Abstract
Cystic fibrosis (CF) is associated with fatty acid alterations characterized by low linoleic and docosahexaenoic acid. It is not clear whether these fatty acid alterations are directly linked to cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction or result from nutrient malabsorption. We hypothesized that if fatty acid alterations are a result of CFTR dysfunction, those alterations should be demonstrable in CF cell culture models. Two CF airway epithelial cell lines were used: 16HBE, sense and antisense CFTR cells, and C38/IB3-1 cells. Wild-type (WT) and CF cells were cultured in 10% fetal bovine serum (FBS) or 10% horse serum. Fatty acid levels were analyzed by GC-MS. Culture of both WT and CF cells in FBS resulted in very low linoleic acid levels. When cells were cultured in horse serum containing concentrations of linoleic acid matching those found in human plasma, physiological levels of linoleic acid were obtained and fatty acid alterations characteristic of CF tissues were then evident in CF compared with WT cells. Kinetic studies with radiolabeled linoleic acid demonstrated in CF cells increased conversion to longer and more-desaturated fatty acids such as arachidonic acid. In conclusion, these data demonstrate that CFTR dysfunction is associated with altered fatty acid metabolism in cultured airway epithelial cells.
Keywords: cystic fibrosis transmembrane conductance regulator, essential fatty acid deficiency, fetal bovine serum, horse serum, arachidonic acid, docosahexaenoic acid, confluence, Δ6-desaturase
Cystic fibrosis (CF) is a monogenetic disease caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) (1). The CF phenotype typically involves pancreatic insufficiency and recurrent pulmonary infections associated with excessive inflammation, ultimately leading to progressive bronchiectasis, respiratory failure, and death. Closely connected with the disease is an altered fatty acid profile. Decreased serum levels of the essential fatty acid linoleic acid in CF patients were first described in the 1960s (2). Two decades later, an increase in arachidonic acid (AA) in bronchial secretions in CF patients (3) and increased arachidonic release from CF lymphocytes (4) were reported, providing support for the theory that the altered fatty acid profile is not exclusively a result of decreased fatty acid absorption secondary to pancreatic insufficiency.
Further studies have described low serum levels of docosahexaenoic acid (DHA) and increased serum levels of palmitoleic acid in CF patients (5) accompanied by altered turnover of essential fatty acids in CF cells (6, 7). Although CF serum does not display large changes in AA levels, AA levels are increased in tissues from CF patients (8) and in CFTR knock-out mice (9). CF is associated with an excessive host inflammatory response independent of infection (10, 11), with these changes in fatty acids levels being postulated to play a role in this altered innate immune response (9, 12). However, reported fatty acid alterations in CF mouse tissues have been variable (13), and cultured human CF cell lines have not to date shown the fatty acid changes characteristic of CF tissues and serum (14). Importantly, fatty acid levels in cultured cells are strongly influenced by the fatty acids present in the serum of the cell culture media. Because fetal bovine serum (FBS), commonly used in cell culture, has very low levels of linoleic acid, normal use of FBS may result in unphysiologic cellular levels of linoleic acid as well as other fatty acids.
The low levels of linoleic acid and some other fatty acids typically seen in CF patients have led to the proposal that this “essential fatty acid deficiency” may play a role in the pathogenesis of CF. In fact, current recommendations for CF patients include consumption of high-fat diets rich in linoleic acid. However, the low linoleic acid levels have also been explained as a result of increased conversion to AA, leading to increased biosynthesis of proinflammatory eicosanoids.
In this study, we tested the following hypotheses: 1) the fatty acid defect of CF is present in cultured airway epithelial cells, and is linked to loss of CFTR function using either antisense strategies or mutations in the CFTR gene; 2) the expression of the CF fatty acid defect is dependent on culture media linoleic acid levels and on the state of cell confluence; and 3) the decreased levels of linoleic acid in CF are caused by enhanced metabolism to AA and other n-6 fatty acids, compared with that in wild-type (WT) cells.
METHODS
Cell culture
16HBE14o- cells, (a human bronchial epithelial cell line originally established by Dr Dieter Gruenert) were stably transfected with WT CFTR (sense cells) or with a short CFTR antisense RNA [antisense cells (CF) by Dr. Pamela Davis (15). The 16HBE antisense cell line has a CF phenotype and does not respond to cAMP agonists with increased chloride secretion, whereas the WT cells demonstrate a significant chloride secretory response (16). These cells were cultured in flasks coated with a LHC basal media coating solution containing 0.01 mg/ml human fibronectin (Gibco; Grand Island, NY), 1% (v/v) Vitrogen-50 (Angiotech Biomaterials; Palo Alto, CA), and 0.1 mg/ml BSA. Cell culture medium consisted of MEM with glutamax, and was supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% serum (FBS or horse serum), and cells were cultured in 5% CO2 at 37°C. After thawing, the cells were cultured in FBS for at least 2 weeks before switching to horse serum. The cell culture medium was changed three times per week.
For experiments, cells were seeded onto 6-well plates. Because sense cells were smaller than the antisense cells, sense cells were plated at 3-fold initial higher density than antisense cells in order to reach confluence at the same time for experimental use. Unless otherwise stated, 100,000 antisense cells/well (9.6 cm2) were seeded and cells were harvested 1–2 days postconfluence. Medium was changed 24 h before harvest. Harvesting at earlier time points resulted in variable fatty acid levels. Linoleic acid in a stock solution of chloroform-methanol 2:1 was dried under nitrogen onto the wall of a glass tube, and then medium containing 10% horse serum was added. The tube was then placed in a water sonicator three times for 5 s each time. The serum provided the albumin that served as carrier protein of the fatty acid. For linoleic acid supplementation experiments, 0–200 μM free linoleic acid was added to the cell culture medium for 1 week.
IB3-1 and C38 cells were obtained from the American Type Culture Collection (Manassas, VA). The IB3-1 cell line is a compound heterozygote bronchial epithelial cell line from a CF patient containing one ΔF508 allele and one W1282× nonsense mutation allele. The CF phenotype present in the IB3-1 cells has been corrected in the C38 cell line with WT CFTR in an adeno-associated viral vector. IB3-1 and C38 cells were cultured in 10% FBS or horse serum for at least 2 weeks before fatty acid analysis. It was noted that when the cells were placed directly in 10% horse serum after having been stored in liquid nitrogen, it took longer for the cells to express the complete CF fatty acid profile than when the cells were cultured in FBS for 2 weeks before changing to horse serum. For experiments, 100,000 cells/well (9.6 cm2) of both cell types were seeded onto 6-well plates and harvested 3 days after confluence was reached. For experiments to increase CFTR expression in IB3-1 cells (17, 18), confluent cells cultured in horse serum for 2 weeks were incubated for 48 h with 2.5 mM sodium butyrate (Chemicon International; Billerica, MA) and 150 μg/ml G418 (Sigma-Aldrich; St .Louis, MO). Longer treatment could not be performed due to cell toxicity.
Western blotting
Cells were scraped from the cell culture flasks and lysed in a cell lysis buffer (Boston Bioproducts; Worcester, MA) containing 1 mM DTT and protease inhibitor cocktail (Sigma-Aldrich). Protein concentration was determined by the Bradford protein assay (Bio-Rad; Hercules, CA). Thirty micrograms of protein/well were separated on a 4–10% Tris-HCl gradient gel and transferred to nitrocellulose membrane for 2 h, and the membrane immunoblotted for CFTR [1:1,000 dilution of rabbit polyclonal primary antibody (Cell Signaling; Danvers, MA)], followed by 1:3,000 of goat anti-rabbit HRP antibody (Calbiochem; San Diego, CA). A mouse β-actin antibody was used as loading control. Chemiluminescence detection was performed using Supersignal Chemiluminescent reagent (Pierce; Rockford, IL).
