Abstract
The non-obese diabetic (NOD) mouse is prone to develop autoimmune disease, including Sjögren’s syndrome. The purpose of this study was to determine if desiccating environmental stress exacerbates the development of Sjögren’s syndrome-like lacrimal keratoconjunctivitis in the NOD.B10.H2b mouse. Four-week-old male mice were used as young controls. Sixteen-week-old male mice were untreated or subjected to desiccating stress with a fan alone or with a fan plus subcutaneous injections of the anticholinergic agent scopolamine for 5 or 10 days to inhibit tear production. Mice spontaneously developed Sjögren’s syndrome-like lacrimal keratoconjunctivitis as they aged. Desiccating stress increased CD4+ and CCR5+ cells and decreased CD8+ cells in the conjunctival epithelium and lacrimal gland. Intraepithelial γδ T cells significantly decreased after 5 days and returned to baseline levels after 10 days in both groups exposed to desiccating stress. These immunopathological changes were accompanied by a decrease in conjunctival goblet cell density. Greater matrix metalloproteinase-9 production, gelatinase activity and loss of epithelial cell membrane CD25 immunoreactivity was noted in the ocular surface epithelia of stressed mice. These findings indicate that desiccating environmental stress aggravates Sjögren’s syndrome-like lacrimal keratoconjunctivitis in the NOD mouse which has defective immunoregulation.
Keywords: desiccating stress, lacrimal keratoconjunctivitis, non-obese diabetic mouse, Sjögren’s syndrome
1. Introduction
Sjögren’s syndrome is a chronic autoimmune disease that affects the lacrimal and salivary glands, resulting in keratoconjunctivitis sicca (KCS) and xerostomia. It is characterized by focal lymphocytic infiltration of these secretory glands (dacryoadenitis and sialoadenitis), hypergammaglobulinaemia and production of autoantibodies, including anti-SS-A/Ro, anti-SS-B/La and anti-muscarinic type-3 acetylcholine receptor (M3R) [1,2]. Immunopathological features of the conjunctiva in Sjögren’s syndrome include increased expression of cytokines, chemokines and immune activation and adhesion molecules [3,4]. An increase in the CD4/CD8 T cell ratio in the conjunctiva has also been observed [5]. These immune/inflammatory changes are accompanied by abnormal proliferation and differentiation of the ocular surface epithelium with a decrease in conjunctival goblet cell density and disruption of corneal barrier function [5–7].
It is now recognized that the ocular surface epithelia and lacrimal glands function as an integrated unit, “the lacrimal functional unit”, which are derived embryologically from the surface ectoderm and are linked by the sensory and autonomic nerves [8]. Sjögren’s syndrome causes severe dysfunction of the lacrimal functional unit. Desiccating ocular surface stress has been found to induce autoreactive T cells that when adoptively transferred to naïve immunodeficient hosts cause Sjögren’s syndrome-like inflammation in the lacrimal gland, cornea, and conjunctiva, but not in other organs, suggesting the existence of shared epitopes among the components of the lacrimal functional unit [9].
Several mouse models have been used to investigate the pathogenesis of Sjögren’s syndrome, such as the non-obese diabetic (NOD) mouse, the MRL/lpr mouse, the NZB/W F1 mouse, the transforming growth factor (TGF)-β1 knockout mouse and the NFS/sld mouse, thymectomised 3 days after birth [10]. Among these, the NOD mouse is the most accurate model, because development of adenitis is accompanied by decreased secretory function in the lacrimal and salivary glands [11]. Lymphocytic infiltration of the exocrine glands of the NOD mouse occurs well before the onset of clinical findings [12]. Although the NOD mouse can also be used as a model for spontaneous type 1 diabetes, the NOD.B10.H2b strain develops Sjögren’s syndrome-like disease, but not diabetes [13].
The histological features of the lacrimal gland in this mouse strain have been previously reported; however, clinical and immunological evaluation of the ocular surface has not been performed [14–17]. It is well recognized that exposure to a desiccating environment, use of medications with anticholinergic side effects (e.g. antihistamines and antidepressants) and laser in situ keratomileusis (LASIK) surgery are risk factors for development of keratoconjunctivitis sicca. Furthermore, there appears to be individual susceptibility to these stresses, because some patients develop mild disease while others have severe manifestations resembling Sjögren’s syndrome. The cause for the variability in this response to environmental stresses has not been established, but it may be due to immunogenetic factors that regulate the severity of the immune response.
The purpose of the present study was to investigate the effects of desiccating environmental stress on the onset and severity of autoimmune lacrimal keratoconjunctivitis in the NOD.B10.H2b mouse strain.
2. Materials and Methods
2.1. Animals
This research protocol was approved by the Baylor College of Medicine Center for Comparative Medicine and it conformed to the standards in the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
NOD.B10.H2b mice were purchased from the Jackson Laboratories (Bar Harbor, ME). Four-week-old male mice served as young controls (4W). Sixteen-week-old male mice were untreated (16W) or subjected to desiccating stress by exposure to an air draft with a fan, without (F) or with (FS) subcutaneous injection of 0.5 mg/0.2 mL of the muscarinic receptor blocker, scopolamine hydrobromide (Sigma-Aldrich, St. Louis, MO) in alternating hindquarters four times a day (8 am, 11 am, 2 pm and 5 pm), as previously reported [9,18]. Mice were euthanized after 5 or 10 days (D) of treatment. Each experimental group studied consisted of four mice (eight eyes) and all experiments were repeated.
2.2. Histology
Eyes and adnexa were surgically excised, fixed in 10% formalin, and embedded in paraffin. Six-μm sections were stained with periodic acid-Schiff (PAS) reagent. Sections from 4 mice of each group were examined and photographed with a microscope equipped with a digital camera (Eclipse E400 with a DMX 1200; Nikon, Garden City, NY). Goblet cell density in the superior and inferior conjunctiva was measured in 3 sections from each eye using image-analysis software (MetaVue 6.24r; Molecular Device) and expressed as the number of goblet cells per 100 μm.
