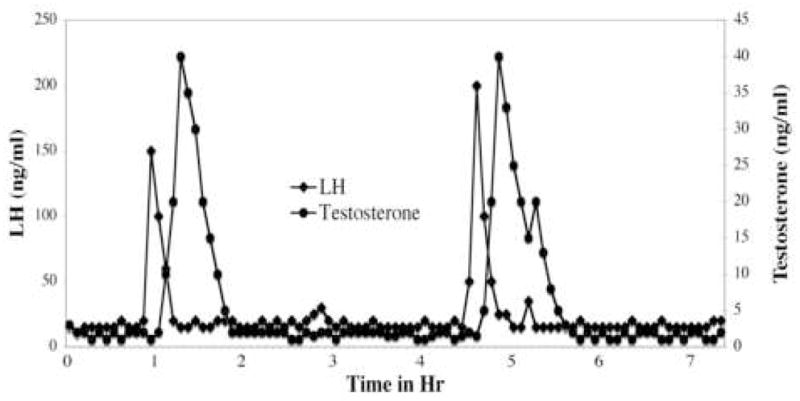
A representative depiction of changes in plasma hormone levels during spontaneous testosterone and LH release in a male house mouse. While interpulse intervals are quite variable, the figure illustrates the dramatic plasma hormone changes during male mouse pulsatile release. Adapted from data collected by Coquelin and Desjardins [4]. See text for more explanation.
The pattern of spontaneous testosterone release was similar to that of the LH [4] that preceded it, with basal levels (1–3 ng/ml) being periodically interspersed with testosterone pulses (30–40 ng/ml). Elevations in testosterone were usually evident within 10 min of LH pulse onset, peaked within 20–30 min of LH pulse onset and returned to baseline in around 60–80 min. Although an LH pulse preceded every testosterone pulse, not every LH pulse produced a testosterone pulse. Occasionally several closely spaced LH pulses preceded a single testosterone pulse. In mice, approximately twice as many LH pulses as testosterone pulses were observed [4] .
To my knowledge, rigorous time sampling of GnRH titers has not been performed in house mice, but in males of other species, GnRH clearly shows pulsatile release. Since an injection of GnRH produces an LH pulse in mice [6], one can reasonably assume that a short-duration GnRH pulse immediately precedes an LH pulse.
This pattern of spontaneous hormone secretion is thought to reflect the activity of an endogenous GnRH pulse generator in the brain that integrates various types of input including feedback from the various hypothalamic/pituitary/gonadal sex hormones to regulate GnRH release [7]. Most of the time the pulse generator keeps GnRH secretion at low levels, but when some integrative threshold is reached (approximately every 3 to 4 hrs in mice), a GnRH pulse is released into the hypophyseal portal system. In the mouse, the signal is amplified as it is translated from one hormone to the next such that each succeeding hormone pulse is of longer duration than the one that precedes and causes it.
Reflexive Sex Hormone Release During Sexual Encounters
In addition, two environmental events associated with mating cause pulsatile sex-hormone secretion in adult male mice [8] (see figure 2).
Figure 2.
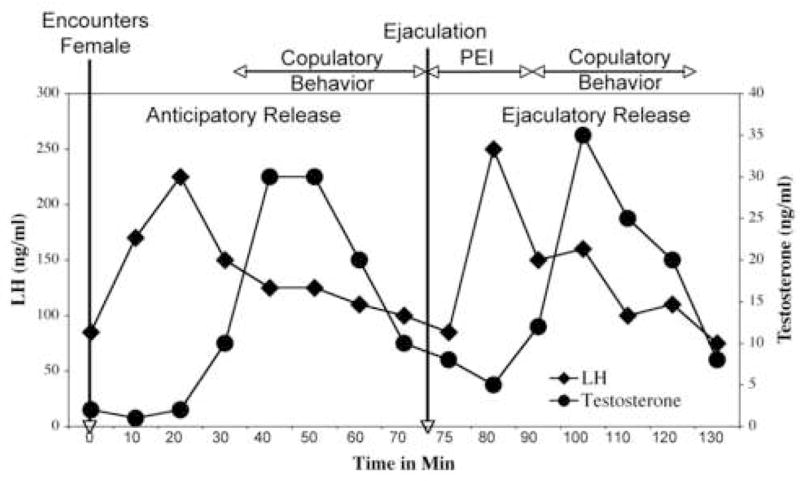
A representative depiction of reflexive sex hormone release in a male house mouse during a mating encounter. Encountering a female causes anticipatory release, while ejaculation causes ejaculatory release. The timing of LH release in relation to ejaculation is adapted from Coquelin and Bronson [8], the approximate timing of copulatory behavior and postejaculatory interval (PEI) in relation to ejaculation adapted from Nyby [27] and the testosterone titers inferred from the time between LH and testosterone release reported by Coquelin and Desjardins [4]. See text for more explanation.
Immediately upon encountering a novel female, a pulsatile sex-hormone release cycle is activated. If courtship and copulation proceed, the male often shows a second cycle of reflexive, pulsatile release immediately after ejaculation. The reflexive release upon first encountering the female, is referred to as “anticipatory” release, and the hormone release following ejaculation, as “ejaculatory” release. The amplitude and duration of both reflexive pulses are highly similar to each other and to that of spontaneous release described earlier. The sexually stimulated hormone pulses would appear to involve exterosensory activation of the GnRH pulse generator.
Other Properties Of Pulsatile Release
Coquelin and Bronson [8] also identify other interesting properties of both endogenously and environmentally regulated LH pulses in mice. For example, anticipatory pulses rapidly habituate with repeated exposure to the same female [9]. This habituation correlates with the decreasing effectiveness over time of the same female to cause sexual arousal.
A refractory period for LH release also follows both spontaneous and reflexive release during which a second LH pulse cannot occur. This refractory period lasts between 25 and 45 minutes and is mediated by events in the brain rather than in the pituitary or peripheral anatomy [6]. The existence of a refractory period also explains why anticipatory and ejaculatory pulses are occasionally not seen at the expected times [6]. For example, if a spontaneous pulse happened just before a male encountered a female, the male would not show the anticipatory pulse. Similarly if a male ejaculates too quickly (before the refractory period for the anticipatory pulse is completed), he will not show the ejaculatory pulse [6].
Stimuli That Cause Anticipatory Testosterone Release
The most powerful stimulus for causing anticipatory testosterone release in mice is a novel female. However, the stimulus female need not be sexually receptive; an ovariectomized female with no circulating ovarian hormones is a reasonably good stimulus [8]. In fact, the female herself need not be physically present, urinary odors from the female are sufficient [8]. A nonvolatile, water-soluble pheromone in female mouse urine [10] detected by the vomeronasal organ [11] accounts for much of the effectiveness of female urine to stimulate reflexive hormone release. Although this female-specific pheromone is not ovary dependent, it is regulated by the pituitary gland [12].
However other sensory cues that predict immediate sexual access to a female also likely contribute [8]. For example, reflexive hormone release in male rats could be conditioned to a previously neutral odor repeatedly associated with access to a female [13]. Volatile ovary-dependent odors in female mouse urine may also, in some circumstances, elicit reflexive testosterone release from male mice [14]. From my own research on the pheromonal elicitation of testosterone release [15] and ultrasonic mating calls [16], I believe that any stimulus (innately recognized or learned as a result of sexual experience) that causes intense sexual arousal is likely to cause reflexive sex hormone release in male mice.
Variability In Reflexive Release
While my survey of the literature was not exhaustive, I found 33 published papers that support reflexive sex hormone release during sexual encounters in non-human male mammals, including mice (12 papers), rats (9 papers), bulls (3 papers), rabbits (4 papers), hamsters (1 paper), and rams (1 paper). However one paper each in rats [17], bulls [18] and rabbits [19] did not find reflexive release following sexual encounters. Moreover some supportive studies showed evidence of reflexive release in some subjects but not in others. In humans the evidence is also mixed where four early papers provided evidence for reflexive release [20; 21; 22; 23] while two did not [24; 25]. The point of presenting this information is that sexual encounters do not invariably lead to reflexive testosterone release. Investigators attempting to examine behaviors occurring during reflexive release should be aware of this issue.
