Abstract
In this article, the formation of prokaryotic and eukaryotic cardiolipin is reviewed in light of its biological function. I begin with a detailed account of the structure of cardiolipin, its stereochemistry, and the resulting physical properties, and I present structural analogs of cardiolipin that occur in some organisms. Then I continue to discuss i) the de novo formation of cardiolipin, ii) its acyl remodeling, iii) the assembly of cardiolipin into biological membranes, and iv) the degradation of cardiolipin, which may be involved in apoptosis and mitochondrial fusion. Thus, this article covers the entire metabolic cycle of this unique phospholipid. It is shown that mitochondria produce cardiolipin species with a high degree of structural uniformity and molecular symmetry, among which there is often a dominant form with four identical acyl chains. The subsequent assembly of cardiolipin into functional membranes is largely unknown, but the analysis of crystal structures of membrane proteins has revealed a first glimpse into the underlying principles of cardiolipin-protein interactions. Disturbances of cardiolipin metabolism are crucial in the pathophysiology of human Barth syndrome and perhaps also play a role in diabetes and ischemic heart disease.
Keywords: tafazzin, mitochondrial biogenesis, phospholipids, molecular species
Cardiolipin is a minor component of bacterial and mitochondrial membranes, which is found in virtually all organisms of the three domains of life: eubacteria, archaebacteria, and eukaryota. Although this suggests some fundamental biological function, its nature has not been fully understood and the function may not be the same in prokaryotes and eukaryotes. It seems that the physical properties of cardiolipin invite a number of interactions that may have implications for the structural organization of biological membranes. Those interactions may cause the segregation of membrane domains, the cross-linking of proteins and their subunits, and the formation of nonbilayer structures. Cardiolipin may trap protons in an acid anion structure, and it has the extraordinary ability to bind to a large variety of unrelated proteins. Some of these phenomena are clearly related to the intrinsic symmetry of the cardiolipin structure, and they seem to be of particular significance for the function of energy-transducing membranes.
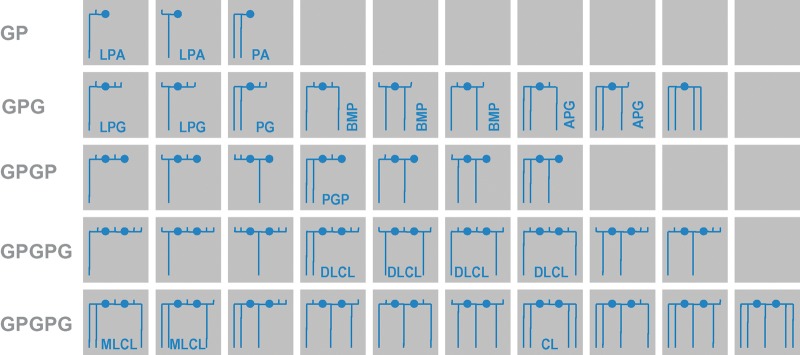
Cardiolipin belongs to a subclass of phospholipids in which backbones and head groups are formed from repeating units of phosphoryl and glycerol moieties (polyglycerophospholipids). Although only a few members of this potentially large family have been discovered in living organisms, including cardiolipin, phosphatidic acid, phosphatidylglycerol, and their lysocompounds, as well as bis(monoacylglycero)phosphate, acylphosphatidylglycerol, and phosphatidylglycerophosphate (Fig. 1), it is conceivable that the era of lipidomics will unearth novel polyglycerophospholipids. What is most intriguing about polyglycerophospholipids is the presence of many hydroxyl groups, which are potential sites for acyl attachment and which can form multiple positional and steric isomers. For instance, phosphatidylglycerol is a positional isomer of bis(monoacylglycero)phosphate, which itself has several isomers. Dilysocardiolipin has four positional isomers, and the complexity would be even greater if one considered all possible steric conformations. In general, the glycerol moieties of polyglycerophospholipids are stereospecific (i.e., they have either R or S conformation). An exception is made by the central glycerol group of cardiolipin and its lysocompounds, in which the steric conformation depends on the number, type, and position of the acyl groups.
Fig. 1.
Family of polyglycerophospholipids. These lipids consist solely of glycerol groups (horizontal lines), phosphate groups (closed circles), and acyl groups (long vertical lines). Free hydroxyl groups are presented as short vertical lines. The backbones may be glycerophosphate (GP), glycerophospho-glycerol (GPG), glycerophospho-glycerophosphate (GPGP), or glycerophospho-glycero-phosphoglycerol (GPGPG). Stereochemical relationships are not shown in this scheme. The identified compounds have R conformation in the GP group, R/S or S/S conformation in the GPG group, R/S conformation in the GPGP group, and R/R/R, R/S/R, or R/R conformation in the GPGPG group. Members of this family include lysophosphatidic acid (LPA), phosphatidic acid (PA), lysophosphatidylglycerol (LPG), phospatidylglycerol (PG), bis(monoacylglycero)phosphate (BMP), acylphosphatidylglycerol (APG), phospatidylglycerophosphate (PGP), dilysocardiolipin (DLCL), monolysocardiolipin (MLCL), and cardiolipin (CL).
Cardiolipin contains two 1,2-diacyl-sn-glycero-3-phosphoryl moieties (also called 3-phosphatidyl groups) linked by a glycerol bridge (Fig. 2) (1). The two phosphatidyl moieties are stereochemically nonequivalent, because one is in pro-R and the other is in pro-S position with respect to the central carbon atom of the glycerol bridge (2). Obviously, the central carbon atom becomes a true chiral center if the two phosphatidyl residues contain different fatty acids (3). The presence of two phosphate groups may give rise to two negative charges, a fact that may become important for protein cross-links and for protein interactions in general. However, in aqueous dispersions with neutral pH, cardiolipin contains a single charge only, because one proton gets trapped in a bicyclic resonance structure formed by the two phosphates and the central hydroxyl group (Fig. 2) (4). Only at the extreme ends of the pH scale is cardiolipin either uncharged (pH < 3) or a divalent anion (pH > 10).
Fig. 2.
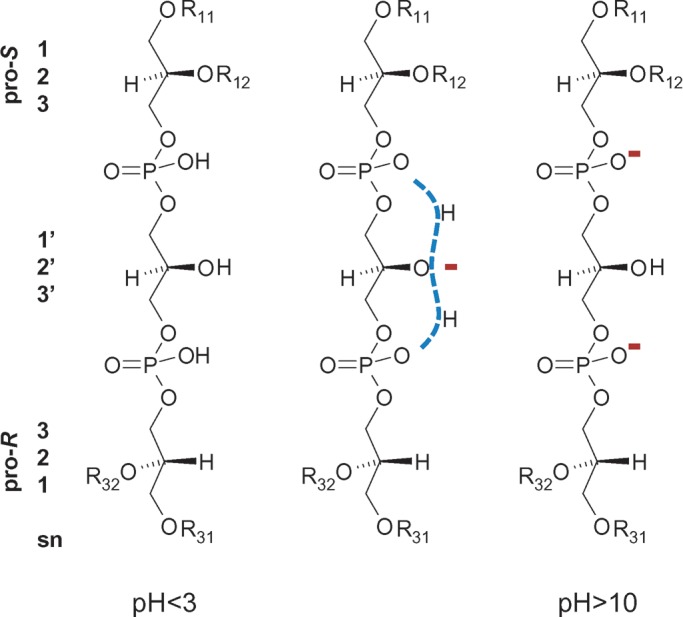
Structure of cardiolipin. Two phosphatidyl residues, both in R conformation, are linked by a central glycerol bridge. One phosphatidyl group is in pro-R and the other in pro-S position with respect to the central carbon atom. Stereochemical relationships are shown on the left side in sn nomenclature. Different ionic states of the two phosphate groups are presented. R11, R12, R31, and R32 are acyl groups.
Aqueous dispersions of cardiolipin and its derivatives display characteristic phase polymorphism, which includes micellar, lamellar, and hexagonal states (5). Phase transitions from micellar to lamellar and from lamellar to hexagonal states are favored by low pH, high ionic strength, and a high number of acyl groups (see Ref. 6 for review). For instance, dilysocardiolipin (two acyl chains) may form micellar and lamellar structures, whereas acylcardiolipin (five acyl chains) always exists in the hexagonal state (7). Cardiolipin (four acyl chains) may exist either in the hexagonal or in the lamellar state. Although hexagonal cardiolipin may play a role in membrane contact zones and other areas where the bilayer structure is perturbed, it is probably fair to say that the majority of mitochondrial and bacterial cardiolipin is in the bilayer phase. In this state, the two phosphatidyl glycerols of cardiolipin are oriented perpendicular to the bilayer surface. The central glycerol group, which is at the water/membrane interface, is oriented parallel to the bilayer surface and has a restricted freedom of motion compared with other phospholipid head groups as a result of its bilateral membrane anchorage (8).
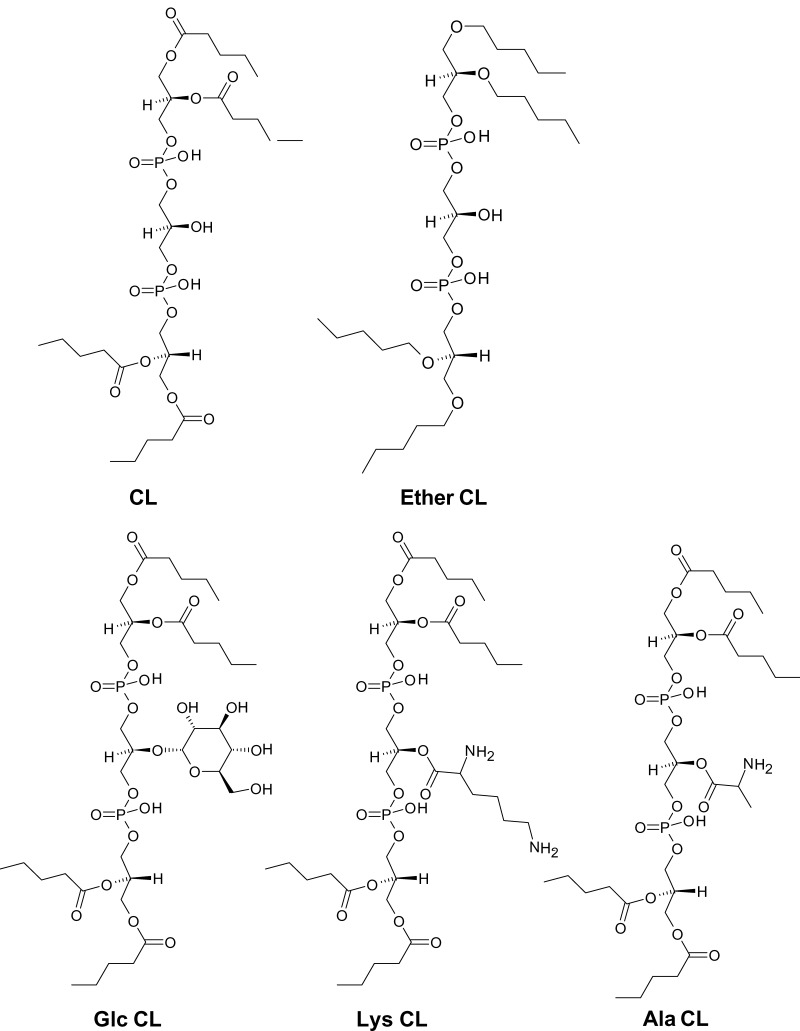
The occurrence of cardiolipin is limited to specific ATP-producing membranes, such as the bacterial plasma membrane (9), mitochondrial membranes (10), and the membranes of hydrogenosomes, a mitochondrion-like organelle from protists (11). The presence of cardiolipin in prokaryotes and eukaryotes has been used as an argument for the endosymbiotic hypothesis, according to which mitochondria were derived from prokaryotes that lived inside a eukaryotic progenitor cell (12). Be that as it may, the role of cardiolipin must have changed during the evolution from prokaryotes to eukaryotes, because mitochondria require a constant level of cardiolipin for baseline activity, whereas prokaryotes accumulate cardiolipin only in specific situations, such as stationary growth or environmental stress. Several structural analogs of cardiolipin have been isolated from some eubacteria and archaebacteria (13–17), as if nature has “experimented” with different chemical variations before settling on the cardiolipin structure (Fig. 3).
