Abstract
Background and purpose
An animal model of selective middle cerebral artery (MCA) occlusion is needed for evaluation of intra-arterial (IA) delivery of thrombolytic agents. We describe a technique for MCA thrombo-occlusion in the rabbit with real-time angiographic documentation of occlusion and thrombolytic recanalization.
Methods
After femoral artery cutdown, a microcatheter was advanced from the internal carotid artery (ICA) to the MCA. MCA occlusion was achieved by IA thrombin and reperfusion by IA plasmin.
Results
The terminal ICA was successfully catheterized in 12 of 13 animals. Stable (2 hour) MCA occlusion was induced and verified angiographically in all 12 animals; two animals also had distal ICA thrombus. Recanalization was achieved rapidly after IA plasmin in 3 of 3 animals.
Conclusions
We describe a new animal model of selective MCA occlusion documented by real-time angiography, and utilized to demonstrate recanalization with IA plasmin.
Keywords: Animal model, ischemic stroke, middle cerebral artery, thrombolysis
Introduction
An animal model of selective MCA occlusion to evaluate IA thrombolytic therapy should be amenable to thrombolysis, allow regional infusions, and provide documentation of angiographic results. We have developed a rabbit stroke model of selective MCA occlusion utilizing microcatheter technology and IA thrombin infusion. We utilize the model for studying IA-delivered thrombolysis with plasmin, using a direct acting fibrinolytic agent.
Methods
Studies were conducted on 13 consecutive New Zealand white rabbits. Animals were sedated with intramuscular ketamine HCl (35 mg/kg) and xylazine (5 mg/kg) (Phoenix Pharmaceuticals, St. Joseph, MO), and anesthetized with isoflurane (Isothesia, Butler Animal Health Supply, Dublin, OH) via endotracheal intubation. A 4F sheath (Terumo Pinnacle, Boston Scientific, Natick, MA) was placed into the right femoral artery and the right common carotid artery (CCA) was catheterized with a Terumo 4F glidecatheter (Terumo Glidecath, Boston Scientific). The terminal right ICA was catheterized with a 1.2F Magic Balt microcatheter and Sorcerer guidewire (BALT extrusion, Montmorency, France). Bovine thrombin (2063 NIH units/mg) (Enzyme Research, South Bend, IN) was reconstituted with saline to 1 NIH unit/μL, and mixed with rabbit brain thromboplastin (1:10 with saline) (Neoplastine® CI PLUS, Diagnostica Stago, Asnieres, France), as 0.3 mL thrombin plus 3.0 mL thromboplastin. MCA occlusion was induced by slow thrombin infusion (10 NIH units/100 μL) into the distal ICA above the posterior communicating artery (P-comm). There was minimal flow of thrombin into the right anterior cerebral artery (ACA), which filled from the contralateral ACA via the anterior communicating artery (A-comm). Plasmin (Talecris Biotherapeutics, Research Triangle Park, NC) (1) was reconstituted as 1 mg/mL sterile non-buffered saline, and administered just proximal to the MCA occlusion. Angiography was performed to document MCA occlusion and recanalization. Animals were euthanised with 1 mL Euthasol (Virbac AH, Inc, Fort Worth, TX), following which the brain was perfused with paraformaldehyde, removed in toto and sectioned for hematoxylin-eosin staining.
Results
The ICA above the P-comm was successfully catheterized in 12 of 13 (93%) animals (Figure 1). Following thrombin infusion, angiography documented MCA occlusion in all 12 animals; one animal also had thrombus in the distal ICA (Table 1). Follow-up angiography at 2 hours showed no spontaneous recanalization; one animal had extension of MCA clot into the ICA. The model was evaluated for thrombolysis in 3 animals treated with IA plasmin into the distal ICA (Figure 2). Post-plasmin angiography showed complete recanalization in all three animals (Table 1). Histology of the ischemic right hemisphere showed vacuolization of the neuropil, diffuse edema and loss of cellular integrity (not shown).
