Abstract
The metabolic fates of radiolabeled sn-2-monoacylglycerol (MG) and oleate (FA) in rat and mouse intestine, added in vivo to the apical (AP) surface in bile salt micelles, or to the basolateral (BL) surface via albumin-bound solution, were examined. Mucosal lipid products were quantified, and the results demonstrate a dramatic difference in the esterification patterns for both MG and FA, depending upon their site of entry into the enterocyte. For both lipids, the ratio of triacylglycerol to phospholipid (TG:PL) formed was approximately 10-fold higher for delivery at the AP relative to the BL surface. Further, a 3-fold higher level of FA oxidation was found for BL compared with AP substrate delivery. Incorporation of FA into individual PL species was also significantly different, with >2-fold greater incorporation into phosphatidylethanolamine (PE) and a 3-fold decrease in the phosphatidylcholine:PE ratio for AP- compared with BL-added lipid. Overnight fasting increased the TG:PL incorporation ratio for both AP and BL lipid addition, suggesting that metabolic compartmentation is a physiologically regulated phenomenon. These results support the existence of separate pools of TG and glycerolipid intermediates in the intestinal epithelial cell, and underscore the importance of substrate trafficking in the regulation of enterocyte lipid metabolism.
Keywords: fatty acid, enterocyte, epithelial cell, lipid metabolism, oxidation, triacylglycerol, phospholipid
sn-2-Monoacylglycerol (MG) and FAs are the hydrolytic products of ingested triacylglycerol (TG), and provide a major source of calories in Western diets. FAs, in addition, provide critical building blocks for membrane biogenesis, are precursors for regulatory second messengers, and are now considered to directly modulate the expression of specific genes (1). Certain MGs may also function outside of the traditionally appreciated lipid metabolic pathways. For example, sn-2-monoarachidonoyl is thought to act as an endogenous ligand for the cannabinoid receptors (2). Thus, the products of dietary fat digestion, once taken up by the intestinal enterocyte, may have diverse metabolic and cellular fates.
It is known that FAs are taken up into the enterocyte across both the apical (AP) plasma membrane as well as across their basolateral (BL) plasma membranes. Further, the intracellular metabolism of FAs is highly dependent upon their site of entry into the cell. In 1975, Gangl and Ockner (3) presented the intriguing finding that luminally derived and plasma-derived palmitic acid had different metabolic fates in the rat enterocyte. Plasma palmitate was primarily oxidized or incorporated into phospholipids (PLs), with relatively low incorporation into TG, whereas palmitate absorbed from the intestinal tract was mainly incorporated into TG. Similar results were shown in humans (4). Studies by Mansbach and Parthasarathy (5) and Mansbach and Dowell (6) further suggested that there are two pools of neutral lipid in the rat enterocyte, dependent in part upon the site of entry of the FA precursors. Our previous studies in Caco-2 cells found a small increase in the ratio of TG to PL for apically compared with basolaterally administered palmitate and oleate (7, 8), supporting the existence of lipid metabolic polarity at the level of the intestinal cell itself.
The hydrolysis of circulating TG-rich lipoproteins by lipoprotein lipase produces sn-2-MG (9, 10), and MG has been shown to bind to albumin with high affinity (11). In Caco-2 cells, we found that basolaterally delivered MG shows a small preferential incorporation into PL, and that AP-delivered MG shows a small preferential incorporation into TG (8, 12). The uptake of MG across the BL surface of the intestinal epithelium has not been previously demonstrated, nor is anything known about the metabolism of plasma MG by the intestine.
In the present studies, our goals were to determine: 1) whether MG is taken up into the intestinal mucosa from the circulation; 2) whether metabolic polarity for MG occurs in vivo; 3) whether, in addition to differences in the relative amount of PL formed from AP versus BL FA, there are also differences in the types of PL formed; 4) whether the degree of enterocyte lipid metabolic polarity of FA is fixed, or can be regulated by physiological state; and 5) the degree to which differential FA metabolism, dependent on entry site, occurs in the mouse, as a platform for further mechanistic studies using transgenic and knockout models. The results show not only that MG is taken up from the circulation, but also that its metabolic fate in rat and mouse intestinal mucosa is dependent on whether it is added via the dietary/AP route or via the bloodstream/BL route. In addition, the incorporation of oleate into PL species is markedly different for dietary compared with bloodstream FA. Finally, the metabolic fate of FA is substantially altered by short-term fasting, indicating regulatable mechanisms of polarized lipid metabolism in the intestinal enterocyte.
MATERIALS AND METHODS
Materials
Oleic acid and sn-2-monoolein were obtained from NuChek Prep, Inc. (Elysian, MN). [3H]oleic acid ([9,10-3H]oleic acid, 26.3 Ci/mmol) and [14C]oleic acid ([1-14C]oleic acid, 54 mCi/mmol) were obtained from Perkin Elmer-New England Nuclear (Stelton, CT). [3H]sn-2-monoolein (sn-2-[9,10 3H]monoolein, 40–60 Ci/mmol) was from American Radiochemical (St. Louis, MO). Authentic neutral lipid and PL standards were purchased from Doosan Serdary Research Laboratories (Toronto, Canada) and Avanti Polar Lipids (Alabaster, AL), respectively. Sodium taurocholate (TC) was purchased from Calbiochem (La Jolla, CA), and FA-free BSA was obtained from Sigma Aldrich (St. Louis, MO). TLC plates (Silica Gel G and Silica Gel K, both 250 μm, 150 Å) were obtained from Whatman (South Plainfield, NJ). All other materials were reagent grade or better.