Fig. 3.
Cardiolipin-like phospholipids from prokaryotes. CL, normal cardiolipin; Ether-CL, tetra-alkylether form of cardiolipin from Halobacterium salinarum; Glc-CL, α-d-glucopyranosyl-cardiolipin from group B Streptococcus; Lys-CL, l-lysyl-cardiolipin from Listeria species; Ala-CL, d-alanyl-cardiolipin from Vagococcus fluvialis.
DE NOVO FORMATION OF CARDIOLIPIN
The biosynthesis of cardiolipin begins with the formation of phosphatidic acid from glycerol-3-phosphate and activated fatty acids. Then, phosphatidic acid reacts with CTP to form the high-energy phosphoanhydride intermediate phosphatidyl-CMP. The activated phosphatidyl group is subsequently transferred to the sn-1 hydroxyl group of another glycerol-3-phosphate to yield phosphatidylglycerophosphate, which in turn is hydrolyzed to phosphatidylglycerol. From there, the pathway diverges into prokaryotic and eukaryotic branches. In prokaryotes, phosphatidylglycerol receives a phosphatidyl group from another phosphatidylglycerol by transesterification, catalyzed by a phospholipase D-type enzyme. In eukaryotes, phosphatidylglycerol receives an activated phosphatidyl group from phosphatidyl-CMP, which is catalyzed by an enzyme that falls into the category of phosphatidyltransferases (Fig. 4). Enzymes of this category also produce phosphatidylserine, phosphatidylinositol, and phosphatidylglycerol. The prokaryotic formation of cardiolipin is a near-equilibrium reaction, whereas the eukaryotic formation has a considerable negative change in free energy.
Fig. 4.
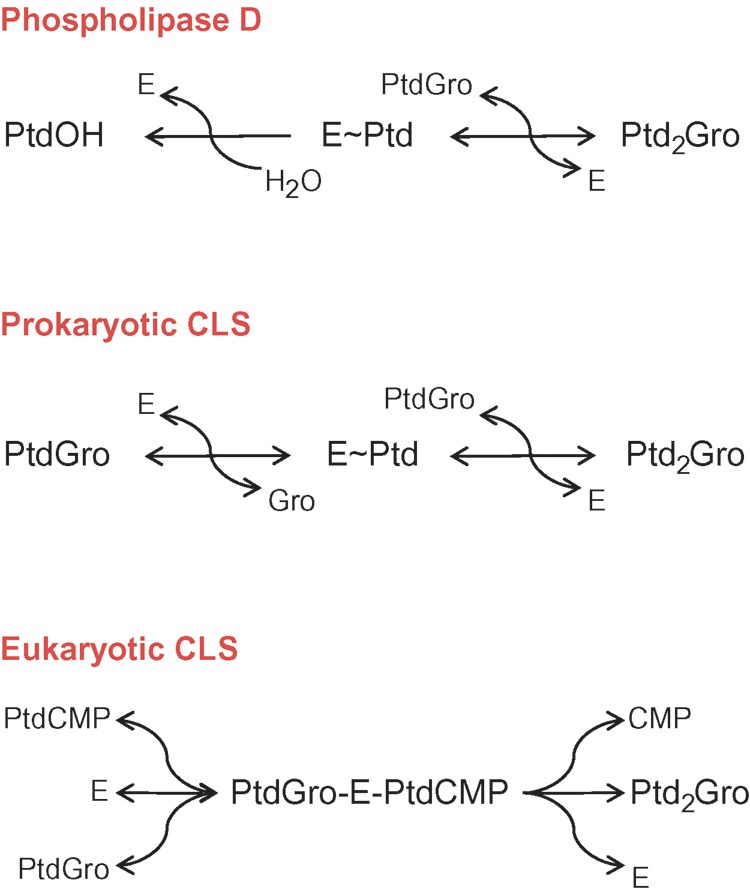
Comparison of reaction mechanisms of phospholipase D and cardiolipin synthase. Prokaryotic cardiolipin synthase is a phospholipase D-type enzyme, in which glycerol replaces water as the phosphatidyl acceptor. Note that both steps of prokaryotic cardiolipin synthase are reversible, whereas the hydrolysis step of phospholipase D is irreversible. Eukaryotic cardiolipin synthase is a phosphatidyltransferase that catalyzes an irreversible reaction. E, enzyme; Gro, glycerol; Ptd, 3-sn-phosphatidyl; PtdOH, phosphatidic acid; PtdGro, phosphatidylglycerol; PtdCMP, phosphatidyl-CMP; Ptd2Gro, cardiolipin.
Prokaryotic cardiolipin synthesis
Bacteria contain variable amounts of cardiolipin depending on their physiologic state. Although cardiolipin is only a trace component during exponential growth, it may increase to become the most dominant phospholipid under certain conditions associated with growth reduction (18). For instance, cardiolipin has been shown to increase in the stationary phase (19), in response to energy deprivation (20), and in response to osmotic stress (21, 22). Accordingly, the expression level of cardiolipin synthase and its catalytic activity increase when Escherichia coli cultures reach the stationary phase (23, 24).
The prokaryotic reaction for cardiolipin synthesis was discovered in the 1970s (25, 26), and the structural gene for the E. coli cardiolipin synthase was discovered in 1978 (27) and was cloned in 1985 (28). The open reading frame encodes a 54.6 kDa polypeptide, but the protein formed in vivo is ∼8 kDa smaller, suggesting some posttranslational modification (28–30). The properties of E. coli cardiolipin synthase have been summarized in a very informative review article (31).
Bacterial cardiolipin synthases belong to the phospholipase D superfamily (Fig. 4). These enzymes attack ester bonds between the phosphoryl group of the phosphatidyl moiety and the alcohol group of the head moiety. Although the alcohol is released, the phosphatidyl moiety remains bound to the enzyme and can either react with water, resulting in hydrolysis, or react with another alcohol, resulting in transphosphatidylation (32). In the case of cardiolipin synthase, transphosphatidylation occurs between two phosphatidylglycerols, one acting as phosphatidyl donor, the other as phosphatidyl acceptor. Because the enzyme does not have strict substrate specificity, mannitol can replace glycerol, a fact that was first suspected when phosphatidylmannitol and bisphosphatidylmannitol were discovered in E. coli cultures grown in the presence of 0.6 M mannitol (33). Subsequent studies confirmed that purified cardiolipin synthase can transfer a phosphatidyl residue from cardiolipin to mannitol (29, 34). This experiment highlights the fact that bacterial cardiolipin synthase may act in the reverse direction, which leads to the decomposition of cardiolipin. Thus, cardiolipin formation in prokaryotes depends not only on the expression level of cardiolipin synthase but also on other factors that affect the transphosphatidylation equilibrium, such as the free energy of cardiolipin in the membrane and the local concentration of glycerol and other alcohols.
Eukaryotic cardiolipin synthesis
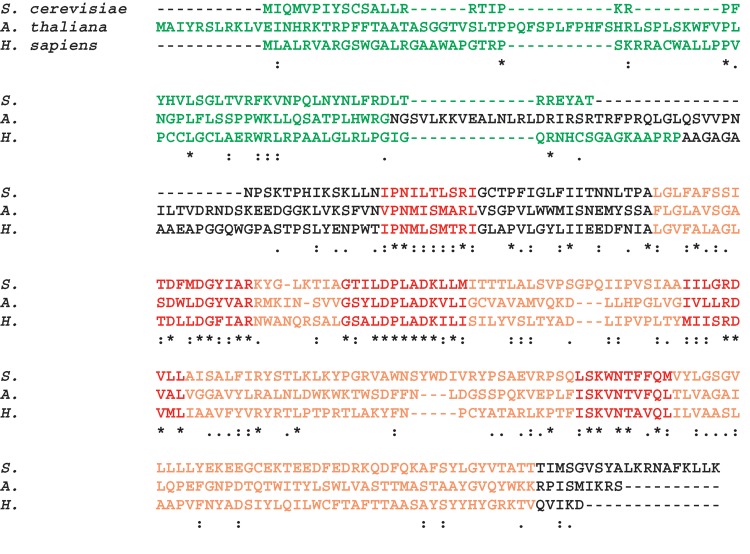
Cardiolipin synthesis from phosphatidyl-CMP and phosphatidylglycerol was first demonstrated in rat liver mitochondria by Hostetler, van den Bosch, and van Deenen (35, 36). Later it was shown that the same reaction is used to form cardiolipin in yeast (37), plants, other fungi, and vertebrate and nonvertebrate animals (38). Genes encoding cardiolipin synthases were identified and the gene products were characterized in yeast (39–41), Arabidopsis (42, 43), and humans (44–46). They are highly homologous and have similar masses, namely 32.0, 38.0, and 32.6 kDa for yeast, Arabidopsis, and human cardiolipin synthase, respectively. After cleavage of the mitochondrial targeting sequence, mature enzymes with masses between 24 and 29 kDa are predicted (Fig. 5). Sequence comparison shows at least five conserved motifs (42). Cardiolipin synthases contain multiple sites for potential hydrophobic interaction, including potential transmembrane domains.
Fig. 5.
Sequence alignment of cardiolipin synthases from Saccharomyces cerevisiae, Arabidopsis thaliana, and Homo sapiens. The alignment was computed with CLUSTAL W. Mitochondrial targeting sequences (shown in green) were predicted with MITOPROT (http://ihg.gsf.de). The section corresponding to the general CDP-alcohol phosphotransferase motif is shown in orange. Motifs that are conserved among cardiolipin synthases are shown in red.
Characterization of cardiolipin synthase
The properties of cardiolipin synthase have been studied in solubilized and partially purified enzyme preparations from rat liver (47) and yeast (48). The Arabidopsis enzyme was also partially purified after expression in E. coli (43). These studies confirmed a number of observations originally made in crude mitochondria, such as the high pH optimum (pH 8–9) and the requirement for specific divalent cations (Mg2+, Mn2+, or Co2+), suggesting that these are intrinsic properties of cardiolipin synthase. Although the cation with the highest stimulatory effect appears to be different for the rat (Co2+), yeast (Mg2+), and Arabidopsis (Mn2+) enzymes, the data support the notion that eukaryotic cardiolipin synthases form a relatively homogeneous family. What is also common to all cardiolipin synthases studied is that the apparent Km value for phosphatidyl-CMP is ∼2 orders of magnitude lower than the Km value for phosphatidylglycerol (43, 47, 48). Cardiolipin synthase has considerable specificity for phosphatidyl-CMP compared with the adenosine, guanosine, and uridine analogs (49), and it does not react when lysophosphatidylglycerol is supplied instead of phosphatidylglycerol (38). The apparent acyl specificity of cardiolipin synthase from humans (44), rat (50, 51), and Arabidopsis (43) has been measured with various molecular species of phosphatidyl-CMP and phosphatidylglycerol. These experiments have clearly established that cardiolipin synthase does not possess the necessary acyl specificity to explain the preferential synthesis of tetralinoleoyl-cardiolipin, the dominant molecular species in many animal and plant tissues, suggesting that another mechanism must exist (see below).