There are, no doubt, multiple reasons for inconsistencies in finding sexually stimulated hormone release. For example, after reviewing the nonhuman literature, Harding [26] concluded that some of the inconsistencies could be explained by the varying methods of collecting hormone from the blood. There is evidence that stress can deactivate the GnRH pulse generator. Many blood-collection techniques, such as venipuncture, heart puncture, tail vein bleeding, and intraocular collection, are stressful and seriously bias the results of multiple measures taken from the same male.
A second important variable may be the extent to which the male is sexually aroused by the reproductive situation. In male mice, sexual interest in the female is accompanied by the emission of ultrasonic mating calls [27; 28] and based upon arguments presented elsewhere [16], I believe these calls to be an excellent index of sexual arousal in male mice. We [15] recently found that anticipatory testosterone release 30 min after stimulus presentation was significantly correlated with the occurrence of mating calls during the first min after stimulus presentation in CF-1 or DBA/2J males. Thus, immediate sexual arousal by the sexually arousing stimulus likely initiates the cascade of neurological/hormonal events leading to later testosterone release.
The intensity of male sexual arousal may also play a role. For example, in mice, reflexive release is optimized when the sexually arousing stimulus is a novel female [9]. A familiar cohabiting female is not as good [29]. It is interesting that in a human study not finding reflexive release, hormone levels were measured after coitus in married couples [24]. In another nonsupportive study [25], male humans were sexually aroused by a pornographic film but, in some cases, experienced only partial erection. Perhaps both stimulus situations in humans were suboptimal for initiating the intensity of sexual arousal necessary for reflexive hormone release. The intensity of emotion is reflected in the accompanying level of sympathetic arousal [30], a point to which I will return in a later section of this paper.
Subtle factors in the testing environment may also play a role in the occurrence of sexual arousal. Mating calls by CF-1 males were substantially diminished if the male encountered a female stimulus in an unfamiliar cage or even when a clean cage top was placed on the male’s otherwise familiar home cage immediately before testing (unpublished observations). Thus, CF-1 males must be in highly familiar surroundings to become sexually aroused. Some failures to demonstrate reflexive testosterone release could reflect inadequate male familiarity with the testing environment.
The refractory period for sex hormone release, mentioned earlier, also contributes. For example if a male mouse had previously exhibited spontaneous release within approximately 25–45 min of encountering a female, anticipatory release would not be observed. As a result, in a familiar testing environment using a highly arousing stimulus, only about 75% of CF-1 males showed evidence of testosterone elevations [15]. Unless an investigator can monitor testosterone levels before and during reflexive release without killing, stressing, or otherwise affecting the male’s behavior and endocrine physiology, large sample sizes are required to correlate the presumed occurrence of reflexive release with reproductive behavior or physiology. An alternative, and more direct, strategy would be to simulate reflexive testosterone release. However, there are issues with this strategy as well, which I will address later.
A final variable is whether the male is genetically capable of reflexive release. For example, male mice from a number of strains clearly showed reflexive testosterone release around 30 min after exposure to female stimuli including CD-1 [31], CF-1 [32], CBF-1 [4], CBA [33], SWR, PT, BALB/C, ICR and A/HE [14]. However, other strains did not, including C3HA/Y, and C57BL/6J [14] and C57Bl/6J x AKR/J (CK) hybrids [15].
In further exploration of whether nonresponsive CK males might show release at a different time, no evidence was found of testosterone elevations 20, 50, 60, or 80 min [15], nor LH elevations 10 min (unpublished finding), after stimulation providing confidence that these males were incapable. At the same time, the males’ precopulatory and copulatory behaviors were not impaired in any obvious way; they readily emitted mating calls to, and copulated with, receptive females. Thus the brain mechanism underlying sexual arousal in CK males appeared to function normally but, for some reason, the GnRH pulse generator was not concomitantly activated.
The genetic variability among mice in reflexive release could reflect genetic changes that occurred as a result of domestication. In the protected environments of domestication, reflexive release may not be as essential to reproductive success, resulting in relaxed natural selection for its occurrence. Thus more genetic variation for reflexive release (i.e. more nonresponders) might be expected in domesticated than in feral populations.
As an aside, mouse strains that do not show reflexive release might provide excellent systems in which to dissect the neuroendocrine pathways that normally intervene between sexual arousal and reflexive hormone release.
Adaptive Function for Reflexive Testosterone Release?
To my knowledge, reflexive release in reproductive situations has been observed in at least some males of every mammalian species examined. Such phylogenetic conservation is usually indicative of an important function.
Some early workers speculated on the function of mating-induced sex-hormone release. For example, Maruniak and Bronson [34] pointed out that the dramatic robustness of this phenomenon suggests some function. At the same time, Bronson and Desjardins [32] found the phenomenon “puzzling” since reflexive hormone release does not contribute much to the overall testosterone budget in adult male mice. Why waste energy on such a mechanism unless it has some independent function?
In mice, reflexive testosterone release by the male coincides with courtship and copulatory behavior [9]. As seen in Figure 2, anticipatory release roughly coincides with the initial mating bout; and ejaculatory release, with the resumption of mating following the post-ejaculatory refractory period. Bronson and Desjardins [32] suggested that LH and testosterone (perhaps in conjunction with elevations in prolactin, corticosterone, epinephrine or norepinephrine) might be important in supporting some aspects of mating through the rapid alteration of neurotransmitter actions in the brain. However, one finding that is difficult to interpret in terms of a functional hypothesis is that male reflexive release is not limited to just sexually receptive females in estrus [12]. Females (or their urine) from any stage of the estrous cycle, as well as ovariectomized females (or their urine), are effective [12]. Perhaps this is a peculiarity associated with domestication [35] and that wild mice would be more selective.
However, reflexive testosterone release also may contribute to long-term testosterone balance in some species. For example, evidence from rats could be interpreted as supporting long-term effects. In this work, male rats housed for several months in isolation from females began to show testicular and accessory gland atrophy relative to males that cohabited with females [36]. Purvis and Haynes subsequently found that 4 days of exposure to female rat urine significantly elevated testosterone levels in male rats [37] suggesting that a female pheromone may be involved in maintaining normal hormone levels. Reflexive release has also been suggested to help promote spermatogenesis [34].
On the other hand, while female urinary pheromones hasten puberty in male mice [38], once puberty is attained, males rapidly habituate their hormonal responses to cohabiting females [9] and thereafter cohabitation has little effect upon male sexual development and hormone release [29]. Thus species variation appears to exist in the regulation and function of reflexive hormone release.
Alternatively, reflexive testosterone release may have indirect benefits to the female that stimulated release [39]. For example, by stimulating a male’s testosterone secretion, the female promotes his release of an androgen-dependent urinary pheromone. In the case of an adult female who has wandered into a male's territory, increased pheromone release may accelerate her own estrous cycling thereby bringing her into receptivity quicker (Whitten Effect) [40]. On the other hand, if she were a prepubertal female, the male pheromone may hasten her sexual maturation through pheromone-mediated puberty acceleration (Vandenbergh Effect) [41].
These different long-term and short-term functions are not mutually exclusive and it is likely that in some species reflexive testosterone release serves multiple functions.
Evidence For Testosterone’s Quick Effects Upon Physiology And Behavior
Much nonhuman research examining the effects of testosterone on nonsexual behavior (i.e. reward, anxiety, learning) has been motivated by attempts to model two clinically important human conditions: hypogonadism and anabolic steroid abuse. However, this research often employs chronic rather than acute treatments, which are not ideal for understanding quick testosterone actions. Furthermore, research relevant to hypogonadism typically examines testosterone’s effects in castrates, which may not be relevant for modeling effects in gonadally intact males. Although research relevant to human anabolic steroid abuse often uses gonadally intact males, the high dosages employed may not be relevant to the effects of endogenous titers. Consequently much research must be interpreted with caution in regards to testosterone’s possible quick effects in normal males.
Despite these caveats, there is research consistent with the hypothesis that causing (or simulating) reflexive testosterone secretion in a male mouse or male rat has quick (possibly nongenomic) effects upon his behavior and physiology.