2.3. Immunohistochemistry
Immunohistochemistry was performed to detect and count the cells in the conjunctival epithelium and stroma that stained positively for CD4, CD8, γδ T-cell receptor (TCR) and CCR5. Cryosections from 4 mice per each group were fixed in acetone at −20°C for 10 minutes. After fixation, endogenous peroxidases were quenched with 0.3% H2O2 in PBS for 10 minutes. The sections were sequentially blocked with avidin/biotin block (Vector Laboratories, Burlingame, CA) for 10 minutes each. After blocking with 20% normal goat (for CD4, CD8, and γδ TCR) or rabbit (for CCR5) serum in PBS for 45 min, monoclonal rat antibody against CD4 (clone H129.9, 10 μg/mL; BD Biosciences), monoclonal rat antibody against CD8 (clone 53-6.7, 3.125 μg/mL; BD Biosciences), monoclonal hamster antibody against γδ TCR (clone GL3, 3.125 μg/mL; BD Biosciences) or polyclonal goat antibody against CCR5 (M20; 1 μg/mL; Santa Cruz, Santa Cruz, CA) were applied and incubated for 1 hour at RT. After washing, the sections were incubated with biotinylated goat ant-rat antibodies (for CD4 and CD8; BD Biosciences), mouse anti-hamster antibody (for γδ TCR; BD Biosciences) and rabbit anti-goat antibody (for CCR5; Vectastain Elite ABC Kit; Vector Laboratories). The samples were finally incubated with NovaRed (Vector Laboratories) peroxidase substrate to give a red stain (2–8 minutes, optimized for each antibody) and counterstained with Mayer’s hematoxylin. Secondary antibody alone and appropriate anti-mouse isotype (BD Biosciences) controls were also performed. Three sections from each animal were examined and photographed with a microscope equipped with a digital camera (Eclipse E400 with a DMX 1200; Nikon). Positively stained cells were counted in the goblet cell rich area of the conjunctiva, over a length of at least 500 μm in the epithelium and to a depth of 75 μm below the epithelial basement membrane in the stroma for a distance of 500 μm using image-analysis software (MetaVue 6.24r; Molecular Device). Results were expressed as the number of positive cells per 100 μm.
2.4. Immunofluorescent staining and laser scanning confocal microscopy
Immunofluorescent staining was performed to evaluate expression of CD25 and matrix metalloproteinase (MMP)-9 in corneal and conjunctival tissue sections. Eyes and adnexa from 4 mice of each group were surgically excised, embedded in OCT™ compound (VWR, Swannee, GA), and flash frozen in liquid nitrogen. Cryosections were fixed in acetone at −20°C for 5 minutes. After blocking with 20% normal goat serum in phosphate buffer solution (PBS) for 45–60 minutes, monoclonal rat antibody against CD25 (clone 7D4; 5 μg/mL; BD Biosciences, San Jose, CA) or polyclonal rabbit antibody against MMP-9 (10 μg/mL; Chemicon, Billerica, MA) were applied, and the sections were incubated for 1 hour at RT. Secondary antibodies, Alexa-Fluor 488 conjugated goat anti-rat IgG or goat anti-rabbit IgG (1:300 dilution) were then applied, and the sections were incubated in a dark chamber for 1 hour, followed by counterstaining with propidium iodide (PI; 2μg/ml in PBS; Sigma-Aldrich, St. Louis, MO) for 10 minutes. Tissues without primary antibody were used as negative controls.
Digital confocal images (512 × 512 pixels) were captured with a laser-scanning confocal microscope (LSM 510, with krypton-argon and He-Ne laser; Carl Zeiss Meditec, Thornwood, NY) with 488-excitation and 543-nm emission filters (LP505 and LP560, respectively; Carl Zeiss Meditec) and were acquired with a 40/1.3x oil-immersion objective. The images were captured with identical photomultiplier tube gain settings and were processed using the microscope system (LSM-PC; Carl Zeiss Meditec) and image analysis (Adobe Photoshop 7.0; Adobe System, San Jose, CA) software.
2.5. In situ zymography
In situ zymography was performed to localize the gelatinase activity in the cornea and conjunctiva, as previously described [18]. Cryosections from 4 mice per each group were thawed and incubated overnight with reaction buffer (0.05M Tris-HCl, 0.15M NaCl, 5mM CaCl2, and 0.2 mM NaN3; pH 7.6), containing 40 μg/mL FITC-labeled DQ gelatin, which was available in a gelatinase/collagenase assay kit (EnzChek Molecular Probes, Eugene, OR). As a negative control, 50 μM 1,10-phenanthroline, a metalloproteinase inhibitor, was added to the reaction buffer before applying the FITC-labeled DQ gelatin. After incubation, the sections were counterstained with PI. Areas of gelatinolytic activity were photographed with a microscope equipped with a digital camera (Eclipse E400 with a DMX 1200; Nikon).
2.6. Measurement of tear volume and tear turnover rate
Four mice (8 eyes) per each group were examined in two different sets of experiments. Tear volume was measured with phenol-red impregnated cotton threads (Zone-Quick, Oasis, Glendora, CA) as previously described [19]. Tear turnover rate was determined using a Fluorotron™ Master Fluorophotometer (Ocumetrics, Moutain View, CA) by measuring the decay of a high molecular weight (MW) fluorescent molecule, Oregon green dextran (OGD; 70,000 MW; Invitrogen, Eugene, OR), according to the manufacturer’s instructions [20]. After anesthesia with an intraperitoneal injection of 97% 2,2,2-tribromoethanol, nine scans at 4-minute intervals were performed after instillation of 0.5 μL of 50 μg/mL OGD. Baseline measurements before administration of OGD was used to substract the background fluorescence.
2.7. Corneal smoothness and permeability
Four mice (8 eyes) per each group were examined in two different sets of experiments. Corneal smoothness was assessed by measuring the distortion of a white ring reflected off the corneal epithelium in digital images, as previously described [7]. Corneal epithelial permeability to OGD was assessed by grading the severity of corneal staining with this fluorescent dye, as previously described [18].
2.8. Statistical Analysis
A sample size of 8 eyes per group was calculated to have a 99% power of detecting a between group difference of 2 CD4+ intraepithelial T cells with an alpha = 0.05. Results are presented as the mean ± standard error of the mean (SEM). Nonparametric comparisons (Corneal smoothness and permeability) were done with the Mann-Whitney U test and parametric comparisons with unpaired t-test, using GraphPad Prism 3.0 software (GraphPad Software; San Diego, CA). p ≤ 0.05 was considered statistically significant.