Fatty acid analysis
Cells grown on 6-well plates were harvested by scraping on ice after rinsing twice with ice-cold PBS. Cells were transferred to a glass tube, and 30 μl of 1 mg/ml 17:0 was added as an internal standard. Lipids were extracted by adding 6 vols of chloroform-methanol (2:1). After centrifugation (800 g for 4 min), the lower phase was transferred to a new tube. The samples were then dried under nitrogen gas and methylated as previously described (19). Briefly, methanolic base (0.5 ml) was added and the sample was vortexed and heated (100°C for 3 min). After cooling at room temperature, BF3 solution (0.5 ml) was added and the sample was vortexed and heated (100°C for 1 min). After cooling, 0.5 ml of hexane was added, and after vortexing, 6.5 ml of a saturated NaCl solution was added. The sample was then centrifuged (800 g for 3 min), and the upper phase was used for quantification of fatty acid methyl esters (FAMEs) with a HP5890 Series II Hewlett-Packard gas chromatograph equipped with a Supelcowax SP-10 capillary column (Supelco, Bellefonte, PA) coupled to a mass spectrometer (HP-5971, Hewlett-Packard, Wilmington, DE). FAME mass was determined by comparing areas of unknown FAMEs to that of a fixed concentration of 17:0 internal standard. The response of the GC-MS in terms of assignment of area units to each fatty acid is variable and is influenced by the properties of the column selected, the inherent ability of the GC-MS instrumentation itself, and the degree of unsaturation of the fatty acid. To correct for this variability, response factors were determined for each individual FAME of the GC-MS response based on known mass ratios of the reference standard mixture.
For radiolabeling experiments, cells were incubated with [14C]18:2n-6 (PerkinElmer Life Sciences, Boston, MA; 4.9 μM, 0.5 μCi) or [3H]20:5n-3 (PerkinElmer Life Sciences; 4.5 μM, 0.5 μCi) in 10% lipid-free serum (Cocalico Biologicals; Reamstown, PA) for 4 h. Radiolabeled lipids in ethanol were dried under nitrogen in a glass tube. Medium was added, and the tube was placed in a water sonicator three times for 5 s each time. Albumin in the serum served as the carrier protein. After the incubation, the cells were rinsed with PBS twice and further incubated for 20 h in 10% serum (with lipids). After washing twice with PBS, cells were scraped off the plate. Lipids were extracted and methylated as above. The samples were then dried under nitrogen and dissolved in acetonitrile and analyzed by HPLC (Waters; Milford, MA). Fatty acids were separated using a binary solvent system. Solvent A consisted of double-distilled H2O with 0.02% H2PO4, and solvent B was 100% acetonitrile (HPLC grade). The solvent program was 76% of acetonitrile for 0.5 min, a linear gradient from 76% to 86% acetonitrile over 10 min, a hold for 20 min, a linear gradient from 86% to 100% acetonitrile over 2 min, a hold for 18 min, followed by reconstitution of the original conditions. Quantification of the elution profile peaks was performed by a scintillation counter coupled to the HPLC. Peaks were identified by comparison of retention times of unlabeled standards detected using ultraviolet detection at 205 nm.
Mouse colony
Mouse tissue for fatty acid compositional analysis was obtained from animals in our colony under protocols approved by the Beth Israel Deaconess Medical Center Animal Care Committee. The mice were housed in a room that controlled for temperature (20–22°C), humidity (30–70%), and light (light 6 AM to 8 PM). Exon 10 cftr−/− UNC transgenic mice from our established breeding colony and their WT littermates were used for the experiments as previously described (9). Tail-clip samples of 14 day-old mice were used for genotype analysis. The mice were weaned at 23 days of age. All CF mice were maintained on Peptamen (Nestle Clinical Nutrition; Deerfield, IL) and water. One week before experiments, WT littermates were put on Peptamen and water.
Tissue preparation
Mice were euthanized with carbon dioxide. The pancreas was then removed, placed in PBS, and homogenized by sonication with an ultrasonic probe (Sonifier Cell Disruptor, Misonix, Inc., Farmingdale, NY). Fatty acids were analyzed as described above for cells.
Statistical analysis
Statistical differences between groups tested were evaluated by using Student's t-test (Microsoft Excel). Data are presented as mean ± standard error of the mean. P < 0.05 was interpreted as indicative of statistical significance.
RESULTS
Characterization of CFTR expression in the airway epithelial cell lines
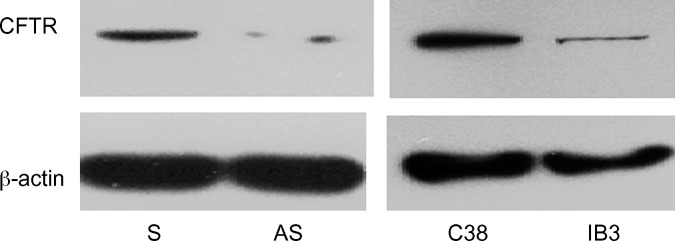
For this study, we used two different CF airway epithelial cell culture systems, one with decreased CFTR function secondary to expression of CFTR antisense RNA (16HBE antisense CFTR), and one with decreased CFTR function secondary to CFTR mutations in cells derived from a CF patient (IB3-1). Figure 1 shows CFTR protein expression in the airway epithelial cell lines used. 16HBE sense cells expressed CFTR, whereas the 16HBE antisense cell line lacked evident CFTR expression. The IB3-1 cells, originating from a CF patient with ΔF508/W1282× CFTR mutations, had significantly lower CFTR protein levels than those of the C38 cells transfected with WT CFTR, as expected.
Fig. 1.
Cystic fibrosis transmembrane conductance regulator (CFTR) expression in airway epithelial cell lines. Western blot analysis of CFTR was performed on cell lysates of 16HBE sense (S) and antisense (AS) CFTR cell lines and C38 and IB3-1 cell lines using a rabbit polyclonal anti-CFTR antibody. Thirty micrograms of protein was loaded per well. A mouse β-actin antibody was used as loading control. Data are representative of two different experiments.
Expression of the fatty acid defect is linked to CFTR dysfunction and is dependent on linoleic acid content in the media
Fatty acid composition of sera
Because the fatty acid composition of different sera, and even different serum lots, show variability, we analyzed the fatty acid content of FBS and horse serum. This was necessary because the fatty acid composition of cells in culture is reflective of what is present in the cell culture media. The fatty acid content of the cell culture media supplemented with 10% FBS or 10% horse serum is shown in Table 1. Ten percent FBS contained very low levels of linoleic acid (5.6 μM) compared with human plasma, which has a linoleic acid concentration of approximately 2.8 mmol/L (20), with the majority in triglycerides and phospholipids. Two different lots of horse serum (A and B) were analyzed and found to contain high levels of linoleic acid (52 mol% and 45 mol%, or 145 μM and 146 μM, respectively, in 10% serum). Lot B was found to contain higher levels of n-3 fatty acids than lot A.
TABLE 1.