Localization of cardiolipin synthase
Eukaryotic cardiolipin synthases are targeted to mitochondria by their N-terminal presequence (Fig. 5). The rat liver enzyme has been shown to reside in the inner mitochondrial membrane (52, 53), and in yeast, cardiolipin synthase appears to be part of a large protein complex (54). The catalytic center of cardiolipin synthase is exposed to the matrix side of the inner membrane because i) the susceptibility of cardiolipin synthase to proteases is similar to that of the matrix enzyme glutamate dehydrogenase; ii) cardiolipin formation is inhibited when the entry of the cofactor Mn2+ into the matrix is blocked by ruthenium red; and iii) a membrane-impermeable inhibitor of cardiolipin synthase is only effective after solubilization of the inner membrane (53).
Regulation of cardiolipin synthase
Not surprisingly, human cardiolipin synthase is most abundantly expressed in tissues that are rich in mitochondria, such as heart, skeletal muscle, and liver (45, 46). Thus, one may presume that cardiolipin synthesis is under the control of transcription factors that regulate mitochondrial biogenesis, although direct evidence for this notion has yet to emerge. Nevertheless, the response of cardiolipin concentration and cardiolipin synthase activity to thyroxin, an endocrine stimulator of mitochondrial biogenesis, clearly supports the concept that cardiolipin formation is controlled by a transcriptional program that acts upon mitochondrial homeostasis (55–57). The expression of MIDAS (for mitochondrial DNA absence sensitivity factor), a novel protein of the mitochondrial intermembrane space, seems to increase mitochondrial mass by specifically upregulating mitochondrial lipids, such as cardiolipin (58). Another factor that has been identified to affect the activity of cardiolipin synthase is the assembly of respiratory complex IV (54). Furthermore, the proton gradient across the inner mitochondrial membrane was shown to affect the rate of cardiolipin formation, which may simply result from the stimulation of cardiolipin synthase by alkaline pH at the matrix side (59). Finally, a large body of evidence has been accumulated in yeast on the regulation of cardiolipin formation by inositol (for reviews, see Refs. 6, 60).
REMODELING OF CARDIOLIPIN
In mitochondria, postsynthetic modifications of cardiolipin entail substantial changes in the acyl composition. This process, originally described in rat liver mitochondria (61), is called “remodeling” and produces in most instances a peculiar form of cardiolipin with four identical acyl residues. This form of cardiolipin cannot be synthesized by the de novo pathway because the molecular species of the precursor lipids are very different from the species of cardiolipin (62), and the substrate specificity of cardiolipin synthase cannot make up for this difference (43, 44, 50). Thus, remodeling has been recognized as an indispensable step to produce mature cardiolipin, although the purpose of remodeling and the remodeling mechanism have remained a matter of controversy. Interest in cardiolipin remodeling has increased recently because disturbances in the molecular composition of cardiolipin have been identified in human disorders, such as Barth syndrome (63), diabetes (64), heart failure (65), and Parkinson syndrome (66). In the case of Barth syndrome, the remodeling defect can be attributed directly to mutations in the enzyme tafazzin (see Ref. 67 for review). However, in diabetes, heart failure, and Parkinson disease, the role of cardiolipin remodeling has not been defined. Thus, a number of interesting questions arise regarding the biological function of cardiolipin remodeling and its mechanism.
Molecular species of cardiolipin
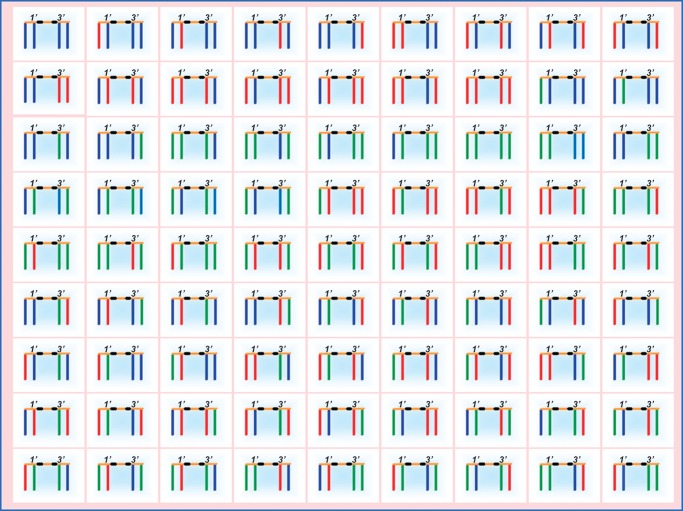
It has been known for some time that the fatty acid profile of cardiolipin is different from the fatty acid profiles of other mitochondrial phospholipids (68). Many authors have stressed that the fatty acids of cardiolipin are highly unsaturated and argued that this may be important for membrane fluidity and protein interaction. However, there is no characteristic degree of unsaturation among cardiolipins from different organisms (3, 69–73), nor is it plausible to postulate that a minor lipid like cardiolipin would determine the overall fluidity of the membrane. Table 1 clearly shows that the dominant fatty acids of various cardiolipins may have anywhere from 14 to 22 carbon atoms and anywhere from 0 to 6 double bonds. In the face of these data, it is difficult to maintain the idea that unsaturation is essential for the function of cardiolipin in mitochondria. What is striking, however, among all of the cardiolipins listed in Table 1 is that they contain only one or two types of major fatty acids, which together account for 60% to 90% of the total fatty acid mass (3, 69–73). This restrictive pattern, a result of acyl-selective remodeling, limits the otherwise enormous structural diversity of cardiolipin molecular species. Cardiolipin can potentially form a huge number of molecular species as a result of different fatty acid combinations, which are a consequence of having four stereochemically distinct acyl positions (Fig. 6). In fact, there are n4 potential molecular species for a cardiolipin with n types of fatty acids (3). Thus, in humans, where n equals ∼14, the number of cardiolipin species could easily be a five-digit figure. In reality, however, the remodeling process generates structural uniformity and also molecular symmetry, because the proportion of symmetric cardiolipins (i.e., those with two identical phosphatidyl residues) increases if only one or two types of fatty acids are present (3). The most widely known form of remodeled cardiolipin is the tetralinoleoyl species, a symmetric molecule that occurs in plants and many animal tissues, but other uniformly substituted species have also been found, for instance tetrapalmitoyl-cardiolipin in rat testis (70), tetrapalmitoleoyl-cardiolipin in insect cells (unpublished data), and tetradocosahexaenoyl-cardiolipin in certain bivalves (73).
TABLE 1.
Dominant fatty acids in various cardiolipins
| Cardiolipin Source | Fatty Acids | References |
|---|---|---|
| Sea urchin sperm (H. pulcherrimus, A. crassispina) | 14:0, 16:0 | 69 |
| Rat testis | 16:0 | 70 |
| Sf9 insect cells | 16:1 | 71 |
| Saccharomyces cerevisiae | 16:1, 18:1 | 3 |
| Human lymphoblasts | 18:1 | 3 |
| Drosophila melanogaster | 16:1, 18:2 | 3 |
| Human heart | 18:2 | 3 |
| Mung bean hypocotyls | 18:2, 18:3 | 3 |
| Manila clam (R. philippinarum) | 20:5, 22:6 | 72 |
| Marine bivalves (P. maximus, C. gigas, M. edulis) | 22:6 | 73 |
H. pulcherrimus, Hemicentrotus pulcherrimus; A. crassispina, Anthocidaris crassispina; R. philippinarum, Ruditapes philippinarum; P. maximus, Pecten maximus; C. gigas, Crassostrea gigas; M. edulis, Mytilus edulis. The dominant fatty acids account for >50% of the total fatty acids of cardiolipin in each tissue. 14:0, myristoyl; 16:0, palmitoyl; 16:1, palmitoleoyl; 18:1, oleoyl or vaccenoyl; 18:2, linoleoyl; 18:3, linolenoyl; 20:5, eicosapentaenoyl; 22:6, docosahexaenoyl.
Fig. 6.
Molecular diversity of cardiolipin species. A total of 81 molecular species can be formed in the presence of three different acyl groups (shown in blue, red, and green). Glycerols are shown in orange, and phosphate groups are shown in black. The diversity arises from the stereochemical nonequivalence of the two phosphatidyl residues in the 1′ and 3′ positions.
Remodeling pathway
The question of how the molecular species of cardiolipin are formed is interesting both from a mechanistic and from a physiologic point of view. Initially, it was assumed that cardiolipin remodeling follows the Lands cycle, in which the original acyl groups are removed by phospholipase A2 and then new acyl groups are attached by acyl-CoA:lysophospholipid acyltransferase (61, 74). Indeed, two enzymes have been characterized that in principle can form cardiolipin from monolysocardiolipin and acyl-CoA. One enzyme is a 74 kDa protein that was purified from pig liver mitochondria (75), and the other one is a 44 kDa protein that was identified in the mouse genome and that was shown by expression studies to catalyze acyl-CoA-dependent reacylation of monolyso- and dilysocardiolipin (76). Because the 44 kDa enzyme is associated with the endoplasmic reticulum, it is probably identical to an activity that was described by Eichberg >30 years ago (77). However, for neither enzyme has a convincing analysis of substrate specificity been presented, which leaves in doubt the actual physiologic function of these enzymes. Hence, the question remains whether they are specific for cardiolipin or they are involved in the reacylation of other lysophospholipids. This question is made more pressing by the fact that the 44 kDa enzyme is localized outside the mitochondria. Neither the 44 kDa nor the 74 kDa enzyme displays an acyl specificity that is even remotely consistent with the fatty acid patterns of cardiolipin in mammalian tissues (74–77). In general, acyltransferases may have broad substrate specificity; for instance, the yeast lysophosphatidylethanolamine acyltransferase was shown to also acylate lysophosphatidic acid (78).
Further studies in isolated liver mitochondria suggested that phospholipid transacylation may be the mechanism of cardiolipin remodeling (79). The transacylase in question was later identified to be tafazzin (71). Because tafazzin mutations cause severe derangements of the cardiolipin species patterns in humans (63, 80–82), yeast (83, 84), and fruit flies (85), it has been widely accepted that tafazzin is in fact involved in cardiolipin remodeling. In all tafazzin mutants, the dominant cardiolipin species were replaced by a large multitude of minor species, suggesting that acyl-specific remodeling gave way to random acyl substitution. This further supports the notion that tafazzin is specifically required to maintain structural uniformity among cardiolipin species.
The role of tafazzin
Enzymatic function
Tafazzin is encoded by the gene G4.5, which carries mutations responsible for Barth syndrome, and was named after a comic character from Italian television (86). Vreken et al. (87) found that tafazzin mutations lead to reduced incorporation of linoleic acid into cardiolipin of human fibroblasts. Eventually, my laboratory demonstrated that purified tafazzin catalyzes the general reaction PLA + LPLB ↔ LPLA + PLB, where PL and LPL represent phospholipids and lysophospholipids with head groups A and B, respectively (71). Although the enzyme shows certain head group preferences in vitro, it appears in principle to react with all phospholipids. Neither CoA nor acyl-CoA is required for the reaction. The acyl group is transferred directly from the phospholipid to the lysophospholipid, rather than forming an acyl enzyme intermediate, because free fatty acids are not released in the absence of an acyl acceptor (71). In contrast, the formation of an acylated enzyme is the transacylation mechanism of phospholipases A. Tafazzin is not homologous to phospholipases but belongs to a different class of enzymes, namely the acyltransferase superfamily (88). Proteins in this family typically contain an HX4D motif that is thought to play a key role in the catalytic mechanism (89).