Copulatory Behavior
Gonadally intact male CF-1 mice were pre-exposed to female urine [42], under conditions that cause reflexive hormone release [4]. Thirty min after encountering the urine (when the reflexive pulse should have been peaking), these males were quicker to begin mounting receptive females than males not receiving urine pre-exposure. However, the increase in mounting behavior could have been caused by many other co-occurring factors. In a second experiment which attempted to mimic a naturally occurring pulse by s.c. injection, gonadally intact male mice began mounting receptive females quicker than did oil-treated males 30 min after receiving 500 μg of testosterone. Thus, elevated testosterone titers did quickly hasten the onset of male mouse sex behavior.
Other work suggests that the quick actions of testosterone in male mice may be mediated by aromatization to estradiol. Aromatase activity can change quickly in the brain (within minutes) in response to both naturally occurring events [43; 44] and to experimental manipulation by aromatase inhibitors [45]. Such events, in turn, rapidly alter estradiol levels in the brain.
Accordingly, two different aromatase inhibitors (Vorozol & ATD), each administered in a single injection, suppressed both precopulatory (anogenital investigation) and copulatory reproductive behavior (intromission and mount frequency) in C57BL/6J male mice during a 30-min test beginning 10 min after injection [46]. Several lines of evidence supported that the inhibitors were working through suppression of brain estradiol synthesis rather than through nonspecific actions. Furthermore, the inhibition of reproductive behavior by ATD could be overcome by high dosages of estradiol. Furthermore, in estrogen-deficient aromatase knock-out (ARKO) mice, the quick behavioral actions of estradiol upon the males’ reproductive behavior were accentuated by a week’s pretreatment with a low dosage of estradiol. Thus, I would predict that gonadally intact, wild-type male mice (with normal brain levels of testosterone and estradiol) would be more sensitive to estradiol’s quick effects than long-term castrates.
Similar data exist for in rats. Pre-exposing a gonadally intact male rat for 10 min to a female rat, which should cause reflexive sex hormone release [47], subsequently reduced the male's mount latency, intromission latency, and ejaculation latency when given access to a second receptive female [48]. Consistent with these shortened latencies being caused by testosterone, gonadally intact male rats injected i.p. with 100 μg of testosterone (simulating a testosterone pulse) exhibited shortened intromission and ejaculation latencies 1 hr after injection [49].
The quick effects of testosterone upon male rat reproductive behavior also may require aromatization to estradiol [50]. A single injection of estradiol (either 20 or 100 μg/kg) to sexually experienced, castrated male rats increased precopulatory (anogenital sniffing) and copulatory sex behavior (mounting) to a receptive female beginning 15 min after injection. A single injection of testosterone (2 or 10 mg/kg) was ineffective. However, the same males received both testosterone and estradiol treatments, with testosterone injections preceding estradiol injections by 1 wk. Although this confound was acknowledged and discounted, the testosterone treatment nonetheless could have affected estradiol responsiveness but not vice versa. The ineffectiveness of testosterone, compared to the previous study [49], may have been due to the males’ castrated status.
Thus in both mice and rats, elevated testosterone or estradiol titers had quick effects in promoting the occurrence of copulatory behavior. Moreover, since estradiol was effective at much lower dosages than testosterone in both species, it is highly plausable that testosterone exerts its quick effects upon reproductive behavior following aromatization to estradiol.
While testosterone’s quick facilitation of reproductive behavior may have been caused via effects upon sexual motivation (through conversion to estradiol), other possibilities also exist (see below).
Penile Reflexes
Similar to male copulatory behavior, penile reflexes are lost in male rats following castration and restored by androgen replacement [51]. However the mechanism of testosterone action for penile reflexes is quite different than for copulatory behavior. Whereas copulatory behavior restoration required testosterone aromatization, penile-reflex restoration required either a direct action of testosterone or its 5α-reduction [52; 53; 54; 55]. In addition, the testosterone dosage necessary to restore penile reflexes was about 5 times higher than that necessary to restore male copulatory behavior [56]. And finally, in contrast to copulatory behavior restoration, penile muscular responses that underlay the penile reflex were altered in as little as 6 min following testosterone injection in long-term castrates [57] and did not depend upon protein synthesis [58].
The evidence was most consistent with penile reflexes being activated by a nongenomic membrane mechanism of testosterone action in the spinal cord [58]. Hart [56] further suggested that while baseline testosterone levels are sufficient to maintain copulatory behavior, pulsatile levels may be necessary to activate penile reflexes. Thus the quick effects of testosterone in promoting sexual performance in male rodents, described earlier, could have been due, in part, to its effects upon penile reflexes.
Reward and Learning
Using a conditioned place preference (CPP) paradigm, s.c. injections of testosterone dissolved in oil were rewarding to gonadally intact male mice [59; 60]. Since the pairing of testosterone with either of the two CPP chambers (black or white) occurred 30 min after injection, testosterone’s effect had to be relatively quick. However, the results did not appear as conclusive as for many drugs of abuse [61], since males were conditioned successfully to the black chamber but not to the white chamber.
Research examining rats demonstrated that the rewarding effects of testosterone occur in the brain. Gonadally intact male rats given either subcutaneous, intra-nucleus accumbens, or intra-preoptic area testosterone treatments became conditioned to prefer the compartment associated with high testosterone titers [62; 63; 64; 65]. Since the pairing of testosterone with a CPP compartment occurred during the 30 min after testosterone administration, testosterone exerted relatively quick effects. Thus testosterone modified affective mood through its quick effects in the brain. (However, see Calderone et al [66] for another interpretation.). Like most rewarding drugs, the rewarding effects of testosterone appeared to be mediated by dopamine release with both D1 and D2 receptors playing a role [67].
More recent evidence indicates that 5α-androstanediol, a reduced metabolite of testosterone, is more rewarding than testosterone [68] suggesting that testosterone may be a prohormone for its 5α-reduced neurosteroid metabolites. Since 5α-androstanediol is a weak androgen receptor agonist, but can act as an allosteric modulator of various ionotropic receptors [68], this steroid has the potential to quickly modulate neurotransmitter activity in the brain resulting in quick changes in behavior.
Another paradigm to study the rewarding effects of a drug is to examine whether animals will self-administer it. Further supporting testosterone being rewarding, gonadally intact male hamsters self-administered testosterone by either oral [69], i.v. or i.c.v. self-administration [70]. In fact, following i.c.v. self-administration, 24% of male hamsters died of apparent overdose several days after a high intake of testosterone. Since the central autonomic effects of testosterone self-administration (depressed locomotion, respiration, and body temperature), as well as self-administration itself, could be blocked with naltrexone, Peters and Wood [71] hypothesized that testosterone’s rewarding effects were mediated, at least in part, through an opioidergic mechanism. Although castrated males self-administered testosterone i.c.v., gonadally intact males did so at lower concentrations [72] indicating that normal endogenous testosterone levels appear necessary to maintain optimal testosterone responsiveness. In addition, hamsters also self-administered drostanolone (a nonaromatizable anabolic steroid) indicating that aromatization to estrogens was not required.
At the same time, Peters and Woods [73] noted that the preference for self-administering testosterone was not as pronounced as for either cocaine or heroin. They suggested that the magnitude of testosterone reinforcement is more similar to mild reinforcers such as caffeine, nicotine, or benzodiazepines.
Perhaps one function of testosterone’s rewarding effect is to enhance a male’s capacity for associative learning during reproductive situations so that sexual performance can be improved more quickly with experience. Consistent with this hypothesis, gonadally intact male rats performed better in hippocampally mediated learning tasks than castrated males and the learning deficits in castrated males could be reversed with treatments of testosterone, or it’s 5α-reduced metabolites, dihydrotestosterone, and 5α-androstanediol, either systemically or directly into the hippocampus [74; 75]. The efficacy of 5α-androstanediol suggests a nongenomic mechanism. However, recent work also implicates the possible importance of estrogen receptor beta as a substrate for 5α-androstanediol action [76].