Total fatty acid composition of different sera
| FBS | HS Lot A | HS Lot B | ||||
|---|---|---|---|---|---|---|
| mol%a | ||||||
| Saturated | ||||||
| 16:0 | 20.17 ± 0.53 | 14.14 ± 0.55 | 11.16 ± 0.39 | |||
| 18:0 | 11.92 ± 0.17 | 18.88 ± 0.61 | 17.01 ± 0.52 | |||
| 20:0 | 0.16 ± 0.00 | 0.32 ± 0.08 | 0.30 ± 0.01 | |||
| n-6 | ||||||
| 18:2n-6 | 7.32 ± 0.19 | 51.64 ± 3.20 | 45.00 ± 0.72 | |||
| 18:3n-6 | 0.11 ± 0.00 | ND | ND | |||
| 20:2n-6 | 0.14 ± 0.02 | 0.20 ± 0.13 | 0.18 ± 0.00 | |||
| 20:3n-6 | 2.78 ± 0.09 | 0.11 ± 0.11 | 0.32 ± 0.08 | |||
| 20:4n-6 | 9.89 ± 0.34 | 0.75 ± 0.22 | 1.28 ± 0.04 | |||
| 22:4n-6 | 0.60 ± 0.04 | ND | 0.01 ± 0.01 | |||
| 22:5n-6 | 0.18 ± 0.01 | ND | ND | |||
| n-3 | ||||||
| 18:3n-3 | 0.55 ± 0.01 | 1.46 ± 0.32 | 4.97 ± 0.26 | |||
| 20:5n-3 | ND | ND | 0.37 ± 0.00 | |||
| 22:5n-3 | 4.68 ± 0.11 | ND | 0.37 ± 0.03 | |||
| 22:6n-3 | 5.38 ± 0.14 | ND | 0.12 ± 0.01 | |||
| n-7 | ||||||
| 16:1n-7 | 6.28 ± 0.20 | 0.88 ± 0.48 | 2.98 ± 0.19 | |||
| 18:1n-7 | 6.13 ± 0.11 | 0.95 ± 0.33 | 0.77 ± 0.08 | |||
| n-9 | ||||||
| 18:1n-9 | 18.73 ± 0.54 | 10.08 ± 0.15 | 13.39 ± 1.23 | |||
| 20:1n-9 | 0.26 ± 0.01 | 0.15 ± 0.15 | 0.34 ± 0.01 | |||
| 20:3n-9 | 0.39 ± 0.01 | ND | 0.05 ± 0.02 | |||
HS, horse serum; ND, not detected. All samples were analyzed at least in duplicate. Data are given as mean ± SEM.
Mol% = mol% of individual fatty acids relative to total fatty acids.
The characteristic fatty acid alterations in CF are demonstrated by incubation of 16HBE14o- cells with physiological levels of linoleic acid
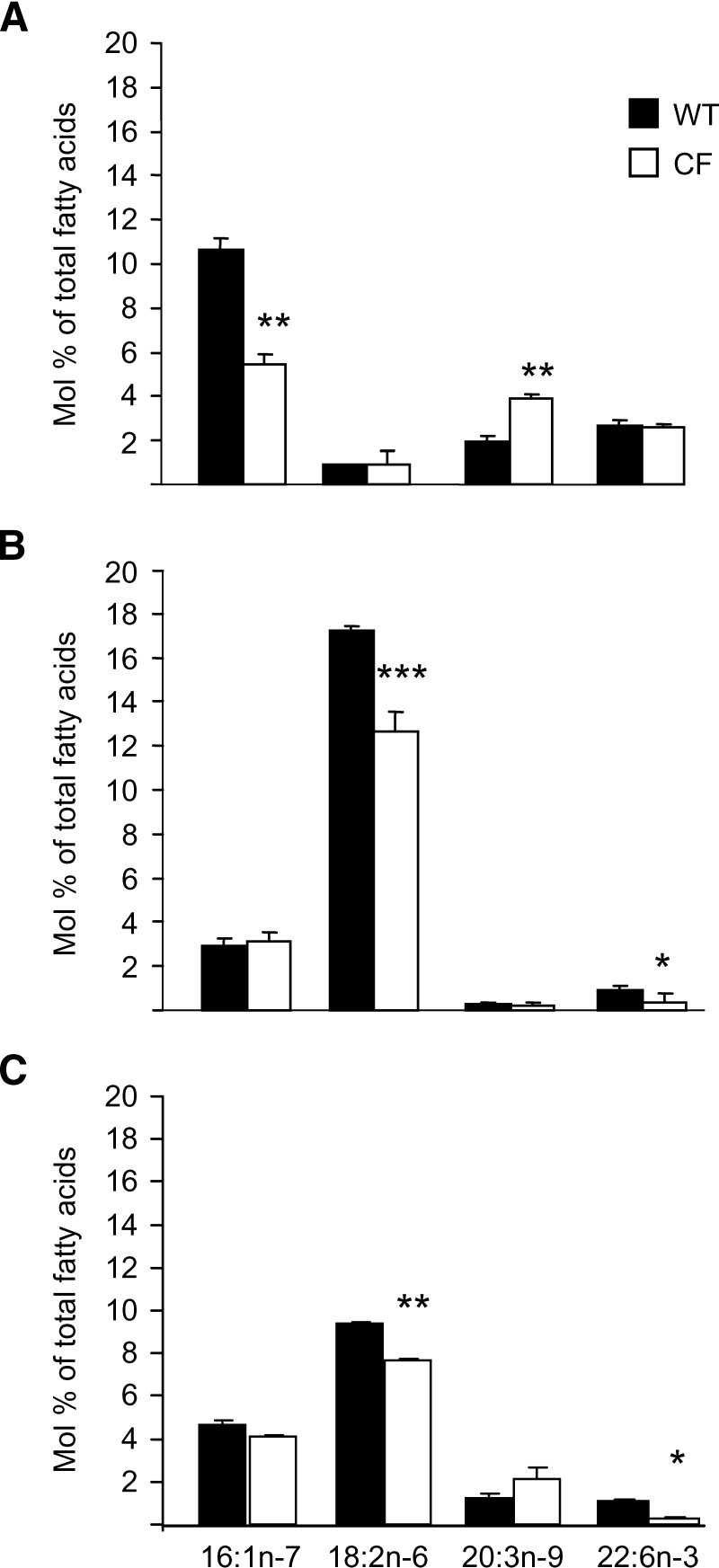
Levels of fatty acids in 16HBE cells cultured in different sera are shown in Fig. 2. The fatty acids shown are those that have been previously reported to be altered in CF. When cultured in FBS, which contains very low levels of linoleic acid (Fig. 2A), both sense (WT) and antisense (CF) 16HBE14o- cells were partially depleted of linoleic acid (18:2n-6) (WT: 0.83 ± 0.09 mol%; CF: 0.92 ± 0.06 mol%). These levels can be compared with physiological levels in mouse pancreas (18.0 mol%) and human nasal epithelial tissue (19.5 mol%) (8). DHA (22:6n-3) levels were not different between WT and CF cells (WT: 2.69 ± 0.25 mol%; CF: 2.57 ± 0.19 mol%) when cultured in FBS. Levels of 16:1n-7 were significantly higher in WT cells, and Mead acid (20:3n-9) was significantly higher in CF cells. Both WT and CF cells cultured in FBS exhibited an essential fatty acid deficiency as defined by a Mead acid/AA ratio >0.2. This ratio was significantly higher in CF cells compared with WT cells (WT: 0.38 ± 0.05 mol%, CF: 0.67 ± 0.05 mol%; P < 0.001).
Fig. 2.
Fatty acid profile of 16HBE cells cultured in different sera. 16HBE cells were cultured in (A) 10% FBS, (B) 10% horse serum lot A, or (C) 10% horse serum lot B. Fatty acids were extracted, methylated, and analyzed by GC-MS. The internal standard was 17:0. Data are expressed as the mean ± SEM of a minimum of three different experiments, with each condition tested in duplicate. * P < 0.05, ** P < 0.01, *** P < 0.001.