3. Results
3.1. Age and desiccating stress worsen lacrimal keratoconjunctivitis
Development of autoimmune lacrimal keratoconjunctivitis is a hallmark of Sjögren’s syndrome. This experiment evaluated the density of lymphocytes positive for CD4, CD8, γδ TCR, and CCR5 in the conjunctivae and lacrimal glands of four groups of NOD.B10.H2b mice: four week old (4W), sixteen week old (16W), sixteen week old mice subjected to an air draft from a fan (F) and sixteen week old mice subjected to an air draft and treated with scopolamine to inhibit tear secretion (FS). The comparative results of these studies are presented in Table 1. In the conjunctiva of the older (16W) group, the number of CD4+ T cells in the epithelium, γδ T cells in the epithelium and stroma, and CCR5+ cells in the stroma significantly increased, while CD4+ T cells in the stroma significantly decreased compared to the 4W group (p < 0.01 for all). Desiccating stress significantly increased the number of CD4+ T cells in the conjunctival epithelium in the FS group (p < 0.05) at 5 days and in both F (p < 0.01) and FS (p < 0.001) groups at 10 days (Fig. 1A). In contrast, the density of CD4+ T cells in the stroma was noted to decrease in the FS group at 5 and 10 days (p < 0.05 for both). Desiccating stress also caused a profound decrease in the density of CD8+ T cells in the conjunctival epithelia and stroma of both F and FS groups at 5 and 10 days (p < 0.001 for the F group at 5 and 10 days and FS group at 5 days; p < 0.01 for the FS group at 10 days) (Fig. 1B). The density of γδ T cells significantly decreased in the epithelia of both F (p < 0.05) and FS (p < 0.001) groups after 5 days, but returned to baseline levels after 10 days (Fig. 1C). The density of CCR5+ cells significantly increased in the epithelium and stroma of the FS group after 10 days of desiccating stress (p < 0.05 for both). The FS group had a higher number of CD4+ T cells in the epithelium at 5 (p < 0.05) and 10 (p < 0.01) days and CCR5+ T cells in the stroma at 10 days (p < 0.01) than the F group.
Table 1.
The density of immune/inflammatory cells in the conjunctiva of 4-week-old untreated mice (4W), 16-week-old untreated mice (16W), and 16-week-old mice subjected to an air draft without (F) or with (FS) systemic scopolamine administration for 5 and 10 days (D) in the NOD.B10.H2b mouse strain
| Area | 4W | 16W | Group | 5Da | 10Da,b | p (F vs. FS) | |
|---|---|---|---|---|---|---|---|
| P (4W vs. 16W) | |||||||
| CD4+ (cells/100 μm) | Epithelium | 1.39±0.20 | 2.13±0.28 | F | 2.26±0.51 | 2.82±0.38** | <0.05 in 5D |
| <0.01 | FS | 3.14±0.78* | 4.30±0.73***† | <0.01 in 10D | |||
| Stroma | 4.66±0.58 | 3.29±0.32 | F | 3.02±0.55 | 3.31±0.61 | ||
| <0.01 | FS | 2.80±0.39* | 2.56±0.62* | ||||
| CD8+ (cells/100 μm) | Epithelium | 1.82±0.54 | 1.33±0.21 | F | 0.26±0.10*** | 0.33±0.12*** | |
| 0.06 | FS | 0.28±0.32*** | 0.58±0.42** | ||||
| Stroma | 2.24±0.77 | 1.58±0.38 | F | 0.23±0.11*** | 0.34±0.13*** | ||
| 0.09 | FS | 0.28±0.27*** | 0.55±0.54** | ||||
| γδ TCR+ (cells/100 μm) | Epithelium | 0.46±0.25 | 1.21±0.40 | F | 0.70±0.33* | 1.56±0.53†† | |
| <0.01 | FS | 0.43±0.29*** | 1.57±0.57††† | ||||
| Stroma | 0.24±0.04 | 0.59±0.26 | F | 0.43±0.12 | 0.52±0.32 | ||
| <0.01 | FS | 0.42±0.22 | 0.54±0.32 | ||||
| CCR5+ (cells/100 μm) | Epithelium | 0.42±0.21 | 0.53±0.13 | F | 0.57±0.16 | 0.62±0.19 | |
| 0.44 | FS | 0.62±0.13 | 0.82±0.14* | ||||
| Stroma | 0.94±0.35 | 1.58±0.31 | F | 1.65±0.38 | 1.81±0.32 | <0.01 in 10D | |
| 0.01 | FS | 2.01±0.69 | 2.96±0.69* | ||||
p<0.05,
p<0.01,
p<0.001 vs. 16W.
p<0.05,
p<0.01,
p<0.001 vs. 5D.
Fig. 1.
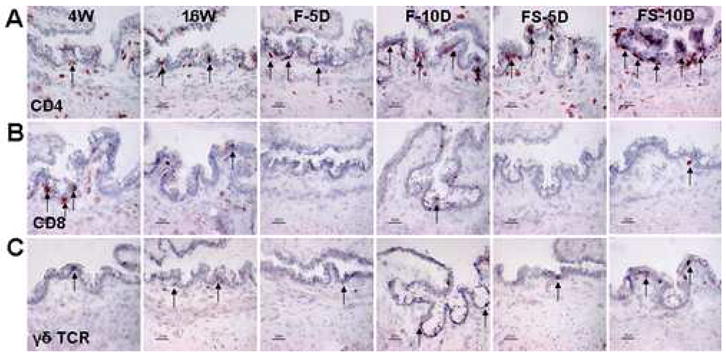
Immunohistochemistry for CD4 (A), CD8 (B), and γδ TCR (C) in the conjunctiva of NOD.B10.H2b mouse mice. Representative staining in 4-week-old untreated mice (4W), 16-week-old untreated mice (16W), and 16-week-old mice subjected to an air draft without (F) or with (FS) systemic scopolamine administration for 10 days (D). The CD4, CD8, and γδ TCR positive cells are indicted by arrows. Original magnification X400, bar = 25μm.
