The genetically encoded fluorescent calcium-indicator protein GCaMP2 was crystallized in the calcium-saturated form. X-ray diffraction data were collected to 2.0 Å resolution and the structure was solved by molecular replacement.
Keywords: green fluorescent proteins, calmodulin, genetically encoded calcium indicators
Abstract
Fluorescent proteins and their engineered variants have played an important role in the study of biology. The genetically encoded calcium-indicator protein GCaMP2 comprises a circularly permuted fluorescent protein coupled to the calcium-binding protein calmodulin and a calmodulin target peptide, M13, derived from the intracellular calmodulin target myosin light-chain kinase and has been used to image calcium transients in vivo. To aid rational efforts to engineer improved variants of GCaMP2, this protein was crystallized in the calcium-saturated form. X-ray diffraction data were collected to 2.0 Å resolution. The crystals belong to space group C2, with unit-cell parameters a = 126.1, b = 47.1, c = 68.8 Å, β = 100.5° and one GCaMP2 molecule in the asymmetric unit. The structure was phased by molecular replacement and refinement is currently under way.
1. Introduction
The green fluorescent protein (GFP) of the jellyfish Aequoria victoria is capable of transducing blue chemiluminescence from the aequorin protein into green fluorescence within the light-producing cells of the organism. Following its discovery (Morin & Hastings, 1971 ▶; Shimomura et al., 1962 ▶) and cloning (Prasher et al., 1992 ▶), it was demonstrated that heterologous expression of the GFP gene results in spontaneous maturation of its p-hydroxybenzylideneimidazolinone chromophore and fluorescence (Chalfie et al., 1994 ▶; Inouye & Tsuji, 1994 ▶). This discovery paved the way for its ubiquitous use in fluorescence microscopy; promoter activity and subcellular localization of proteins could be visualized, among numerous other applications. More recently, engineered color variants of GFP and fluorescent protein-based sensors have dramatically expanded the utility of fluorescent proteins in the study of biology (Baird et al., 1999 ▶; Giepmans et al., 2006 ▶).
The genetically encoded calcium indicator GCaMP was introduced in 2001 by insertion of a circularly permuted enhanced EGFP (cpEGFP) domain between calmodulin (CaM) at the C-terminus and a CaM target peptide (M13) from the myosin light-chain kinase at the N-terminus (Nakai et al., 2001 ▶). Upon addition of calcium to GCaMP, an increase in green fluorescence is observed. Subsequent rounds of protein engineering have produced improvements in the brightness and folding efficiency at 310 K of GCaMP (Diez-Garcia et al., 2005 ▶; Ohkura et al., 2005 ▶; Tallini et al., 2006 ▶). The current version (GCaMP2) displays a fluorescence intensity increase of fourfold to fivefold when going from depleted to saturating levels of calcium ions. GCaMP2 is 451 amino acids in length, with a molecular weight of 50 657 Da. Prior to the M13 peptide is an N-terminal affinity and epitope tag encoded by the pRSET vector with sequence MRGSHHHHHHGMASMTGGQQMGRDLYDDDDKD.
The goal of this structure determination is to guide the rational structure-based improvement of this genetically encoded calcium indicator to allow additional applications in imaging, as well as to establish structural principles that could aid the development of novel sensor proteins. The structure would represent the first for a genetically encoded calcium sensor, as well as the first structure of a circularly permuted fluorescent protein.
