Abstract
The purpose of this study was to investigate the mechanisms by which carotenoids [xanthophylls vs. β-carotene(β-C)] are taken up by retinal pigment epithelial (RPE) cells. The human RPE cell line, ARPE-19, was used. When ARPE-19 cells were fully differentiated (7–9 weeks), the xanthophylls lutein (LUT) and zeaxanthin (ZEA) were taken up by cells to an extent 2-fold higher than β-C (P < 0.05). At 9 weeks, cellular uptakes were 1.6, 2.5, and 3.2%, respectively, for β-C, LUT, and ZEA. Similar extents were observed when carotenoids were delivered in either Tween 40 or “chylomicrons” produced by Caco-2 cells. Differentiated ARPE-19 cells did not exhibit any detectable β-C 15,15′-oxygenase activity or convert exogenous β-C into vitamin A. When using specific antibodies against the lipid transporters cluster determinant 36 (CD36) and scavenger receptor class B type I (SR-BI), cellular uptake of β-C and ZEA were significantly decreased (40–60%) with anti-SR-BI but not with anti-CD36. Small interfering RNA transfection for SR-BI led to marked knockdown of SR-BI protein expression (∼90%), which resulted in decreased β-C and ZEA uptakes by 51% and 87%, respectively. Thus, the present data show that RPE cells preferentially take up xanthophylls versus the carotene by a process that appears to be entirely SR-BI-dependent for ZEA and partly so for β-C. This mechanism may explain, in part, the preferential accumulation of xanthophylls in the macula of the retina.
Keywords: carotenoids, xanthophylls, cellular uptake, scavenger receptor class B type I, small interfering RNAs, ARPE-19 cells
Several lines of evidence suggest a protective role of the xanthophylls lutein (LUT) and zeaxanthin (ZEA) against age-related macular degeneration (AMD), the leading cause of blindness among elderly people (1–3). Carotenoids are not synthesized in the human body and therefore must be obtained from the diet. Of the 50 carotenoids typically consumed in the human diet, only 20 of them are found in human blood and tissues, and β-carotene (β-C), α-carotene, lycopene, β-cryptoxanthin, LUT, and ZEA are the six most predominant (4). The carotene β-C is the most common carotenoid found in the human diet and body and the most studied carotenoid for its provitamin A activity. LUT and ZEA are widely distributed in plants, mainly in dark green leafy vegetables (e.g., kale, spinach, broccoli, zucchini, green pea) and in yellow to orange fruits and vegetables (e.g., carrot, papaya, squash, peach) (5, 6). In foods, LUT and ZEA can be recovered in different forms that partly determine their bioavailability (free molecule, bound to proteins, or esterified at one or both hydroxyl groups of the ionone rings) (5). The daily xanthophyll intake varies between 0.5 and 2 mg/day in Western countries (7, 8).
The xanthophylls account for 20–30% of total carotenoids in human plasma, and the ratio of LUT to ZEA is consistently between 4:1 and 5:1 (7, 9). Interestingly, in the human retina, LUT and ZEA represent ∼80% of the total carotenoid content of the retina, while β-C is found in trace amounts (10, 11). These xanthophylls are preferentially accumulated in the macula region of the retina to form a yellow spot (also named macula lutea) (12, 13), and they are thus referred to as macular pigment. The highest xanthophyll concentration of the body was reported in the most central part (fovea) of the macula at 1 mM (14). This foveal xanthophyll concentration is >1,000-fold higher than the typical plasma xanthophyll concentration reported in the literature (between 0.1 and 0.6 μM) (15–17), suggesting a selective uptake of xanthophylls by retinal tissue. The physiological significance of the presence of these xanthophyll pigments in the retina and particularly in the macula remains uncertain. LUT and ZEA are believed to help filter out damaging blue light, to improve visual acuity by attenuating light scattering and chromatic aberrations, or to quench harmful photochemically induced free radicals (18–21). A direct neuroprotection of xanthophylls on photoreceptors was also reported recently (22). These different activities of the xanthophylls may contribute to reducing the risk of AMD.
How can LUT and ZEA accumulate preferentially to other carotenoids in the macula? Whenever a tissue exhibits a highly selective uptake for a compound, it is likely that one or more specific binding proteins are involved in the process. For instance, retinoids are highly concentrated in the retina as a result of the combined actions of retinoid binding proteins (23) and of a membrane receptor recognizing plasma retinol binding protein (24). For carotenoids, much less is known about carotenoid binding proteins in the mammalian eye. Yemelyanov, Katz, and Bernstein (25) reported that LUT and ZEA bind saturably and specifically to solubilized membrane proteins from human retina, and the most highly purified preparations contained two major protein bands at 25 and 55 kDa that consistently coeluted with endogenous LUT and ZEA. Although these data provided the first evidence for the existence of specific xanthophyll binding protein(s) in the vertebrate retina, definitive identification and purification of the protein(s) clearly remain to be performed. Several recent reports have indicated that the intestinal transport of carotenoids is a facilitated process mediated by the scavenger receptor class B type I (SR-BI) in intestinal Caco-2 cells in culture (26, 27) and in mice (28). Furthermore, the molecular basis for the blindness of a Drosophila mutant, ninaD, is a defect in the cellular uptake of carotenoids caused by a mutation in the ninaD gene that encodes for a scavenger receptor with a high homology to mammalian scavenger receptors [i.e., SR-BI and cluster determinant 36 (CD36)] (29). This suggests that SR-BI could be involved in carotenoid uptake in vertebrate eyes as well.
In the human retina, the retinal pigment epithelium (RPE) has been shown to play critical roles in the physiology of the underlying photoreceptors (i.e., functioning to transport nutrients from the vascular choroid). Therefore, RPE cells could be an important transfer point for LUT and ZEA uptake by the neural retina from the circulating blood. Dunn et al. (30) have developed and characterized ARPE-19, a spontaneously arising human RPE cell line with differentiated structural and functional properties similar to those of RPE cells in vivo. In the current study, we demonstrate that xanthophylls are preferentially taken up by ARPE-19 cells and that SR-BI is involved in xanthophyll transport into RPE cells.
MATERIALS AND METHODS
Chemicals
All-trans β-C (type IV, >95% purity), Tween 40, and other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO). β-C stock solution in hexane contained mainly all-trans β-C (cis isomers < 2%) as determined by HPLC. All-trans-LUT and all-trans-ZEA (>99.9% purity by TLC) were from Indofine Chemical Co., Inc. (Hillsborough, NJ). The rabbit polyclonal antibody against SR-BI (anti-SR-BI) and the mouse monoclonal antibody against human CD36 (anti-CD36) were purchased from Novus Biologicals (Littleton, CO). Nonimmunized rabbit anti-IgG was provided by DakoCytomation Corp. (Carpinteria, CA).
Cell culture
ARPE-19 cells (human retinal pigment epithelial cells, passages 18–25) were obtained from the American Type Culture Collection (Rockville, MD). ARPE-19 cells at a seeding density of 1.7 × 106 cells/cm2 were grown in a 1:1 mixture of DMEM and Ham's F12 with 2.5 mM l-glutamine supplemented with 10% FBS and 1.5 g/l sodium bicarbonate (Gibco, Life Technologies, Inc.) and incubated at 37°C in a humidified atmosphere of 5% CO2. The medium was changed every 48 or 72 h.
Caco-2 cells (passages 30–40) were obtained from the American Type Culture Collection and grown on Transwells (24 mm diameter, 3 μm pore size; Corning Costar Corp., Cambridge, MA) for 3 weeks in the presence of complete medium [DMEM plus 20% heat-inactivated FBS, 1% nonessential amino acids, and 1% antibiotics (Gibco) as described previously] (31). On the day of the experiment, the complete medium was replenished by the corresponding serum-free medium for either ARPE-19 or Caco-2 cells.
Delivery of carotenoids to cells
β-C, LUT, and ZEA were delivered individually to cells using the “Tween” method (32) unless specified otherwise. In a sterilized glass tube, required amounts of the carotenoid in hexane (for a final concentration of 1 μmol/l) and of Tween 40 in acetone (to obtain final concentrations of 0.1% and 0.05%, respectively, with Caco-2 and ARPE-19 cells) were introduced, solvents were evaporated, and the dried residue was solubilized in serum-free medium.