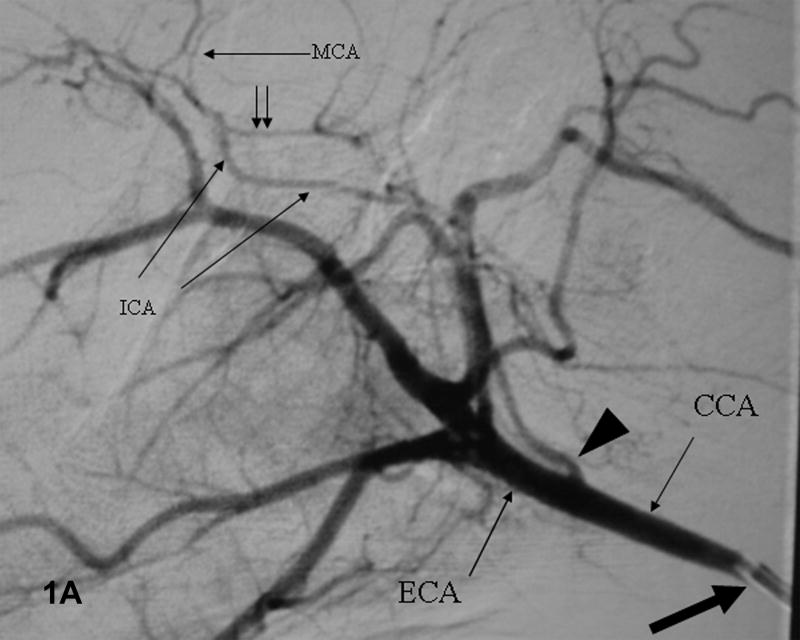
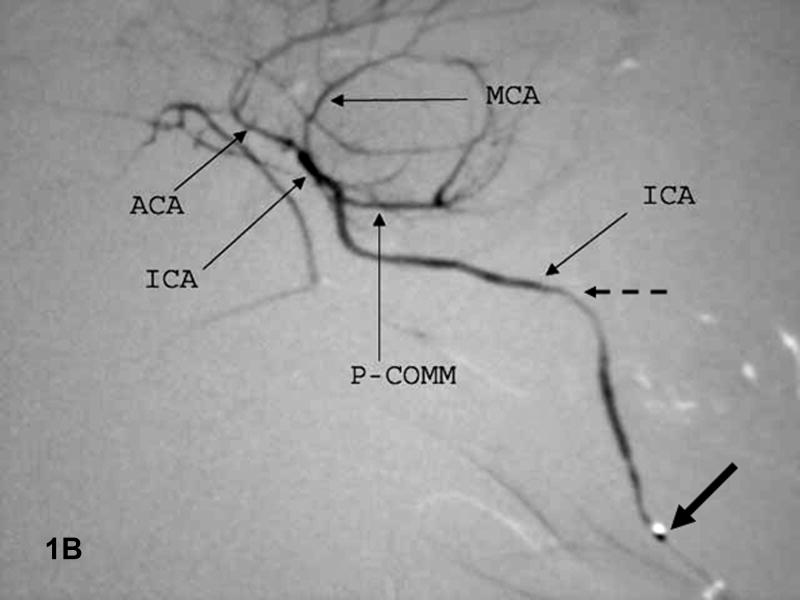
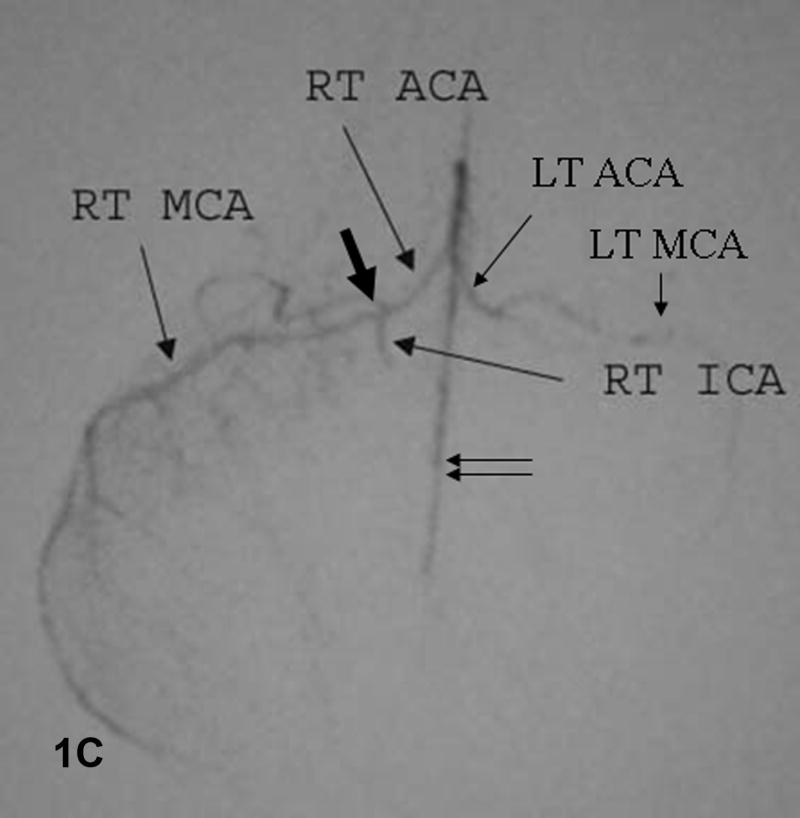
Figure 1. Baseline angiograms.