The CD4/CD8 ratio in the 4W and 16W groups was 0.85 ± 0.45 and 1.62 ± 0.25 (p < 0.01) in the conjunctival epithelium and 1.97 ± 0.70 and 2.17 ± 0.47 (p = 0.58) in the conjunctival stroma, respectively. Compared with the 16W group, the CD4/CD8 ratio in the epithelia of the F and SF groups increased to 8.90 ± 3.32 and 8.42 ± 4.37 after 5 days and to 9.80 ± 2.30 and 9.07 ± 5.01 after 10 days of desiccating stress, respectively (p < 0.01 for all). The ratio in the stromas of the F and FS groups also increased to 13.06 ± 4.69 and 13.01 ± 6.61 after 5 days and 9.34 ± 2.05 and 7.05 ± 2.93 after 10 days of desiccating stress, respectively (p < 0.01 for all).
The lacrimal gland in the older 16W group showed a higher number of CD4+ and CCR5+ cells and a lower number of CD8+ cells than the 4W group. Following desiccating stress, CD4+ T cell infiltration in the lacrimal gland tissue increased markedly, especially in the FS group. In contrast, CD8+ cells were barely detected in the lacrimal glands of both groups exposed to desiccating stress. The pattern of CCR5+ cell infiltration in the lacrimal gland was similar to the ocular surface (Fig. 2).
Fig. 2.
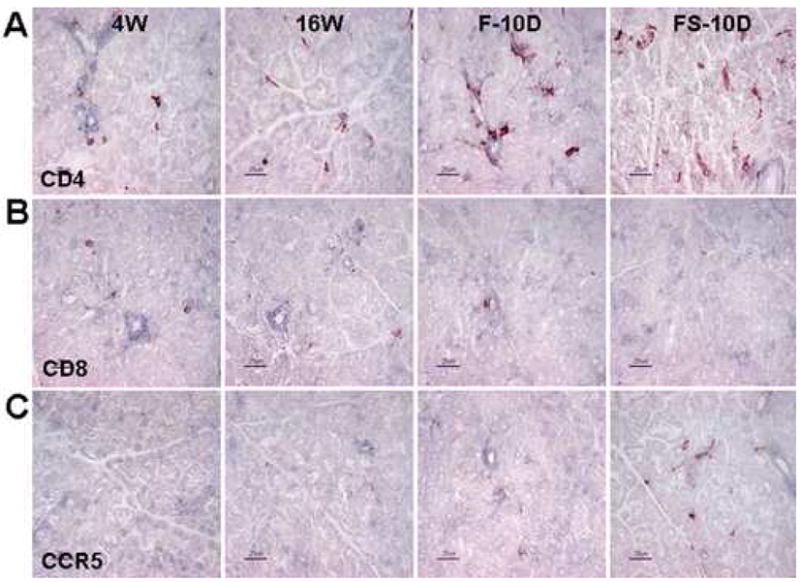
Immunohistochemistry for CD4 (A), CD8 (B), and CCR5 (C) in the lacrimal gland of NOD.B10.H2b mice. Representative staining in 4-week-old untreated mice (4W), 16-week-old untreated mice (16W), and 16-week-old mice subjected to an air draft without (F) or with (FS) systemic scopolamine administration for 10 days (D). Original magnification X400, bar = 25μm.
3.2. MMP-9 expression and gelatinase activity on the ocular surface
Increased MMP-9 protein and activity has been observed in the tears of patients with dry eye, with the highest levels observed in Sjögren’s syndrome [21] MMP-9 has been found to cleave cell membrane proteins from the ocular surface epithelia, including occludin and IL-2rα (CD25) [6]. Similar to human Sjögren’s syndrome, MMP-9 production and gelatinase activity have been noted to increase in the salivary glands and saliva of NOD.B10.H2b mice [22]. Based on these findings, MMP-9 production and gelatinase activity were evaluated in the ocular surface epithelia. CD25 immunoreactivity as a marker of MMP-9 activity was also assessed.
Minimal staining for MMP-9 and intense staining for CD25 were observed in the corneal and conjunctival epithelia in the 4W group. MMP-9 staining increased and CD25 staining decreased in the cornea and conjunctival epithelia of the aged (16W) mice. Desiccating stress induced a progressive increase in MMP-9 immunreactivity and a decrease in CD25 immunoreactivity, which were most prominent in the FS group at 10 days (Fig. 3A, 3B, 4A and 4B). Gelatinase activity in the cornea and conjunctiva showed a similar pattern to levels of MMP-9 protein (Fig. 3C and 4C).
Fig. 3.
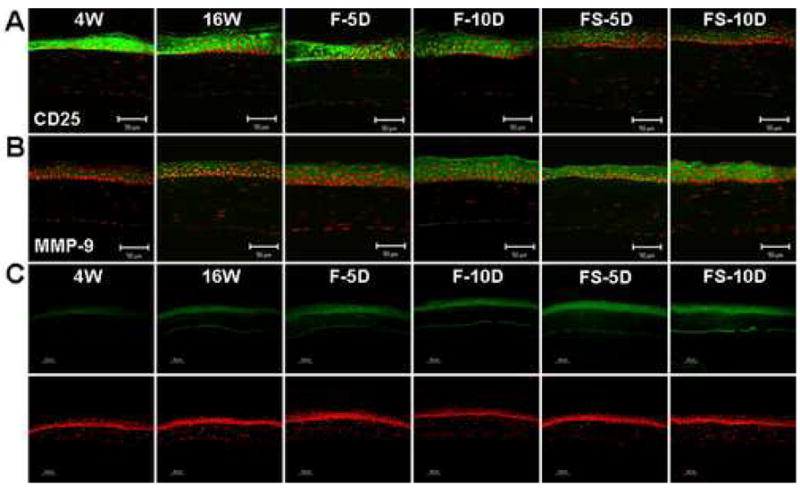
CD25 and MMP-9 expression and gelatinolytic activity in the corneal epithelium of NOD.B10.H2b mice. (A and B) Immunofluorescent staining and laser scanning confocal microscopy in corneal tissue sections stained with antibodies (green) to CD25 (A) and MMP-9 (B). (C) In situ zymogram showing gelatinolytic activity (green) in the corneal epithelium. Nuclei were counterstained with propidium iodide (red). Representative staining in 4-week-old untreated mice (4W), 16-week-old untreated mice (16W), and 16-week-old mice subjected to an air draft without (F) or with (FS) systemic scopolamine administration for 5 and 10 days (D). Original magnification X400, bar = 50 μm.