Because the linoleic acid levels in the cells were well below physiologic levels when cultured in FBS, we hypothesized that increasing linoleic acid levels into the physiologic range by incubating cells in horse serum would be critical to demonstrating the fatty acid defect linked to loss of CFTR function. Because horse serum fatty acid composition is variable, results were obtained from cells incubated in two lots of horse serum, lot A (Fig. 2B) and lot B (Fig. 2C). Levels of linoleic acid were significantly increased in cells cultured in horse serum compared with FBS (Fig. 2B, C), and approximated the physiologic levels of linoleic acid seen in mouse and human tissues. CF cells cultured in horse serum lot A (Fig. 2B) had lower levels of linoleic acid (WT: 17.2 ± 0.91 mol%, CF: 12.6 ± 0.96 mol%; P < 0.01) and DHA (WT: 0.91 ± 0.18 mol%, CF: 0.37 ± 0.14 mol%; P < 0.05) compared with WT. Hence, in the presence of physiologic levels of linoleic acid, CF cells displayed the characteristic fatty acid alterations that were seen in nasal epithelial tissue from CF patients (8) [mol% linoleic acid WT: 18.0, CF: 11.4 (P < 0.05); DHA WT: 2.0, CF: 1.1 (P < 0.05)] and in pancreas from exon 10 cftr−/− knock-out mice [mol% linoleic acid WT: 17.4 ± 1.1, CF: 12.3 ± 0.5 (P < 0.05); DHA WT: 1.6 ± 0.2, CF: 0.8 ± 0.2 (P < 0.05)]. Levels of AA were often increased in the CF cells, but this finding was variable. Furthermore, culturing the cells in horse serum decreased 16:1n-7 levels compared with cells cultured in FBS. Mead acid levels also decreased significantly, and the cells were no longer essential fatty acid-deficient (Mead acid/AA ratio was 0.02 for both WT and CF cells). Cells cultured in horse serum lot B to assess for variability in fatty acid composition of the media resulted in lower linoleic acid levels than in horse serum lot A. The characteristic fatty acid alterations of CF, however, were present in cells grown in both lots of horse serum.
Table 2 shows the complete fatty acid analyses by GC-MS obtained from 16HBE cells incubated in horse serum lot A. Among n-6 pathway fatty acids, linoleic acid (18:2n-6), and its elongation product, 20:2n-6, were both decreased, whereas AA (20:4n-6) was increased in the CF cells. Among metabolites downstream from AA, both 22:4n-6 and 22:5n-6 fatty acids were also decreased. In the CF cells, among n-3 fatty acids, eicosapentaenoic acid [(EPA) 20:5n-3] was increased, whereas DHA (22:6n-3) was decreased.
TABLE 2.
Fatty acid composition of 16HBE cells cultured in 10% horse serum lot A
| WT | CF | |||
|---|---|---|---|---|
| Mol%a | ||||
| Saturated | ||||
| 16:0 | 16.95 ± 0.70 | 18.90 ± 0.80 | ||
| 18:0 | 16.35 ± 0.95 | 17.93 + 0.65 | ||
| 20:0 | 0.36 ± 0.06 | 0.17 ± 0.01c | ||
| 22:0 | 0.11 ± 0.02 | 0.08 ± 0.02 | ||
| 24:0 | 0.40 ± 0.14 | 0.06 ± 0.06 | ||
| n-6 | ||||
| 18:2n-6 | 17.20 ± 0.91 | 12.61 ± 0.96c | ||
| 18:3n-6 | 0.25 ± 0.04 | 0.35 ± 0.05 | ||
| 20:2n-6 | 0.61 ± 0.06 | 0.15 ± 0.04d | ||
| 20:3n-6 | 1.22 ± 0.13 | 1.21 ± 0.15 | ||
| 20:4n-6 | 11.14 ± 0.59 | 14.82 ± 0.62c | ||
| 22:4n-6 | 2.51 ± 0.17 | 1.39 ± 0.12d | ||
| 22:5n-6 | 1.34 ± 0.33 | 0.08 ± 0.04c | ||
| n-3 | ||||
| 18:3n-3 | ND | ND | ||
| 20:5n-3 | 0.15 ± 0.04 | 0.66 ± 0.09d | ||
| 22:5n-3 | 2.68 ± 0.18 | 3.28 ± 0.41 | ||
| 22:6n-3 | 0.91 ± 0.19 | 0.37 ± 0.13b | ||
| n-7 | ||||
| 16:1n-7 | 2.88 ± 0.40 | 3.11 ± 0.44 | ||
| 18:1n-7 | 3.37 ± 0.19 | 2.52 ± 0.13c | ||
| n-9 | ||||
| 18:1n-9 | 19.73 ± 0.60 | 20.45 ± 1.26 | ||
| 20:1n-9 | 0.24 ± 0.03 | 0.05 ± 0.01d | ||
| 20:3n-9 | 0.27 ± 0.10 | 0.24 ± 0.14 | ||
WT, wild-type; CF, cystic fibrosis. Data are given as mean ± SEM.
Mol% = mol% of total fatty acids.
P < 0.05 comparing WT cells with CF cells.
P < 0.01 comparing WT cells with CF cells.
P < 0.001 comparing WT cells with CF cells.
Similar fatty acid alterations are present in airway cells with the ΔF508 mutation
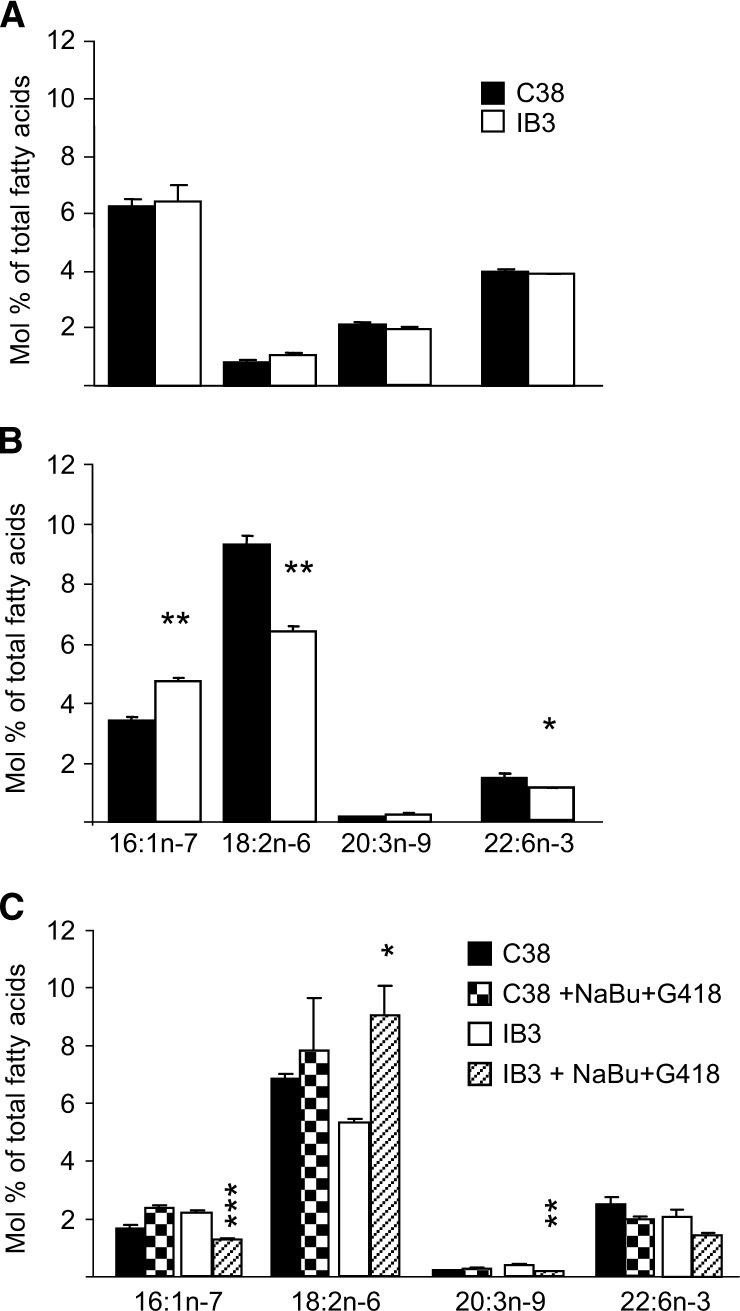
The results described above in 16HBE cells reflect a loss of CFTR function secondary to expression of CFTR antisense RNA. To demonstrate that CFTR mutations also dysregulate fatty acid metabolism, the cell line pair of IB3-1 and C38 cell lines was used. The C38 cell line is identical to the IB3-1 ΔF508/W1282× cell line, except that it is transfected with WT CFTR (21). Figure 3 shows the fatty acid profile of C38/IB3-1 cells cultured in FBS (Fig. 3A) and horse serum (Fig. 3B). There were no differences in 16:1n-7, linoleic acid, Mead acid, or DHA levels in cells cultured in FBS. After the cells were cultured in horse serum (lot A) for 8 weeks, linoleic acid was decreased in the IB3-1 ΔF508/W1282× cells, whereas 16:1n-7 was increased compared with the C38 (WT-CFTR-corrected) cells (Fig. 3B). Thus, dysregulation of fatty acid metabolism is also present in the ΔF508/W1282× IB3-1 cell line, indicating that either loss of CFTR function secondary to antisense suppression or decreased expression of CFTR resulting from CFTR mutations both lead to characteristic alterations in fatty acid metabolism.