A. Lateral view. Catheter (thick arrow) is in the CCA. Arrowhead indicates the ICA take-off and double arrows the P-comm. B. Lateral view. The microcatheter tip (thick arrow) is in the ICA which narrows significantly at the skull base (dashed arrow). C. Submental vertex view. The microcatheter is in the ICA above the P-comm. ICA bifurcation into the MCA and ACA noted by the thick arrow, distal ACA by double arrows. The left ACA and MCA fill via the A-comm.
Table 1. Results.
| Procedure | # (%) |
|---|---|
| Animals studied | 13 |
| Catheter into CCA | 13 (100%) |
| Catheter into ICA above P-comm | 12 (92%) |
| Thrombin infusion into ICA | 12 |
| MCA occlusion | 12 (100%) |
| Thrombus in MCA+ICA | 1 (8%) |
| Followup angiogram (2 hours) | 12 |
| MCA occlusion | 12 (100%) |
| Thrombus in MCA+ICA | 2 (17%) |
| IA infusion of plasmin | 3 |
| Successful thrombolysis | 3 (100%) |
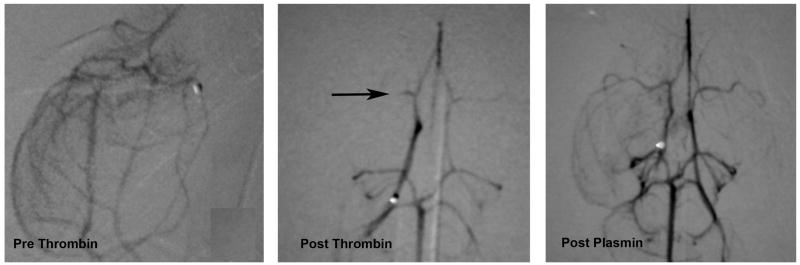
Figure 2. Thrombin-induced MCA occlusion and plasmin-induced recanalization.
MCA fills normally at baseline (left panel), is absent after IA thrombin (center panel) and is recanalized after IA plasmin (right panel).
Discussion
We describe the technique and feasibility of selective MCA thrombo-occlusion in the rabbit, using angiography to confirm both occlusion and thrombolytic-induced reperfusion. All 12 animals that were catheterized to the terminal ICA had successful MCA thrombo-occlusion, with no spontaneous recanalization after 2 hours. Occlusion was limited to the MCA in 10, with distal ICA occlusion also present in 2. All three animals treated with IA plasmin showed rapid angiographic recanalization.
The major advantages of our model are reliable occlusion of the target artery, real-time angiographic confirmation of vessel status, and occlusive material that is amenable to thrombolysis. Prior attempts to access the distal ICA were hindered by catheter size (2), as selective occlusion of the MCA is unreliable without access to the ICA beyond the P-comm. Injection of clots into the rabbit CCA fails to occlude a cerebral artery or terminates outside of the ICA circulation in 25% (3,4). Use of thrombin-induced clot that can be dissolved with thrombolytic agents provides an advantage over suture occlusions (5-7) or injection of particulates (8,9). Our demonstration of rapid lysis of MCA clot with plasmin, a newly-developed direct-acting thrombolytic (10,11), warrants further study to assess safety and efficacy for eventual IA clinical use.
Further characterization is needed to assess the extent and variation of induced ischemic damage, since even reliable MCA occlusion may not cause similar sized infarcts. Slow delivery of coagulant mixture may result in more complete occlusion of distal MCA branches than occurs in human stroke, where proximal MCA occlusion allows some collateral flow from the ACA. Stroke induction under general anesthesia may have a neuroprotective effect.
Conclusions
We describe a new technique for angiographically confirmed MCA thrombo-occlusion in the rabbit with a high degree of success and demonstrate the utility of this model for IA-induced thrombolytic recanalization.