Preparation of lipids for bloodstream administration
Stock solutions were prepared by drying the appropriate lipids under nitrogen and then adding 0.5% (final vol) ethanol, followed by a 1:1 mixture of mouse serum:0.85% NaCl. For experiments using rats, 1 ml of labeled lipid mixture containing 13–14 μCi of [14C]oleate (231–259 nmol), 75 μCi of [3H]oleate (2.9 nmol), or 65–107 μCi of [3H]monoolein (587–966 nmol) was administered to each animal. For mice, 150 μl of lipid solution containing 7.5 μCi of [14C]oleate (140 nmol) or [3H]monoolein (125 nmol) was used.
Preparation of lipids for intraduodenal administration
Stock solutions were prepared by drying the appropriate lipids under nitrogen and then adding 0.5% (final vol) ethanol, followed by 10 mM sodium TC in 0.85% NaCl. For experiments using rats, 1 ml of TC micelles containing 3–4 μCi of [14C]oleate (46–69 nmol), 5 μCi [3H]oleate (4 nmol), or 2–4 μCi [3H]monoolein (26–39 nmol) was administered to each animal. For mice, 150 μl of TC micelles containing 1.5–3 μCi of [14C]oleate (28–52 nmol) or 1–2 μCi [3H]monoolein (12–28 nmol) was used.
Animals, diet, and surgical procedures
Male Sprague-Dawley rats (Taconic Farms; Germantown, NY) were used for several experiments, as indicated. The majority of the present studies were performed using C57BL/6J mice obtained from Jackson Laboratories (Bar Harbor, ME). For rats, animals weighed approximately 325 g and were 2–3 months old. Mice were used at 3–4 months of age and 25–30 g body weight. Experiments were performed in the fed state, typically between 8 AM and 11 AM when food had been present overnight, except for studies of fasting, when food was withheld as indicated prior to experimentation. Animals were fed Purina 5015 rodent chow (60% carbohydrate, 12% fat, 28% protein by kcal). This research was conducted in conformity with the Public Health Service Policy on Humane Care and Use of Laboratory Animals, and the studies were approved by the Rutgers University Animal Use Protocol Review Committee of the Laboratory Animals Services Department.
Animals were anesthetized with ketamine-xylazine-ace promazine (80:100:150 mg/kg, respectively). The peritoneum was exposed for rapid access to the intestine. For administration of lipids via the bloodstream, the tail vein was used for rats, and for mice the jugular vein was exposed. A bolus of radiolabeled lipids, described above, was injected at time zero. For administration of lipids via the gastrointestinal tract, the stomach and small intestine were exposed. A small incision was made using microsurgical scissors <1 cm below the pyloric sphincter, and an 18 gauge blunted needle was inserted and secured in place with surgical string. A bolus of radiolableled lipids, described above, was injected at time zero.
At exactly 2 min after either mode of lipid infusion, the intestine, from pylorus to cecum, was harvested, flushed with 60 ml ice-cold saline, and opened longitudinally. For rats, the intestine was divided into proximal and distal segments of equal length. Intestinal mucosa was scraped using a glass slide, and samples were placed immediately into polypropylene tubes in dry ice-acetone and were then placed in a −70°C freezer. To ensure that the radiolabeled lipids were not modified in the lumen or bloodstream during the 2 min experimental period, blood and intestinal contents were sampled at 2 min and extracted lipids were subjected to TLC and phosphorimager (or scintillation counting) analysis. This was especially important for the sn-2-MG, where isomerization to the sn-1-isomer could have been a concern, or lipolysis might have taken place prior to cellular uptake. It was consistently found that the administered labeled lipids remained in their intact form, i.e., the lipids taken up during 2 min of AP or BL administration were the FA and sn-2-MG species that were administered. Separation of MG isomers by TLC was performed as described previously (13). On average, ∼0.5 to 1% of the injected radiolabeled lipid was recovered in intestinal mucosa, and ∼50% was taken up into the mucosa from intraduodenal administration.
Lipid extraction and metabolite analysis
Intestinal samples were homogenized by 20 strokes of a Dounce homogenizer (0.1–0.15 mm clearance) on ice, using a Wheaton overhead stirrer at 5,000 rpm on ice in 20 ml/g mucosa of 10 mM phosphate buffer containing 150 mM NaCl at pH 7.4 (PBS). Lipids were extracted using chloroform-methanol (2:1; v/v) by the method of Folch, Lees, and Sloane-Stanley (14). Samples were diluted to 1 mg protein/ml of PBS, and all extractions were performed on 1 mg of tissue protein within 1 day following the experiment. Lipid extracts and known standards were spotted on Silica Gel G TLC plates and developed using a nonpolar solvent system (hexane-diethyl ether-acetic acid, 70:30:1; v/v) to separate the lipid classes, and the plates were dried and exposed to iodine vapors to visualize and identify the lipid spots. In the case of tritiated lipids, the spots corresponding to each lipid were scraped into scintillation vials containing 5 ml of ReadySafe scintillation fluid (Beckman Coulter; Fullerton, CA), vortexed vigorously, and counted the following day. For 14C-labeled lipids, the TLC plate was exposed to a phosphorimager screen overnight, and the percent of total lipid extract radioactivity present in each lipid class (excluding oxidation products) was analyzed using a Storm 840 phosphorimager. For FA, the amount of unincorporated label (range 30% to 50% of mucosal counts) was excluded from calculations.