Fig. 3.
Fatty acid profile in C38/IB3-1 cells. C38 [wild-type (WT)] and IB3-1 (CF ΔF508/W1282×) cells were cultured in (A) 10% FBS, (B) 10% horse serum lot A, or (C) 10% horse serum lot B and incubated with and without sodium butyrate (2.5 mM) and G418 (150 μg/ml) for 48 h before harvest. Fatty acids were extracted, methylated, and analyzed by GC-MS. The internal standard was 17:0. Data are expressed as the mean ± SEM and are representative of a minimum of three different experiments, with each condition tested in triplicate. * P < 0.05, ** P < 0.01, *** P < 0.001.
ΔF508-CFTR can be rescued by incubating cells with sodium butyrate (NaBu) (17), whereas G418 treatment allows read-through of the premature stop codon, resulting in increased CFTR expression and activity in cells with the W1282× CFTR mutation (18). This combination of NaBu and G418 was used to determine whether increased CFTR expression corrects the fatty acid alterations in the IB3-1 cells. Figure 3C shows that levels of 16:1n-7 and 20:3n-9 were selectively decreased and linoleic acid was selectively increased in IB3-1 cells after incubation with NaBu and G418 for 48 h. DHA was not altered with the treatment in either C38 or IB3-1 cells.
Having demonstrated that the fatty acid alterations are present in both CF cell lines, we used the 16HBE cells for the remainder of the studies.
Cell confluence affects fatty acid alterations in CF cells
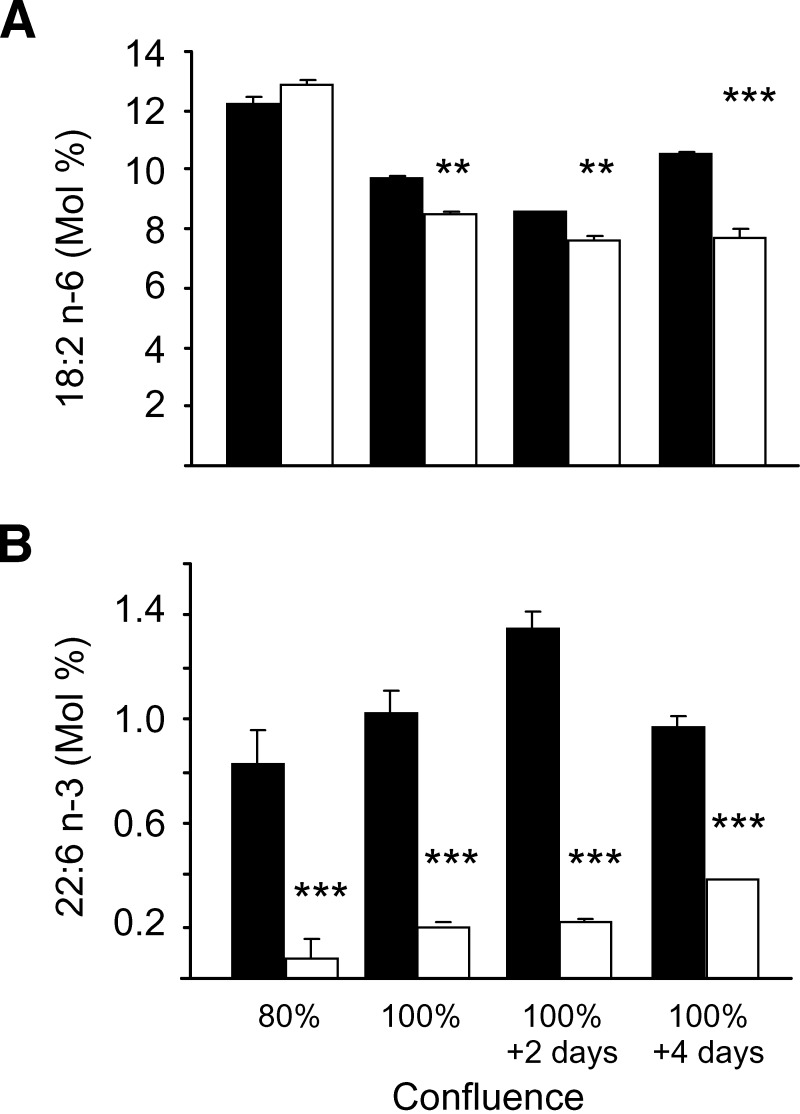
For experiments described thus far, cells were harvested at 24–48 h after 100% confluence. Because the degree of cell contact can affect fatty acid metabolism (22, 23), the effect of cell density on fatty acid levels in 16HBE cells was examined (Fig. 4). Cells were harvested at 80% confluence, 100% confluence, and 2 and 4 days after the cells reached confluence, which produced more-densely packed monolayers. At 80% confluence, there was no difference in linoleic acid levels between WT and CF cells. At confluence, linoleic acid levels decreased in both WT and CF cells, but to a greater degree in CF cells. Decreased levels of DHA were present in the CF cells at all levels of confluence. Similar results were found using the C38/IB3-1 cells (data not shown).
Fig. 4.
The effect of confluency on fatty acids in sense and antisense CFTR 16HBE cells. Cells were cultured in 10% horse serum lot B to 80%, 100% confluency, and 2 days and 4 days past confluency. Cells were scraped, and fatty acids were extracted, methylated, and analyzed by GC-MS. Data are expressed as mean ± SEM and are representative of two different experiments, with each condition tested in triplicate. A: Mole percent of linoleic acid. B: Mole percent of docosahexaenoic acid. Closed bars, WT; open bars, CF. ** P < 0.01, *** P < 0.001.