Anxiolysis
A series of experiments examining house mice [77] supported the hypotheses that reflexive testosterone release reduces male anxiety (in terms of behavior on an elevated plus-maze) and that this anxiolysis is mediated by the conversion of testosterone to neurosteroids that interact with GABAA receptors. For example, exposure to opposite-sex conspecifics for 10 min significantly reduced both male and female anxiety 20 min later compared to control mice not receiving this exposure. Male exposure to female urine was also anxiolytic. Thus, urinary pheromones of female mice likely initiated the events leading to male anxiolysis. Testosterone’s effects in shortening the latency for copulatory behaviors in mice and rats also could have been mediated by anxiety reduction.
Supporting the idea that anxiolysis was mediated by heightened testosterone titers, the anxiolytic effect of testosterone was dose dependent with a 250 μg s.c. injection required. Thus, testosterone elevations were associated with reduced male anxiety consistent with a rapid mechanism of testosterone action. Testosterone levels had to be well above baseline levels in order to induce anxiolysis. A high dosage of 5α-dihydrotestosterone was more anxiolytic than a high dosage of estradiol benzoate suggesting that testosterone action requires 5α-reduction. These results were consistent with an earlier abstract that both testosterone and dihydrotestosterone appeared anxiolytic in male mice [78].
In addition, androsterone and 3α-androstandione, 3α, 5α-reduced metabolites of testosterone, were both anxiolytic at a lower dosage than testosterone; supporting the notion that testosterone is converted into neurosteroid metabolites for anxiolytic activity. And finally, picrotoxin and bicucculine, noncompetitive and competitive antagonists of the GABAA receptor respectively, blocked the anxiolytic effects of testosterone. These antagonists can be toxic at higher dosages, which complicates the interpretation of their effects. Nonetheless, the working hypothesis was that testosterone’s anxiolytic effect in male mice required conversion to neurosteroid metabolites acting as allosteric modulators of ionotropic receptors in the brain.
Similar results were found in male rats. Bitran and his colleagues [79; 80; 81] demonstrated that 2 different anabolic-androgenic steroids, testosterone propionate, and dianabol, both reduced anxiety and potentiated GABA-stimulated chloride flux in intact male rats. Another laboratory also found castration of male rats to be anxiogenic and testosterone replacement therapy, anxiolytic [82].
Edinger and Frye [74; 83] found testosterone, dihydrotestosterone, and 3α-androstanediol all to be anxiolytic in castrated male rats when administered systemically or via intrahippocampal incannulation. Placement into the hippocampus 2 hrs before testing indicated a relatively quick mechanism of action. Indomethacin (which blocks the conversion of dihydrotestosterone to 3α-androstanediol) also blocked the anxiolytic effects of dihydrotestosterone, suggesting that the 3α, 5α-metabolites of testosterone are the active molecules.
However, conflicting data exist on the mechanism of testosterone’s anxiolytic action in rats. Fernandez-Guasti and Martinez-Mota [84], using burying behavior to measure anxiolysis, found that while testosterone reduced anxiety in castrated male rats, two 3α, 5α-reduced metabolites of testosterone (3α-androstanediol and androsterol) did not. Moreover, while flutamide (an androgen receptor blocker) blocked the anxiolytic effects of testosterone, flumazenil (a GABAA receptor blocker) did not. Thus testosterone appeared to reduce anxiety through intracellular androgen receptors. The reasons for these differences are not clear but could be due to the different rat strains (Wistar vs Long Evans) or different anxiety assays used. Further research will be necessary to resolve this issue.
Analgesia
Since anxiolytic drugs are often also analgesic, it is interesting that castration of male rats makes them more sensitive to pain which is reversed in castrates by testosterone propionate [85]. Edinger and Fry [74] further found that systemically administered testosterone, dihydrotestosterone, or 3α-androstanediol all increased analgesia in the tail flick and paw lick paradigms. When administered intrahippocampally, these three steroids were also analgesic in the tail flick paradigm but only testosterone and 3α-androstanediol were analgesic in the paw lick paradigm. Although the systemic treatments were chronic silastic implants, the hippocampal treatments were infused (as cyclodextrin solutions) just before a given day’s testing indicating relatively quick hormone actions.
Converging Evidence And Unresolved Issues
For the rodent responses described here (anxiolysis, reward, learning, analgesia & penile reflexes) the preponderance of evidence implicates their quick (possibly nongenomic) activation by either testosterone or its neurosteroid metabolites. At one time, nongenomic and genomic steroid effects were thought distinguishable by their time frame of action and by their receptor location. Nongenomic effects involved membrane receptors and took seconds or minutes while genomic effects involved traditional intracellular steroid receptors and took hours or days. However, we now know that temporal overlap exists for some genomic and nongenomic actions and that the distinction between nongenomic membrane receptors and genomic intracellular ones also is not always valid (See McEwen [86] for an excellent discussion of these issues). Ultimately, classifying the quick responsiveness to testosterone (or its metabolites) as genomic or nongenomic requires identifying the appropriate receptors and their mechanism of action.
Data from several labs converge on the rewarding, anxiolytic, analgesic actions of testosterone being mediated by the reduced neurosteroid metabolites of testosterone perhaps working as positive allosteric modulators of GABAA receptors in the brain. This conclusion seems plausible since other GABAA agonists (i.e. benzodiazepines, barbiturates, alcohol) produce responses similar to those described for testosterone upon reward, anxiolysis, and analgesia. Although testosterone’s quick effect upon penile reflexes likely occurs in the spinal cord, rather than the brain, testosterone’s reduced metabolites appear involved here as well.
However, testosterone’s quick effect upon male reproductive behavior appears quite different in involving aromatization and activation of estrogenic pathways [87; 88]. Outside the CNS, testosterone also exerts quick effects through G-protein coupled receptors [89], and such effects inside the CNS cannot be ruled out. Some steroids also interact with voltage-gated ion channels and specific membrane receptors [2]. And finally one cannot rule out genomic actions of testosterone at classical androgen receptors [82]. It is likely that the quick responses to testosterone are carried out by multiple mechanisms of testosterone action at different locations of the brain and spinal cord with their relative importance perhaps differing across strains and species.
Although more work is required to discriminate among these, and perhaps other, possibilities, the actions of testosterone described in this review certainly appear to differ from the slow and prolonged estrogenic actions of testosterone in organizing and activating the mammalian brain [90].
Another issue critical for testosterone’s relevance in normal male behavior is the dosage required to produce quick effects. Many experiments modeling human anabolic steroid abuse employ dosages much higher than those necessary to restore reproductive behaviors in castrated males. If dosages beyond the physiological range are required, as has been suggested for reward in rats [91], an argument could be made against these testosterone-mediated responses being important in normal behavior (although not affecting their validity for understanding human anabolic steroid abuse).
However, there are a number of considerations in deciding whether a testosterone dosage is in the physiological or pharmacological range. One obvious factor would be whether exogenously administered testosterone exceeds the amplitude of pulsatile release in a given species. However, this amplitude must be determined empirically, and may not be apparent from mean hormone titers commonly reported in the literature.
To illustrate this potential problem of interpretation, individually housed, gonadally intact male mice typically have serum levels around 1–3 ng/ml [15]. This mean is a “snapshot” reflecting that, at any given time, approximately 75% of the males are experiencing baseline levels while the other 25% are experiencing a testosterone pulse, although not necessarily at peak levels. On the other hand, peak pulsatile amplitudes in individual males can reach as much as 40 times higher than mean values (40 ng/ml) [4].
In addition, dosages necessary for responsiveness in castrated males may overestimate the amount required for gonadally intact males. As noted earlier, normal endogenous testosterone titers in hamsters appear necessary to maintain full sensitivity to testosterone’s rewarding effect [72]. And male mice that are unable to synthesize estradiol (ARKO mice), require pretreatment with estradiol to exhibit good responsiveness to its quick effects on reproductive behavior [46]. Thus several lines of evidence suggest that normal brain levels of testosterone (or its metabolites) are necessary to maintain full responsiveness to the quick effects of testosterone (or its metabolites).