Analysis of PL species
The one-dimensional TLC procedure described by Vaden et al. (15) was used. Briefly, K5 silica gel plates were prewashed in chloroform-methanol (1:1; v/v). Plates were wetted by immersion in 1.8% boric acid in 100% ethanol, dried at room temperature for 5 min, and baked at 100°C for 20 min. The concentration zone was divided into several lanes by scraping parallel lines at 1.5 cm intervals. Aliquots of the total lipid extract were applied to the concentration zone lanes, allowed to dry, and developed using chloroform-ethanol-water-triethylamine (30:35:7:35; v/v). The plate was dried at room temperature in a fume hood for 30 min and then run for a second time in a different tank with the same solvent system, to achieve higher resolution (15). Authentic PL standards were used to identify PL species. After development, radiolableled PLs were detected as above.
FA oxidation
To determine how much of the administered radioactive FA was oxidized, the method of Ontko and Jackson (16) was used with minor modification. Briefly, 1 ml of 1 mg protein/ml sample homogenate was placed in a 15 ml disposable plastic tube. A 0.5 ml Eppendorf tube containing tissue paper soaked in 1 M benzethonium hydroxide was also placed in the 15 ml tube to trap 14CO2. The samples were acidified with 0.3 ml 3M perchloric acid, capped tightly, and incubated overnight at 37°C with shaking. The radioactivity of the tissue paper was determined, the acidified sample was vortexed and centrifuged at 2,800 rpm for 10 min, and radioactivity of the supernatant was determined. Total oxidation was calculated by adding total radioactivity of the supernatant (acid-soluble products, ketones, and TCA intermediates) plus tissue paper (CO2). This number was divided by the amount of radioactivity contained in 1 mg of sample to yield percent oxidation.
Statistical methods
Unless otherwise indicated, data are shown as mean ± SE. Statistical comparisons were performed using Microsoft Excel, and significance was determined by independent, two-tailed Student's t-tests, with P values of 0.05 or lower considered as significantly different.
RESULTS
MG and FA metabolism in rat small-intestinal mucosa
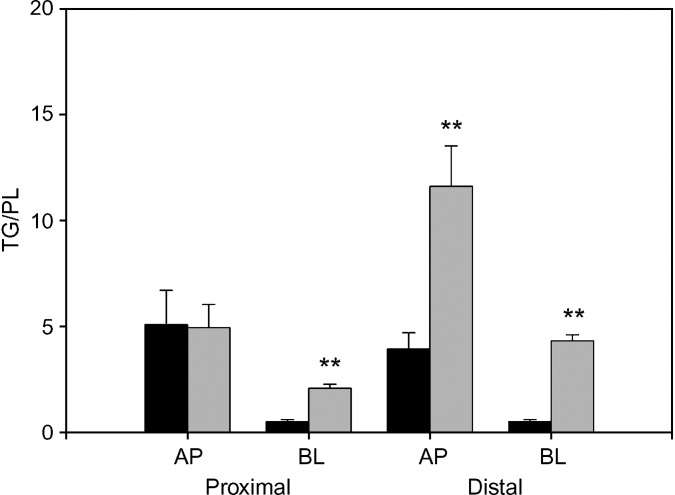
In the present studies, we examined whether sn-2-MG metabolism was dependent upon its site of entry into the absorptive enterocytes, and whether oleic acid demonstrated metabolic polarity similar to that reported for the saturated FA palmitate (3). Labeled lipids were administered to anesthetized male rats as described above. Analysis of radiolabeled metabolites 2 min following administration showed that the major esterified product of apically delivered oleate was TG, whereas the major esterified product of basally delivered oleate was PL (Table 1). Thus, the TG:PL ratios differed by approximately 10-fold. In the proximal half of rat small intestine, TG:PL was 5.1 ± 1.6 for dietary oleate, and 0.53 ± 0.07 for bloodstream oleate (P < 0.01). Essentially no difference in relative incorporation of AP or BL oleate was found between proximal and distal segments of the intestine, despite the known lower absorptive capacity of the distal small intestine (17). The mass of mucosal lipid species following the 2 min administration of tracer lipids at the AP or BL poles of the epithelium were not different (data not shown).
TABLE 1.