The HX4D group was proposed to act as a charge relay that abstracts a proton from the free hydroxyl group of the glycerol moiety, enabling this hydroxyl group to engage an acyl ester bond. This is the likely mechanism by which acyl groups are transferred from the thioester bond of acyl-CoA to the hydroxyl ester bond of phospholipids in the catalytic cycle of acyl-CoA-dependent acyltransferases (90). This mechanism may also apply to tafazzin, in which case the acyl group in transit would be under the simultaneous influence of two hydroxyl groups and the HX4D charge relay (Fig. 7). Whichever structure the catalytic intermediate has, it must possess internal symmetry to account for the fact that the reaction may proceed in either direction. For instance, it is easy to see from Fig. 7 that the intermediate may react to become either a phospholipid with head group A and a lysophospholipid with head group B or vice versa. Reversibility also demands symmetry of the substrate sites, because the phospholipid binding site may become the lysophospholipid binding site and vice versa. Thus, the enzyme must have two identical substrate sites, which raises the question whether its active form is a dimer. Although there is no direct evidence to support this idea, a dimeric nature of yeast tafazzin is at least consistent with its migration behavior in nondenaturing gel electrophoresis (91).
Fig. 7.
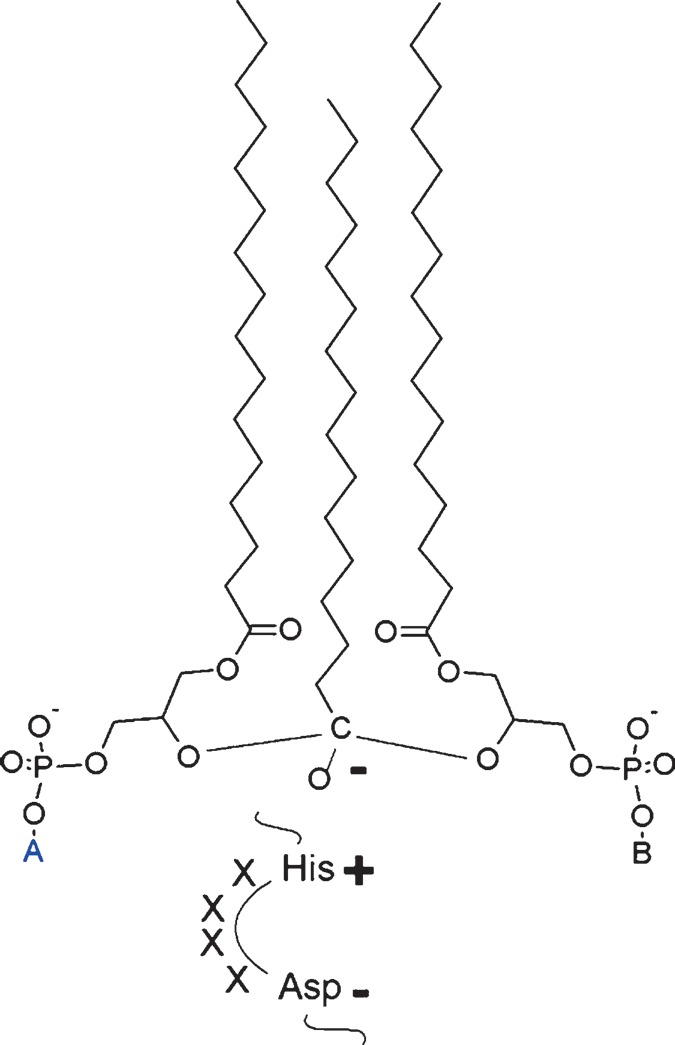
Hypothetical structure of the catalytic intermediate at the active site of tafazzin. In this model, one acyl group is under the simultaneous influence of two lysophospholipid hydroxyl groups. The structure is stabilized by a histidine-aspartate charge relay in accordance with the current model of acyltransferase catalysis. A and B are phospholipid head groups. The catalytic process may progress to form either phospholipid A and lysophospholipid B or lysophospholipid A and phospholipid B.
Another important question concerns the regiospecificity of tafazzin, because the complete remodeling of cardiolipin requires the turnover of both sn-1 and sn-2 acyl residues. Recently, we found that tafazzin is equally active with the substrates sn-1-monolysocardiolipin and sn-2-monolysocardiolipin (unpublished data), suggesting that tafazzin is able to remodel all fatty acyl residues of cardiolipin. Because tafazzin can also transfer acyl groups from cardiolipin (CL) to monolysocardiolipin (MLCL), it can essentially function as a positional isomerase: CL + 1-MLCL ↔ 2-MLCL + CL
Role in cardiolipin remodeling
It is clear from the above that tafazzin plays a central role in cardiolipin remodeling and that it may catalyze several transacylation reactions, which may be used for cardiolipin remodeling (Fig. 8). These reactions differ from the deacylation-reacylation cycle (Lands cycle), which is the remodeling pathway for other phospholipids. Whereas the Lands cycle consists of two largely irreversible steps, catalyzed by phospholipase A2 and acyl-CoA-dependent acyltransferase, respectively, cardiolipin remodeling is a near-equilibrium chemical reaction catalyzed by a single enzyme, tafazzin. However, phospholipids that participate in the transacylation equilibrium may at the same time be substrates of phospholipase A2, and lysophospholipids may be substrates of other acyltransferases. In that sense, the Lands cycle may be involved indirectly in cardiolipin remodeling, because it provides the necessary turnover of fatty acids (Fig. 8).
Fig. 8.
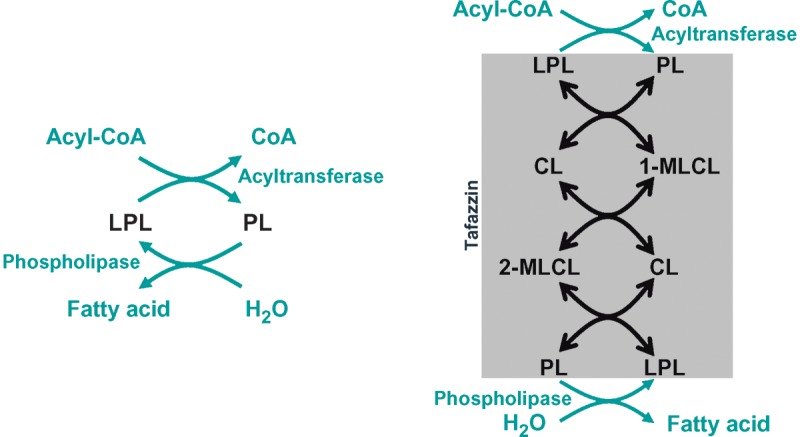
Remodeling pathways. Left: Phospholipid remodeling by the deacylation-reacylation cycle. Right: Cardiolipin remodeling by phospholipid transacylation. See text for details. CL, cardiolipin; LPL, lysophospholipid; MLCL, monolysocardiolipin; PL, phospholipid.
The notion of transacylation raises questions with regard to acyl specificity. Although previous studies have shown remarkable specificity in tafazzin-catalyzed transacylations (71, 79), the idea that tafazzin determines the fatty acid profile of cardiolipin is conceptually problematic and contradicts experimental evidence. First, the reversible nature of the tafazzin reaction makes it difficult to envision the selection of molecular species based on enzyme specificity. Second, the apparent acyl specificity of tafazzin seems to depend on the environment of the enzyme. For instance, the expression of human tafazzin in yeast prompts the formation of yeast cardiolipin rather than human cardiolipin (83). However, it is possible that acyl specificity arises as a consequence of the chemical equilibrium between molecular species, because tafazzin may allow those species to accumulate that make the least contribution to the overall free energy of the membrane. Further details of this concept will be presented in a future study.
ASSEMBLY OF CARDIOLIPIN INTO FUNCTIONAL MEMBRANES
After de novo synthesis and remodeling, cardiolipin has to be assembled into various membrane subcompartments and protein complexes. Very little is known about this process and the biological function of cardiolipin in general, partly because it has been difficult to pinpoint a single effect of cardiolipin that could be regarded as absolutely essential. However, cardiolipin induces a number of changes in the physical properties of membranes, some of which may be key to understanding its biological function. For instance, even small amounts of cardiolipin decrease the lateral interaction within the monolayer leaflet, which decreases the energy required to stretch the membrane and therefore could favor the creation of membrane folds (92). Also, cardiolipin has the ability to form clusters (93) and nonbilayer structures (5–7). The potential significance of nonbilayer structures is supported by the absolute requirement for phosphatidylethanolamine, another phospholipid with nonbilayer properties, in cardiolipin-deficient yeast (94). The ability of cardiolipin to trap protons (Fig. 2), may have implications for the distribution of the proton-motive force in energy-converting membranes. Finally, cardiolipin interacts strongly with many different proteins, which is perhaps its most important property. The structural basis of these physical interactions is not clearly understood, but it seems reasonable to consider structural symmetry (3), charge distribution (4), and the presence of a bulky hydrophobic tail in combination with a small immobilized head group (8, 95) as key factors.
Assembly of bacterial membranes
Bacterial membranes contain low steady-state levels of cardiolipin, which increase only during the stationary growth phase and under certain conditions of environmental stress (18–22). The increase in cardiolipin formation seems to be driven by higher expression of cardiolipin synthase (23, 24). Recent studies confirmed that bacteria with cardiolipin synthase deficiency are more vulnerable to osmotic stress and organic solvents (96, 97). The mechanism by which cardiolipin stabilizes bacterial membranes is not known, but an interesting observation was made recently, namely that cardiolipin localizes to the polar and septal regions of the cytoplasmic membrane (98–100). This localization of cardiolipin may help to maintain the proper spatial segregation of proteins, including the osmosensory transporter that was also shown to localize to the poles (100). The accumulation of cardiolipin at the bacterial poles can be explained on the basis of lipid self-organization, because cardiolipin clusters have a high intrinsic curvature and therefore have lower energy when they are located in curved membrane regions (101). Thus, the localization of cardiolipin to bacterial poles may be the result of its spontaneous tendency to form homogeneous clusters.
Assembly of mitochondrial membranes
Transport of cardiolipin within mitochondria
Although the de novo synthesis of cardiolipin occurs in the inner leaflet of the inner mitochondrial membrane (53), subsequent remodeling must take place at a site where tafazzin is localized. In yeast, tafazzin was shown to be integrated into the outer leaflet of the inner membrane and the inner leaflet of the outer membrane (102). Thus, cardiolipin must be translocated from the inner to the outer leaflet of the inner membrane for remodeling to occur. Finally, cardiolipin must be moved to its various destinations, which include both leaflets of the inner membrane and also to some extent the outer membrane, where small amounts of cardiolipin have been found. This implies that there must be mechanisms in place that allow the transfer of cardiolipin both across the inner membrane and between the inner and the outer membranes. Two mechanisms have been identified that could, at least in theory, facilitate cardiolipin translocation. First, phospholipid scramblase-3 has been shown to promote the accumulation of cardiolipin in the outer mitochondrial membrane (103, 104). It is possible that this is a result of cardiolipin translocation from the inner to the outer leaflet of the inner membrane, because scramblases are generally known to facilitate the transmembrane movement of phospholipids. Second, mitochondrial creatine kinase and nucleoside diphosphate kinase, two enzymes of the intermembrane space, have been shown to transfer lipids between membranes (105). This activity is cardiolipin-dependent and, in the case of creatine kinase, requires an octameric aggregation state. At the same time, creatine kinase induces the formation of cardiolipin clusters (106). Because these kinases are part of the contact sites between the inner and the outer membranes (107), it is possible that they play a role in intermembrane lipid transfer in vivo.
Assembly of cardiolipin into protein complexes
In mitochondria, a certain portion of cardiolipin is bound to proteins (108), and in some cases, like that of the ADP-ATP carrier, cardiolipin is essential for the stability of the quaternary protein structure (109, 110). Therefore, the questions arise how cardiolipin is incorporated into these protein complexes and which role cardiolipin plays in the overall process of complex assembly. In this regard, it is helpful to remember the ubiquitous nature of cardiolipin-protein interactions (i.e., the fact that many structurally unrelated proteins are able to engage in a strong binding with cardiolipin) (6). Thus, the structure of cardiolipin must provide a flexible force field that can adapt to a variety of protein surfaces. In that sense, cardiolipin behaves similar to molecular chaperones. A general role of lipids in the folding of proteins has been proposed (111), and cardiolipin specifically has been shown to promote the folding of the mitochondrial matrix enzyme rhodanese (112).