Yet another issue in interpreting whether dosages are pharmacological is the method of administering testosterone. In some studies cited here, testosterone was administered s.c. dissolved in oil or inside silastic capsules. This method results in slow and prolonged absorption. While useful for studying mechanisms of testosterone action that require sustained hormone levels, this method may, for several reasons, overestimate the amount necessary for testosterone’s effects. First of all, the rewarding effect (i.e. abuse potential) of a drug is enhanced by quick absorption, which can account for some of the difference between different drugs in the same class (e.g. heroin vs methadone) or the same drug administered differently (e.g. smoking of crack cocaine vs chewing, drinking or snorting cocaine) [92]. And empirically, lower overall amounts of androgen were required to maintain androgen-sensitive behavior and physiology in rats if administered in a pulsatile, rather than a more continuously available, fashion [93]. Thus administration that better mimics endogenous testosterone’s abrupt, pulsatile rise in plasma should provide a more realistic estimate of appropriate dosage for its quick effects (perhaps through i.p. or i.v. administration and/or the use of cyclodextrin).
And finally the brain has the enzymatic machinery to produce de novo, and modify, many of the steroids produced by the peripheral endocrine glands [2; 94]. Such “neurosteroids” are thought to work in a paracrine or autocrine fashion, and in some cases can achieve local brain concentrations substantially higher than in the blood. In fact, such high concentrations are thought, in some cases, to be necessary for nongenomic effects [2].
It is possible that testosterone produced by peripheral endocrine glands, or its neurosteroid metabolites, must augment endogenous neurosteroids to attain brain concentrations necessary for nongenomic effects. If endogenous neurosteroid levels are low (perhaps due to castration or to some other aspect of being in an artificial laboratory setting), it may be necessary to provide exogenous testosterone dosages that exceed normal blood levels to observe effects. Furthermore, castration/adrenalectomy reduces testosterone in the brain to undetectable levels suggesting that testosterone itself (unlike some other steroids in the brain) may be solely of peripheral endocrine origin [94].
A Heuristic Model For Investigating Testosterone’s Quick Effects On Behavior
Building upon functional arguments developed by Hart [56], some of the physiological (i.e. penile reflex activation and analgesia) and motivational changes (i.e. lowered anxiety, enhanced positive affect/reward) that occur immediately prior to and during reproduction may be costly or maladaptive to maintain outside of reproductive situations. Perhaps these changes have become linked in some male mammals to a physiological state (reflexive testosterone release) that insures their occurrence at the appropriate time.
In Figure 3, I present a hypothetical (and speculative) model that attempts to integrate how I envision spontaneous and reflexive testosterone release interacting with the CNS to regulate male reproductive behavior and physiology.
Figure 3.
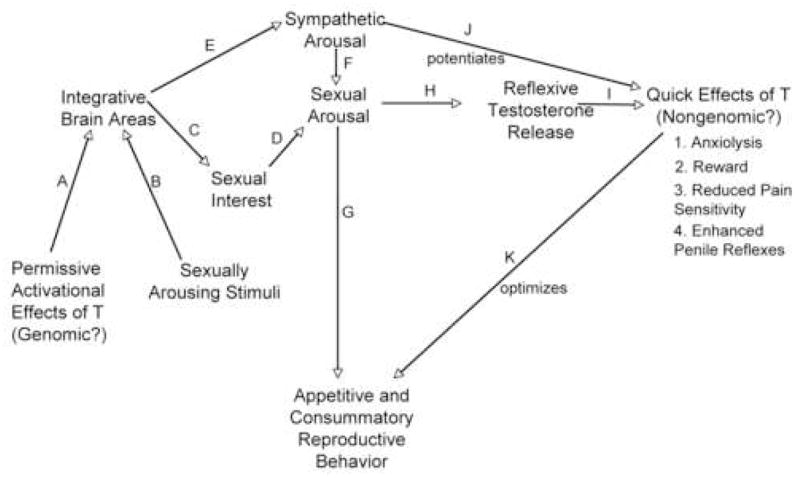
A hypothetical model of how spontaneous and reflexive testosterone release might affect adult male reproductive physiology and behavior. See the text for an explanation of the various events (A-K) are hypothesized to be involved.
In order for an adult male to be capable of expressing normal levels of male-typical sexual motivation and reproductive behavior, the male’s brain must be organized early in development by testosterone. The model presented here assumes that such organizational events have already occurred. The model also reflects my own biases about the nature and measurement of sexual arousal (See Sachs [95] for a good discussion of the complexities and a different perspective).
In order for an adult male to express many male-typical behaviors, the organized areas of his brain also must be activated by testosterone (designated by A in Fig. 3). This activation occurs largely via spontaneous testosterone release since such release accounts for the vast majority of the male’s adult testosterone budget. However, as noted earlier, in some species such as the Norway rat, reflexive testosterone might also make a contribution.
However the activational effects of testosterone in adulthood do not cause reproductive behavior to occur. Rather testosterone release simply permits sex behavior to occur if the male also encounters the appropriate sexually arousing stimuli (B). In mice, pheromones produced by the female are probably of greatest importance although other stimuli that are innately recognized or are learned as a result of experience also likely contribute.
In the presence of appropriate stimulation, the male’s motivation to perform sexual behavior (sexual arousal) increases. Sexual arousal could be considered to be an emotion and one perspective [30] is that emotions consist of two components. One component (C & D) determines the emotion’s specificity (i.e. sexual, aggressive, fearful, etc) while a second component (E & F: sympathetic arousal), common to most emotions, determines its intensity. The intensity of sexual arousal would be affected both by the salience of the sexually arousing stimuli and also by normal fluctuations in the male’s internal state. For example, freshly collected female urine is more salient for stimulating male-typical behaviors than urine voided for 24 hr [96] and how recently the male had engaged in sexual activity could also affect his sexual arousability.
If the male becomes highly sexually aroused and a receptive female is also present, the male will then engage in male-typical appetitive and copulatory reproductive behaviors (G).
We now come to the even more speculative part of the model. I hypothesize, based upon research already described, that intense sexual arousal also causes reflexive testosterone release (H). However, one problem with the model as presented so far is that it fails to differentiate between the effects of spontaneous and reflexive release. It does not make much adaptive sense for a male rodent to experience reward, anxiolysis, reduced pain sensitivity, and enhanced penile reflexes during high testosterone titers outside of reproductive or agonistic situations (i.e. during spontaneous release).
However, one fundamental difference between reflexive and spontaneous pulses is that reflexive release, unlike spontaneous release, is also typically accompanied by sympathetic arousal. Consequently, I hypothesize that male sympathetic arousal during reproduction synergizes with, and potentiates, the quick and transitory effects of reflexive testosterone release upon physiology and behavior (J). However, the mechanism by which sympathetic arousal would accomplish this potentiation of testosterone action is not known.
While the quick effects of testosterone are hypothesized to enhance or optimize successful reproduction (J), these effects are not absolutely necessary. As pointed out earlier, several mouse strains that do not exhibit sexually stimulated reflexive release nonetheless breed successfully in the laboratory. On the other hand, enhanced sexual learning, reduced anxiety, a lowered pain threshold, and enhanced penile reflexes may be more critical for successful reproduction in natural situations than under benign laboratory conditions.
Mice, Rats or Other Species?
Although males of many mammalian species show reflexive testosterone release associated with sexual arousal, I believe mice to be a model species for examining nongenomic effects of testosterone upon behavior.
One reason relates to species differences in the “peaks and valleys” of testosterone secretion. For example, basal testosterone levels in the rat are higher than the mouse, while testosterone peaks are lower [4; 5]. Thus the testosterone “signal” appears more distinct in the mouse than the rat. In addition, the various sex hormones in the mouse also appear more tightly linked than in some other species. For example, while a single LH pulse is often sufficient to stimulate testosterone release in the mouse, more LH signal (several LH pulses in quick succession) is usually necessary to stimulate testosterone release in the rat and human [5]. There also is a good understanding of the environmental signals (i.e. pheromones) and sensory detection (i.e. vomeronasal system) that trigger reflexive testosterone release in the mouse. And finally the treasure chest of genetic variability that exists within the house mouse (many genetically characterized inbred strains as well as knockouts, conditional knockouts, knockdowns, knockins, as well as a sequenced genome), should greatly aid in genetically characterizing the mechanisms involved in mammalian reflexive testosterone release.
At the same time, the validity and generality of testosterone’s quick effects upon physiology and behavior ultimately will be determined by their occurrence in other species as well.