β-C 15, 15′-oxygenase 1 activity assay in ARPE-19 cells
β-C central cleavage enzyme activity was assayed according to the protocol of During et al. (32, 33) using an enzyme preparation (supernatant S-9) from 7 week differentiated ARPE-19 cells obtained as previously described (32). The product of the reaction (retinal) was analyzed by HPLC. The HPLC system was equipped with a 114M pump (Beckman Instruments, Inc.), a UV-970 UV-Vis absorbance variable-wavelength detector (Jasco, Tokyo, Japan), a 250 μl sample loop, and GOLD System for analyses (Beckman). Retinal was eluted on a TSK gel ODS-80Ts C18 reverse-phase column (5 μm particle size, 4.6 × 150 mm) (TosoHaas, Montgomeryville, PA) attached to a precolumn (2 × 20 mm) of Pelliguard LC-18 (Supelco, Inc., Bellefonte, PA) using acetonitrile-water (90:10, v/v) containing 0.1% ammonium acetate as mobile phase (flow rate of 1.0 ml/min). Retinal was monitored at 380 nm, and its retention time was ∼7.5 min. Retinal formed during the enzyme reaction was quantified from its peak area using a standard reference curve obtained using varying amounts of purified retinal.
Small interfering RNA inhibition
Gene expression of the lipid transporter SR-BI in ARPE-19 cells was blocked using the small interfering RNA (siRNA) or RNA interference (RNAi). Using the BLOCK-iT™ RNAi Designer, a double-stranded Stealth™ RNA (RNAi_1850; sense, 5′-UCA ACA AGC ACU GUU CUG GAA CCU U; antisense, 5′-AAG GUU CCA GAA CAG UGC UUG UUG A) was designed specifically for SR-BI (accession number NM_005505) (Invitrogen Corp., Life Technologies, Carlsbad, CA). A Stealth™ RNAi negative control (Medium GC Duplex; 48% GC; Invitrogen Corp.) was used as a control in RNAi transfection experiments. RNAi transfection into ARPE-19 cells was done using Lipofectamine 2000 (LP2000) (Invitrogen Corp.). Briefly, cells (passages 8–10) were platted on six-well plates (Corning Costar Corp.) at a high density (12.5 × 104 cells/cm2) to reach 40–60% confluence (24 h later) on the day of transfection. In a tube, RNAi-LP2000 complexes were formed by adding 5 μl of LP2000 and 250 pmol of RNAi in Opti-MEM® I Reduced Serum Medium (Gibco) and incubated for 20 min at room temperature. Then, RNAi-LP2000 complexes were added to cells and the cells were placed in a CO2 incubator at 37°C for 72 h. Cells were then incubated with carotenoids at 2 μM for 1 h using the mode of delivery described above.
Western blot analysis of SR-BI
ARPE-19 cells were plated on 25 cm2 flasks at 12.5 × 104 cells/cm2 and then transfected with one of the following treatments: LP2000 alone, RNAi (or Stealth™ RNAi negative control)-LP2000 complexes, or RNAi_1850-LP2000 complexes. At 72 h after transfection, medium was removed, cells were washed three times with a saline solution, and proteins were extracted by using a total protein extraction kit (Chemicon International, Inc.). Protein concentration of samples was then determined by the Bio-Rad Protein Assay (Bio-Rad Laboratories), and 25 μg of proteins was used for Western blot analysis. Western blot analyses were done according to the NuPAGE® technical guide (Invitrogen Corp.). Proteins were separated by SDS-PAGE, under reducing conditions, on a 4–12% NuPAGE® Novex Bis-Tris gel using the NuPAGE MOPS running buffer. After electrophoresis, proteins were transferred onto a 0.45 μm nitrocellulose membrane. Blotted membranes were then incubated with the anti-human IgG primary antibodies against SR-BI at 1:500, and immunodetection was performed using an anti-rabbit IgG secondary antibody according to the WesternBreeze Chromogenic kit (Invitrogen Corp.).
Carotenoid extraction and analysis by HPLC
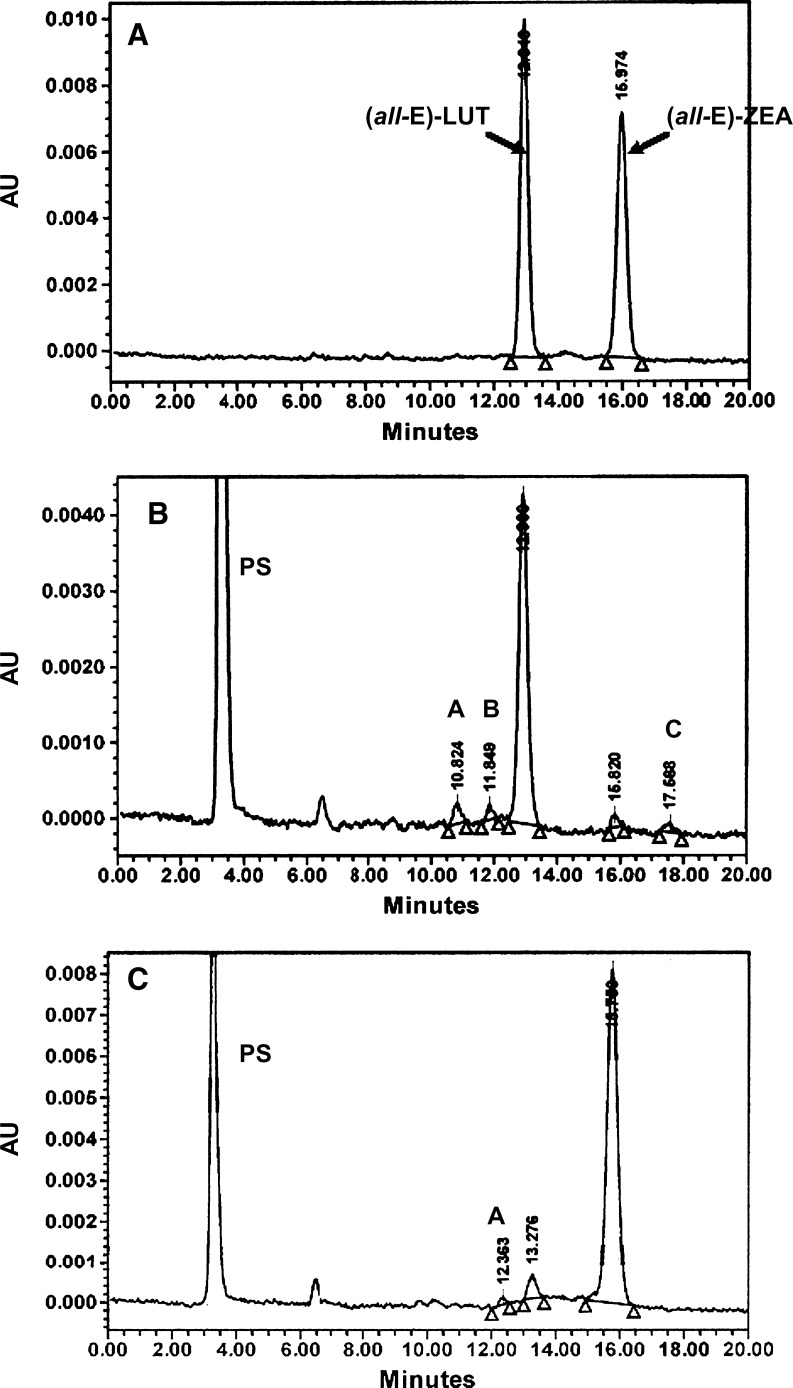
Carotenoid extractions from cells and media were performed as described previously (34, 35). Carotenoids were analyzed using a Waters HPLC system equipped with a model 717 Plus autosampler, a model 996 photo diode array detector, and a Millenium32 chromatography manager (Waters T system; Milford, MA). For β-C analyses, a TSK gel ODS 120-A C18 reverse-phase column, 4.6 × 250 mm, 5 μm (TosoHaas), was used with methanol-dichloromethane (84:16, v/v) at 1 ml/min as mobile phase. For xanthophyll analyses, column C30 Type Carotenoid, 4.6 × 250 mm, 3 μm (YMC, Inc., Milford, MA) was used with methanol-methyl-tert-butyl-ether (90:10, v/v) at 0.9 ml/min as mobile phase. Under these conditions, the two standards, LUT and ZEA, are well separated, with retention times of ∼12.5 min and ∼15.5 min, respectively (Fig. 1A). Carotenoids were monitored at 450 nm and quantified from their peak areas using external standard curves established for each carotenoid tested. Average recoveries of carotenoids in cells and media after incubation (16 or 20 h) were as follows: 98% for β-C, 94% for LUT, and 85% for ZEA.