Determination of the enzymatic steps modulated by loss of CFTR function
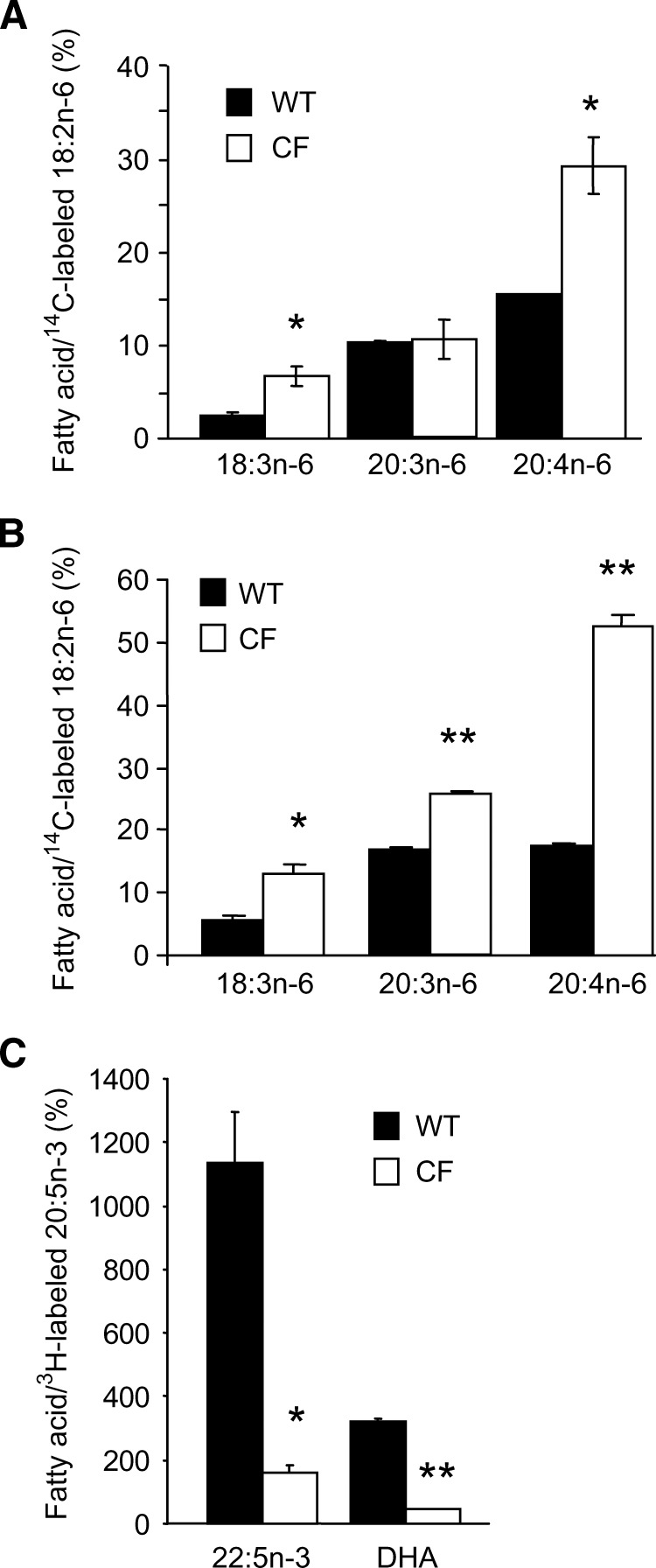
We hypothesized that the fatty acid alterations in CF are due to increased enzymatic activity in the desaturation steps that convert linoleic acid to AA. Although we have demonstrated that endogenous total levels of linoleic acid and AA are affected by the amount of linoleic acid in the media (horse serum vs. FBS), alterations in specific enzymes should be detectable under all conditions. This hypothesis was tested by incubating cells grown in either horse serum or FBS with radiolabeled linoleic acid (18:2n-6) and studying the conversion to downstream metabolites. Figure 5A shows that the synthesis of 18:3n-6, the first downstream fatty acid formed from linoleic acid through the action of Δ6-desaturase, was increased in CF cells compared with WT cells cultured in horse serum. Although levels of radiolabeled AA were increased in CF cells, the fact that the fold increase of AA did not differ from the fold increase of 18:3 indicates that this is due to an increase in Δ6-desaturase activity in the setting of loss of CFTR function. This result is consistent with Δ6-desaturase activity being the rate-limiting enzyme in the conversion of linoleic acid to AA (24, 25). This increased metabolic activity was present also when the cells were cultured in FBS (Fig. 5B).
Fig. 5.
Analysis of fatty acid metabolism. For analysis of the n-6 pathway, 16HBE cells were incubated with [14C]linoleic acid (4.9 μM, 0.5 μCi) for 4 h in lipid-free cell culture media, washed twice, and incubated for 20 h in serum-containing media. Lipids were extracted and methylated. Fatty acids were then separated by HPLC, and radioactivity was quantified by scintillation counting, as described in the Methods section. Results are shown for cells cultured in 10% horse serum (A) and in 10% FBS (B). Data are representative of two experiments, with each condition tested in triplicate and expressed as mean ± SEM. C: Results from analysis of n-3 fatty acid synthesis in16HBE cells cultured in 10% horse serum, where cells were incubated with [3H]eicosapentaenoic acid (4.5 μM, 0.5 μCi) and analyzed as above. Data are expressed as mean ± SEM, n = 2. * P < 0.05, ** P < 0.01.
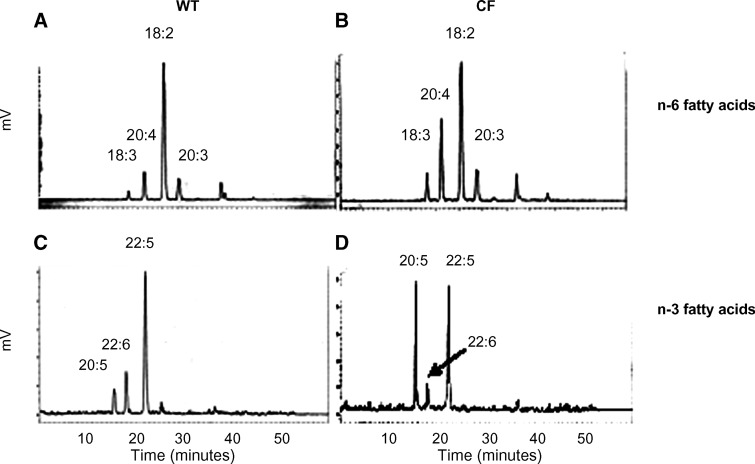
The low levels of DHA, the final fatty acid in the n-3 pathway, in CF cell lines suggest decreased activity in one or more enzymes of the n-3 pathway. The conversion rate in the n-3 fatty acid pathway was examined by incubating the cells with radiolabeled 20:5n-3 (EPA). The formation of 22:5n-3 was significantly decreased in the CF cells (Fig. 5C), consistent with inhibition of the first elongation step. Although this may account for the low DHA levels, there may be inhibition of the enzymatic steps distal to 22:5n-3, including the Δ6-desaturase and the enzymes involved in β oxidation. However, this could not be assessed, owing to nondetectable levels of 24:5n-3 and 24:6n-3. Figure 6 shows the separation of n-6-radiolabeled and n-3-radiolabeled fatty acids by HPLC.
Fig. 6.
HPLC chromatograms of fatty acid metabolism in sense and antisense CFTR 16HBE cells. WT and cystic fibrosis (CF) cells were labeled with [14C]18:2n-6 or [3H]20:5n-3 in lipid-free cell culture media for 4 h and further incubated for 20 h. After extraction and methylation, fatty acids were separated by HPLC and radioactivity was counted for each peak. A: N-6 fatty acid metabolism in WT cells. B: N-6 fatty acid metabolism in CF cells. C: N-3 fatty acid metabolism in WT cells. D: N-3 fatty acid metabolism in CF cells.
DISCUSSION
The presence of fatty acid alterations in this model of cultured CF airway epithelial cells clearly establishes that loss of CFTR function, either through antisense suppression or through CFTR gene mutations, is associated with alterations in fatty acid composition. Our data suggest that the changes occur through increased conversion of linoleic acid to AA in the n-6 pathway and through decreased conversion of EPA to DHA in the n-3 pathway. Thus, the characteristic low linoleic acid and DHA levels observed in CF patients are not solely related to impaired uptake of dietary fatty acids from the gastrointestinal tract, but are at least partly the result of altered fatty acid metabolism in cells. The results of this study also demonstrate that the altered fatty acid composition of airway epithelial cell lines with defective CFTR function appears only in the presence of adequate amounts of linoleic acid in the cell. Adequate amounts of linoleic acid can be provided by growing the cultured cells in horse serum, whereas culturing in FBS with very low levels of linoleic acid leads to essential fatty acid-deficient cells. We cannot exclude the possibility that other differences between horse serum and FBS, such as lower AA and DHA levels, are required for the appearance of the CF fatty acid profile. Involvement of serum AA levels, however, appears less likely, because linoleic acid is efficiently converted to AA in both WT and CF cells. The amount of DHA in the serum may be involved in the expression of low DHA levels in CF cells. Cells cultured in horse serum, which has extremely low levels of DHA, are entirely dependent on the metabolic conversion of 18:3n-3 to produce DHA. FBS, on the other hand, directly provides the cells with high levels of DHA, perhaps explaining why decreased DHA levels are not seen in CF cells cultured in FBS. A combination treatment with NaBu and G418, to increase CFTR expression in IB3-1 cells, led to normalization of 16:1n-7 and 18:2n-6 levels, whereas there were no changes in the C38 cells with the same treatment. These results confirm an effect of CFTR on fatty acid metabolism. DHA levels were not corrected with the treatment. This may be due to the relatively short duration of treatment with NaBu and G418, which could not be prolonged, owing to cell toxicity.