Acknowledgments
This work was supported by a grant from NIMH (R15 MH065956 to JGN). I thank Murray Itzkowitz and 2 anonymous reviewers for critically reading earlier versions of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.McEwen BS. Steroid hormone actions on the brain: When is the genome involved? Hormones and Behavior. 1994;28:396–405. doi: 10.1006/hbeh.1994.1036. [DOI] [PubMed] [Google Scholar]
- 2.Schlichter R, Keller AF, De Roo M, Breton JD, Inquimbert P, Poisbeau P. Fast nongenomic effects of steroids on synaptic transmission and role of endogenous neurosteroids in spinal pain pathways. J Mol Neurosci. 2006;28:33–51. doi: 10.1385/jmn:28:1:33. [DOI] [PubMed] [Google Scholar]
- 3.Wehling M, Losel R. Non-genomic steroid hormone effects: membrane or intracellular receptors? J Steroid Biochem Mol Biol. 2006;102:180–3. doi: 10.1016/j.jsbmb.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Coquelin A, Desjardins C. Luteinizing hormone and testosterone secretion in young and old male mice. Am J Physiol. 1982;243:E257–63. doi: 10.1152/ajpendo.1982.243.3.E257. [DOI] [PubMed] [Google Scholar]
- 5.Ellis GB, Desjardins C. Male rats secrete luteinizing hormone and testosterone episodically. Endocrinology. 1982;110:1618–1627. doi: 10.1210/endo-110-5-1618. [DOI] [PubMed] [Google Scholar]
- 6.Coquelin A, Bronson F. Episodic release of luteinizing hormone in male mice: antagonism by a neural refractory period. Endocrinology. 1981;109:1605–1610. doi: 10.1210/endo-109-5-1605. [DOI] [PubMed] [Google Scholar]
- 7.Vadakkadath Meethal S, Atwood CS. The role of hypothalamic-pituitary-gonadal hormones in the normal structure and functioning of the brain. Cell Mol Life Sci. 2005;62:257–70. doi: 10.1007/s00018-004-4381-3. [DOI] [PubMed] [Google Scholar]
- 8.Coquelin A, Bronson F. Secretion of luteinizing hormone in male mice: factors that influence release during sexual encounters. Endocrinology. 1980;106:1224–1229. doi: 10.1210/endo-106-4-1224. [DOI] [PubMed] [Google Scholar]
- 9.Coquelin A, Bronson F. Release of luteinizing hormone in male mice during exposure to females: habituation of the response. Science. 1979;206:1099–1101. doi: 10.1126/science.573924. [DOI] [PubMed] [Google Scholar]
- 10.Singer A, Clancy A, Macrides F, Agosta W, Bronson F. Chemical properties of a female mouse pheromone that stimulates gonadotropin secretion in males. Biology of Reproduction. 1988;38:193–199. doi: 10.1095/biolreprod38.1.193. [DOI] [PubMed] [Google Scholar]
- 11.Clancy AN, Coquelin A, Macrides F, Gorski RA, Noble EP. Sexual behavior and aggression in male mice: Involvement of the vomeronasal system. J Neurosci. 1984;4:2222–2229. doi: 10.1523/JNEUROSCI.04-09-02222.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston RE, Bronson F. Endocrine control of female mouse odors that elicit luteinizing hormone surges and attraction to males. Biology of Reproduction. 1982;27:1174–1180. doi: 10.1095/biolreprod27.5.1174. [DOI] [PubMed] [Google Scholar]
- 13.Graham JM, Desjardins C. Classical conditioning: induction of luteinizing hormone and testosterone secretion in anticipation of sexual activity. Science. 1980;210:1039–41. doi: 10.1126/science.7434016. [DOI] [PubMed] [Google Scholar]
- 14.Amstislavskaya TG, Khrapova MV. Effect of genotype on behavioral and hormonal components of sexual activation of male mice. Bull Exp Biol Med. 2002;133:475–7. doi: 10.1023/a:1019865822028. [DOI] [PubMed] [Google Scholar]
- 15.James P. Department of Biological Sciences. Lehigh University; Bethlehem, PA: 2005. Reflexive testosterone release in male mice: roles of genotype and sexual arousal; p. 156. Ph.D. Dissertation. [Google Scholar]
- 16.Nyby JG. Auditory communication among adults. In: Willott JF, editor. Handbook of mouse auditory research: From behavior to molecular biology. CRC Press; Boca Raton, London, N.Y., Washington, D.C; 2001. pp. 3–18. [Google Scholar]
- 17.Balin MS, Schwartz NB. Effects of mating on serum LH, FSH, and prolactin and accessory tissue weight in male rats. Endocrinol. 1976;98:522–6. doi: 10.1210/endo-98-2-522. [DOI] [PubMed] [Google Scholar]
- 18.Gombe S, Hall EC, McEntee K, Hansel W, Pickett BW. Regulation of blood levels of LH in bulls: influence of age, breed, sexual stimulation and temporal fluctuations. Journal of Reproduction and Fertility. 1973;35:493. doi: 10.1530/jrf.0.0350493. [DOI] [PubMed] [Google Scholar]
- 19.Younglai EV, Moor Bc, Dimond P. Effects of sexual activity on luteinizing hormone and testosterone levels in the adult male rabbit. Journal of Endocrinology. 1976;76:183–191. doi: 10.1677/joe.0.0690183. [DOI] [PubMed] [Google Scholar]
- 20.Anon. Effects of sexual activity on beard growth in man. Nature. 1970;226:869–870. doi: 10.1038/226869a0. [DOI] [PubMed] [Google Scholar]
- 21.Fox CA, Ismail AAA, Love DN, Kirkham KE, Loraine JA. Studies on the relationship between plasma testosterone levels and human sexual activity. Journal of Endocrinology. 1972;52:51. doi: 10.1677/joe.0.0520051. [DOI] [PubMed] [Google Scholar]
- 22.LaFerla JJ, Anderson DL, Schalch DS. Psychoendocrine response to sexual arousal in human males. Psychosomatic Medicine. 1978;40:166–172. doi: 10.1097/00006842-197803000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Pirke KM, Kockott G, Dittmar F. Psychosexual stimulation and plasma testosterone in man. Archives of Sexual Behavior. 1974;3:577–584. doi: 10.1007/BF01541140. [DOI] [PubMed] [Google Scholar]
- 24.Stearns EL, Winter JSD, Faiman C. Effects of coitus on gonadotropin, prolactin and sex steroid levels in man. Journal of Clinical Endocrinology and Metabolism. 1973;37:687. doi: 10.1210/jcem-37-5-687. [DOI] [PubMed] [Google Scholar]
- 25.Lincoln GA. Luteinizing hormone and testosterone in man. Nature. 1974;252:232–233. doi: 10.1038/252232a0. [DOI] [PubMed] [Google Scholar]
- 26.Harding CF. Social modulation of circulating hormone levels in the male. American Zoologist. 1981;21:223–231. [Google Scholar]
- 27.Nyby J. Ultrasonic vocalizations during sex behavior of male house mice (Mus musculus): a description. Behav Neural Biol. 1983;39:128–34. doi: 10.1016/s0163-1047(83)90722-7. [DOI] [PubMed] [Google Scholar]
- 28.Sales GD. Ultrasound and mating behaviour in rodents with some observations on other behavioral situation. Journal of Zoology, London. 1972;168:149–164. [Google Scholar]
- 29.Maruniak J, Coquelin A, Bronson F. The release of LH in male mice in response to female urinary odors: characteristics of the response in young males. Biology of Reproduction. 1978;18:251–255. doi: 10.1095/biolreprod18.2.251. [DOI] [PubMed] [Google Scholar]
- 30.Schachter S, Singer JE. Cognitive, social, and physiological determinants of emotional state. Psychol Rev. 1962;69:379–99. doi: 10.1037/h0046234. [DOI] [PubMed] [Google Scholar]
- 31.Bartke A, Dalterio S. Evidence for episodic secretion of testosterone in laboratory mice. Steroids. 1975;26:749–756. doi: 10.1016/0039-128x(75)90107-5. [DOI] [PubMed] [Google Scholar]
- 32.Bronson F, Desjardins C. Endocrine responses to sexual arousal in male mice. Endocrinology. 1982;111:1286–1291. doi: 10.1210/endo-111-4-1286. [DOI] [PubMed] [Google Scholar]