Metabolism of apically and basolaterally added FA in rat small intestine
| Proximal |
Distal |
|||
|---|---|---|---|---|
| BL (n = 9) | AP (n = 9) | BL (n = 6) | AP (n = 8) | |
| CE | 8.7 ± 1.7 | 12.9 ± 3.4 | 11.8 ± 3.2 | 14.4 ± 3.3 |
| TG | 14.9 ± 1.7 | 47.9 ± 5.9b | 22.1 ± 3.5 | 46.7 ± 6.0a |
| DG | 18.6 ± 3.5 | 16.0 ± 3.0 | 12.6 ± 2.7 | 21.6 ± 5.6 |
| MG | 15.9 ± 3.0 | 9.1 ± 2.3 | 10.1 ± 0.7 | 10.0 ± 2.3 |
| PL | 31.9 ± 3.7 | 10.9 ± 1.5b | 48.7 ± 5.9 | 12.2 ± 1.4b |
| TG:PL | 0.5 ± 0.1 | 5.1 ± 1.6b | 0.6 ± 0.1 | 3.9 ± 0.8b |
BL, basolateral; AP, apical; CE, cholesteryl ester; TG, triacylglycerol; DG, diacylglycerol; MG, monoacylglycerol; PL, phospholipid. Incorporation of [14C]18:1 into male rat small-intestinal mucosa 2 min after administration was determined as described in Materials and Methods. Results are means ± SE for the percent of total mucosa-associated labeled FA incorporated into each lipid species. 100% = 0.9 nmol for proximal BL sample, 24.9 nmol for proximal AP sample, 1.0 nmol for distal BL sample, and 8.9 nmol for distal AP sample (averages ± 10%).
a P < 0.05, b P < 0.01 versus BL.
The acute metabolic fate of sn-2-monoolein was similarly examined, and the results show clearly that a dramatic metabolic compartmentation occurs depending upon the site of MG entry into the enterocyte. The results in Table 2 show that the TG:PL ratio for MG metabolites in the proximal half of the rat small-intestinal mucosa was 0.44 ± 0.05 for bloodstream MG, and 2.0 ± 0.5 for luminal MG (P < 0.05). This approximately 5-fold difference reflected both a small increase in TG and, particularly, a decrease in MG incorporation into PL in the MG delivered via the AP surface (10.6 ± 0.7% for BL PL, 3.0 ± 0.2% for AP PL, P < 0.01).
TABLE 2.
Metabolism of apically and basolaterally added MG in male rat small intestine
| Proximal |
Distal |
|||
|---|---|---|---|---|
| BL | AP | BL | AP | |
| CE | 4.6 ± 1.9 | 1.9 ± 0.5 | 3.5 ± 0.8 | 3.0 ± 1.0 |
| TG | 4.6 ± 0.7 | 6.1 ± 1.1 | 6.4 ± 0.7 | 10.0 ± 3.1 |
| FA | 27.7 ± 7.9 | 20.9 ± 7.8 | 19.0 ± 5.5 | 21.8 ± 6.7 |
| DG | 36.1 ± 5.9 | 56.3 ± 7.6 | 44.7 ± 4.6 | 51.4 ± 7.2 |
| MG | 16.5 ± 4.9 | 11.8 ± 2.6 | 13.9 ± 3.2 | 9.8 ± 1.6 |
| PL | 10.6 ± 0.7 | 3.0 ± 0.2b | 12.5 ± 2.3 | 4.1 ± 0.8a |
| TG:PL | 0.4 ± 0.1 | 2.0 ± 0.5a | 0.5 ± 0.1 | 2.5 ± 0.6a |
Incorporation of [3H]sn-2-18:1 into rat small-intestinal mucosa 2 min after administration was determined as described in Materials and Methods. Results are means ± SE (n = 4 per group) for the percent of total mucosa-associated labeled MG incorporated into each lipid species. 100% = 17.1 nmol for proximal BL sample, 138.2 nmol for proximal AP sample, 18.0 nmol for distal BL sample, and 44.9 nmol for distal AP sample (averages ± 10%).
a P < 0.05, b P < 0.01 versus BL.