Lower linoleic acid levels in CF than in WT cells suggests that loss of CFTR function leads to alterations in the enzymes regulating the conversion of linoleic acid to downstream metabolites such as AA in the n-6 fatty acid pathway. This conclusion is supported by our studies using radiolabeled linoleic acid, which showed an increase in the rate of conversion of linoleic acid to 18:3n-6 and 20:4n-6 in CF cells. The enzyme Δ6-desaturase is responsible for the conversion of 18:2 to 18:3 and is considered the rate-limiting step in the conversion from linoleic acid to AA (24, 25). Radiolabeling experiments demonstrated an increase in Δ6-desaturase activity in CF airway cells independent of whether horse serum or FBS was used in the cell culture medium. These data suggest that culturing cells in FBS leads to deprivation of linoleic acid in both WT and CF cells, thereby masking the characteristic decrease in linoleic acid levels in CF cells.
AA is the precursor of proinflammatory eicosanoids. Increased AA levels in the plasma membrane of tumor cells have been associated with increased prostaglandin production (26). The increased prostaglandin levels present in CF patients could possibly be explained by increased production of AA. AA levels may also be increased because of decreased conversion to 22:4n-6 and 22:5n-6 fatty acids, downstream from AA, since levels of both were significantly decreased (to 40% and 6.0% of WT levels, respectively) in the CF 16HBE cells. This finding contrasts with our recent study in CF mouse pancreas, in which AA and the terminal fatty acid 22:5n-6 were both increased (27). Hence, the fatty acid alterations in CF appear to be cell or tissue specific.
The n-6 and n-3 fatty acid pathways compete for the same enzymes. Thus, we would expect increased Δ6-desaturase activity also in the n-3 pathway. Levels of 18:4n-3 were not detectable, but 20:5n-3 (EPA) was increased 4.4-fold in CF compared with WT cells, consistent with increased Δ6-desaturase activity. With a decrease in the second elongation step in the n-3 pathway in the CF cells (conversion of 20:5n-3 to 22:5n-3), the same reasoning predicts a decrease in the conversion from 20:4n-6 (AA) to 22:4n-6. This is confirmed by decreased levels of 22:4n-6 in the CF cells, as well as by low levels of 22:5n-6, the final fatty acid in the n-6 pathway. Thus, altered enzyme activities in CF do not discriminate between n-6 and n-3 pathways in this cell culture model.
The identification of culture conditions necessary to reveal the fatty acid composition changes in CFTR-defective cells explains the variable fatty acid levels observed by other groups. Dragomir et al. (14) analyzed fatty acid composition in two WT and two CF unmatched airway cell lines, as well as in baby hamster kidney cells transfected with WT and ΔF508 CFTR, all cultured in 10% FBS. Their results resembled the results for cells cultured in FBS in our study, in that cellular levels of linoleic acid were very low. Thus, their study demonstrated no consistent alterations in fatty acid composition in the CF cells.
In addition to the importance of culturing cells in the presence of linoleic acid, we found that the state of cell confluence affected the expression of the characteristic CF-associated fatty acid alterations. Decreased levels of linoleic acid in CF cells were evident only after the cells had reached confluence. The degree of confluence of cultured cells affects both gene expression (28) and protein modifications associated with the state of differentiation (29). Sood et al. (30) showed that CFTR mRNA expression as well as protein expression increased with cell density in intestinal epithelial cell lines. Similarly, enzymes regulating fatty acid utilization are affected, based on two recent reports. Bailleux et al. (22) observed that the attainment of confluency with the establishment of cell-to-cell contacts controlled the activation of the cytosolic form of phospholipase A2. This was a result of a stoichiometric change in the interaction of the phospholipase A2 with the protein p11. Jiang et al. (23) found that confluent human umbilical vein endothelial cells expressed low levels of cyclooxygenase-2, whereas higher levels of this enzyme were seen in a subconfluent stage. The higher levels of cyclooxygenase-2 resulted in enhanced release of prostaglandin E2.
Although this is the first cell culture model of CF cell lines to show the altered fatty acid levels characteristic of CF, studies of fatty acid metabolism by other groups have shown increased conversion of linoleic acid to AA in CF cell lines. Bhura-Bandali et al. (6) demonstrated in ΔF508-CFTR pancreatic epithelial cells enhanced conversion of linoleic acid to AA compared with cells with WT CFTR. This is in agreement with our cell culture data.
In summary, our data demonstrate that the low linoleic acid levels in CF are not due to an essential fatty acid deficiency, but rather are the result of CFTR loss of function leading to increased conversion of linoleic acid to AA and, additionally, a decrease in the formation of DHA. In addition, variability in fatty acid alterations in CF cell culture can be explained by different levels of linoleic acid in the media as well as the degree of cell confluence. However, it is important to note that the CF-associated dysregulation of fatty acid metabolism is present independent of cell culture condition, as shown by radiolabeling experiments. This cell culture model provides a more physiological system in which to study fatty acids and fatty acid metabolites, and to show how such changes modulate cell function. These data also suggest that strategies to limit rather than increase linoleic acid levels in the diet of CF patients may be of therapeutic benefit.
Acknowledgments
The authors thank Dr. Seth Alper for his advice and critical reading of the manuscript.
Abbreviations
AA, arachidonic acid
CF, cystic fibrosis
CFTR, cystic fibrosis transmembrane conductance regulator
DHA, docosahexaenoic acid
EPA, eicosapentaenoic acid
FAME, fatty acid methyl ester
FBS, fetal bovine serum
WT, wild type
Published, JLR Papers in Press, April 25, 2008.
Footnotes
This study was funded by National Institutes of Health Grant R01 DK-52765 (S.D.F.) and The Cystic Fibrosis Foundation (FREEDM06A0).