2. Experimental procedures and results
2.1. Protein expression and purification
For general DNA manipulation, Escherichia coli strain XL-1 (Stratagene, La Jolla, USA) was used. Plasmid pRSETA (Invitrogen) harboring gcamp2 was a kind gift from Karel Svoboda (Janelia Farm Research Campus). GCaMP2 was recombinantly produced in E. coli strain BL21 (DE3) (Novagen, Madison, USA) in ZYM-5052 medium using the method of Studier (2005 ▶). 2 l cultures were directly inoculated from a single colony and grown at 310 K until the OD600nm reached 0.5, after which protein production was allowed to continue at 298 K for 48 h. Cells were harvested by centrifugation (20 min, 5000g, 277 K), resuspended in lysis buffer (20 mM Tris–HCl, 1 M NaCl pH 8.0) and disrupted by subsequent freezing at 253 K, thawing in water at room temperature and sonication on ice (2 min at 20 W). Insoluble material was removed by centrifugation (45 min, 35 000g, 277 K). The resulting cell-free extract (CFE) was slowly mixed with 5 ml His-Select nickel-affinity gel (Sigma) at 277 K for 60 min. After mixing, the resin was allowed to settle in a 20 ml disposable column. Contaminant proteins were eluted by washing the resin with 10 ml wash buffer (20 mM Tris–HCl, 1 M NaCl, 20 mM imidazole pH 8.0). GCaMP2 was eluted in 20 mM Tris–HCl, 1 M NaCl, 300 mM imidazole pH 8.0 and subsequently dialyzed into 20 mM Tris–HCl, 100 mM NaCl, 2 mM CaCl2 in the dark. After dialysis, GCaMP2 was concentrated using an Amicon Ultra centrifugal filter device with a 10 000 Da molecular-weight cutoff (Millipore, USA) to a concentration of 12 mg ml−1. SDS–PAGE analysis of the purified protein sample indicated that the protein was >95% pure (Fig. 1 ▶).
Figure 1.

SDS–PAGE analysis of the purified GCaMP2 protein used for crystallization. Molecular weights of protein markers (in kDa) are indicated on the left.
2.2. Crystallization
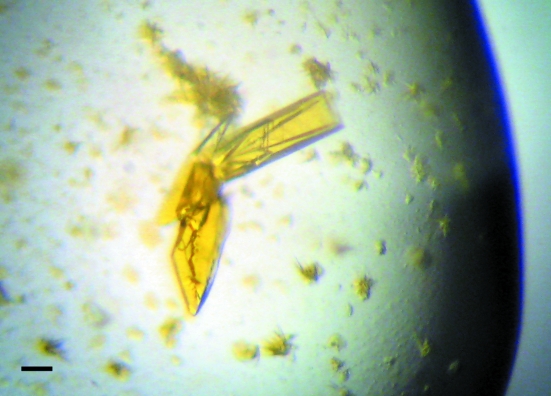
Crystals of calcium-saturated GCaMP2 were grown at 298 K using the hanging-drop vapor-diffusion technique and resulted from sparse-matrix crystallization screening using commercially available screens (Hampton Research). 1.5 µl purified GCaMP2 (12 mg ml−1 in 20 mM Tris–HCl pH 8, 100 mM NaCl, 2 mM CaCl2) was mixed with 1.5 µl precipitant solution [0.2 M lithium sulfate monohydrate, 0.1 M Tris–HCl pH 8.5, 30%(w/v) polyethylene glycol 4000; Crystal Screen condition No. 17, Hampton Research] on a siliconized glass cover slip and sealed above a reservoir of 700 µl precipitant solution in a VDX-format crystallization tray. Crystals appeared after approximately 5 d as clusters of yellow-green rectangular plates (Fig. 2 ▶) and were used for data collection without optimization.
Figure 2.
Crystals of calcium-saturated GCaMP2. The scale bar is 100 µm in length.
2.3. Data collection and processing
Prior to data collection, GCaMP2 crystals were soaked for 30 s in a cryoprotectant solution consisting of the precipitant solution supplemented with 15%(v/v) glycerol. Crystals were then mounted in a fiber loop and cooled to 100 K under a stream of nitrogen gas for data collection. X-ray diffraction data were collected on a rotating copper-anode home X-ray source equipped with a Saturn 92 CCD detector (Rigaku/Molecular Structure Corporation). The crystals belonged to space group C2, with unit-cell parameters a = 126.1, b = 47.1, c = 68.8 Å, β = 100.54°, and diffracted to a maximum resolution of 2.0 Å (Fig. 3 ▶). Data processing, integration and scaling were performed using d*TREK (Pflugrath, 1999 ▶) from the CrystalClear software suite (Rigaku/Molecular Structure Corporation). Data statistics are presented in Table 1 ▶.
Figure 3.
Representative X-ray diffraction pattern from a single GCaMP2 crystal. The smaller and larger circles correspond to Bragg spacings of 3.0 and 2.0 Å, respectively.
Table 1. X-ray data-collection and processing statistics.