Metabolic fate of sn-2-monoolein and oleate in mouse small intestine
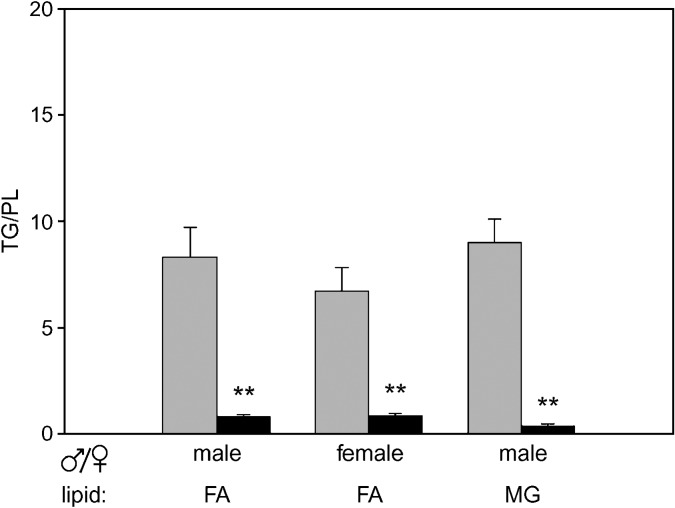
We wished to establish the degree to which metabolic divergence of MG and FA metabolism occurred in mouse intestine, because our ultimate goal is to understand the underlying mechanisms and regulatory controls for this lipid compartmentation. The results in Table 3 show that marked differences in relative partitioning of both substrates occur in the mouse intestine depending upon where they enter the enterocyte layer. Addition of oleate at the AP membrane resulted in more than 3-fold greater incorporation into TG than addition at the BL membrane (48.1 ± 4.2% for AP TG, 15.8 ± 1.8% for BL TG, P < 0.01). Essentially opposite results were found for oleate incorporation into PL, where approximately 3-fold greater incorporation into PL was observed for basolaterally added relative to apically added oleate (20.2 ± 1.4% for BL PL, 7.7 ± 0.6% for AP PL, P < 0.01). For monoolein, similar and somewhat greater metabolic differences were found, with 4-fold greater incorporation of the BL MG label into PL (17.7 ± 4.6%), relative to AP MG (4.6 ± 0.9%; P < 0.01), and almost 7-fold greater incorporation of AP MG label into TG (40.2 ± 7.6%), relative to basal MG (6.1 ± 1.9%; P < 0.01). To determine whether there was a gender difference in this apparent divergence in lipid trafficking, we compared the metabolism of apically and basolaterally added oleate in male and female mouse intestinal mucosa. Figure 1 depicts the TG:PL ratios for FA and MG incorporation in male mice, and for FA in female mice. As noted, large differences are observed for both the FA and MG substrates in male mouse intestine. The dramatic differences in metabolic fate of dietary oleate relative to plasma-derived oleate were found in both male and female mice.
TABLE 3.
Metabolism of apically and basolaterally added FA and MG in mouse small intestine
| FA |
MG |
||||
|---|---|---|---|---|---|
| BL (n = 27) | AP (n = 24) | BL (n = 6) | AP (n = 5) | ||
| CE | 10.0 ± 0.9 | 3.7 ± 0.4b | CE | 4.0 ± 2.1 | 1.2 ± 0.3 |
| TG | 26.8 ± 2.3 | 68.5 ± 3.6b | TG | 6.1 ± 1.9 | 40.2 ± 7.6b |
| DG | 18.2 ± 1.2 | 9.3 ± 1.4b | FA | 38.1 ± 6.3 | 22.9 ± 2.6 |
| MG | 8.1 ± 0.9 | 6.0 ± 1.6 | DG | 22.4 ± 4.8 | 16.8 ± 5.3 |
| PL | 37.0 ± 2.2 | 12.5 ± 1.2b | MG | 11.6 ± 1.6 | 14.3 ± 1.6 |
| TG:PL | 0.8 ± 0.1 | 7.5 ± 1.3b | PL | 17.7 ± 4.6 | 4.6 ± 0.9a |
| TG:PL | 0.4 ± 0.1 | 9.0 ± 1.1b | |||
Incorporation of [14C]18:1 or [3H]sn-2-18:1 into male mouse small-intestinal mucosa 2 min after administration was determined as described in Materials and Methods. Results are means ± SE for the percent of total mucosa-associated labeled FA or MG incorporated into each lipid species. 100% = 1.2 nmol for FA BL sample, 22.4 nmol for FA AP sample, 0.2 nmol for MG BL sample, and 14.1 nmol for MG AP sample (averages ± 10%).
a P < 0.05, b P < 0.01 versus BL.
Fig. 1.
Effect of gender on metabolic compartmentation of FA and monoacylglycerol (MG) in mouse small intestine. Incorporation of [14C]18:1 or [3H]sn-2-18:1 into triacylglycerol (TG) relative to phospholipid (PL) following 2 min incubation at the apical (AP) (gray) or basolateral (BL) (black) surface of the intestine was determined as described under Materials and Methods. Results are means ± SE for the percent total mucosal label incorporated into TG relative to that incorporated into PL. n = 24–27 for male FA, 5–6 for female FA, and 5–6 for male MG. ** P < 0.01 versus AP.
Apically and basolaterally added oleate incorporation into different PL species
We examined whether, in addition to the quantitative difference in PL formed, there might also be a qualitative difference in the PL species in which the fatty acyl chains become enriched. Analysis of radiolabel incorporation into male mouse intestinal PLs, shown in Table 4, indicates that the incorporation of oleate into specific PLs is strongly dependent on its site of entry into the enterocyte. Whereas phosphatidylcholine (PC) and phosphatidylethanolamine (PE) were the two most-abundant labeled species for both incubation conditions, their relative distributions were considerably different. A >2-fold greater incorporation was found for apically added FA into PE relative to basolaterally added FA, with substantially decreased incorporation into PC and PI for AP FA relative to BL FA. Thus, the PC:PE ratio for BL FA was 2.2 ± 0.2, whereas for AP FA it was 0.7 ± 0.04 (P < 0.01). In female mouse intestine, similar results were observed (Table 4), where once again the largest difference appears to be in the incorporation of labeled fatty acyl chains into PE, which is more than 2-fold greater for dietary delivery than for bloodstream delivery of the 18:1 substrate (P < 0.01).
TABLE 4.