Fig. 1.
HPLC profiles of a standard lutein (LUT) and zeaxanthin (ZEA) mixture (A), an extract from 7 week differentiated ARPE-19 cells after 20 h of incubation with 1 μM LUT (B), and an extract from 7 week differentiated ARPE-19 cells after 20 h of incubation with 1 μM ZEA (C). HPLC conditions were as follows: a YMC C30 column (250 × 4.6 mm, 3 μm), methanol-methyl-tert-butyl-ether (90:10, v/v) as mobile phase, and a flow rate at 0.9 ml/min (see Materials and Methods). A, B, and C, unidentified peaks; AU, absorbance units; PS, peak solvent.
Statistical analysis
Values are means ± SD. Statistical analyses of the results were assessed using Statview, version 5.0 (SAS Institute, Cary, NC). Data were tested for homogeneity of variances by Bartlett's test and then analyzed by one-way ANOVA coupled with the posthoc Fisher's least significant difference test to identify means with significant differences. Relationships between two variables were examined by simple or logarithmic regression analyses. The choice of the regression (simple vs. logarithmic or exponential) was determined by the squared value of the regression coefficient (R2); the regression given the highest R2 value was chosen. P values of <0.05 were considered significant.
RESULTS
Xanthophyll HPLC profiles of extracts from ARPE-19 cells
After incubation with all-trans-LUT, differentiated ARPE-19 cells had a typical xanthophyll profile of 96 ± 3% all-trans-LUT, 4 ± 2% all-trans-ZEA, and 0–2% unidentified peaks, as shown on Fig. 1B, while the 20 h cell culture medium (data not shown) contained 86 ± 5% all-trans-LUT, 3 ± 1% all-trans-ZEA, and 11 ± 5% unidentified peaks (against 96 ± 1% LUT and 4 ± 1% ZEA only in the 0 h medium) (values are from four independent experiments, n = 4). The unidentified peaks present in the medium probably correspond to cis isomers of xanthophylls. Indeed, our HPLC conditions (C30 column) allow us to separate all-trans (all-E) from cis (Z) isomers of xanthophylls, as demonstrated in a previous study using the same HPLC conditions (36). When cells were incubated with all-trans-ZEA, all-trans-ZEA was the main peak detected in cells with all-trans-LUT and another unidentified peak present in trace amounts as shown on Fig. 1C, while the 20 h cell culture medium (data not shown) contained 92 ± 3% all-trans-ZEA and 8 ± 3% all-trans-LUT (against 96 ± 2% ZEA and 3 ± 2% LUT at 0 h incubation) (n = 4). Thus, during incubation at 37°C, xanthophylls can be spontaneously isomerized and that isomerization was greater in cell culture media than in cells. All-trans-LUT is more subject to isomerization than all-trans-ZEA. Note that, under our analytical conditions, we were not able to distinguish between the configurational isomers (3R,3′R)-ZEA and (3R,3′S)-meso-ZEA, which presumably eluted together. To separate these two compounds, the use of a derivatizing method or a chiral HPLC column would be required (37).
Differentiation stage of ARPE-19 cells affects the cellular carotenoid uptake
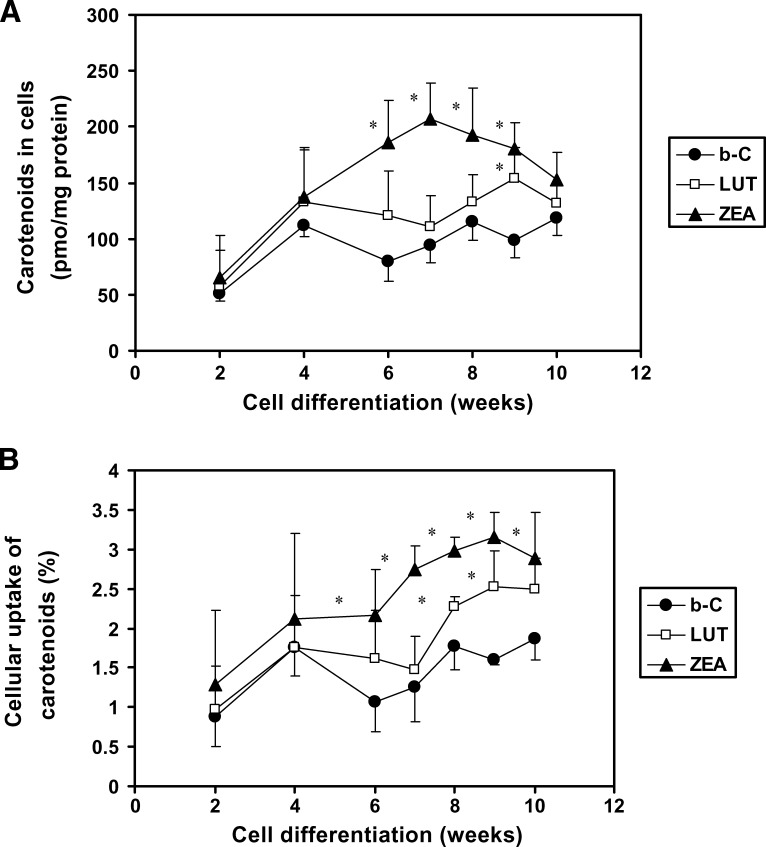
Previous data have shown that ARPE-19 cells, when cultured under the same conditions as used in the present study, started to develop RPE characteristics at around 5 weeks of a 12 week total culture period (38). Therefore, the purpose of this study was to see whether the stage of differentiation of ARPE-19 cells (from 2 to 10 weeks after confluence) could affect the cellular uptake of carotenoids. When data were expressed per milligram of protein (Fig. 2A), the uptake of the three carotenoids all increased in a similar manner up to 4 weeks of differentiation. After that, we observed an ∼2-fold higher uptake for ZEA from 6 to 9 weeks (P < 0.02) and 1.6-fold higher uptake for LUT at 9 weeks (P < 0.05) compared with that of β-C at comparable stages of differentiation (Fig. 2A). A similar tendency was observed when data were expressed as percentage uptake of the initial dose of carotenoid added to the cell culture medium (Fig. 2B). For instance, at 9 weeks of differentiation, the percentage uptake was 1.6% for β-C, 2.5% for LUT, and 3.2% for ZEA (Fig. 2B). Note that RPE cell uptake of ZEA was higher than that of LUT, in agreement with a previous report (39). In sum, when ARPE-19 cells are well differentiated, the xanthophylls LUT and ZEA are better taken up by cells than the carotene β-C.
Fig. 2.
Effects of the differentiation stage of ARPE-19 cells on the cellular uptake of β-carotene [β-C (b-C)], LUT, and ZEA. Cells were plated on six-well plates to reach confluence after 7 days, the starting point of the experiment. At different points of differentiation (up to 10 weeks), cells were incubated with β-C, LUT, or ZEA at 1 μM for 18 h. After incubation, cells were analyzed for their carotenoid content. Values are expressed as picomoles of carotenoid per milligram of protein (A) or as percentage of carotenoid recovered in cells from the initial amount added to the cell culture media (B). Data are means ± SD of three independent experiments. * P < 0.05 compared with β-C at a given time point.