Further insight into the nature of cardiolipin-protein interactions may be derived from crystal structures. Tightly bound cardiolipin has been identified in crystals of mitochondrial complex III (113), complex IV (114), and the ADP-ATP carrier (115) as well as in crystallized prokaryotic proteins, such as the photoreaction center (116), the trimeric formate dehydrogenase N (117), and succinate dehydrogenase (118). In all of these protein crystals, the head group of cardiolipin forms strong hydrophilic interactions with a number of amino acid residues, involving electrostatic forces, hydrogen bonds, and water molecules. The acyl chains, however, remain flexible and interact with the protein surface by van der Waals forces at multiple sites. Cardiolipin sits typically at monomer interfaces of oligomeric assemblies and appears to mediate the contact between two monomers or the contact between the protein surface and the bilayer. In complex III, the cardiolipin head group was suggested to be an integral part of the proton uptake pathway, implying its direct participation in catalysis (113). The ability of cardiolipin to interact with proteins also seems to play a role in the formation of supercomplexes, because cardiolipin deficiency decreases the stability of these large supramolecular aggregates (119–122).
The question of whether certain molecular species of cardiolipin are preferred for protein binding has been addressed in a purified preparation of the ADP-ATP carrier (68) and in crystallized complex IV (114). Although tetralinoleoyl-cardiolipin was the sole species in complex IV and the dominant species in the ADP-ATP carrier, an absolute requirement for symmetric cardiolipin cannot be postulated, because these proteins remain active in tafazzin mutants, in which symmetric cardiolipin virtually disappears (123, 124).
MODIFICATION AND DEGRADATION OF CARDIOLIPIN
Like any other biological compound, cardiolipin requires continuous turnover to maintain the steady state. Very few details are known about the degradation of cardiolipin, except during apoptosis, when the cardiolipin concentration declines rapidly. A large number of research papers have dealt with the issue of cardiolipin in apoptosis, but true conceptual progress has been hampered by inadequate experimental techniques and careless overinterpretations. Nevertheless, a few consistent facts have emerged over the years, which highlight an important new role of cardiolipin in programmed cell death. In addition, cardiolipin can be hydrolyzed by phospholipases, and this too may have intriguing functional implications.
Modification of cardiolipin by oxidation
The cellular content of cardiolipin decreases acutely in various models of apoptosis (125), ischemia (126), and ischemia/reperfusion (127). The decline in mitochondrial cardiolipin was considered important because it correlated with the release of the apoptotic trigger cytochrome c from the mitochondrial compartment (125, 126, 128). Because cytochrome c is known to be attached to cardiolipin on the outer face of the inner membrane, these data suggested that the mobilization of cytochrome c is caused directly by cardiolipin deficiency. This idea was supported by a completely different approach, in which the reduction of cardiolipin by RNA interference knockdown of cardiolipin synthase resulted in the detachment of cytochrome c from the inner mitochondrial membrane (129). The mechanism of cardiolipin degradation during apoptosis and ischemia was felt to be the result of oxidative damage, partly because a similar combination of cardiolipin decrease and cytochrome c release could be achieved when mitochondria were forced to produce reactive oxygen species (130) and partly because cardiolipin hydroperoxide was detected in apoptotic cells (131). Eventually, the specific formation of oxidized cardiolipin in response to apoptotic stimulation was demonstrated by mass spectrometry, and it was shown that this oxidation is catalyzed by the peroxidase activity of cardiolipin-bound cytochrome c (125).
Hence, cardiolipin oxidation is a critical step in apoptosis, as it sets cytochrome c free into the intermembrane space. However, for cytochrome c to be released into the cytosol, where it can trigger the caspase cascade, additional steps are required, including mitochondrial fragmentation, cristae remodeling, and outer membrane permeabilization (132). To what extent cardiolipin is involved in outer membrane permeabilization and whether it interacts specifically with Bcl-2 proteins have remained open questions (for review, see Ref. 133). It is also unclear whether oxidation is a requirement for the apparent redistribution of cardiolipin during apoptosis, which results in gradual mass transfer toward the mitochondrial periphery (i.e., from the inner leaflet of the inner membrane to the outer membrane) (125). Furthermore, the chemical structure of oxidatively modified cardiolipin is still unknown. We have resolved homologous series of oxidized cardiolipins by mass spectrometry, but only after in vitro oxidation of pure cardiolipin (134). Despite all the excitement about apoptosis-induced oxidation of cardiolipin, it is important to realize that this is probably not a universal phenomenon, because cardiolipins from many mitochondria contain primarily saturated or monounsaturated acyl chains, which are very resistant to oxidative modification (Table 1).
Degradation of cardiolipin by phospholipases
Phospholipase A
Mitochondria contain members of the families of calcium-independent phospholipases A2 (iPLA2), like iPLA2β and iPLA2γ (135, 136), and cytosolic phospholipases A2 (cPLA2), like cPLA2β3 (137), which in theory are able to deacylate cardiolipin to monolysocardiolipin and dilysocardiolipin. Further deacylation probably requires a lysophospholipase. The idea that deacylation is in fact a possible fate of cardiolipin has been supported by labeling experiments in isolated mitochondria (61) and by the detection of monolysocardiolipin in tafazzin-deficient tissues (84, 138). Although monolysocardiolipin may be an intermediate of the remodeling pathway, it may also be an intermediate of the degradation pathway. Monolysocardiolipin accumulates in some models of apoptosis, for instance in Fas-treated liver (139) and in tBid-treated isolated mitochondria (140), which is consistent with the hypothesis that oxidized cardiolipin either undergoes spontaneous deacylation or is more susceptible to phospholipase A2. Of course, it is also possible that phospholipase A2 is activated directly during apoptosis, without requiring cardiolipin oxidation. Together, these data support the notion that the degradation of cardiolipin occurs by deacylation and that this pathway is activated during apoptosis and certain other conditions.
Phospholipase D
Another reaction that can degrade cardiolipin is catalyzed by phospholipase D. Recently, a novel phospholipase D was discovered that appeared to be specific for cardiolipin and that promoted mitochondrial fusion (141). The active enzyme forms a dimer at the outer surface of the outer mitochondrial membrane, with the catalytic site far away from the membrane surface, so that it has access to the outer membrane of an opposing mitochondrion. Membrane fusion may be facilitated by phosphatidic acid, a product of cardiolipin hydrolysis by phospholipase D. A rationale for the cardiolipin specificity of this enzyme is provided by its homology to bacterial cardiolipin synthases (141). It can be seen from Fig. 4 that bacterial cardiolipin synthase and phospholipase D share similar reaction sequences, which differ only in the use of either water or glycerol as the phosphatidyl acceptor. Mitochondrial phospholipase D is an intriguing enzyme that may link the fission-fusion equilibrium of mitochondria to the metabolism of cardiolipin.
CONCLUSION AND OUTLOOK
Once considered an obscure minor phospholipid, cardiolipin has now attracted the curiosity of researchers from diverse fields, including membrane biogenesis, lipid-protein interaction, apoptosis, lipidomics, and mitochondrial physiology. The exponential increase of publications on cardiolipin provides impressive evidence for the new interest in this subject. I have tried to give an overview on the current knowledge of the life cycle of cardiolipin. For the sake of clarity, I have focused on what I consider the most compelling data and have not attempted an encyclopedic review of the literature. I believe that cardiolipin research is in the midst of an evolution that promises to give new insight into fundamental questions of cell biology. There is, first, the process of cardiolipin remodeling, which to date has yielded surprising results likely to expand our concept of how phospholipids acquire a particular composition of molecular species. Then, there is the strong, but nonspecific, interaction of cardiolipin with proteins, for which a satisfying explanation has yet to be found. Most importantly, however, cardiolipin may hold the key to a better understanding of the mitochondrial ultrastructure, physiology, and biogenesis and of the role that mitochondria play in cell differentiation and cell death. This, in turn, may give unexpected insight into human disease processes, such as Barth syndrome, cardiomyopathies in general, and perhaps the metabolic syndrome.
Acknowledgments
Dr. Ashim Malhotra created Fig. 6.
Published, JLR Papers in Press, December 12, 2007.
Footnotes
Research in the author's laboratory has been supported by grants from the National Institutes of Health (Grant R01 HL-078788), the American Heart Association (Grant 0350126N), and the Barth Syndrome Foundation.