- 33.Amstislavskaya TG, Popova NK. Female-induced sexual arousal in male mice and rats: behavioral and testosterone response. Horm Behav. 2004;46:544–50. doi: 10.1016/j.yhbeh.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Maruniak J, Bronson F. Gonadotropic responses of male mice to female urine. Endocrinology. 1976;99:963–969. doi: 10.1210/endo-99-4-963. [DOI] [PubMed] [Google Scholar]
- 35.Price EO. Behavioral aspects of animal domestication. Q Rev Biol. 1984;59:1–32. [Google Scholar]
- 36.Drori D, Folman Y. Effect of cohabitation on the reproductive system, kidney and body composition of male rats. Journal of Reproduction and Fertility. 1964;8:351–359. doi: 10.1530/jrf.0.0080351. [DOI] [PubMed] [Google Scholar]
- 37.Purvis K, Haynes NB. Effect of the odour of female rat urine on plasma testosterone concentrations in male rats. Journal of Reproduction and Fertility. 1978;53:63–65. doi: 10.1530/jrf.0.0530063. [DOI] [PubMed] [Google Scholar]
- 38.Vandenbergh JG. The influence of the social environment on sexual maturation in male mice. J Reprod Fertil. 1971;24:383–90. doi: 10.1530/jrf.0.0240383. [DOI] [PubMed] [Google Scholar]
- 39.Bronson FH. The reproductive ecology of the house mouse. The Quarterly Review of Biology. 1979;54:265–299. doi: 10.1086/411295. [DOI] [PubMed] [Google Scholar]
- 40.Clancy A, Singer AG, Macrides F, Bronson FH, Agosta WC. Experiential and endocrine dependence of gonadotropin responses in male mice to conspecific urine. Biol Reprod. 1988;38:183–191. doi: 10.1095/biolreprod38.1.183. [DOI] [PubMed] [Google Scholar]
- 41.Macrides F, Bartke A, Dalterio S. Strange females increase plasma testosterone levels in male mice. Science. 1975;189:1104–1106. doi: 10.1126/science.1162363. [DOI] [PubMed] [Google Scholar]
- 42.James PJ, Nyby JG. Testosterone rapidly affects the expression of copulatory behavior in house mice (Mus musculus) Physiol Behav. 2002;75:287–94. doi: 10.1016/s0031-9384(01)00666-7. [DOI] [PubMed] [Google Scholar]
- 43.Balthazart J, Baillien M, Ball GF. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology. 2006;147:359–66. doi: 10.1210/en.2005-0845. [DOI] [PubMed] [Google Scholar]
- 44.Balthazart J, Baillien M, Charlier TD, Ball GF. Calcium-dependent phosphorylation processes control brain aromatase in quail. Eur J Neurosci. 2003;17:1591–606. doi: 10.1046/j.1460-9568.2003.02598.x. [DOI] [PubMed] [Google Scholar]
- 45.Balthazart J, Baillien M, Ball GF. Rapid and reversible inhibition of brain aromatase activity. J Neuroendocrinol. 2001;13:63–73. doi: 10.1046/j.1365-2826.2001.00598.x. [DOI] [PubMed] [Google Scholar]
- 46.Taziaux M, Keller M, Bakker J, Balthazart J. Sexual behavior activity tracks rapid changes in brain estrogen concentrations. J Neurosci. 2007;27:6563–72. doi: 10.1523/JNEUROSCI.1797-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamel F, Mock EJ, Wright WW, Frankel AI. Alterations in plasma concentrations of testosterone, LH, and prolactin associated with mating in the male rat. Hormones and Behavior. 1975;6:277–288. doi: 10.1016/0018-506x(75)90014-8. [DOI] [PubMed] [Google Scholar]
- 48.De Jonge FH, Oldenburger WP, Louwerse AL, Van de Poll NE. Changes in male copulatory behavior after sexual exciting stimuli: Effects of medial amygdala lesions. Physiology and Behavior. 1992;52:327–332. doi: 10.1016/0031-9384(92)90279-b. [DOI] [PubMed] [Google Scholar]
- 49.Malmnas CO. Short-latency effect of testosterone on copulatory behaviour and ejaculation in sexually experienced intact male rats. Journal of Reproduction and Fertility. 1977;51:351–4. doi: 10.1530/jrf.0.0510351. [DOI] [PubMed] [Google Scholar]
- 50.Cross E, Roselli CE. 17beta-estradiol rapidly facilitates chemoinvestigation and mounting in castrated male rats. Am J Physiol. 1999;276:R1346–50. doi: 10.1152/ajpregu.1999.276.5.R1346. [DOI] [PubMed] [Google Scholar]
- 51.Hart BL. Activation of sexual reflexes of male rats by dihydrotestosterone but not estrogen. Science. 1979;155:1283–1284. doi: 10.1016/0031-9384(79)90129-x. [DOI] [PubMed] [Google Scholar]
- 52.Hart BL, Wallach SJ, Melese-d' PY. Hospital, Differences in responsiveness to testosterone of penile reflexes and copulatory behavior of male rats. Horm Behav. 1983;17:274–83. doi: 10.1016/0018-506x(83)90026-0. [DOI] [PubMed] [Google Scholar]
- 53.Gray GD, Smith ER, Davidson JM. Hormonal regulation of penile erection in castrated male rats. Physiology and Behavior. 1980;24:463–468. doi: 10.1016/0031-9384(80)90237-1. [DOI] [PubMed] [Google Scholar]
- 54.O'Hanlon JK, Meisel RL, Sachs BD. Estradiol maintains castrated male rats' sexual reflexes in copula, but not ex copula. Behav Neural Biol. 1981;32:269–73. doi: 10.1016/s0163-1047(81)90645-2. [DOI] [PubMed] [Google Scholar]
- 55.Meisel RL, O'Hanlon JK, Sachs BD. Differential maintenance of penile responses and copulatory behavior by gonadal hormones in castrated male rats. Horm Behav. 1984;18:56–64. doi: 10.1016/0018-506x(84)90050-3. [DOI] [PubMed] [Google Scholar]
- 56.Hart BL. Role of testosterone secretion and penile reflexes in sexual behavior and sperm competition in male rats: a theoretical contribution. Physiol Behav. 1983;31:823–7. doi: 10.1016/0031-9384(83)90279-2. [DOI] [PubMed] [Google Scholar]
- 57.Sachs BD, Leipheimer RE. Rapid effect of testosterone on striated muscle activity in rats. Neuroendocrinology. 1988;48:453–8. doi: 10.1159/000125049. [DOI] [PubMed] [Google Scholar]
- 58.Meisel RL, Leipheimer RE, Sachs BD. Anisomycin does not disrupt the activation of penile reflexes by testosterone in rats. Physiol Behav. 1986;37:951–6. [PubMed] [Google Scholar]
- 59.Arnedo MT, Salvador A, Martinez-Sanchis S, Pellicer O. Similar rewarding effects of testosterone in mice rated as short and long attack latency individuals. Addict Biol. 2002;7:373–9. doi: 10.1080/1355621021000005955. [DOI] [PubMed] [Google Scholar]
- 60.Arnedo MT, Salvador A, Martinez-Sanchis S, Gonzalez-Bono E. Rewarding properties of testosterone in intact male mice: A pilot study. Pharmacology Biochemistry and Behavior. 2000;65:327–332. doi: 10.1016/s0091-3057(99)00189-6. [DOI] [PubMed] [Google Scholar]
- 61.Schechter MD, Calcagnetti DJ. Continued trends in the conditioned place preference literature from 1992 to 1996, inclusive, with a cross-indexed bibliography. Neurosci Biobehav Rev. 1998;22:827–46. doi: 10.1016/s0149-7634(98)00012-8. [DOI] [PubMed] [Google Scholar]
- 62.Alexander GM, Packard MG, Hines M. Testosterone has rewarding affective properties in male rats: Implication for the biological bases of sexual motivation. Behavioral Neuroscience. 1994;108:424–428. doi: 10.1037//0735-7044.108.2.424. [DOI] [PubMed] [Google Scholar]
- 63.King BE, Packard MG, Alexander GM. Affective properties of intra-medial preoptic area injections of testosterone in male rats. Neuroscience Letters. 1999;269:149–152. doi: 10.1016/s0304-3940(99)00440-1. [DOI] [PubMed] [Google Scholar]
- 64.Packard MG, Cornell AH, Alexander GM. Rewarding Affective Properties of Intra-Nucleus Accumbens Injections of Testosterone. Behavioral Neuroscience. 1997;111:219. doi: 10.1037//0735-7044.111.1.219. [DOI] [PubMed] [Google Scholar]