Fig. 4.
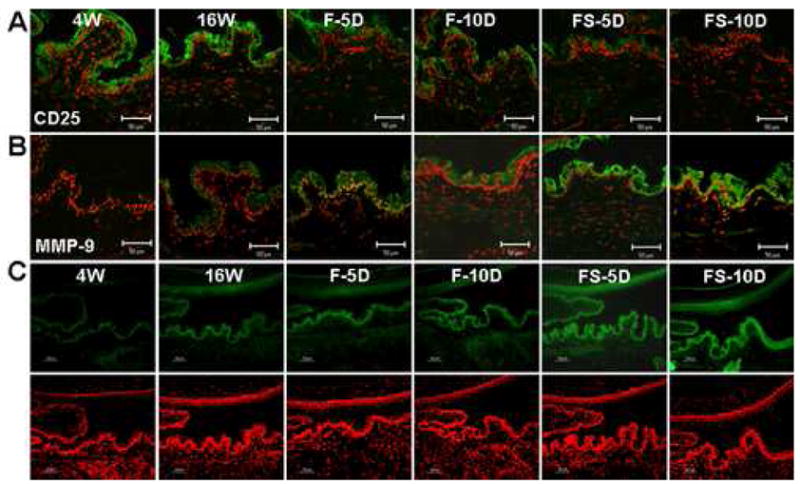
CD25 and MMP-9 expression and gelatinolytic activity in the conjunctival epithelium of NOD.B10.H2b mice. (A and B) Immunofluorescent staining and laser scanning confocal microscopy in conjunctival tissue sections stained with antibodies (green) to CD25 (A) and MMP-9 (B). (C) In situ zymography showing gelatinolytic activity (green) in the conjunctival epithelium. Nuclei were counterstained with propidium iodide (red). Representative staining in 4-week-old untreated mice (4W), 16-week-old untreated mice (16W), and 16-week-old mice subjected to an air draft without (F) or with (FS) systemic scopolamine administration for 5 and 10 days (D). Original magnification X400, bar = 50 μm.
3.3. Age and Desiccating Stress Worsen Keratoconjunctivitis Sicca
To determine if the immunopathological changes in the ocular surface and lacrimal glands of elderly and environmentally stressed mice altered tear function and produced ocular surface disease, several parameters of dry eye were measured. Tear volume (p < 0.01) and tear turnover rate (p = 0.06) were found to decrease with age in NOD.B10.H2b mice. Compared with the 16W group, there was a significant decrease in tear volume of the FS group at 5 (p < 0.01) and 10 (p < 0.001) days (Table 2). Although there was no significant change in tear turnover rate after 5 days of desiccating stress, the rates in both stressed groups significantly decreased compared with the 16W group after 10 days (p < 0.05 for the F group; p < 0.01 for the SF group) (Table 2).
Table 2.
Tear volume, corneal smoothness, corneal permeability, tear turnover rate, and conjunctival goblet cell density in 4-week-old untreated mice (4W), 16-week-old untreated mice (16W), and 16-week-old mice subjected to an air draft without (F) or with (FS) systemic scopolamine administration for 5 and 10 days (D) in the NOD.B10.H2b mouse strain
| 4W | 16W | Group | 5Da | 10Da,b | p (F vs. FS) | ||
|---|---|---|---|---|---|---|---|
| p (4W vs. 16W) | |||||||
| Tear volume (μL) | 0.06±0.01 | 0.05±0.01 | F | 0.04±0.01 | 0.04±0.01 | <0.01 in 5D | |
| <0.01 | FS | 0.03±0.01** | 0.02±0.01*** | <0.05 in 10D | |||
| Tear turnover rate (%) | 5.21±1.93 | 3.28±1.54 | F | 3.15±1.61 | 1.31±0.52*† | ||
| 0.06 | FS | 2.03±1.10 | 1.05±0.41** | ||||
| Corneal smoothness score | 0.25±0.43 | 1.00±0.60 | F | 1.13±0.64 | 1.17±0.30 | <0.05 in 5D | |
| <0.01 | FS | 2.13±1.13* | 2.80±1.39** | <0.01 in 10D | |||
| Corneal permeability score | 0.40±0.69 | 2.50±1.00 | F | 3.17±1.40 | 3.83±1.40* | ||
| <0.01 | FS | 3.88±0.96** | 4.83±0.94***† | ||||
| Goblet cell density (cells/100 μm) | 5.07±0.86 | 4.18±0.21 | F | 3.84±0.44 | 3.56±0.32** | <0.01 in 10D | |
| <0.05 | FS | 3.76±0.14** | 2.82±0.34***†† | ||||
p<0.05,
p<0.01,
p<0.001 vs. 16W.
p<0.05,
p<0.01,
p<0.001 vs. 5D.
Corneal surface irregularity and corneal uptake of the fluorescent dye OGD significantly increased in older mice (p < 0.01 for both) (Table 2). The corneal smoothness score significantly increased in the FS group after 5 (p < 0.05) and 10 (p < 0.01) days. Compared with baseline, the corneal permeability score in the F and FS groups increased after 5 (p = 0.37 and p < 0.01, respectively) days and after 10 (p < 0.05 and p < 0.001, respectively) days.
3.4. Desiccating Stress Decreased Conjunctival Goblet Cell Density
Conjunctival goblet cell density is recognized to decrease in human Sjögren’s syndrome [23]. The number of PAS-positive goblet cells was noted to decrease with age in NOD.B10.H2b mice (p < 0.05) (Table 2). Compared with the 16W group (4.18 ± 0.21 cells/100 μm), goblet cell density in the F and FS groups decreased to 3.84 ± 0.44 (p = 0.08) and 3.76 ± 0.14 cells/100 μm (p < 0.01) after 5 days and 3.56 ± 0.32 (p < 0.01) and 2.82 ± 0.34 cells/100 μm (p < 0.001) after 10 days, respectively.
These findings indicate that the immunopathological changes in the conjunctiva and lacrimal gland are associated with functional alterations of tear production.