References
- 1.LeCocq J., and C. E. Ballou. 1964. On the structure of cardiolipin. Biochemistry. 3 976–980. [DOI] [PubMed] [Google Scholar]
- 2.Powell G. L., and J. Jacobus. 1974. The nonequivalence of the phosphorus atoms in cardiolipin. Biochemistry. 13 4024–4026. [DOI] [PubMed] [Google Scholar]
- 3.Schlame M., M. Ren, Y. Xu, M. L. Greenberg, and I. Haller. 2005. Molecular symmetry in mitochondrial cardiolipins. Chem. Phys. Lipids. 138 38–49. [DOI] [PubMed] [Google Scholar]
- 4.Kates M., J-Y. Syz, D. Gosser, and T. H. Haines. 1993. pH-dissociation characteristics of cardiolipin and its 2′-deoxy analogue. Lipids. 28 877–882. [DOI] [PubMed] [Google Scholar]
- 5.Dahlberg M. 2007. Polymorphic phase behavior of cardiolipin derivatives studied by coarse-grained molecular dynamics. J. Phys. Chem. 111 7194–7200. [DOI] [PubMed] [Google Scholar]
- 6.Schlame M., D. Rua, and M. L. Greenberg. 2000. The biosynthesis and functional role of cardiolipin. Prog. Lipid Res. 39 257–288. [DOI] [PubMed] [Google Scholar]
- 7.Powell G. L., and D. Marsh. 1985. Polymorphic phase behavior of cardiolipin derivatives studied by 31P NMR and X-ray diffraction. Biochemistry. 24 2902–2908. [DOI] [PubMed] [Google Scholar]
- 8.Allegrini P. R., G. Pluschke, and J. Seelig. 1984. Cardiolipin conformation and dynamics in bilayer membranes as seen by deuterium magnetic resonance. Biochemistry. 23 6452–6458. [Google Scholar]
- 9.Dowhan W. 1997. Molecular basis for membrane phospholipid diversity: why are there so many lipids? Annu. Rev. Biochem. 66 199–232. [DOI] [PubMed] [Google Scholar]
- 10.Daum G. 1985. Lipids of mitochondria. Biochim. Biophys. Acta. 822 1–42. [DOI] [PubMed] [Google Scholar]
- 11.De Andrade Rosa I., M. Einicker-Lamas, R. R. Bernardo, L. M. Previatto, R. Mohana-Borges, J. A. Morgado-Diaz, and M. Benchimol. 2006. Cardiolipin in hydrogenosomes: evidence of symbiotic origin. Eukaryot. Cell. 5 784–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin W., M. Hoffmeister, C. Rotte, and K. Henze. 2001. An overview of endosymbiotic models for the origins of eukaryotes, their ATP-producing organelles (mitochondria and hydrogenosomes), and their heterotrophic lifestyle. Biol. Chem. 382 1521–1539. [DOI] [PubMed] [Google Scholar]
- 13.Corcelli A., M. Colella, G. Mascolo, F. P. Fanizzi, and M. Kates. 2000. A novel glycolipid and phospholipid in the purple membrane. Biochemistry. 39 3318–3326. [DOI] [PubMed] [Google Scholar]
- 14.Lattanzio V. M. T., A. Corcelli, G. Mascolo, and A. Oren. 2002. Presence of two novel cardiolipins in the halophilic archeal community in the crystallizer brines from the salterns of Margherita di Savoia (Italy) and Eliat (Israel). Extremophiles. 6 437–444. [DOI] [PubMed] [Google Scholar]
- 15.Fischer W. 1977. The polar lipids of group B streptococci. I. Glucosylated diphosphatidylglycerol, a novel glycophospholipid. Biochim. Biophys. Acta. 487 74–88. [DOI] [PubMed] [Google Scholar]
- 16.Fischer W., and D. Arneth-Seifert. 1998. d-Alanylcardiolipin, a major component of the unique lipid pattern of Vagococcus fluvialis. J. Bacteriol. 180 2093–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peter-Katalinic J., and W. Fischer. 1998. α-d-Glucopyranosyl-, d-alanyl- and l-lysylcardiolipin from Gram-positive bacteria: analysis by fast atom bombardment mass spectrometry. J. Lipid Res. 39 2286–2292. [PubMed] [Google Scholar]
- 18.Shibuya I. 1992. Metabolic regulations and biological functions of phospholipids in Escherichia coli. Prog. Lipid Res. 31 245–299. [DOI] [PubMed] [Google Scholar]
- 19.Short S. A., and D. C. White. 1971. Metabolism of phosphatidylglycerol, lysophosphatidyl-glycerol and cardiolipin of Staphylococcus aureus. J. Bacteriol. 108 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koch H. U., R. Haas, and W. Fischer. 1984. The role of lipoteichoic acid biosynthesis in membrane lipid metabolism of growing Staphylococcus aureus. Eur. J. Biochem. 138 357–363. [DOI] [PubMed] [Google Scholar]
- 21.Kanemasa Y., T. Yioshioka, and H. Hayashi. 1972. Alteration of the phospholipid composition of Staphylococcus aureus cultures in medium containing NaCl. Biochim. Biophys. Acta. 280 444–450. [PubMed] [Google Scholar]
- 22.Catucci L., N. Depalo, V. M. T. Lattanzio, A. Agostiano, and A. Corcelli. 2004. Neosynthesis of cardiolipin in Rhodobacter sphaeroides under osmotic stress. Biochemistry. 43 15066–15072. [DOI] [PubMed] [Google Scholar]
- 23.Heber S., and B. E. Tropp. 1991. Genetic regulation of cardiolipin synthase in Escherichia coli. Biochim. Biophys. Acta. 1129 1–12. [DOI] [PubMed] [Google Scholar]
- 24.Hiraoka S., H. Matsuzaki, and I. Shibuya. 1993. Active increase in cardiolipin synthesis in the stationary phase and its physiological significance in Escherichia coli. FEBS Lett. 336 221–224. [DOI] [PubMed] [Google Scholar]
- 25.De Siervo A. J., and M. R. J. Salton. 1971. Biosynthesis of cardiolipin in the membranes of Micrococcus lysodeikticus. Biochim. Biophys. Acta. 239 280–292. [DOI] [PubMed] [Google Scholar]
- 26.Hirschberg C. B., and E. P. Kennedy. 1972. Mechanism of enzymatic synthesis of cardiolipin in Escherichia coli. Proc. Natl. Acad. Sci. USA. 69 648–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pluschke G., Y. Hirota, and P. Overath. 1978. Function of phospholipids in Escherichia coli. Characterization of a mutant deficient in cardiolipin synthesis. J. Biol. Chem. 253 5048–5055. [PubMed] [Google Scholar]
- 28.Ohta A., T. Obara, Y. Asami, and I. Shibuya. 1985. Molecular cloning of the cls gene responsible for cardiolipin synthesis in Escherichia coli and phenotypic consequences of its amplification. J. Bacteriol. 163 506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shibuya I., and S. Hiraoka. 1992. Cardiolipin synthase from Escherichia coli. Methods Enzymol. 209 321–330. [DOI] [PubMed] [Google Scholar]
- 30.Ragolia L., and B. E. Tropp. 1994. The effects of phosphoglycerides on Escherichia coli cardiolipin synthase. Biochim. Biophys. Acta. 1214 323–332. [DOI] [PubMed] [Google Scholar]
- 31.Tropp B. E. 1997. Cardiolipin synthase from Escherichia coli. Biochim. Biophys. Acta. 1348 192–200. [DOI] [PubMed] [Google Scholar]
- 32.Waite M. 1999. The PLD superfamily: insight into catalysis. Biochim. Biophys. Acta. 1439 187–197. [DOI] [PubMed] [Google Scholar]
- 33.Shibuya I., S. Yamagoe, C. Miyazaki, H. Matsuzaki, and A. Ohta. 1985. Biosynthesis of novel acidic phospholipid analogs in Escherichia coli. J. Bacteriol. 161 473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiraoka S., K. Nukui, N. Uetake, A. Ohta, and I. Shibuya. 1991. Amplification and substantial purification of cardiolipin synthase of Escherichia coli. J. Biochem. 110 443–449. [DOI] [PubMed] [Google Scholar]
- 35.Hostetler K. Y., H. van den Bosch, and L. L. M. van Deenen. 1971. Biosynthesis of cardiolipin in liver mitochondria. Biochim. Biophys. Acta. 239 113–119. [DOI] [PubMed] [Google Scholar]
- 36.Hostetler K. Y., H. van den Bosch, and L. L. M. van Deenen. 1972. The mechanism of cardiolipin biosynthesis in liver mitochondria. Biochim. Biophys. Acta. 260 507–513. [DOI] [PubMed] [Google Scholar]
- 37.Tamai K. T., and M. L. Greenberg. 1990. Biochemical characterization and regulation of cardiolipin synthase in Saccharomyces cerevisiae. Biochim. Biophys. Acta. 1046 214–222. [DOI] [PubMed] [Google Scholar]
- 38.Schlame M., S. Brody, and K. Y. Hostetler. 1993. Mitochondrial cardiolipin in diverse eukaryotes. Comparison of biosynthetic reactions and molecular species. Eur. J. Biochem. 212 727–735. [DOI] [PubMed] [Google Scholar]
- 39.Jiang F., H. S. Rizavi, and M. L. Greenberg. 1997. Cardiolipin is not essential for the growth of Saccharomyces cerevisiae on fermentable or non-fermentable carbon sources. Mol. Microbiol. 26 481–491. [DOI] [PubMed] [Google Scholar]
- 40.Tuller G., C. Hrastnik, G. Achleitner, U. Schiefthaler, F. Klein, and G. Daum. 1998. YDL142c encodes cardiolipin synthase (Cls1p) and is non-essential for aerobic growth of Saccharomyces cerevisiae. FEBS Lett. 421 15–18. [DOI] [PubMed] [Google Scholar]
- 41.Chang S. C., P. N. Heacock, E. Mileykovskaya, D. R. Voelker, and W. Dowhan. 1998. Isolation and characterization of the gene (CLS1) encoding cardiolipin synthase in Saccharomyces cerevisiae. J. Biol. Chem. 273 14933–14941. [DOI] [PubMed] [Google Scholar]
- 42.Katayama K., I. Sakurai, and H. Wada. 2004. Identification of an Arabidopsis thaliana gene for cardiolipin synthase located in mitochondria. FEBS Lett. 577 193–198. [DOI] [PubMed] [Google Scholar]
- 43.Nowicki M., F. Müller, and M. Frentzen. 2005. Cardiolipin synthase of Arabidopsis thaliana. FEBS Lett. 579 2161–2165. [DOI] [PubMed] [Google Scholar]
- 44.Houtkooper R. H., H. Akbari, H. van Lenthe, W. Kulik, R. J. A. Wanders, M. Frentzen, and F. M. Vaz. 2006. Identification and characterization of human cardiolipin synthase. FEBS Lett. 580 3059–3064. [DOI] [PubMed] [Google Scholar]
- 45.Lu B., F. Y. Xu, Y. J. Jiang, P. C. Choy, G. M. Hatch, C. Grunfeld, and K. R. Feingold. 2006. Cloning and characterization of a cDNA encoding human cardiolipin synthase (hCLS1). J. Lipid Res. 47 1140–1145. [DOI] [PubMed] [Google Scholar]
- 46.Chen D., X-Y. Zhang, and Y. Shi. 2006. Identification and functional characterization of hCLS1, a human cardiolipin synthase localized in mitochondria. Biochem. J. 398 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlame M., and K. Y. Hostetler. 1991. Solubilization, purification, and characterization of cardiolipin synthase from rat liver mitochondria. Demonstration of its phospholipid requirement. J. Biol. Chem. 266 22398–22403. [PubMed] [Google Scholar]
- 48.Schlame M., M. Zhao, D. Rua, D. Haldar, and M. L. Greenberg. 1995. Kinetic analysis of cardiolipin synthase: a membrane enzyme with two glycerophospholipid substrates. Lipids. 30 633–640. [DOI] [PubMed] [Google Scholar]
- 49.Poorthuis B. J. H. M., and K. Y. Hostetler. 1976. Studies on nucleotide diphosphate diacylglycerol specificity of acidic phospholipid biosynthesis in rat liver subcellular fractions. Biochim. Biophys. Acta. 431 408–415. [DOI] [PubMed] [Google Scholar]
- 50.Hostetler K. Y., J. M. Galesloot, P. Boer, and H. van den Bosch. 1975. Further studies on the formation of cardiolipin and phosphatidylglycerol in rat liver mitochondria. Effect of divalent cations and the fatty acid composition of CDP-diglyceride. Biochim. Biophys. Acta. 380 382–389. [DOI] [PubMed] [Google Scholar]
- 51.McMurray W. C., and E. C. Jarvis. 1980. Partial purification of diphosphatidylglycerol synthase from rat liver mitochondrial membranes. Can. J. Biochem. 58 771–776. [DOI] [PubMed] [Google Scholar]