- 65.Packard MG, Schroeder JP, Alexander GM. Expression of testosterone conditioned place preference is blocked by peripheral or intra-accumbens injection of α-Flupenthixol. Hormones and Behavior. 1998;34:39–47. doi: 10.1006/hbeh.1998.1461. [DOI] [PubMed] [Google Scholar]
- 66.Caldarone BJ, Stock HS, Abrahamsen GC, Boechler ML, Svare BS, Rosellini RA. Nonassocative processes and place preferences conditioned by testosterone. The Psychological Record. 1996;46:373. [Google Scholar]
- 67.Schroeder JP, Packard MG. Role of dopamine receptor subtypes in the acquisition of a testosterone conditioned place preference in rats. Neuroscience Letters. 2000;282:17–20. doi: 10.1016/s0304-3940(00)00839-9. [DOI] [PubMed] [Google Scholar]
- 68.Frye CA, Park D, Tanaka M, Rosellini R, Svare B. The testosterone metabolite and neurosteroid 3α-androstanediol may mediate the effects of testosterone on conditioned place preference. Psychoneuroendocrinology. 2001;26:731–750. doi: 10.1016/s0306-4530(01)00027-0. [DOI] [PubMed] [Google Scholar]
- 69.Johnson LR, Wood RI. Oral testosterone self-administration in male hamsters. Neuroendocrinology. 2001;73:285–92. doi: 10.1159/000054645. [DOI] [PubMed] [Google Scholar]
- 70.Wood RI, Johnson LR, Chu L, Schad C, Self DW. Testosterone reinforcement: intravenous and intracerebroventricular self-administration in male rats and hamsters. Psychopharmacology (Berl) 2004;171:298–305. doi: 10.1007/s00213-003-1587-7. [DOI] [PubMed] [Google Scholar]
- 71.Peters KD, Hom SM, Wood RI. Testosterone and chemosensory detection in male Syrian hamster. Horm Behav. 2004;46:341–8. doi: 10.1016/j.yhbeh.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 72.DiMeo AN, Wood RI. Circulating androgens enhance sensitivity to testosterone self-administration in male hamsters. Pharmacol Biochem Behav. 2004;79:383–9. doi: 10.1016/j.pbb.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 73.Peters KD, Wood RI. Androgen dependence in hamsters: overdose, tolerance, and potential opioidergic mechanisms. Neuroscience. 2005;130:971–81. doi: 10.1016/j.neuroscience.2004.09.063. [DOI] [PubMed] [Google Scholar]
- 74.Edinger KL, Frye CA. Testosterone's analgesic, anxiolytic, and cognitive-enhancing effects may be due in part to actions of its 5alpha-reduced metabolites in the hippocampus. Behav Neurosci. 2004;118:1352–64. doi: 10.1037/0735-7044.118.6.1352. [DOI] [PubMed] [Google Scholar]
- 75.Edinger KL, Lee B, Frye CA. Mnemonic effects of testosterone and its 5alpha-reduced metabolites in the conditioned fear and inhibitory avoidance tasks. Pharmacol Biochem Behav. 2004;78:559–68. doi: 10.1016/j.pbb.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 76.Edinger KL, Frye CA. Androgens' effects to enhance learning may be mediated in part through actions at estrogen receptor-beta in the hippocampus. Neurobiol Learn Mem. 2007;87:78–85. doi: 10.1016/j.nlm.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aikey JL, Nyby JG, Anmuth DM, James PJ. Testosterone rapidly reduces anxiety in male house mice (Mus musculus) Horm Behav. 2002;42:448–60. doi: 10.1006/hbeh.2002.1838. [DOI] [PubMed] [Google Scholar]
- 78.Domek D, Niekrasz I, Granica A, Seale T. The potent, rapid and behavior-specific actions of androgens. Society for Neuroscience Abstracts. 1992;18:814. [Google Scholar]
- 79.Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3a-hydroxy-5a{B}-pregnan-20-one: Endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Research. 1991;561:157–161. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- 80.Bitran D, Kellogg CK, Hilvers RJ. Treatment with an anabolic-androgenic steroid affects anxiety-related behavior and alters the sensititivy of cortical GABAA receptors in the rat. Hormones and Behavior. 1993;27:568–583. doi: 10.1006/hbeh.1993.1041. [DOI] [PubMed] [Google Scholar]
- 81.Bitran D, Hilvers R, Frye C, Erskine M. Chronic anabolic-androgenic steroid treatment affects brain GABA(A) receptor-gated chloride ion transport. Life Sciences. 1996;62:573–583. doi: 10.1016/0024-3205(95)02326-7. [DOI] [PubMed] [Google Scholar]
- 82.Gonzalez MI, Farabollini F, Albonetti E, Wilson CA. Interactions between 5-hydroxytryptamine (5-HT) and testosterone in the control of sexual and nonsexual behaviour in male and female rats. Pharmacology, Biochemistry and Behavior. 1994;47:591–601. doi: 10.1016/0091-3057(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 83.Edinger KL, Frye CA. Testosterone's anti-anxiety and analgesic effects may be due in part to actions of its 5alpha-reduced metabolites in the hippocampus. Psychoneuroendocrinology. 2005;30:418–30. doi: 10.1016/j.psyneuen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 84.Fernandez-Guasti A, Martinez-Mota L. Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinology. 2005;30:762–70. doi: 10.1016/j.psyneuen.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 85.Pednekar JR, Mulgaonker VK. Role of testosterone on pain threshold in rats. Indian Journal of Physiology and Pharmacology. 1995;39:423–4. [PubMed] [Google Scholar]
- 86.McEwen BS. Genome and Hormones: Gender Differences in Physiology: Invited Review: Estrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- 87.Balthazart J, Baillien M, Cornil CA, Ball GF. Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiol Behav. 2004;83:247–70. doi: 10.1016/j.physbeh.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 88.Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behav Brain Res. 2006;166:110–23. doi: 10.1016/j.bbr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 89.Lieberherr M, Grosse B. Androgens increase intracellular calcium concentration and inositol 1,4,5-trisphosphate and diacylglycerol formation via a pertussis toxin-sensitive G-protein. J Biol Chem. 1994;269:7217–23. [PubMed] [Google Scholar]
- 90.Naftolin F. Brain aromatization of androgens. J Reprod Med. 1994;39:257–61. [PubMed] [Google Scholar]
- 91.Frye CA. Some rewarding effects of androgens may be mediated by actions of its 5alpha-reduced metabolite 3alpha-androstanediol. Pharmacol Biochem Behav. 2007;86:354–67. doi: 10.1016/j.pbb.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O'Brien CP. Drug addiction and drug abuse. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. McGraw-Hill; New York: 2006. pp. 607–628. [Google Scholar]
- 93.Taylor GT, Weiss J, Pitha J. Testosterone in a cyclodextrin-containing formulation: behavioral and physiological effects of episode-like pulses in rats. Pharm Res. 1989;6:641–6. doi: 10.1023/a:1015922019038. [DOI] [PubMed] [Google Scholar]
- 94.LaGrange AH, Kelly MJ. Neuroactive Steroids. In: Henry H, Norman A, editors. Encyclopedia of Hormones. Elsevier; 2003. pp. 8–19. [Google Scholar]
- 95.Sachs BD. A contextual definition of male sexual arousal. Horm Behav. 2007;51:569–78. doi: 10.1016/j.yhbeh.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 96.Sipos M, Alterman L, Nyby J, Perry B, Vandenberg J. An ephemeral pheromone of female house mice: degradation by oxidation. Animal Behaviour. 1995 [Google Scholar]