The mode of carotenoid delivery to ARPE-19 cells does not affect cellular carotenoid uptake
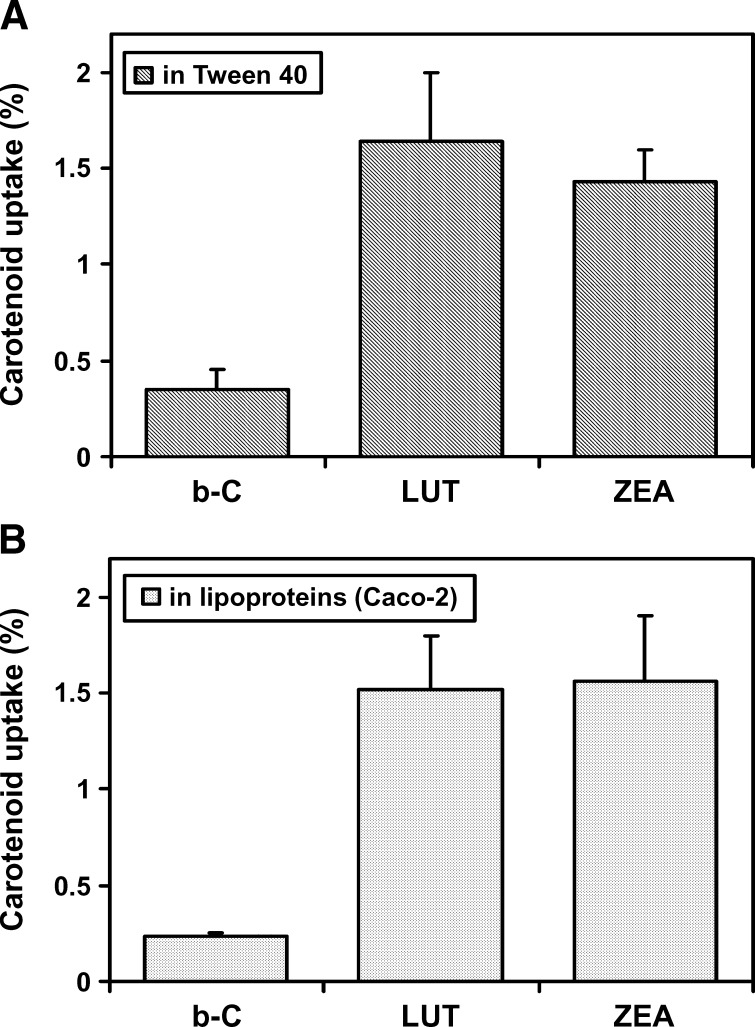
β-C, LUT, and ZEA were delivered to ARPE-19 cells via either a carotenoid-detergent micellar suspension (Fig. 3A) or a more physiological mode of delivery, the carotenoid-enriched lipoproteins produced by Caco-2 cells (Fig. 3B). We reported previously that, under oleic acid-taurocholate supplementation in the presence of a carotenoid, highly differentiated Caco-2 cells were able to produce and secrete chylomicrons, but no HDL, and 90% of the total amount of the secreted carotenoid was associated with chylomicrons (31). Therefore, in the present experiment (Fig. 3B), we conducted a coculture in which 3 week differentiated Caco-2 cells on membrane were placed on the top of 7 week differentiated ARPE-19 cells. At time 0 of the experiment, the carotenoid (10 μM) and oleic acid-taurocholate were added at the apical side of the Caco-2 cells. After 20 h of incubation, carotenoids were analyzed in media and both types of cells. As expected, ∼10% of the initial dose of the carotenoid passed through Caco-2 cells, making a basolateral concentration of ∼1 μM (Fig. 3B), comparable with that used with the Tween 40 experiment (Fig. 3A). Note that in the experiment of Fig. 3B, we assume that, in the basolateral medium, carotenoids were associated with large lipoproteins secreted by Caco-2. Identical uptakes for each of the three carotenoids by the ARPE-19 cells were observed when the carotenoid was delivered in either Tween 40 or lipoproteins (Fig. 3). Both LUT and ZEA exhibited higher uptake rates (∼1.5%) compared with those of β-C (0.3%), confirming that xanthophylls are preferentially taken up by ARPE-19 cells.
Fig. 3.
Effects of the mode of delivery of carotenoids to ARPE-19 cells on the cellular uptake of β-C (b-C), LUT, and ZEA. A: β-C, LUT, or ZEA at 1 μM was solubilized in Tween 40 as described previously (32) and detailed in Materials and Methods. The resulting carotenoid-Tween suspension was applied to 7 week differentiated ARPE-19 cells. B: At time 0 of the experiment, 3 week differentiated Caco-2 cell monolayers on inserts were placed on top of 7 week differentiated ARPE-19 cells, and β-C, LUT, or ZEA at 10 μM in Tween 40 was applied at the apical side of Caco-2 cells in the presence of oleic acid and taurocholate to produce carotenoid-enriched chylomicrons (31). Carotenoids were thus delivered to ARPE-19 cells via lipoproteins produced by Caco-2 cells at a final concentration of 1 μM. After 18 h of exposure with one of the carotenoids (either solubilized in Tween 40 or carried by lipoproteins), carotenoid contents in cells and cell culture media were determined by HPLC. Data are means ± SD of two independent experiments.
β-C is not converted into retinoids in differentiated ARPE-19 cells
Since cellular β-C content was significantly lower than those of the xanthophylls, it might be possible that in ARPE-19 cells the β-C was metabolized to vitamin A. Thus, we used two different approaches to study β-C metabolism: a) determination of β-carotene 15,15′-oxygenase (BCO1) activity, and b) measurement of retinol formed by cells after 24 h of incubation with β-C. Differentiated ARPE-19 cells did not exhibit any central cleavage enzyme activity for β-C (data not shown), in agreement with another study (40) reporting no mRNA transcript of BCO1 in ARPE-19 cells. Moreover, we were unable to detect any vitamin A (retinol) in cells incubated with β-C (data not shown).
Effects of incubation time and concentration on cellular ZEA uptake
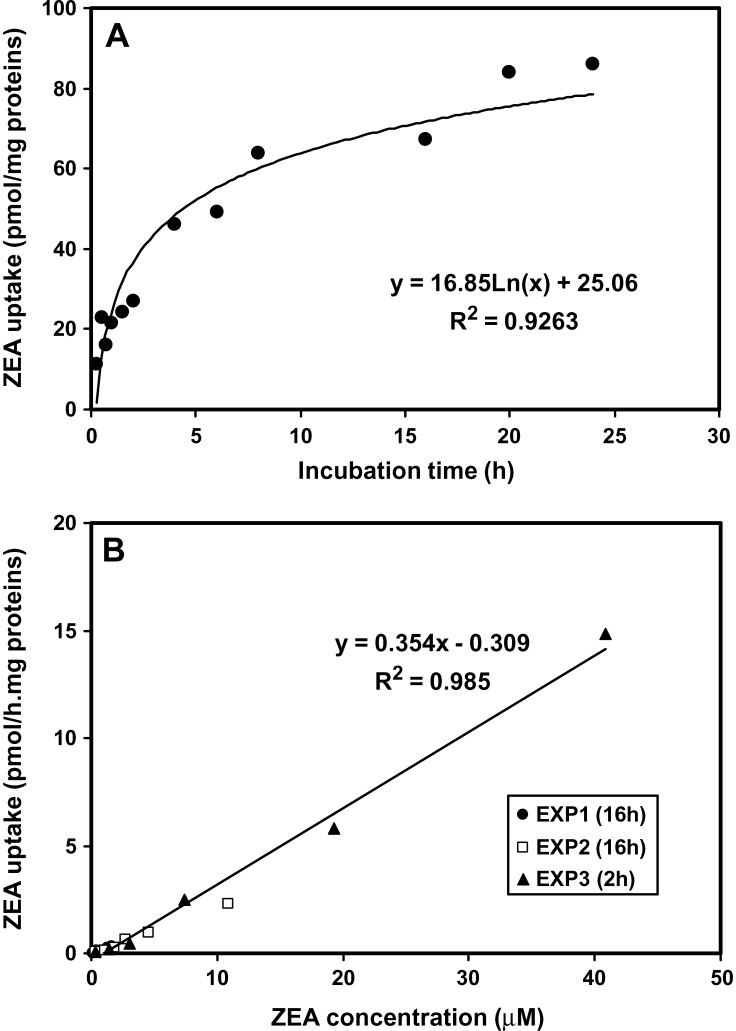
These studies were conducted on 7 week differentiated ARPE-19 cells incubated with ZEA at varying concentrations (from 0.4 to 40 μM) for different incubation times (from 15 min to 24 h) (Fig. 4). At 1 μM ZEA, the cellular uptake of ZEA as a function of the incubation time increased following a logarithmic regression: y = 25.1 + 16.9.Ln(x) (n = 12 points, R2 = 0.926, P < 0.0001), indicating that the process of ZEA uptake by ARPE-19 cells slows down with the time. The steepest slope of the curve was observed between 15 min and 2 h and corresponded to an initial rate of 9.1 pmol ZEA taken up per milligram of protein per hour (Fig. 4A). The cellular uptake of ZEA as a function of its initial concentration followed a linear regression: y = 0.354x − 0.309 (n = 18 points, R2 = 0.985, P < 0.0001) (Fig. 4B). When we increased ZEA concentration up to 40 μM and incubated cells for 2 or 16 h, we observed that retinal ZEA uptake increased in a roughly linear manner. Thus, under our present conditions, there was no saturation of ZEA uptake within the initial ZEA concentration up to 40 μM.