4. Discussion
These studies investigated the effects of age and desiccating stress on the development of Sjögren’s syndrome-like autoimmune inflammation in the conjunctiva and lacrimal gland of NOD.B10.H2b mice. We found that these mice developed Sjögren’s syndrome-like autoimmune inflammation in the ocular surface and lacrimal gland. Inflammation developed spontaneously in the ocular surface epithelia and lacrimal glands of these mice as they aged to 16 weeks. Particularly, the CD4+/CD8+ T cell ratio was noted to increase in the conjunctival epithelia and lacrimal glands of mice subjected to desiccating stress. Furthermore, increased MMP-9 production and gelatinase activity and decreased CD25 immunoreactivity were observed in the ocular surface epithelia of stressed mice. These pathological changes were accompanied by reduce tear volume and tear turnover rate, decrease conjunctival goblet cell density and disruption of corneal epithelial barrier function in 16-week-old mice subjected to desiccating stress. These findings suggest that desiccating environmental stress aggravates lacrimal keratoconjunctivitis in the NOD mouse.
An animal model mimicking human Sjögren’s syndrome lacrimal keratoconjunctivitis is a valuable tool to investigate the multiple factors that have been implicated in the pathogenesis of this condition. Desiccating stress by environmental and pharmacological means has been used to induce dry eye in several different mouse strains, including C57BL/6, BALB/c, CBA and 129SvEv/CD-1 [5,18,24]. In contrast to these strains, we found that the NOD.B10.H2b strain spontaneously develops autoimmune lacrimal keratoconjunctivitis with age. Serum autoantibodies to acinar and ductal epithelial cells of exocrine glands, 52-kDa SS-A/Ro, M3R, 120-kDa α-foldrin and LPG10 have been previously detected in NOD mice [10,25]. Histopathologically, the autoimmune inflammation in the secretory glands of NOD mice accelerates acinar cell loss via apoptosis and necrosis and ductal epithelial hyperplasia by local production of cytotoxic auto- and paracrine factors [16,26]. It is characterized by glandular infiltration by T cells and B cells, primarily in periductal and perivascular areas [12].
Age and sex differences in the exocrine gland inflammation have been observed in the NOD mouse [14,15,27,28]. The severity of sialoadenitis is worse in females, whereas inflammation in the lacrimal glands is far worse in males [27]. Changes in the exocytotic pathway and abnormalities in acinar cells, such as mitochondrial deterioration, cytoplasmic vacuoles and lipid accumulation occur at 1 month of age in the lacrimal glands of males [14,15]. Lymphocytic infiltration of the lacrimal gland is first detected about 6–10 weeks in males and around 30 weeks in females, though secretory dysfunction is generally detectable by 4 months in males [14,28]. Complement C3 and interleukin-4 (IL-4) have been found to play a critical role in the onset of this autoimmune exocrinopathy [17,29].
Similar to other mouse strains and humans with dry eye, increased gelatinolytic activity was noted on the ocular surface of NOD mice. The expression of CD25, the IL-2 receptor alpha chain, was noted to be inversely correlated with MMP-9 expression and gelatinase activity in the ocular surface epithelia of NOD mice. MMP-9 has previously been shown to proteolytically cleave CD25 from the surface of lymphocytes [30]. A similar phenomenon may be occurring on the ocular surface epithelia of NOD mice.
Derangements in the balance between effector and regulatory T cell responses underlie the pathogenesis of autoimmune diseases [31]. We have previously reported that desiccating stress-induced lacrimal keratoconjunctivitis was mediated by CD4+ T cells and was mitigated by CD4+CD25+ natural regulatory T cells [9]. In this study, an increase in CD4+ T cells and CCR5+ cells was accompanied by a decrease in CD8+ T cells in the ocular surface epithelium and lacrimal gland, resulting in an increased CD4/CD8 ratio. Intraepithelial γδ T cells have been identified in the gut, genital tract, tongue and skin (where they are called dendritic epidermal T cells) of mice [32,33]. They are believed to play an immunoregulatory role in the skin, both initiating and inhibiting inflammation. Until now, the distribution and role of γδ T cells in the ocular surface epithelia has not been investigated. Our results indicate that similar to other mucosal tissues, γδ T cells are also present in the conjunctival epithelia of the mice. We found that desiccating stress caused a decrease in the number of intraepithelial γδ T cells, in contrast to αβ TCR CD4+ T cells.
The change in the density of CD4+, CD8+, CCR5+ and γδ T cells in the lacrimal functional unit of NOD mice indicates that desiccation induces Sjögren’s syndrome-like autoimmune inflammation not only in the ocular surface, but also in the lacrimal gland, suggesting that there may be shared epitopes in these tissues. The effects of desiccating stress on salivary gland immunopathology in the NOD strain were not investigated in this study. Our group had previously reported that desiccating stress promoted development of auto reactive CD4+ T cells in BALB/c mice that were capable of producing lacrimal keratoconjunctivitis, but not salivary gland inflammation when adoptively transferred to nude mouse recipients [9].
The mechanisms responsible for the development of autoimmune diseases remain unclear; however, several hypotheses such as inflammation, disturbances in apoptosis, infection, genetic background and aberrant glandular water transport have been suggested. Based on the concept of molecular mimicry, environmental factors like xenobiotics or chemical compounds foreign to a living organism as well as bacteria and viruses have been also implicated in the pathogenesis of several autoimmune diseases [34,35]. In this study, we found that the F group, which was exposed to an air draft without cholinergic inhibition of tear production, worsened the severity of the lacrimal keratoconjuctivitis, though to a lesser degree than mice subjected to an air draft and pharmacological inhibition of tear secretion. This indicates that environment alone may trigger an autoimmune reaction, potentially initiating or aggravating the autoimmune lacrimal keratoconjunctivitis in Sjögren’s syndrome.