Incorporation of apically and basolaterally added oleic acid into phospholipid species in mouse small-intestinal mucosa
| Male |
Female |
|||
|---|---|---|---|---|
| BL (n = 14) | AP (n = 13) | BL (n = 4) | AP (n = 4) | |
| PA | 4.1 ± 1.0 | 3.4 ± 0.4 | 5.5 ± 1.6 | 1.4 ± 0.7 |
| PE | 19.2 ± 2.0 | 42.4 ± 2.5b | 19.4 ± 3.8 | 46.0 ± 1.7b |
| PG | 5.5 ± 1.2 | 2.7 ± 0.5a | 4.9 ± 2.1 | 0.5 ± 0.1 |
| PS | 5.7 ± 0.8 | 3.3 ± 0.8 | 4.3 ± 4.3 | 0.9 ± 0.1 |
| PI | 16.9 ± 2.8 | 10.7 ± 1.1 | 14.5 ± 1.0 | 8.5 ± 0.4a |
| PC | 38.0 ± 2.9 | 30.3 ± 1.7a | 40.9 ± 5.8 | 41.2 ± 2.1 |
| SM | 4.6 ± 1.0 | 3.9 ± 0.8 | 5.7 ± 2.8 | 0.5 ± 0.1 |
| LPC | 5.8 ± 1.2 | 6.1 ± 0.8 | 5.1 ± 1.7 | 1.1 ± 0.2 |
| PC:PE | 2.19 ± 0.24 | 0.73 ± 0.04b | 2.28 ± 0.39 | 0.90 ± 0.08a |
Incorporation of [14C]18:1 into phospholipid species at 2 min following administration either into the duodenum (AP) or bloodstream (BL), as described in Materials and Methods. Results are means ± SE for the percent of total mucosa-associated labeled FA incorporated into each PL species. 100% = 0.23 nmol for male BL sample, 1.72 nmol for male AP sample, 0.25 nmol for female BL sample, and 1.81 nmol for female AP sample.
a P < 0.05, b P < 0.01 versus BL.
Effect of site of administration of [14C]18:1 on oxidation in intestinal mucosa
Whereas the majority of exogenous FAs are used by the intestine for anabolic purposes, particularly in the fed state, a small fraction are also oxidized, primarily in mitochondria. We determined the percent of mucosal oleic acid that was oxidized when administered at the AP versus the BL surfaces, and the results show that, as for FA anabolic reactions, catabolism too was markedly influenced by the cellular point of entry. Whereas 5.1 ± 0.6% of dietary oleate in the mouse mucosa was oxidized to CO2 and acid-soluble products, for bloodstream-delivered oleate, 12.1 ± 1.3% was oxidized (P < 0.01). Following 18 h fasting, the fraction of mucosal FA that was oxidized increased for BL-delivered oleate (28.6 ± 2.2%) but not for AP-derived oleate (2.6 ± 0.4%); thus, the difference in AP versus BL FA oxidation was maintained or even greater in the fasted state (P < 0.001).
Effect of overnight fasting on [14C]oleate metabolism in rat intestinal mucosa
To determine whether the partitioning of FA into different metabolites was a fixed aspect of enterocyte metabolism or could be regulated by physiological state, animals were fasted overnight, and the incorporation of labeled oleate into esterified products was determined. As shown in Fig. 2, fasting resulted in a large increase in the incorporation of FA into TG relative to PL. The increase was quite marked for bloodstream-delivered oleate, where the proximal intestine TG:PL ratio rose 4-fold, from 0.5 ± 0.1 in the fed state to 2.1 ± 0.2 after fasting (P < 0.01). The increase in the distal small intestine was especially large, where TG:PL rose over 7-fold, from 0.6 ± 0.1 in the fed state to 4.3 ± 0.3 after overnight fasting (P < 0.01). For apically administered oleate, an increase in TG:PL was also observed, but only in the distal half of the small intestine.
Fig. 2.
Effect of short-term fasting on the metabolic fate of apically and basolaterally added oleate by small-intestinal mucosa. Rats were either in the fed state (black) or had been without food overnight (gray). Incorporation of [14C]18:1 into TG relative to PL following 2 min incubation was determined as described in Materials and Methods. Results are means ± SE for the percent total mucosal label incorporated into TG relative to that incorporated into PL. n = 7–10 per group. ** P < 0.01 versus fed animals.
As noted above for mouse intestine, in rats we also found different degrees of oxidation; for BL oleate, 11% was oxidized and for AP oleate, 4% was oxidized. Further, the increase in oxidation following an overnight fast was observed for bloodstream oleate, which rose to almost 30% of mucosal FA oxidized, but not for dietary oleate, which remained at ≤5% (data not shown).
DISCUSSION
The AP uptake of diet-derived MG is well appreciated; however, it was not established whether plasma MG was available to the intestinal cell (17), or whether compartmentation of MG metabolism occurs. Here, we show for the first time that circulating MG is taken up into the intestinal mucosal cell layer. Moreover, we demonstrate a marked metabolic compartmentation of MG metabolism in both rat and mouse small-intestinal mucosa.