Fig. 4.
Kinetics of ZEA uptake in differentiated ARPE-19 cells as a function of the incubation time (A) and the initial concentration of ZEA (B). At 7 to 8 weeks of differentiation, cells were incubated with ZEA at 1 μM for varying times (15 min up to 24 h) or with varying concentrations of ZEA (0.37 up to 40 μM) for 2 or 16 h. After incubation, cells were analyzed for their ZEA content by HPLC analysis. Data are duplicates from three independent experiments.
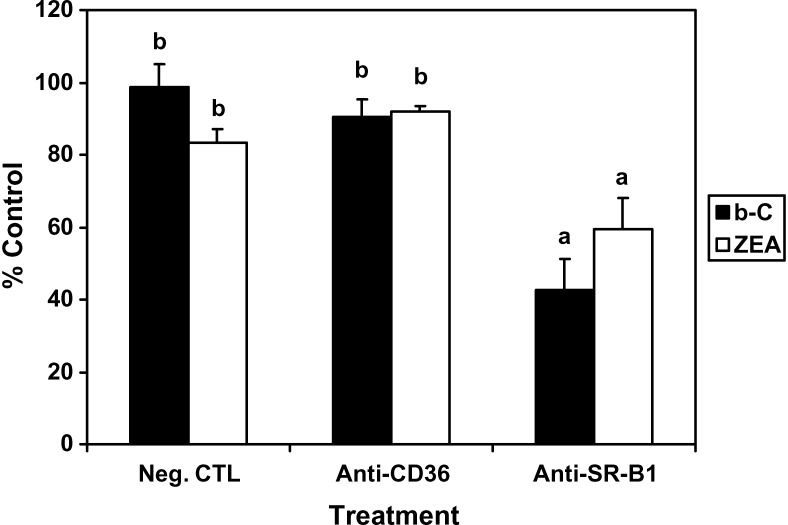
The blocking antibody against SR-BI diminished carotenoid uptake by ARPE-19 cells
Human RPE cells express both scavenger receptors SR-BI and CD36 (41, 42). The purpose of this experiment was to test the possibility that these two protein transporters could participate in the carotenoid transport into RPE using specific antibodies targeting each of the proteins (anti-CD36 and anti-SR-BI, respectively). Compared with the negative control (nonimmunized rabbit IgG), anti-SR-BI pretreatment resulted in a significant decrease of the cellular uptake of β-C and ZEA by 58% and 41%, respectively, while anti-CD36 pretreatment did not have any effect (Fig. 5). The present data suggest that SR-BI, but not CD36, is involved in the carotenoid transport into RPE cells, as described previously in intestinal cells (26, 27).
Fig. 5.
Effects of antibodies against scavenger receptor class B type I (SR-BI; anti-SR-BI) and cluster determinant 36 (CD36; anti-CD36) on β-C (b-C) and ZEA uptake by ARPE-19 cells. At 7 weeks of differentiation, cells were preincubated with nonimmunized rabbit anti-IgG, anti-CD36, or anti-SR-BI at 8 μg protein/ml for 2 h, followed by incubation with β-C or ZEA in Tween 40 for 18 h. The absolute control experiment corresponded to cells incubated with the carotenoid alone (without pretreatment). The negative control (Neg. CTL) corresponded to the nonimmunized rabbit anti-IgG pretreatment. After incubation with carotenoids, cells were harvested and their carotenoid contents analyzed by HPLC. Data are expressed as percentage of the absolute control and presented as means ± SD of three independent experiments. a,b P < 0.001.
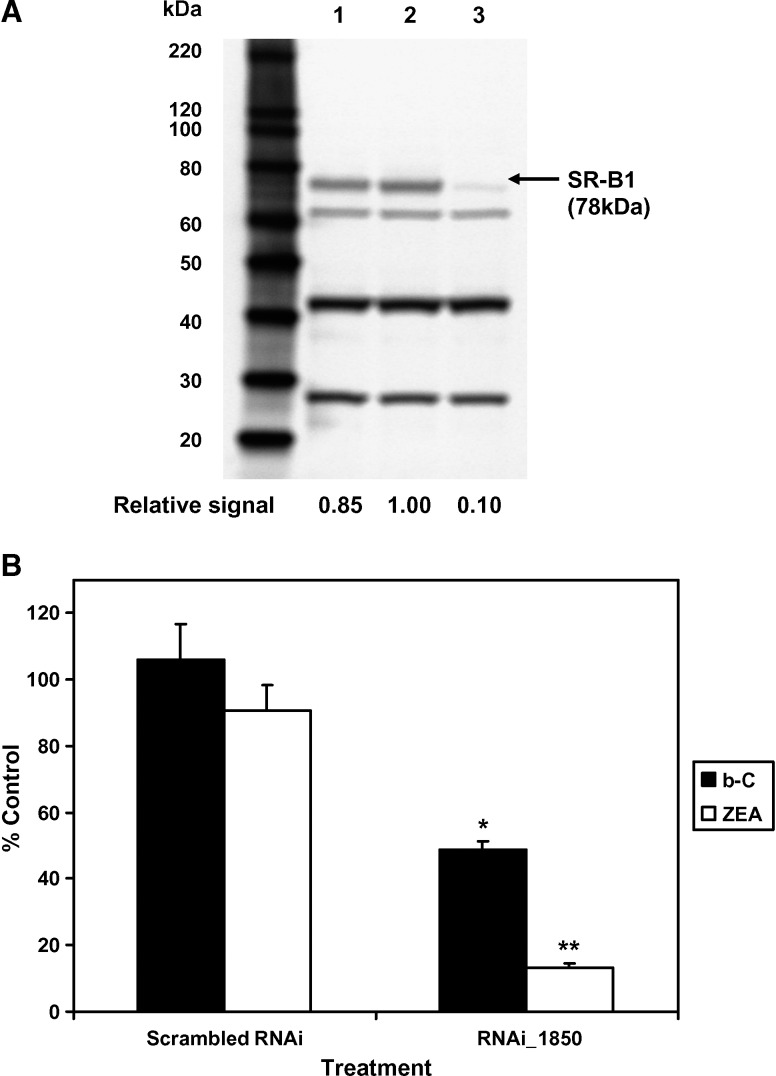
SR-BI gene silencing resulted in a marked inhibition of carotenoid uptake by ARPE-19 cells
To further examine the role of SR-BI, we used RNAi or siRNA to specifically block the expression of SR-BI. In this experiment, we used only one siRNA of 25 nucleotides; ARPE-19 cells were thus transfected with RNAi_1850-LP2000 complexes (see protocol in Materials and Methods), and the expression of the targeted protein was then analyzed by Western blotting (Fig. 6A). When compared with SR-BI expression levels in either control cells treated with the scrambled RNAi or cells treated with LP2000 only (lanes 1 and 2, respectively, in Fig. 6A), the expression levels of SR-BI were significantly lower in cells treated with RNAi_1850-LP2000 complexes (Fig. 6A, lane 3). A complete inhibition of SR-BI expression by RNAi_1850 was almost achieved (∼90% knockdown), indicating a high efficiency of the cationic lipid (LP2000)-mediated delivery of the siRNA to ARPE-19 cells. Note that in Caco-2 cells, knockdown of SR-BI by RNAi_1850 and others was incomplete (∼40%) (43).