There are several potential mechanisms by which desiccating stress may expose autoantigens in the ocular surface and/or lacrimal gland epithelia to the immune system and promote the development of lacrimal keratoconjunctivitis. This could be a direct effect of dryness or it could result from the increased protease activity that we detected in response to dryness. Cleavage of cell surface proteins by gelatinases, such as MMP-9 could expose antigenic epitopes. As an example, we found that desiccating stress caused loss of cell membrane CD25 from the ocular surface epithelia. Another possibility is that desiccating stress alters the local immunoregulatory environment. The conjunctival epithelium in humans and mice contains a repertoire of intraepithelial lymphocytes (predominantly CD8 T cells in humans and a mixture of γ δ and CD8 T cells in mice). These intraepithelial T cells have been postulated to have an immunoregulatory role [32]. We found a significant decrease in both CD8+ and γ δ intraepithelial T cells after 5 days of desiccating stress that may have tipped the balance to allow entry of CD4+ effector T cells into the conjunctival epithelium. Finally, desiccating stress could alter the production of immunoregulatory cytokines. We have found that conjunctival goblet cells produce TGF-β2 and there is a decrease in immunoreactive TGF-β2 in the conjunctival epithelium in dry eye that accompanies loss of goblet cells in this condition [36]. TGF-β has been linked to the retention of lymphocytes within mucosal epithelia due to its unique ability to up-regulate CD103 on mucosally homed T cells [37].
Our study indicates that the NOD mouse strain develops spontaneous dry eye and Sjögren’s syndrome-like autoimmune inflammation in the ocular surface and lacrimal gland. Desiccating environmental stress significantly worsens this process. These findings indicate that environmental factors may exacerbate autoimmunity in susceptible individuals.
A number of immunoregulatory defects have been identified in NOD mice that may be responsible for their susceptibility to develop an autoimmune response to ocular surface antigens that are exposed during aging and desiccating environmental stress. These include impaired negative selection of autoreactive T cells in the thymus, defective apoptosis of effector T cells, and reduced number and function of regulatory T cells [38–40]. This model provides a good framework for determining which of these immunoregulatory defects increases susceptibility of NOD mice to develop autoimmune lacrimal keratoconjunctivitis and it may provide clues regarding susceptibility factors for development of Sjögren’s syndrome in humans.
Acknowledgments
This study was supported by NIH Grant EY 11915 (SCP), an unrestricted grant from Research to Prevent Blindness, The Oshman Foundation, The William Stamps Farish Fund and an unrestricted grant from Allergan, Inc.
Footnotes
Financial interests or commercial relationship: no
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harley JB, Alexander EL, Bias WB, Fox OF, Provost TT, Reichlin M, et al. Anti-Ro (SS-A) and anti-La (SS-B) in patients with Sjögren’s syndrome. Arthritis Rhuem. 1986;29:196–206. doi: 10.1002/art.1780290207. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen KH, Brayer J, Cha S, Diggs S, Yasunari U, Hilal G, et al. Evidence for antimuscarinic acetylcholine receptor antibody-mediated secretory dysfunction in nod mice. Arthritis Rhuem. 2000;43:2297–2306. doi: 10.1002/1529-0131(200010)43:10<2297::AID-ANR18>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 3.Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren’s syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19:201–211. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- 4.Rolando M, Barabino S, Mingari C, Moretti S, Giuffrida S, Calabria G. Distribution of conjunctival HLA-DR expression and the pathogenesis of damage in early dry eye. Cornea. 2005;24:951–954. doi: 10.1097/01.ico.0000157421.93522.00. [DOI] [PubMed] [Google Scholar]
- 5.De Paiva CS, Villarreal AL, Corrales RM, Rahman HT, Chang VY, Farley WJ, et al. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Invest Ophthalmol Vis Sci. 2007;48:2552–2560. doi: 10.1167/iovs.07-0069. [DOI] [PubMed] [Google Scholar]
- 6.Pflugfelder SC, Farley W, Luo L, Chen LZ, de Paiva CS, Olmos LC, et al. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Patho. 2005;166:61–71. doi: 10.1016/S0002-9440(10)62232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Paiva CS, Corrales RM, Villarreal AL, Farley W, Li DQ, Stern ME, et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest Ophthalmol Vis Sci. 2006;47:2847–2856. doi: 10.1167/iovs.05-1281. [DOI] [PubMed] [Google Scholar]
- 8.Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17:584–589. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Niederkorn JY, Stern ME, Pflugfelder SC, De Paica CS, Corrales RM, Gao J, et al. Desiccating stress induces T cell-mediated Sjögren’s syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006;176:3950–3957. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- 10.Van Blokland SC, Versnel MA. Pathogenesis of Sjögren’s syndrome: characteristics of different mouse models for autoimmune exocrinopathy. Clin Immunol. 2002;103:111–124. doi: 10.1006/clim.2002.5189. [DOI] [PubMed] [Google Scholar]
- 11.Brayer JB, Humphreys-Beher MG, Peck AB. Sjögren’s syndrome: immunological response underlying the disease. Arch Immunol Ther Exp. 2001;49:353–360. [PubMed] [Google Scholar]
- 12.Jonsson MV, Delaleu N, Brokstad KA, Berggreen E, Skarstein K. Impaired salivary gland function in NOD mice: association with changes in cytokine profile but not with histopathologic changes in the salivary gland. Arthritis Rheum. 2006;54:2300–2305. doi: 10.1002/art.21945. [DOI] [PubMed] [Google Scholar]