References
- 1.Riordan J. R., J. M. Rommens, B. Kerem, N. Alon, R. Rozmahel, Z. Grzelczak, J. Zielenski, S. Lok, N. Plavsic, J. L. Chou, et al. 1989. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 245 1066–1073. [DOI] [PubMed] [Google Scholar]
- 2.Kuo P. T., N. N. Huang, and D. R. Bassett. 1962. The fatty acid composition of the serum chylomicrons and adipose tissue of children with cystic fibrosis of the pancreas. J. Pediatr. 60 394–403. [DOI] [PubMed] [Google Scholar]
- 3.Gilljam H., B. Strandvik, A. Ellin, and L. G. Wiman. 1986. Increased mole fraction of arachidonic acid in bronchial phospholipids in patients with cystic fibrosis. Scand. J. Clin. Lab. Invest. 46 511–518. [DOI] [PubMed] [Google Scholar]
- 4.Carlstedt-Duke J., M. Bronnegard, and B. Strandvik. 1986. Pathological regulation of arachidonic acid release in cystic fibrosis: the putative basic defect. Proc. Natl. Acad. Sci. USA. 83 9202–9206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strandvik B., E. Gronowitz, F. Enlund, T. Martinsson, and J. Wahlstrom. 2001. Essential fatty acid deficiency in relation to genotype in patients with cystic fibrosis. J. Pediatr. 139 650–655. [DOI] [PubMed] [Google Scholar]
- 6.Bhura-Bandali F. N., M. Suh, S. F. Man, and M. T. Clandinin. 2000. The deltaF508 mutation in the cystic fibrosis transmembrane conductance regulator alters control of essential fatty acid utilization in epithelial cells. J. Nutr. 130 2870–2875. [DOI] [PubMed] [Google Scholar]
- 7.Ulane M. M., J. D. Butler, A. Peri, L. Miele, R. E. Ulane, and V. S. Hubbard. 1994. Cystic fibrosis and phosphatidylcholine biosynthesis. Clin. Chim. Acta. 230 109–116. [DOI] [PubMed] [Google Scholar]
- 8.Freedman S. D., P. G. Blanco, M. M. Zaman, J. C. Shea, M. Ollero, I. K. Hopper, D. A. Weed, A. Gelrud, M. M. Regan, M. Laposata, et al. 2004. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N. Engl. J. Med. 350 560–569. [DOI] [PubMed] [Google Scholar]
- 9.Freedman S. D., M. H. Katz, E. M. Parker, M. Laposata, M. Y. Urman, and J. G. Alvarez. 1999. A membrane lipid imbalance plays a role in the phenotypic expression of cystic fibrosis in cftr(−/−) mice. Proc. Natl. Acad. Sci. USA. 96 13995–14000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zahm J. M., D. Gaillard, F. Dupuit, J. Hinnrasky, D. Porteous, J. R. Dorin, and E. Puchelle. 1997. Early alterations in airway mucociliary clearance and inflammation of the lamina propria in CF mice. Am. J. Physiol. 272 C853–C859. [DOI] [PubMed] [Google Scholar]
- 11.Tirouvanziam R., S. de Bentzmann, C. Hubeau, J. Hinnrasky, J. Jacquot, B. Peault, and E. Puchelle. 2000. Inflammation and infection in naive human cystic fibrosis airway grafts. Am. J. Respir. Cell Mol. Biol. 23 121–127. [DOI] [PubMed] [Google Scholar]
- 12.Freedman S. D., D. Weinstein, P. G. Blanco, P. Martinez-Clark, S. Urman, M. Zaman, J. D. Morrow, and J. G. Alvarez. 2002. Characterization of LPS-induced lung inflammation in cftr−/− mice and the effect of docosahexaenoic acid. J. Appl. Physiol. 92 2169–2176. [DOI] [PubMed] [Google Scholar]
- 13.Werner A., M. E. Bongers, M. J. Bijvelds, H. R. de Jonge, and H. J. Verkade. 2004. No indications for altered essential fatty acid metabolism in two murine models for cystic fibrosis. J. Lipid Res. 45 2277–2286. [DOI] [PubMed] [Google Scholar]
- 14.Dragomir A., L. Hjelte, L. Hagenfeldt, and G. M. Roomans. 2004. Heparin can improve the viability of transfected cystic fibrosis cell lines in vitro. Life Sci. 75 2203–2216. [DOI] [PubMed] [Google Scholar]
- 15.Kube D., U. Sontich, D. Fletcher, and P. B. Davis. 2001. Proinflammatory cytokine responses to P. aeruginosa infection in human airway epithelial cell lines. Am. J. Physiol. Lung Cell. Mol. Physiol. 280 L493–L502. [DOI] [PubMed] [Google Scholar]
- 16.Rajan S., G. Cacalano, R. Bryan, A. J. Ratner, C. U. Sontich, A. van Heerckeren, P. Davis, and A. Prince. 2000. Pseudomonas aeruginosa induction of apoptosis in respiratory epithelial cells: analysis of the effects of cystic fibrosis transmembrane conductance regulator dysfunction and bacterial virulence factors. Am. J. Respir. Cell Mol. Biol. 23 304–312. [DOI] [PubMed] [Google Scholar]
- 17.Cheng S. H., S. L. Fang, J. Zabner, J. Marshall, S. Piraino, S. C. Schiavi, D. M. Jefferson, M. J. Welsh, and A. E. Smith. 1995. Functional activation of the cystic fibrosis trafficking mutant delta F508-CFTR by overexpression. Am. J. Physiol. 268 L615–L624. [DOI] [PubMed] [Google Scholar]
- 18.Rowe S. M., K. Varga, A. Rab, Z. Bebok, K. Byram, Y. Li, E. J. Sorscher, and J. P. Clancy. 2007. Restoration of W1282X CFTR activity by enhanced expression. Am. J. Respir. Cell Mol. Biol. 37 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alvarez J. G., and B. T. Storey. 1995. Differential incorporation of fatty acids into and peroxidative loss of fatty acids from phospholipids of human spermatozoa. Mol. Reprod. Dev. 42 334–346. [DOI] [PubMed] [Google Scholar]
- 20.Kuriki K., K. Tajima, and S. Tokudome. 2006. Accelerated solvent extraction for quantitative measurement of fatty acids in plasma and erythrocytes. Lipids. 41 605–614. [DOI] [PubMed] [Google Scholar]
- 21.Flotte T. R., S. A. Afione, R. Solow, M. L. Drumm, D. Markakis, W. B. Guggino, P. L. Zeitlin, and B. J. Carter. 1993. Expression of the cystic fibrosis transmembrane conductance regulator from a novel adeno-associated virus promoter. J. Biol. Chem. 268 3781–3790. [PubMed] [Google Scholar]
- 22.Bailleux A., D. Wendum, F. Audubert, A. M. Jouniaux, K. Koumanov, G. Trugnan, and J. Masliah. 2004. Cytosolic phospholipase A2-p11 interaction controls arachidonic acid release as a function of epithelial cell confluence. Biochem. J. 378 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang H., A. S. Weyrich, G. A. Zimmerman, and T. M. McIntyre. 2004. Endothelial cell confluence regulates cyclooxygenase-2 and prostaglandin E2 production that modulate motility. J. Biol. Chem. 279 55905–55913. [DOI] [PubMed] [Google Scholar]
- 24.Marcel Y. L., K. Christiansen, and R. T. Holman. 1968. The preferred metabolic pathway from linoleic acid to arachidonic acid in vitro. Biochim. Biophys. Acta. 164 25–34. [DOI] [PubMed] [Google Scholar]
- 25.Bernert J. T., and H. Sprecher. 1975. Studies to determine the role rates of chain elongation and desaturation play in regulating the unsaturated fatty acid composition of rat liver lipids. Biochim. Biophys. Acta. 398 354–363. [DOI] [PubMed] [Google Scholar]
- 26.Kokoglu E., Y. Tuter, K. S. Sandikci, Z. Yazici, E. Z. Ulakoglu, H. Sonmez, and E. Ozyurt. 1998. Prostaglandin E2 levels in human brain tumor tissues and arachidonic acid levels in the plasma membrane of human brain tumors. Cancer Lett. 132 17–21. [DOI] [PubMed] [Google Scholar]
- 27.Ollero M., M. Laposata, M. M. Zaman, P. G. Blanco, C. Andersson, J. Zeind, Y. Urman, G. Kent, J. G. Alvarez, and S. D. Freedman. 2006. Evidence of increased flux to n-6 docosapentaenoic acid in phospholipids of pancreas from cftr(−/−) knockout mice. Metabolism. 55 1192–1200. [DOI] [PubMed] [Google Scholar]
- 28.Lai J. Y., and M. R. Pittelkow. 2004. Culture confluence regulates gene expression of normal human keratinocytes. Wound Repair Regen. 12 613–617. [DOI] [PubMed] [Google Scholar]
- 29.Xu F., and Z. J. Zhao. 2001. Cell density regulates tyrosine phosphorylation and localization of focal adhesion kinase. Exp. Cell Res. 262 49–58. [DOI] [PubMed] [Google Scholar]
- 30.Sood R., C. Bear, W. Auerbach, E. Reyes, T. Jensen, N. Kartner, J. R. Riordan, and M. Buchwald. 1992. Regulation of CFTR expression and function during differentiation of intestinal epithelial cells. EMBO J. 11 2487–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]