Fig. 6.
Effects of the small interfering RNA inhibition of SR-BI expression on the cellular uptake of β-C (b-C) and ZEA in ARPE-19 cells. A: Immunoblots of SR-BI expression (using 25 μg total protein/well) in cells treated under the following conditions: lane 1, scrambled RNA interference (RNAi); lane 2, Lipofectamine 2000 (LP2000) only; lane 3, RNAi_1850. The first lane represents protein standards (MagicMark XP Standard from 60 to 220 kDa). B: Cellular uptake of carotenoids (expressed as percentage of control cells treated with LP2000 only) after incubation of 7 week differentiated cells with a carotenoid at 2 μM for 1 h at 72 h after transfection with a RNAi against SR-BI. Data are means ± SD of three independent experiments for each compound tested. * P < 0.005, ** P < 0.0001 compared with the negative control.
Knockdown of SR-BI resulted in a significant decrease of the carotenoid uptake by ARPE-19 cells (Fig. 6B). With RNAi_1850 treatment, cellular uptakes of β-C and ZEA were reduced by 51% and 87%, respectively, compared with the control treatment (LP2000 only) (P < 0.05). Note that the extent of inhibition of ZEA uptake was quantitatively similar to the extent of SR-BI knockdown, suggesting that xanthophylls exclusively use the protein transporter SR-BI to enter RPE cells.
DISCUSSION
Several in vivo observations suggest a selective mechanism for carotenoid accumulation in the human retina in favor of xanthophylls versus others. The present data suggest that this selective uptake could take place at least in RPE cells; in vivo, RPE cells form a cell monolayer that nutrients (e.g., carotenoids) from the blood vessels of the choroid must cross to reach the photoreceptors. Here, we show that highly differentiated human ARPE-19 cells preferentially accumulated the xanthophylls LUT and ZEA compared with β-C, suggesting that carotenoid uptake by RPE cells is mediated by one or more specific transporter(s).
Several xanthophyll binding proteins have been identified and proposed to play a role in the selective uptake of xanthophylls by retinal tissues in mammals. The first one was retinal tubulin, which exhibited carotenoid binding properties (44), but with less specificity and affinity than other xanthophyll binding proteins discovered later. Two membrane-associated xanthophyll binding proteins (25 and 55 kDa) partially purified from human retina and macula preparations coeluted with endogenous LUT and ZEA, but these proteins still remain to be identified (25). The glutathione S-transferase GSTP1 (23 kDa) was reported to act as a specific xanthophyll binding protein in human macula (45), but this water-soluble protein seems to differ from the 25 kDa protein described earlier by the same group (25). Although these proteins have shown strong xanthophyll binding activity, their physiological roles as carriers remain to be elucidated. However, these proteins may play a role in the selective accumulation of xanthophylls in retinal tissues.
In the present study, we show that SR-BI is involved in carotenoid transport in RPE cells. Several lines of evidence point to SR-BI as a potential carrier of carotenoids in human retina. First, the SR-BI transporter is involved in the intestinal absorption of carotenoids (26–28), while others, such as ABCA1 and Niemann-pick C1 Like 1, are not (43). Second, in Drosophila, mutation in the gene ninaD encoding a protein receptor that has high homology to the two mammalian receptors, SR-BI and CD36, was associated with a loss of carotenoid accumulation in the eye (29). Finally, both SR-BI and CD36 receptors are expressed in human RPE cells (41, 42). The participation of SR-BI in carotenoid uptake by RPE cells was demonstrated using two distinct approaches. In the first, antibodies targeted against SR-BI and CD36 proteins were used to block the activity of transporters. In the presence of anti-SR-BI, ZEA and β-C accumulation in differentiated ARPE-19 cells was decreased by 40–60%, while anti-CD36 did not have any effect. In the second approach, the siRNA silencing technique was used to target mRNA molecules of a specific protein, resulting in their degradation and consequently the reduction of protein expression. The use of siRNA against SR-BI resulted in a 90% inhibition of SR-BI protein expression that was associated with 87% reduction of ZEA uptake by ARPE-19 cells, while β-C uptake was decreased only by ∼50%. These data indicate that, in human RPE cells, xanthophyll uptake is entirely dependent on SR-BI and other mechanisms are possibly involved in β-C uptake.
Note that CD36 and SR-BI display a polarized distribution in RPE cells related to their biological activities: SR-BI localized at the choroidal (basolateral) interface is implicated in lipoprotein uptake, and CD36 at the photoreceptor (apical) interface of RPE cells is involved in the phagocytosis of rod outer segments (46). Thus, it is possible that the anti-CD36 treatment may not have worked by simply not reaching the protein. Thus, a possible role of CD36 in retinal carotenoid transport cannot be excluded. In the present study, although the participation of the transporter SR-BI was clearly demonstrated, xanthophylls accumulated in RPE cells in a concentration-dependent manner without saturation for concentrations much higher (up to 40 μM) than the physiological level, a fact that could partly explain why, in some retinal tissues, the concentration of xanthophylls can reach up to 1 mM (14).
Vitamin A (or retinol) is essential for vision; retinoids indeed participate in the visual cycle that occurs partly in the photoreceptors and continues in the adjacent RPE. In that process, the recycling of rhodopsin from opsin requires a constant supply of 11-cis-retinal. Thus, local β-C cleavage into retinal could contribute to the maintenance of a steady-state level of retinoids in RPE cells under conditions of high demand (i.e., photobleaching). Although the intestine and the liver are known as the main sites of carotenoid metabolism that could largely supply the retina in retinoids, there is little knowledge about whether and to what extent carotenoids (particularly xanthophylls) might be metabolized in RPE. Discrepancies exist among published studies: some of them reported high levels of mRNA expression and/or activity of BCO1 in human RPE cells and tissues (47, 48), while others indicated low and variable expression levels of BCO1 in the human retina and RPE-choroid tissues and in ARPE-19 cells (40). In agreement with the latter study (40), we failed to show any BCO1 activity in ARPE-19 cells. In addition, β-C recoveries in cells plus media after 20 h of incubation were quite high (98%), demonstrating little metabolism under our experimental conditions. Finally, cellular uptake of retinol (33 ± 4%) was much higher than that of β-C (1.7 ± 1.1%; means ± SD of three to five independent experiments) in ARPE-19 cells (data not shown), suggesting that dietary vitamin A is certainly a better source of retinoids for human retina than β-C. All together, it is still unclear whether BCO1 activity plays a role in retinoid supply in vivo and thus whether dietary β-C can promote eye health. A recent human trial reported that β-C supplementation, previously thought to be useful in slowing or preventing vision loss from AMD, may not be as effective as thought before (49).
In the present study, carotenoids were delivered to cells using a Tween 40 method established earlier (32) that does not require any solvent, in contrast to the synthetic micelle ethanol/Tween 40 preparation proposed recently to study cellular uptake of LUT and ZEA by ARPE-19 cells (39). Nevertheless, to make sure that our delivery method was appropriate for these kinds of studies, we compared it with a more “physiological” mode of delivery in which the carotenoid was associated with triglyceride-rich chylomicrons (31). Note that the biologically relevant vehicles for carotenoid transport in fasting plasma are the circulating LDLs and HDLs, but in the postprandial state, RPE cells can also be exposed to carotenoids associated with chylomicrons. Here, we found no difference in the cellular uptake of carotenoids by ARPE-19 cells when comparing the two methods (Tween 40 vs. chylomicrons).