- 52.Hostetler K. Y., and H. van den Bosch. 1972. Subcellular and submitochondrial localization of the biosynthesis of cardiolipin and related phospholipids in rat liver. Biochim. Biophys. Acta. 260 380–386. [DOI] [PubMed] [Google Scholar]
- 53.Schlame M., and D. Haldar. 1993. Cardiolipin is synthesized on the matrix side of the inner membrane in rat liver mitochondria. J. Biol. Chem. 268 74–79. [PubMed] [Google Scholar]
- 54.Zhao M., M. Schlame, D. Rua, and M. L. Greenberg. 1998. Cardiolipin synthase is associated with a large complex in yeast mitochondria. J. Biol. Chem. 273 2402–2408. [DOI] [PubMed] [Google Scholar]
- 55.Paradies G., and F. M. Ruggiero. 1988. Effect of hyperthyroidism on the transport of pyruvate in rat heart. Biochim. Biophys. Acta. 935 79–86. [DOI] [PubMed] [Google Scholar]
- 56.Cao S. G., P. Cheng, A. Angel, and G. M. Hatch. 1995. Thyroxine stimulates phosphatidylglycerolphosphate synthase activity in rat heart mitochondria. Biochim. Biophys. Acta. 1256 241–244. [DOI] [PubMed] [Google Scholar]
- 57.Paradies G., and F. M. Ruggiero. 1990. Stimulation of phosphate transport in rat liver mitochondria by thyroid hormones. Biochim. Biophys. Acta. 1019 133–136. [DOI] [PubMed] [Google Scholar]
- 58.Nakashima-Kamimura N., S. Asoh, Y. Ishibashi, Y. Mukai, Y. Shidara, H. Oda, K. Munakata, Y. Goto, and S. Ohta. 2005. MIDAS/GPP34, a nuclear gene product, regulates total mitochondrial mass in response to mitochondrial dysfunction. J. Cell Sci. 118 5357–5367. [DOI] [PubMed] [Google Scholar]
- 59.Gohil V. M., P. Hayes, S. Matsuyama, H. Schagger, M. Schlame, and M. L. Greenberg. 2004. Cardiolipin biosynthesis and mitochondrial respiratory chain function are interdependent. J. Biol. Chem. 279 42612–42618. [DOI] [PubMed] [Google Scholar]
- 60.Li G., S. Chen, M. N. Thompson, and M. L. Greenberg. 2007. New insights into the regulation of cardiolipin biosynthesis in yeast: implications for Barth syndrome. Biochim. Biophys. Acta. 1771 432–441. [DOI] [PubMed] [Google Scholar]
- 61.Schlame M., and B. Rustow. 1990. Lysocardiolipin formation and reacylation in isolated rat liver mitochondria. Biochem. J. 272 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rustow B., M. Schlame, H. Rabe, G. Reichmann, and D. Kunze. 1989. Species pattern of phosphatidic acid, diacylglycerol, CDP-diacylglycerol and phosphatidylglycerol synthesized de novo in rat liver mitochondria. Biochim. Biophys. Acta. 1002 261–263. [DOI] [PubMed] [Google Scholar]
- 63.Schlame M., J. A. Towbin, P. M. Heerdt, R. Jehle, S. DiMauro, and T. J. J. Blanck. 2002. Deficiency of tetralinoleoyl-cardiolipin in Barth syndrome. Ann. Neurol. 51 634–637. [DOI] [PubMed] [Google Scholar]
- 64.Han X., J. Yang, K. Yang, Z. Zhao, D. R. Abendschein, and R. W. Gross. 2007. Alterations in myocardial cardiolipin content and composition occur at the very earliest stages of diabetes: a shotgun lipidomics study. Biochemistry. 46 6417–6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sparagna G. C., A. J. Chicco, R. C. Murphy, M. R. Bristow, C. A. Johnson, M. L. Rees, M. L. Maxey, S. A. McCune, and R. L. Moore. 2007. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J. Lipid Res. 48 1559–1570. [DOI] [PubMed] [Google Scholar]
- 66.Ellis C. E., E. J. Murphy, D. C. Mitchell, M. Y. Golovko, F. Scaglia, G. C. Barcelo-Coblijn, and R. L. Nussbaum. 2005. Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking α-synuclein. Mol. Cell. Biol. 25 10190–10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schlame M., and M. Ren. 2006. Barth syndrome, a human disorder of cardiolipin metabolism. FEBS Lett. 580 5450–5455. [DOI] [PubMed] [Google Scholar]
- 68.Schlame M., K. Beyer, M. Hayer-Hartl, and M. Klingenberg. 1991. Molecular species of cardiolipin in relation to other mitochondrial phospholipids. Is there an acyl specificity of the interaction between cardiolipin and the ADP/ATP carrier? Eur. J. Biochem. 199 459–466. [DOI] [PubMed] [Google Scholar]
- 69.Mita M., and N. Ueta. 1989. Fatty chain composition of phospholipids in sea urchin spermatozoa. Comp. Biochem. Physiol. 92B 319–322. [DOI] [PubMed] [Google Scholar]
- 70.Wang H-Y. J., S. N. Jackson, and A. S. Woods. 2007. Direct MALDI-MS analysis of cardiolipin from rat organ sections. J. Am. Soc. Mass Spectrom. 18 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu Y., A. Malhotra, M. Ren, and M. Schlame. 2006. The enzymatic function of tafazzin. J. Biol. Chem. 281 39217–39224. [DOI] [PubMed] [Google Scholar]
- 72.Kraffe E., P. Soudant, Y. Marty, and N. Kervarec. 2005. Docosahexaenoic acid- and eicosapentaenoic acid-enriched cardiolipin in the Manila clam Ruditapes philippinarum. Lipids. 40 619–625. [DOI] [PubMed] [Google Scholar]
- 73.Kraffe E., P. Soudant, Y. Marty, N. Kervarec, and P. Jehan. 2002. Evidence of a tetradocosahexaenoic cardiolipin in some marine bivalves. Lipids. 37 507–514. [DOI] [PubMed] [Google Scholar]
- 74.Ma B. J., W. A. Taylor, V. W. Dolinsky, and G. M. Hatch. 1999. Acylation of monolysocardiolipin in rat heart. J. Lipid Res. 40 1837–1845. [PubMed] [Google Scholar]
- 75.Taylor W. A., and G. M. Hatch. 2003. Purification and characterization of monolysocardiolipin acyltransferase from pig liver mitochondria. J. Biol. Chem. 278 12716–12721. [DOI] [PubMed] [Google Scholar]
- 76.Cao J., Y. Liu, J. Lockwood, P. Burn, and Y. Shi. 2004. A novel cardiolipin-remodeling pathway revealed by a gene encoding an endoplasmic reticulum-associated acyl-CoA:lysocardiolipin acyltransferase (ALCAT1) in mouse. J. Biol. Chem. 279 31727–31734. [DOI] [PubMed] [Google Scholar]
- 77.Eichberg J. 1974. The reacylation of deacylated derivatives of diphosphatidylglycerol by microsomes and mitochondria from rat liver. J. Biol. Chem. 249 3423–3429. [PubMed] [Google Scholar]
- 78.Riekhof W. R., J. Wu, J. L. Jones, and D. R. Voelker. 2007. Identification and characterization of the major lysophosphatidylethanolamine acyltransferase in Saccharomyces cerevisiae. J. Biol. Chem. 282 28344–28352. [DOI] [PubMed] [Google Scholar]
- 79.Xu Y., R. I. Kelley, T. J. J. Blanck, and M. Schlame. 2003. Remodeling of cardiolipin by phospholipid transacylation. J. Biol. Chem. 278 51380–51385. [DOI] [PubMed] [Google Scholar]
- 80.Schlame M., R. I. Kelley, A. Feigenbaum, J. A. Towbin, P. M. Heerdt, T. Schieble, R. J. A. Wanders, S. DiMauro, and T. J. J. Blanck. 2003. Phospholipid abnormalities in children with Barth syndrome. J. Am. Coll. Cardiol. 42 1994–1999. [DOI] [PubMed] [Google Scholar]
- 81.Valianpour F., R. J. A. Wanders, H. Overmars, P. Vreken, A. H. van Gennip, F. Baas, B. Plecko, R. Santer, K. Becker, and P. G. Barth. 2002. Cardiolipin deficiency in X-linked cardioskeletal myopathy and neutropenia (Barth syndrome, MIM 302060): a study in cultured skin fibroblasts. J. Pediatr. 141 729–733. [DOI] [PubMed] [Google Scholar]
- 82.Kuijpers T. W., N. A. Maianski, A. T. J. Tool, K. Becker, B. Plecko, F. Valianpour, R. J. A. Wanders, R. Pereira, J. van Hove, A. J. Verhoeven, et al. 2004. Neutrophils in Barth syndrome (BTHS) avidly bind annexin-V in the absence of apoptosis. Blood. 103 3915–3923. [DOI] [PubMed] [Google Scholar]
- 83.Vaz F. M., R. H. Houtkooper, F. Valianpour, P. G. Barth, and R. J. A. Wanders. 2003. Only one splice variant of the human TAZ gene encodes a functional protein with a role in cardiolipin metabolism. J. Biol. Chem. 278 43089–43094. [DOI] [PubMed] [Google Scholar]
- 84.Gu Z., F. Valianpour, S. Chen, F. M. Vaz, G. A. Hakkaart, R. J. A. Wanders, and M. L. Greenberg. 2004. Aberrant cardiolipin metabolism in the yeast taz1 mutant: a model for Barth syndrome. Mol. Microbiol. 51 149–158. [DOI] [PubMed] [Google Scholar]
- 85.Xu Y., M. Condell, H. Plesken, I. Edelman-Novemsky, J. Ma, M. Ren, and M. Schlame. 2006. A Drosophila model of Barth syndrome. Proc. Natl. Acad. Sci. USA. 103 11584–11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bione S., P. D'Adamo, E. Maestrini, A. K. Gedeon, P. A. Bolhuis, and D. Toniolo. 1996. A novel X-linked gene, G4.5. is responsible for Barth syndrome. Nat. Genet. 12 385–389. [DOI] [PubMed] [Google Scholar]
- 87.Vreken P., F. Valianpour, L. G. Nijtmans, L. A. Grivell, B. Plecko, R. J. A. Wanders, and P. G. Barth. 2000. Defective remodeling of cardiolipin and phosphatidylglycerol in Barth syndrome. Biochem. Biophys. Res. Commun. 279 378–382. [DOI] [PubMed] [Google Scholar]
- 88.Neuwald A. F. 1997. Barth syndrome may be due to an acyltransferase deficiency. Curr. Biol. 7 R465–R466. [DOI] [PubMed] [Google Scholar]
- 89.Lewin T. M., P. Wang, and R. A. Coleman. 1999. Analysis of amino acid motifs diagnostic for the sn-glycerol-3-phosphate acyltransferase reaction. Biochemistry. 38 5764–5771. [DOI] [PubMed] [Google Scholar]
- 90.Heath R. J., and C. O. Rock. 1998. A conserved histidine is essential for glycerolipid acyltransferase catalysis. J. Bacteriol. 180 1425–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brandner K., D. U. Mick, A. E. Frazier, R. D. Taylor, C. Meisinger, and P. Rehling. 2005. Taz1, an outer mitochondrial membrane protein, affects stability and assembly of inner membrane protein complexes: implications for Barth syndrome. Mol. Biol. Cell. 16 5202–5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nichols-Smith S., S-Y. Teh, and T. L. Kuhl. 2004. Thermodynamic and mechanical properties of model mitochondrial membranes. Biochim. Biophys. Acta. 1663 82–88. [DOI] [PubMed] [Google Scholar]
- 93.Mileykovskaya E., W. Dowhan, R. L. Birke, D. Zheng, L. Lutterodt, and T. H. Haines. 2001. Cardiolipin binds nonyl acridine orange by aggregating the dye at exposed hydrophobic domains on bilayer surfaces. FEBS Lett. 507 187–190. [DOI] [PubMed] [Google Scholar]
- 94.Gohil V. M., M. N. Thompson, and M. L. Greenberg. 2005. Synthetic lethal interaction of the mitochondrial phosphatidylethanolamine and cardiolipin biosynthetic pathways in Saccharomyces cerevisiae. J. Biol. Chem. 280 35410–35416. [DOI] [PubMed] [Google Scholar]
- 95.Lewis R. N. A. H., D. Zweytick, G. Pabst, K. Lohner, and R. N. McElhaney. 2007. Calorimetric, X-ray diffraction, and spectroscopic studies of the thermotropic phase behavior and organization of tetramyristoyl cardiolipin membranes. Biophys. J. 92 3166–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lopez C. S., A. F. Alice, H. Heras, E. A. Rivas, and C. Sanchez-Rivas. 2006. Role of anionic phospholipids in the adaptation of Bacillus subtilis to high salinity. Microbiology. 152 605–616. [DOI] [PubMed] [Google Scholar]
- 97.Bernal P., J. Munoz-Rojas, A. Hurtado, J. L. Ramos, and A. Segura. 2007. A Pseudomonas putida cardiolipin synthesis mutant exhibits increased sensitivity to drugs related to transport functionality. Environ. Microbiol. 9 1135–1145. [DOI] [PubMed] [Google Scholar]