Acknowledgments
Grant Support: Grants # RO 1 HL 07451 and T32 HL 66992 from the National Heart, Lung, and Blood Institute, and P50 NS 44378 from the National Institute of Neurological Diseases and Stroke, and from Talecris Biotherapeutics, Research Triangle Park, North Carolina.
Footnotes
Disclosures: Dr. Marder serves as a consultant to Talecris Biotherapeutics, which provided an unrestricted grant for pursuance of this study.
Dr. Vandeberg is an employee of Talecris Biotherapeutics.
References
- 1.Novokhatny V, Taylor K, Zimmerman TP. Thrombolytic potency of acid-stabilized plasmin: superiority over tissue-type plasminogen activator in an in vitro model of catheter-assisted thrombolysis. J Thromb Haemost. 2003;1:1034–41. doi: 10.1046/j.1538-7836.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 2.Kirchhof K, Welzel T, Zoubaa S, Lichy C, Sikinger M, de Ruiz HL, Sartor K. New method of embolus preparation for standardized embolic stroke in rabbits. Stroke. 2002;33:2329–2333. doi: 10.1161/01.str.0000027436.82700.73. [DOI] [PubMed] [Google Scholar]
- 3.Lapchak PA, Araujo DM, Song D, Zivin JA. The nonpeptide glycoprotein IIb/IIIa platelet receptor antagonist sm-20302 reduces tissue plasminogen activator-induced intracerebral hemorrhage after thromboembolic stroke. Stroke. 2002;33:147–52. doi: 10.1161/hs0102.100530. [DOI] [PubMed] [Google Scholar]
- 4.Lyden PD, Madden KP, Clark WM, Sasse KC, Zivin JA. Incidence of cerebral hemorrhage after treatment with tissue plasminogen activator or streptokinase following embolic stroke in rabbits [corrected] Stroke. 1990;21:1589–93. doi: 10.1161/01.str.21.11.1589. [DOI] [PubMed] [Google Scholar]
- 5.Takano K, Tatlisumak T, Bergmann AG, Gibson DG, 3rd, Fisher M. Reproducibility and reliability of middle cerebral artery occlusion using a silicone-coated suture (Koizumi) in rats. J Neurol Sci. 1997;153:8–11. doi: 10.1016/s0022-510x(97)00184-6. [DOI] [PubMed] [Google Scholar]
- 6.Koizumi J, Yoshida Y, Nakazawa T, Ooneda G. Experimental studies of ischemic brain edema I: a new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn J Stroke. 1986;8:1–8. [Google Scholar]
- 7.Schmid-Elsaesser R, Zausinger S, Hungerhuber E, Baethmann A, Reulen HJ. A critical reevaluation of the intraluminal thread model of focal cerebral ischemia: Evidence of inadvertent premature reperfusion and subarachnoid hemorrhage in rats by laser-doppler flowmetry. Stroke. 1998;29:2162–70. doi: 10.1161/01.str.29.10.2162. [DOI] [PubMed] [Google Scholar]
- 8.Mayzel-Oreg O, Omae T, Kazemi M, Li F, Fisher M, Cohen Y, Sotak CH. Microsphere-induced embolic stroke: An MRI study. Magn Reson Med. 2004;51:1232–38. doi: 10.1002/mrm.20100. [DOI] [PubMed] [Google Scholar]
- 9.Gerriets T, Li F, Silva MD, Meng X, Brevard M, Sotak CH, Fisher M. The macrosphere model: Evaluation of a new stroke model for permanent middle cerebral artery occlusion in rats. J Neurosci Methods. 2003;122:201–11. doi: 10.1016/s0165-0270(02)00322-9. [DOI] [PubMed] [Google Scholar]
- 10.Marder VJ, Landskroner K, Novokhatny V, et al. Plasmin induces local thrombolysis without causing hemorrhage: A comparison with tissue plasminogen activator in the rabbit. Thromb Haemost. 2001;86:739–45. [PubMed] [Google Scholar]
- 11.Stewart D, Kong M, Novokhatny V, Jesmok G, Marder VJ. Relevance of plasma cogualtion for safety during plasmin administration: Comparison with TPA in a model of fibrinolytic hemorrhage. Blood. 2003;101:3002–7. doi: 10.1182/blood-2002-08-2546. [DOI] [PubMed] [Google Scholar]