Recently, a mechanism of lipid transport in the retina was proposed by Tserentsoodol et al. (46, 50), based on their observations made with cholesterol. This mechanism would involve both LDLs and HDLs and their respective receptors, the LDL receptor and class B scavenger receptors. Circulating LDLs and HDLs would enter in the retina via the RPE cells (and possibly Müller cells) and thus contribute to the supply of cholesterol and other lipophilic substances in the retina (46). Regarding carotenoids, it is known that the more nonpolar carotenoids, such as β-C, associate predominantly with LDLs, while the more polar carotenoids, the xanthophylls, are equally distributed between LDLs and HDLs (51). Thus, it is possible that in vivo xanthophylls preferentially accumulate in RPE cells because of their association with HDLs, a known ligand for SR-BI.
The present study indicates that a) RPE cells accumulate preferentially xanthophylls versus β-C, b) the transporter SR-BI is involved in both xanthophyll and β-C uptake, and c) xanthophyll uptake by RPE cells is entirely SR-BI-dependent. These data provide new mechanistic information about the preferential accumulation of the xanthophylls in the human eye and strongly suggest the participation of the transporter SR-BI. Further investigations will be necessary to confirm this suggestion, since the human ARPE-19 cells may not fully recapitulate the properties of the RPE in vivo.
Abbreviations
AMD, age-related macular degeneration
β-C, β-carotene
BCO1, β-carotene 15,15′-oxygenase
CD36, cluster determinant 36
LP2000, Lipofectamine 2000
LUT, lutein
RNAi, RNA interference
RPE, retinal pigment epithelium
siRNA, small interfering RNA
SR-BI, scavenger receptor class B type I
ZEA, zeaxanthin
Published, JLR Papers in Press, April 19, 2008.
Footnotes
The authors acknowledge with thanks the financial support of National Institutes of Health Grants R01 DK-044498 and R01 HL-49879.
This work was presented in part at the Annual Meeting of FASEB, Experimental Biology '03, San Diego, CA, April 2003 (abstract No. 456.16).
References
- 1.Moeller S. M., N. Parekh, L. Tinker, C. Ritenbaugh, B. Blodi, R. B. Wallace, and J. A. Mares. 2006. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-Related Eye Disease Study (CAREDS): ancillary study of the Women's Health Initiative. Arch. Ophthalmol. 124 1151–1162. [DOI] [PubMed] [Google Scholar]
- 2.Snodderly D. M. 1995. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am. J. Clin. Nutr. 62 (6Suppl.) 1448S–1461S. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarieh M., S. Sacu, and A. Wedrich. 2003. The role of the carotenoids, lutein and zeaxanthin, in protecting against age-related macular degeneration: a review based on controversial evidence. Nutr. J. 11 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker R. S. 1989. Carotenoids in human blood and tissues. J. Nutr. 119 101–104. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin, T. W., and G. Britton. 1988. Distribution and analysis of carotenoids. In Plant Pigments, T.W. Goodwin, editor. Academic Press, New York. 61–127.
- 6.Holden J. M., A. L. Eldridge, G. R. Beecher, M. I. Buzzard, S. Bhagwat, C. S. Davis, L. W. Douglass, S. Gebhardt, D. Haytowitz, and S. Schakel. 1999. Carotenoid content of US foods: an update of the database. J. Food Compos. Anal. 12 169–196. [Google Scholar]
- 7.Curran-Celentano J., B. R. Hammond, Jr., T. A. Ciulla, D. A. Cooper, L. M. Pratt, and R. B. Danis. 2001. Relation between dietary intake, serum concentrations, and retinal concentrations of lutein and zeaxanthin in adults in a Midwest population. Am. J. Clin. Nutr. 74 796–802. [DOI] [PubMed] [Google Scholar]
- 8.Broekmans W. M., I. A. Klopping-Ketelaars, C. R. Schuurman, H. Verhagen, H. van den Berg, F. J. Kok, and G. van Poppel. 2002. Fruits and vegetables increase plasma carotenoids and vitamins and decrease homocysteine in humans. J. Nutr. 130 1578–1583. [DOI] [PubMed] [Google Scholar]
- 9.Stacewicz-Sapuntzakis M., P. E. Bowen, and J. A. Mares-Perlman. 1993. Serum reference values for lutein and zeaxanthin using a rapid separation technique. Ann. N. Y. Acad. Sci. 691 207–209. [DOI] [PubMed] [Google Scholar]
- 10.Handelman G. J., D. M. Snodderly, A. J. Adler, M. D. Russett, and E. A. Dratz. 1992. Measurement of carotenoids in human and monkey retinas. Methods Enzymol. 213 220–230. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz H. H., C. L. Poor, E. T. Gugger, and J. W. Erdman, Jr. 1993. Analysis of carotenoids in human and animal tissues. Methods Enzymol. 214 102–116. [DOI] [PubMed] [Google Scholar]
- 12.Bone R. A., J. T. Landrum, and S. L. Tarsis. 1985. Preliminary identification of the human macular pigment. Vision Res. 25 1531–1535. [DOI] [PubMed] [Google Scholar]
- 13.Handelman G. J., E. A. Dratz, C. C. Reay, and J. G. van Kuijk. 1988. Carotenoids in the human macula and whole retina. Invest. Ophthalmol. Vis. Sci. 29 850–855. [PubMed] [Google Scholar]
- 14.Landrum J. T., R. A. Bone, L. L. Moore, and C. M. Gomez. 1999. Analysis of zeaxanthin distribution within individual human retinas. Methods Enzymol. 299 457–467. [DOI] [PubMed] [Google Scholar]
- 15.The Eye Disease Case-Control Study Group. 1992. Risk factors for neovascular age-related macular degeneration. Arch. Ophthalmol. 110: 1701–1708. [DOI] [PubMed]
- 16.Bone R. A., J. T. Landrum, Z. Dixon, Y. Chen, and C. M. Llerena. 2000. Lutein and zeaxanthin in the eyes, serum and diet of human subjects. Exp. Eye Res. 71 239–245. [DOI] [PubMed] [Google Scholar]
- 17.Schalch W., W. Cohn, F. M. Barker, W. Kopcke, J. Mellerio, A. C. Bird, A. G. Robson, F. F. Fitzke, and F. J. van Kuijk. 2007. Xanthophyll accumulation in the human retina during supplementation with lutein or zeaxanthin—the LUXEA (LUtein Xanthophyll Eye Accumulation) study. Arch. Biochem. Biophys. 458 128–135. [DOI] [PubMed] [Google Scholar]
- 18.Haegerstrom-Portnoy G. 1988. Short-wavelength-sensitive-cone sensitivity loss with aging: a protective role for macular pigment? J. Opt. Soc. Am. A. 5 2140–2144. [DOI] [PubMed] [Google Scholar]
- 19.Schalch W. 1992. Carotenoids in the retina—a review of their possible role in preventing or limiting damage caused by light and oxygen. EXS. 62 280–298. [DOI] [PubMed] [Google Scholar]
- 20.Sperling H. G., C. Johnson, and R. S. Harwerth. 1980. Differential spectral photic damage to primate cones. Vision Res. 20 1117–1125. [DOI] [PubMed] [Google Scholar]
- 21.Reading V. M., and R. A. Weale. 1974. Macular pigment and chromatic aberration. J. Opt. Soc. Am. 64 231–234. [DOI] [PubMed] [Google Scholar]
- 22.Chucair A. J., N. P. Rotstein, J. P. SanGiovanni, A. During, E. Y. Chew, and L. E. Politi. 2007. Lutein and zeaxanthin protect photoreceptors from apoptosis induced by oxidative stress. Relation with docosahexaenoic acid. Invest. Ophthalmol. Vis. Sci. 48 5168–5177. [DOI] [PubMed] [Google Scholar]
- 23.Saari J. C. 2000. Biochemistry of visual pigment regeneration. Invest. Ophthalmol. Vis. Sci. 41 337–348. [PubMed] [Google Scholar]