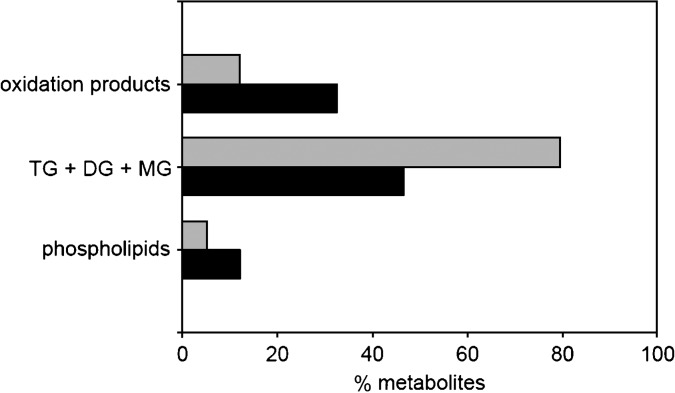
In agreement with previous findings for the saturated FA palmitate (3, 4), a striking degree of metabolic polarity, based upon delivery site, was found for intestinal metabolism of the monounsaturated FA oleate. Figure 3 summarizes the overall incorporation of oleic acid into TG and its esterification intermediates, PL, and oxidation products, added to the intestine from the dietary or plasma routes. The results highlight the striking divergence for dietary compared with bloodstream FA metabolism. These findings establish the fundamental nature of this metabolic divergence within a single cell, and suggest a conserved and potentially important mechanism for channeling of dietary and plasma lipids to specific subcellular sites within the enterocyte.
Fig. 3.
Overall metabolic fate of oleic acid in mouse intestinal mucosa. Data from Table 3 and Results are combined to illustrate the relative amounts of mucosa-associated labeled oleate incorporated into oxidation products, TG and its esterification intermediates, and phospholipids, for addition at the AP (gray) or BL (black) surface of the mouse proximal intestine. DG, diacylglycerol.
The percent of mucosal oleate oxidized by the intestinal mucosa was small; only 5% of that taken up from the intestinal lumen was oxidized, and about 13% of that taken up from the plasma. In terms of overall ATP generation by small-intestinal mucosa, it is worth noting that the oxidative breakdown of FA is not of quantitative importance, accounting for only 3% of CO2 production (18). Thus, the observation that a small but measurable fraction of FA is oxidized by intestinal mucosa points to a role for the oxidative metabolites formed that may not be directly related to ATP supply. It has been shown that during a steady-state infusion of luminal triolein in the rat, a very small percent of plasma FA is oxidized (6), in contrast to the levels consistently found here in a low-fat-fed state in mice, and by Gangl and Ockner (3) in rats under similar conditions. Nevertheless, it is possible that even though the fractional level of FA oxidized is low under high-fat feeding conditions, the absolute production of oxidative metabolites is maintained. Whether high-fat infusion or high-fat feeding alters the metabolic compartmentation of lipids presented to the enterocyte is currently under investigation.
The basic mechanisms underlying the cellular polarity of enterocyte lipid metabolism are not known, but several possibilities may be considered. The substantial oxidation of BL lipids, relative to AP lipids, may be considered to arise, at least in part, from the polarized distribution of subcellular organelles in the enterocyte. Ultrastructural examination shows that mitochondria are distributed in both the AP and BL regions of the enterocyte, whereas the endoplasmic reticulum (ER), responsible for anabolic metabolism of lipids, is primarily localized to the AP cytoplasm (19). This would mean that lipids entering from the BL surface would initially encounter the oxidative mitochondria, whereas lipids entering from the dietary surface would encounter both mitochondria and the abundant ER. A consideration of the rates at which FA and MG might come upon different regions of the cell does not strongly support this mechanism of lipid metabolic polarity, however. Using the Stokes-Einstein relationship, the calculated diffusion coefficients (D) for oleic acid and monoolein at 37°C, taking into account the 8-fold increased viscosity of cytoplasm relative to water (20), are 5.8 × 10−7 and 5.4 × 10−7 cm2/sec, respectively. If the length of a columnar absorptive cell is taken at ∼25 μm, the lipid monomers could traverse such a distance in <3 s, with diffusion time = (distance)2/4D. If the lipids are present not as free monomers but, as is more likely, bound to ∼15 kDa fatty acid binding proteins (FABPs), the calculated diffusion times for FA and MG to traverse the enterocyte cytoplasmic length would be approximately 10 s The present experiments were conducted following a 120 s incubation, suggesting that in the absence of specific targeting mechanisms, homogenous distributions of both substrates could be expected.
The metabolic divergence in TG:PL ratios for BL compared with AP delivery of MG and FA may be based upon the existence of divergent pathways for TG synthesis. Unlike most cell types, which primarily or solely contain the so-called glycerol-3-phosphate (G3P) pathway of TG synthesis, the small intestine can synthesize TG via both the G3P pathway and the CoA-dependent MG pathways of acylglycerol synthesis, both of which are ER localized (17, 21, 22) These are thought to contribute about 20% and 80%, respectively, to total TG levels in the secreted chylomicron (21, 22). When the FA and sn-2-MG levels are low, the G3P pathway becomes a major route for synthesis of TG in the enterocyte (21). The final reaction in both the MG and G3P pathways is the conversion of diacylglycerol (DG) to TG via diacylglycerol acyltransferase (DGAT). The intestine expresses at least two genes that encode for enzymes with DGAT activity, DGAT1 and DGAT2 (23, 24). It is not yet clear whether the DGAT activities in the MG and G3P pathways are distinct, or whether intestinal DGATs might be involved in the metabolic compartmentation of AP and BL lipids. In this regard, Zammit and colleagues (25, 26) reported the presence of two pools of DGAT in liver microsomes, cytosol-facing and latent, facing the ER lumen. It is thus possible that separate pools of DG exist, which are metabolized at different rates, and/or with varying amounts of DG converted to TG, relative to PL.