Values in parentheses are for the highest resolution shell.
| Space group | C2 |
| Unit-cell parameters (Å, °) | a = 126.1, b = 47.1, c = 68.8, α = 90, β = 100.5, γ = 90 |
| Temperature (K) | 100 |
| Wavelength (Å) | 1.5418 |
| Resolution range (Å) | 30–2.00 (2.07–2.00) |
| Unique reflections | 25763 (2315) |
| Redundancy | 11.5 (7.7) |
| Completeness (%) | 95.0 (86.1) |
| Rmerge† (%) | 12.6 (56.5) |
| Average I/σ(I) | 11.3 (3.3) |
R
merge = 
 , where I
i(hkl) is the ith observation of reflection hkl and 〈I(hkl)〉 is the weighted average intensity for all observations i of reflection hkl.
, where I
i(hkl) is the ith observation of reflection hkl and 〈I(hkl)〉 is the weighted average intensity for all observations i of reflection hkl.
2.4. Structure determination
Cell-content analysis (Collaborative Computational Project, Number 4, 1994 ▶) revealed that one molecule of GCaMP2 was present in the asymmetric unit, with a Matthews coefficient of 2.04 Å3 Da−1 and a corresponding solvent content of 39.8%. The structure of GCaMP2 was solved by molecular replacement using the program Phaser (McCoy et al., 2005 ▶) by sequentially searching for the GFP domain (using PDB entry 1ema) and the CaM–M13 complex (PDB code 1cdl) using data between 20 and 2.5 Å resolution. The top translation function for the GFP domain gave a Z score of 18.1, while the CaM–M13 complex gave a translation-function Z score of 37.0. Strong positive difference density peaks at the expected positions of the calcium ions bound to the CaM domain, which were not included in the molecular-replacement models, indicated the correctness of the solution. Rebuilding and refinement of the GCaMP2 model is currently under way and details of the structure will be reported separately.
Acknowledgments
This work was supported by NIH–COBRE Grant 1P20RR16439-01 and the University of Puerto Rico, Río Piedras Campus.
References
- Baird, G. S., Zacharias, D. A. & Tsien, R. Y. (1999). Proc. Natl Acad. Sci. USA, 96, 11241–11246. [DOI] [PMC free article] [PubMed]
- Chalfie, M., Tu, Y., Euskirchen, G., Ward, W. W. & Prasher, D. C. (1994). Science, 263, 802–805. [DOI] [PubMed]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763.
- Diez-Garcia, J., Matsushita, S., Mutoh, H., Nakai, J., Ohkura, M., Yokoyama, J., Dimitrov, D. & Knopfel, T. (2005). Eur. J. Neurosci.22, 627–635. [DOI] [PubMed]
- Giepmans, B. N., Adams, S. R., Ellisman, M. H. & Tsien, R. Y. (2006). Science, 312, 217–224. [DOI] [PubMed]
- Inouye, S. & Tsuji, F. I. (1994). FEBS Lett.341, 277–280. [DOI] [PubMed]
- McCoy, A. J., Grosse-Kunstleve, R. W., Storoni, L. C. & Read, R. J. (2005). Acta Cryst. D61, 458–464. [DOI] [PubMed]
- Morin, J. G. & Hastings, J. W. (1971). J. Cell. Physiol.77, 313–318. [DOI] [PubMed]
- Nakai, J., Ohkura, M. & Imoto, K. (2001). Nature Biotechnol.19, 137–141. [DOI] [PubMed]
- Ohkura, M., Matsuzaki, M., Kasai, H., Imoto, K. & Nakai, J. (2005). Anal. Chem.77, 5861–5869. [DOI] [PubMed]
- Pflugrath, J. W. (1999). Acta Cryst. D55, 1718–1725. [DOI] [PubMed]
- Prasher, D. C., Eckenrode, V. K., Ward, W. W., Prendergast, F. G. & Cormier, M. J. (1992). Gene, 111, 229–233. [DOI] [PubMed]
- Shimomura, O., Johnson, F. H. & Saiga, Y. (1962). J. Cell. Comp. Physiol.59, 223–239. [DOI] [PubMed]
- Studier, F. W. (2005). Protein Expr. Purif.41, 207–234. [DOI] [PubMed]
- Tallini, Y. N. et al. (2006). Proc. Natl Acad. Sci. USA, 103, 4753–4758.