- 24.Kawaguchi R., J. Yu, J. Honda, J. Hu, J. Whitelegge, P. Ping, P. Wiita, D. Bok, and H. Sun. 2007. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 315 820–825. [DOI] [PubMed] [Google Scholar]
- 25.Yemelyanov A. Y., N. B. Katz, and P. S. Bernstein. 2001. Ligand-binding characterization of xanthophyll carotenoids to solubilized membrane proteins derived from human retina. Exp. Eye Res. 72 381–392. [DOI] [PubMed] [Google Scholar]
- 26.Reboul E., L. Abou, C. Mikail, O. Ghiringhelli, M. Andre, H. Portugal, D. Jourdheuil-Rahmani, M. J. Amiot, D. Lairon, and P. Borel. 2005. Lutein transport by Caco-2 TC-7 cells occurs partly by a facilitated process involving the scavenger receptor class B type I (SR-BI). Biochem. J. 387 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.During A., H. D. Dawson, and E. H. Harrison. 2005. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J. Nutr. 135 2305–2312. [DOI] [PubMed] [Google Scholar]
- 28.Van Bennekum A., M. Werder, S. T. Thuahnai, C. H. Han, P. Duong, D. L. Williams, P. Wettstein, G. Schulthess, M. C. Phillips, and H. Hauser. 2005. Class B scavenger receptor-mediated intestinal absorption of dietary beta-carotene and cholesterol. Biochemistry. 44 4517–4525. [DOI] [PubMed] [Google Scholar]
- 29.Kiefer C., E. Sumser, M. F. Wernet, and J. Von Lintig. 2002. A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila. Proc. Natl. Acad. Sci. USA. 99 10581–10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunn K. C., A. E. Aotaki-Keen, F. R. Putkey, and L. M. Hjelmeland. 1996. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp. Eye Res. 62 155–169. [DOI] [PubMed] [Google Scholar]
- 31.During A., M. M. Hussain, D. W. Morel, and E. H. Harrison. 2002. Carotenoid uptake and secretion by CaCo-2 cells: beta-carotene isomer selectivity and carotenoid interactions. J. Lipid Res. 43 1086–1095. [DOI] [PubMed] [Google Scholar]
- 32.During A., G. Albaugh, and J. C. Smith. 1998. Characterization of β-carotene 15,15′-dioxygenase activity in TC7 clone of human intestinal cell line Caco-2. Biochem. Biophys. Res. Commun. 249 467–474. [DOI] [PubMed] [Google Scholar]
- 33.During A., A. Nagao, C. Hoshino, and J. Terao. 1996. Assay of β-carotene 15,15′-dioxygenase activity by reverse-phase high-pressure liquid chromatography. Anal. Biochem. 241 199–205. [DOI] [PubMed] [Google Scholar]
- 34.Barua A. B., and J. A. Olson. 1998. Reversed-phase gradient high-performance liquid chromatographic procedure for simultaneous analysis of very polar to nonpolar retinoids, carotenoids and tocopherols in animal and plant samples. J. Chromatogr. B Biomed. Sci. Appl. 707 69–79. [DOI] [PubMed] [Google Scholar]
- 35.Barua A. B., D. Kostic, and J. A. Olson. 1993. New simplified procedures for the extraction and simultaneous high-performance liquid chromatographic analysis of retinol, tocopherols and carotenoids in human serum. J. Chromatogr. 617 257–264. [DOI] [PubMed] [Google Scholar]
- 36.Aman R., J. Biehl, R. Carle, J. Conrad, U. Beifuss, and A. Schieber. 2005. Application of HPLC coupled with DAD, APcI-MS and NMR to the analysis of lutein and zeaxanthin stereoisomers in thermally processed vegetables. Food Chem. 92 753–763. [Google Scholar]
- 37.Khachik F., C. J. Spangler, J. C. Smith, Jr., L. M. Canfield, A. Steck, and H. Pfander. 1997. Identification, quantification, and relative concentrations of carotenoids and their metabolites in human milk and serum. Anal. Chem. 69 1873–1881. [DOI] [PubMed] [Google Scholar]
- 38.Janssen J. J., E. D. Kuhlmann, A. H. van Vugt, H. J. Winkens, B. P. Janssen, A. F. Deutman, and C. A. Driessen. 2000. Retinoic acid delays transcription of human retinal pigment neuroepithelium marker genes in ARPE-19 cells. Neuroreport. 11 1571–1579. [PubMed] [Google Scholar]
- 39.Lornejad-Schäfer M. R., C. Lambert, D. E. Breithaupt, H. K. Biesalski, and J. Frank. 2007. Solubility, uptake and biocompatibility of lutein and zeaxanthin delivered to cultured human retinal pigment epithelial cells in tween40 micelles. Eur. J. Nutr. 46 79–86. [DOI] [PubMed] [Google Scholar]
- 40.Bhatti R. A., S. Yu, A. Boulanger, R. N. Fariss, Y. Guo, S. L. Bernstein, S. Gentleman, and T. M. Redmond. 2003. Expression of β-carotene 15,15′ monooxygenase in retina and RPE-choroid. Invest. Ophthalmol. Vis. Sci. 44 44–49. [DOI] [PubMed] [Google Scholar]
- 41.Ryeom S. W., J. R. Sparrow, and R. L. Silverstein. 1996. CD36 participates in the phagocytosis of rod outer segments by retinal pigment epithelium. J. Cell Sci. 109 387–395. [DOI] [PubMed] [Google Scholar]
- 42.Duncan K. G., K. R. Bailey, J. P. Kane, and D. M. Schwartz. 2002. Human retinal pigment epithelial cells express scavenger receptors BI and BII. Biochem. Biophys. Res. Commun. 292 1017–1022. [DOI] [PubMed] [Google Scholar]
- 43.During A., and E. H. Harrison. 2007. Mechanisms of provitamin A (carotenoid) and vitamin A (retinol) transport into and out of intestinal Caco-2 cells. J. Lipid Res. 48 2283–2294. [DOI] [PubMed] [Google Scholar]
- 44.Bernstein P. S., N. A. Balashov, E. D. Tsong, and R. R. Rando. 1997. Retinal tubulin binds macular carotenoids. Invest. Ophthalmol. Vis. Sci. 38 167–175. [PubMed] [Google Scholar]
- 45.Bhosale P., A. J. Larson, J. M. Frederick, K. Southwick, C. D. Thulin, and P. S. Bernstein. 2004. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J. Biol. Chem. 279 49447–49454. [DOI] [PubMed] [Google Scholar]
- 46.Tserentsoodol N., N. V. Gordiyenko, I. Pascual, J. W. Lee, S. J. Fliesler, and I. R. Rodriguez. 2006. Intraretinal lipid transport is dependent on high density lipoprotein-like particle and class B scavenger receptors. Mol. Vis. 12 1319–1333. [PubMed] [Google Scholar]
- 47.Yan W., G. F. Jang, F. Haeseleer, N. Esumi, J. Chang, M. Kerrigan, M. Campochiaro, P. Campochiaro, K. Palczewski, and D. J. Zack. 2001. Cloning and characterization of a human β,β-carotene-15,15′-dioxygenase that is highly expressed in the retinal pigment epithelium. Genomics. 72 193–202. [DOI] [PubMed] [Google Scholar]
- 48.Chichili G. R., D. Nohr, M. Schäffer, J. von Lintig, and H. K. Biesalski. 2005. β-Carotene conversion into vitamin A in human retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 46 3562–3569. [DOI] [PubMed] [Google Scholar]
- 49.Christen W. G., J. E. Manson, R. J. Glynn, J. M. Gaziano, E. Y. Chew, J. E. Buring, and C. H. Hennekens. 2007. Beta carotene supplementation and age-related maculopathy in a randomized trial of US physicians. Arch. Ophthalmol. 125 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tserentsoodol N., J. Sztein, M. Campos, N. V. Gordiyenko, R. N. Fariss, J. W. Lee, S. J. Fliesler, and I. R. Rodriguez. 2006. Uptake of cholesterol by the retina occurs primarily via a low density lipoprotein receptor-mediated process. Mol. Vis. 12 1306–1318. [PubMed] [Google Scholar]
- 51.Romanchick J. E., D. W. Morel, and E. H. Harrison. 1995. Distributions of carotenoids and a-tocopherol among lipoproteins do not change when human plasma is incubated in vitro. J. Nutr. 125 2610–2617. [DOI] [PubMed] [Google Scholar]