- 13.Robinson CP, Yamachika S, Bounous DI, Brayer J, Jonsson R, Holmdahl R, et al. A novel NOD-derived murine model of primary Sjögren’s syndrome. Arthritis Rheum. 1998;41:150–156. doi: 10.1002/1529-0131(199801)41:1<150::AID-ART18>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 14.Ding C, MacVeigh M, Pidgeon M, da Costa SR, Wu K, Hamm-Alvarez SF, et al. Unique ultrastructure of exorbital lacrimal gland in male NOD and BALB/c mice. Curr Eye Res. 2006;31:13–22. doi: 10.1080/02713680500428613. [DOI] [PubMed] [Google Scholar]
- 15.da Costa SR, Wu K, Veigh MM, Pidgeon M, Ding C, Schechter JE, et al. Male NOD mouse external lacrimal glands exhibit profound changes in the exocytotic pathway early in postnatal development. Exp Eye Res. 2006;82:33–45. doi: 10.1016/j.exer.2005.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tensing EK, Törnwall J, Hukkanen M, Nordström DC, Konttinen YT. The protein kinase C system in focal adenitis of the lacrimal gland in the non-obese diabetic mouse model for Sjögren’s syndrome. Acta Ophthalmol Scand. 2004;82:569–573. doi: 10.1111/j.1600-0420.2004.00310.x. [DOI] [PubMed] [Google Scholar]
- 17.Gao J, Killedar S, Cornelius JG, Nguyen C, Cha S, Peck AB. Sjögren’s syndrome in the NOD mouse model in an interleukin-4 time-dependent, antibody isotype-specific autoimmune disease. J Autoimmun. 2006;26:90–103. doi: 10.1016/j.jaut.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 18.De Paiva CS, Corrales RM, Villarreal AL, Farley WJ, Li DQ, Stern ME, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83:526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Villareal AL, Farley W, Pflugfelder SC. Effect of topical ophthalmic epinastine and olopatadine on tear volume in mice. Eye Contact Lens. 2006;32:272–276. doi: 10.1097/01.icl.0000224360.10319.b1. [DOI] [PubMed] [Google Scholar]
- 20.Sorbara L, Simpson T, Vaccari S, Jones L, Fonn D. Tear turnover rate is reduced in patients with symptomatic dry eye. Cont Lens Anterior Eye. 2004;27:15–20. doi: 10.1016/j.clae.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–2292. [PubMed] [Google Scholar]
- 22.Yamachika S, Nanni JM, Nguyen KH, Garces L, Lowry JM, Robinson CP, et al. Excessive synthesis of matrix metalloproteinases in exocrine tissues of NOD mouse models for Sjögren’s syndrome. J Rheumatol. 1998;25:2371–2380. [PubMed] [Google Scholar]
- 23.Pflugfelder SC, Tseng SC, Yoshino K, Monroy D, Felix C, Reis BL. Correlation of goblet cell density and mucosal epithelial membrane mucin expression with rose bengal staining in patients with ocular irritation. Ophthalmology. 1997;104:223–235. doi: 10.1016/s0161-6420(97)30330-3. [DOI] [PubMed] [Google Scholar]
- 24.Dursun D, Wang M, Monroy D, Li DQ, Lokeshwar BL, Stern ME, et al. A mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2002;43:632–638. [PubMed] [Google Scholar]
- 25.Esch TR, Poveromo JD, Aikins MC, Levanos VA. A novel lacrimal gland autoantigen in the NOD mouse model of Sjögren’s syndrome. Scand J Immunol. 2002;55:304–310. doi: 10.1046/j.1365-3083.2002.01042.x. [DOI] [PubMed] [Google Scholar]
- 26.Fox RI, Kang HI, Ando D, Abrams J, Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjögren’s syndrome. J Immunol. 1994;152:5532–5539. [PubMed] [Google Scholar]
- 27.Toda I, Sullivan BD, Rocha EM, Da Silveira LA, Wickham LA, Sullivan DA. Impact of gender on exocrine gland inflammation in mouse model of Sjögren’s syndrome. Exp Eye Res. 1999;69:355–366. doi: 10.1006/exer.1999.0715. [DOI] [PubMed] [Google Scholar]
- 28.Hunger RE, Carnaud C, Vogt I, Mueller C. Male gonadal environment paradoxically promotes dacryoadenitis in nonobese diabetic mice. J Clin Invest. 1998;101:1300–1309. doi: 10.1172/JCI1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen C, Cornelius J, Singson E, Killedar S, Cha S, Peck AB. Role of complement and B lymphocytes in Sjögren’s syndrome-like autoimmune exocrinopathy of NOD.B10.H2b mice. Mol Immunol. 2006;43:1332–1339. doi: 10.1016/j.molimm.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Sheu BC, Hsu SM, Ho HN, Lien HC, Huang SC, Lin RH. A novel role of metalloproteinase in caner-mediated immunosuppression. Cancer Res. 2001;61:237–242. [PubMed] [Google Scholar]
- 31.Abbas AK, Lohr J, Knoechel B. Balancing autoaggressive and protective T cell responses. J Autoimmun. 2007;28:59–61. doi: 10.1016/j.jaut.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 33.Itohara S, Farr AG, Lafaille JJ, Bonneville M, Takagaki Y, Hass W, et al. Homing of a gamma delta thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature. 1990;343:754–757. doi: 10.1038/343754a0. [DOI] [PubMed] [Google Scholar]
- 34.Rieger R, Gershwin ME. The X and why of xenobiotics in primary biliary cirrhosis. J Autoimmun. 2007;28:76–84. doi: 10.1016/j.jaut.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rieger R, Leung PSC, Jeddeloh MR, Kurth MJ, Nantz MH, Lam KS, et al. Identification of 2-nonynoic acid, a cosmetic component, as a potential trigger of primary biliary cirrhosis. J Autoimmun. 2006;27:7–16. doi: 10.1016/j.jaut.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Pflugfelder SC, De Paiva CS, Villarreal AL, Stern ME. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and TGF-β2 Production. Cornea. 2007 doi: 10.1097/ICO.0b013e318158f6dc. (in press) [DOI] [PubMed] [Google Scholar]
- 37.Shibahara T, Si-Tahar M, Shaw SK, Madara JL. Adhesion molecules expressed on homing lymphocytes in model intestinal epithelia. Gastroenterology 1. 2000;18:289–298. doi: 10.1016/s0016-5085(00)70211-3. [DOI] [PubMed] [Google Scholar]
- 38.Aoki CA, Borchers AT, Ridgway WM, Keen CL, Ansari AA, Gershwin ME. NOD mice and autoimmunity. Autoimmne Rev. 2005;4:373–379. doi: 10.1016/j.autrev.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Motta V, Lejon K, Holmberg D. The NOD allele of the Idd5 locus on chromosome 1 mediates a non-cell –autonomous defect in negative selection of T cells. J Autoimmun. 2007;28:16–223. doi: 10.1016/j.jaut.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 40.O’Brien BA, Geng X, Orteu CH, Huang Y, Ghoreishi M, Zhang Y, et al. A deficiency in the in vivo clearance of apoptotic cells is a feature of the NOD mouse. J Autoimmun. 2006;26:104–115. doi: 10.1016/j.jaut.2005.11.006. [DOI] [PubMed] [Google Scholar]