- 98.Mileykovskaya E., and W. Dowhan. 2000. Visualization of phospholipid domains in Escherichia coli by using the cardiolipin-specific fluorescent dye 10-N-nonyl acridine orange. J. Bacteriol. 182 1172–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kawai F., M. Shoda, R. Harashima, Y. Sadaie, H. Hara, and K. Matsumoto. 2004. Cardiolipin domains in Bacillus subtilis Marburg membranes. J. Bacteriol. 186 1475–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Romantsov T., S. Helbig, D. E. Culham, C. Gill, L. Stalker, and J. M. Wood. 2007. Cardiolipin promotes polar localization of osmosensory transporter ProP in Escherichia coli. Mol. Microbiol. 64 1455–1465. [DOI] [PubMed] [Google Scholar]
- 101.Huang, K. C., R. Mukhopadhyay, and N. S. Wingreen. A curvature-mediated mechanism for localization of lipids to bacterial poles. PLOS Comput. Biol. 2: e151. Epub ahead of print. October 4, 2006; doi: 10.1371/journal.pcbi.0020151. [DOI] [PMC free article] [PubMed]
- 102.Claypool S. M., J. M. McCaffery, and C. M. Koehler. 2006. Mitochondrial mislocalization and altered assembly of a cluster of Barth syndrome mutant tafazzins. J. Cell Biol. 174 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu J., J. Chen, Q. Dai, and R. M. Lee. 2003. Phospholipid scramblase 3 is the mitochondrial target of protein kinase C δ-induced apoptosis. Cancer Res. 63 1153–1156. [PubMed] [Google Scholar]
- 104.Liu J., Q. Dai, J. Chen, D. Durrant, A. Freeman, T. Liu, D. Grossman, and R. M. Lee. 2003. Phospholipid scramblase 3 controls mitochondrial structure, function, and apoptotic response. Mol. Cancer Res. 1 892–902. [PubMed] [Google Scholar]
- 105.Epand R. F., U. Schlattner, T. Wallimann, M-L. Lacombe, and R. M. Epand. 2007. Novel lipid transfer property of two mitochondrial proteins that bridge the inner and outer membranes. Biophys. J. 92 126–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Epand R. F., M. Tokarska-Schlattner, U. Schlattner, T. Walliman, and R. M. Epand. 2007. Cardiolipin clusters and membrane domain formation induced by mitochondrial proteins. J. Mol. Biol. 365 968–980. [DOI] [PubMed] [Google Scholar]
- 107.Adams V., W. Bosch, J. Schlegel, T. Walliman, and D. Brdiczka. 1989. Further characterization of contact sites from mitochondria of different tissues: topology of peripheral kinases. Biochim. Biophys. Acta. 981 213–225. [DOI] [PubMed] [Google Scholar]
- 108.Schlame M., L. Horvath, and L. Vigh. 1990. Relationship between lipid saturation and lipid-protein interaction in liver mitochondria modified by catalytic hydrogenation with reference to cardiolipin molecular species. Biochem. J. 265 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beyer K., and M. Klingenberg. 1985. ADP/ATP carrier protein from beef heart mitochondria has high amounts of tightly bound cardiolipin, as revealed by 31P nuclear magnetic resonance. Biochemistry. 24 3821–3826. [DOI] [PubMed] [Google Scholar]
- 110.Beyer K., and B. Nuscher. 1996. Specific cardiolipin binding interferes with labeling of sulfhydryl residues in the adenosine diphosphate/adenosine triphosphate carrier protein from beef heart mitochondria. Biochemistry. 35 15784–15790. [DOI] [PubMed] [Google Scholar]
- 111.Bogdanov M., and W. Dowhan. 1999. Lipid-assisted protein folding. J. Biol. Chem. 274 36827–36830. [DOI] [PubMed] [Google Scholar]
- 112.Zardeneta G., and P. M. Horowitz. 1993. Physical characterization of a reactivatable liposome-bound rhodanese folding intermediate. Biochemistry. 32 13941–13948. [DOI] [PubMed] [Google Scholar]
- 113.Lange C., J. H. Nett, B. L. Trumpower, and C. Hunte. 2001. Specific roles of protein-phospholipid interactions in the yeast cytochrome bc1 complex structure. EMBO J. 20 6591–6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shinzawa-Itoh K., H. Aoyama, K. Muramoto, H. Terada, T. Kurauchi, Y. Tadehara, A. Yamasaki, T. Sugimura, S. Kurono, K. Tsujimoto, et al. 2007. Structure and physiological roles of 13 integral lipids of bovine heart cytochrome c oxidase. EMBO J. 26 1713–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pebay-Peyroula E., G. Dahout, R. Kahn, V. Trezeguet, G. J. Lauquin, and G. Brandolin. 2003. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature. 426 39–44. [DOI] [PubMed] [Google Scholar]
- 116.McAuley K. E., P. K. Fyfe, J. P. Ridge, N. W. Isaacs, R. J. Cogdell, and M. R. Jones. 1999. Structural details of an interaction between cardiolipin and an integral membrane protein. Proc. Natl. Acad. Sci. USA. 96 14706–14711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jormakka M., S. Tornroth, B. Byrne, and S. Iwata. 2002. Molecular basis of proton motive force generation: structure of formate dehydrogenase N. Science. 295 1863–1868. [DOI] [PubMed] [Google Scholar]
- 118.Yankovskaya V., R. Horsefield, S. Tornroth, C. Luna-Chavez, H. Miyoshi, C. Leger, B. Byrne, G. Cecchini, and S. Iwata. 2003. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science. 299 700–704. [DOI] [PubMed] [Google Scholar]
- 119.Zhang M., E. Mileykovskaya, and W. Dowhan. 2002. Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J. Biol. Chem. 277 43553–43556. [DOI] [PubMed] [Google Scholar]
- 120.Pfeiffer K., V. Gohil, R. A. Stuart, C. Hunte, U. Brandt, M. L. Greenberg, and H. Schägger. 2003. Cardiolipin stabilizes respiratory chain supercomplexes. J. Biol. Chem. 278 52873–52880. [DOI] [PubMed] [Google Scholar]
- 121.Zhang M., E. Mileykovskaya, and W. Dowhan. 2005. Cardiolipin is essential for organization of complexes III and IV into a supercomplex in intact yeast mitochondria. J. Biol. Chem. 280 29403–29408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McKenzie M., M. Lazarou, D. R. Thorburn, and M. T. Ryan. 2006. Mitochondrial respiratory chain supercomplexes are destabilized in Barth syndrome patients. J. Mol. Biol. 361 462–469. [DOI] [PubMed] [Google Scholar]
- 123.Ma L., F. M. Vaz, Z. Gu, R. J. A. Wanders, and M. L. Greenberg. 2004. The human TAZ gene complements mitochondrial dysfunction in yeast taz1Δ mutant. Implications for Barth syndrome. J. Biol. Chem. 279 44394–44399. [DOI] [PubMed] [Google Scholar]
- 124.Xu Y., J. J. Sutachan, H. Plesken, R. I. Kelley, and M. Schlame. 2005. Characterization of lymphoblast mitochondria from patients with Barth syndrome. Lab. Invest. 85 823–830. [DOI] [PubMed] [Google Scholar]
- 125.Kagan V. E., V. A. Tyurin, I. Jiang, Y. Y. Tyurina, V. B. Ritov, A. A. Amoscato, A. N. Osipov, N. A. Belikova, A. A. Kapralov, V. Kini, et al. 2005. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 1 223–232. [DOI] [PubMed] [Google Scholar]
- 126.Lesnefsky E. J., T. J. Slabe, M. S. K. Stoll, P. E. Minkler, and C. L. Hoppel. 2001. Myocardial ischemia selectively depletes cardiolipin in rabbit heart subsarcolemmal mitochondria. Am. J. Physiol. 280 H2770–H2778. [DOI] [PubMed] [Google Scholar]
- 127.Paradies G., G. Petrosillo, M. Pistolese, N. Di Venosa, A. Federici, and F. M. Ruggiero. 2004. Decrease in mitochondrial complex I activity in ischemic/reperfused rat heart: involvement of reactive oxygen species and cardiolipin. Circ. Res. 94 53–59. [DOI] [PubMed] [Google Scholar]
- 128.Ostrander D. B., G. C. Sparagna, A. A. Amoscato, J. B. McMillin, and W. Dowhan. 2001. Decreased cardiolipin synthesis corresponds with cytochrome c release in palmitate-induced cardiomyocyte apoptosis. J. Biol. Chem. 276 38061–38067. [DOI] [PubMed] [Google Scholar]
- 129.Choi S-Y., F. Gonzalvez, G. M. Jenkins, C. Slomianny, D. Chretien, D. Arnoult, P. X. Petit, and M. A. Frohman. 2006. Cardiolipin deficiency releases cytochrome c from the inner mitochondrial membrane and accelerates stimuli-elicited apoptosis. Cell Death Differ. 14 597–606. [DOI] [PubMed] [Google Scholar]
- 130.Petrosillo G., F. M. Ruggiero, and G. Paradies. 2003. Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB J. 17 2202–2208. [DOI] [PubMed] [Google Scholar]
- 131.Nomura K., H. Imai, T. Koumura, T. Kobayashi, and Y. Nakagawa. 2000. Mitochondrial phospholipid hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia-induced apoptosis. Biochem. J. 351 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Heath-Engel H. M., and G. C. Shore. 2006. Mitochondrial membrane dynamics, cristae remodeling and apoptosis. Biochim. Biophys. Acta. 1763 549–560. [DOI] [PubMed] [Google Scholar]
- 133.Ott M., B. Zhivotovsky, and S. Orrenius. 2007. Role of cardiolipin in cytochrome c release from mitochondria. Cell Death Differ. 14 1243–1247. [DOI] [PubMed] [Google Scholar]
- 134.Schlame M., I. Haller, L. R. Sammaritano, and T. J. J. Blanck. 2001. Effect of cardiolipin oxidation on solid-phase immunoassay for antiphospholipid antibodies. Thromb. Haemost. 86 1475–1482. [PubMed] [Google Scholar]
- 135.Williams S. D., and R. A. Gottlieb. 2002. Inhibition of mitochondrial calcium-independent phospholipase A2 (iPLA2) attenuates mitochondrial phospholipid loss and is cardioprotective. Biochem. J. 362 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mancuse D. J., X. Han, C. M. Jenkins, J. J. Lehman, N. Sambandam, H. F. Sims, J. Yang, W. Yan, K. Yang, K. Green, et al. 2007. Dramatic accumulation of triglycerides and precipitation of cardiac hemodynamic dysfunction during brief caloric restriction in transgenic myocardium expressing human calcium-independent phospholipase A2γ. J. Biol. Chem. 282 9216–9227. [DOI] [PubMed] [Google Scholar]
- 137.Ghosh M., R. Loper, M. H. Gelb, and C. C. Leslie. 2006. Identification of the expressed form of human cytosolic phospholipase A2β (cPLA2β). cPLA2β3 is a novel variant localized to mitochondria and early endosomes. J. Biol. Chem. 281 16615–16624. [DOI] [PubMed] [Google Scholar]
- 138.Valianpour F., V. Mitsakos, D. Schlemmer, J. A. Towbin, J. M. Taylor, P. G. Ekert, D. R. Thorburn, A. Munnich, R. J. A. Wanders, P. G. Barth, et al. 2005. Monolysocardiolipins accumulate in Barth syndrome but do not lead to enhanced apoptosis. J. Lipid Res. 46 1182–1195. [DOI] [PubMed] [Google Scholar]
- 139.Esposti M. D., I. M. Cristea, S. J. Gaskell, Y. Nakao, and C. Dive. 2003. Proapoptotic Bid binds to monolysocardiolipin, a new molecular connection between mitochondrial membranes and cell death. Cell Death Differ. 10 1300–1309. [DOI] [PubMed] [Google Scholar]
- 140.Tyurin V. A., Y. Y. Tyurina, A. N. Osipov, N. A. Belikova, L. V. Basova, A. A. Kapralov, H. Bayir, and V. E. Kagan. 2007. Interactions of cardiolipin and lyso-cardiolipins with cytochrome c and tBid: conflict or assistance in apoptosis. Cell Death Differ. 14 872–875. [DOI] [PubMed] [Google Scholar]
- 141.Choi S-Y., P. Huang, G. M. Jenkins, D. C. Chan, J. Schiller, and M. A. Frohman. 2006. A common lipid links Mfn-mediated mitochondrial fusion and SNARE-regulated exocytosis. Nat. Cell Biol. 8 1255–1262. [DOI] [PubMed] [Google Scholar]