The concept of spatially distinct lipid precursor pools is supported by reports showing that the MG pathway is primarily associated with smooth ER, thought to be the main site of chylomicron assembly (27), whereas the G3P pathway is localized to the rough ER (28). Indeed, Mansbach and Arnold (29, 30) have proposed that there are two pools of TG associated with the enterocyte ER, one formed primarily from diet-derived precursors, the other from endogenous sources, which would include FA taken up via the BL surface. It is also possible that the albumin-bound BL-administered lipid may redistribute to circulating lipoproteins, and that endocytosed lipids are metabolized differently from those that enter as monomers.
The simultaneous expression within the enterocyte of intestinal and liver-type FABPs (IFABP and LFABP) can also be considered as a potential source of metabolic compartmentation for their ligands, particularly because the FABPs are thought to be involved in specific ligand targeting (31). However, immunocytochemical studies showed no large-scale differences in FABP localization (32), and it has been reported that both enterocyte FABPs are located in the AP pole of the enterocyte in the fasted state, and are distributed throughout the cell cytoplasm upon fat feeding (33). Moreover, neither IFABP nor LFABP is highly expressed in crypt cells, where lipid metabolic polarity was also observed (3). Taken together, these findings suggest that the FABPs are not a major contributing factor to the observed metabolic differences between AP and BL substrates, and preliminary studies in LFABP and IFABP knockout mice indicate that AP versus BL metabolic compartmentation of FA is largely maintained (unpublished observations).
Compartmentation of FA and MG metabolism may also be related to the acyl CoA synthetases (ACSs), because both anabolic and catabolic metabolism of FA, and the conversion of MG to DG, require activation to their CoA derivatives. ACS5 is highly expressed in the small intestine (34), ACS3 is expressed at a low level (35), and ACS1, with widespread tissue distribution, is also found (36). In the liver, Coleman and colleagues (37, 38) have demonstrated distinct subcellular distributions and functional properties for different ACS forms. Inhibitor studies, furthermore, have suggested that ACS5 is functionally linked to PL synthesis and FA β-oxidation, and that ACS1 may be linked to TG synthesis (39). Thus, the ACSs could play a role in the partitioning of FA between anabolic and catabolic pathways (38) and in distinguishing the pathways for TG and PL biosynthesis.
The relative amounts of PL and TG formed from AP versus BL addition of FA are not fixed but, rather, can be modified by physiological state. Perhaps surprisingly, a significant increase in the amount of TG formed relative to PL was observed following an 18 h fast. This could be envisioned as a mechanism of ensuring that the intestine continues to deliver necessary calories, in the form of TG, to the rest of the body when nutrient uptake from the diet is absent. It has been shown in rats that approximately 20% of mucosal protein is lost upon fasting (40), and it is possible that the enzymes of TG synthesis are preserved relative to the PL synthetic enzymes. Replenishment of PL might also occur during fasting from the AP absorption and subsequent acylation of lysoPC formed from biliary PC present in the intestinal lumen (41), sparing FA for incorporation into TG.
The large increase in the amount of labeled PE formed for AP relative to BL addition of oleate may also arise because of different pools of DG within the cell. Both PC and PE can be synthesized from DG by the actions of CDP-choline-1,2 diacylglycerol cholinephosphotransferase and CDP-ethanolamine-1,2 diacylglycerol ethanolaminephosphotransferase, respectively (42). PE can also be converted to PC by successive methylations using S-adenosyl methionine; however, this pathway is not thought to play an important role in the intestine (43, 44). The present results would suggest that the putative DG pool that is generated from the AP addition of substrates is formed via monoacylglycerolacyltransferase action and is used primarily for TG; however, the small amount of PL synthesized from AP delivery of FA, probably from that same DG pool, is converted more to PE than to PC. Because the dietary lipids are thought to be used primarily for chylomicron formation (6), the addition of PE could be important for formation of the prechylomicron transport vesicles that bud off the ER and fuse with the Golgi (45, 46), inasmuch as PE is known to be important for bilayer deformation and membrane fusion and fission events (47).
In summary, we show here for the first time the uptake of plasma MG into the intestinal mucosa, and that metabolic segregation of MGs, as well as FAs, occurs and is markedly dependent upon their site of entry into the enterocyte. These results strongly support the existence of separate pools, not only of TG but also of glycerolipid intermediates. The results also show that the degree of metabolic compartmentation is physiologically regulated, underscoring the importance of lipid trafficking in the regulation of intracellular metabolism. Interestingly, it has been proposed that in the hepatocyte, also a polarized cell, plasma FAs are directed toward β-oxidation and ketogenesis, whereas endogenously generated FAs from cytosolic TG storage pools are mobilized for very low density lipoprotein formation (48).
The nonuniform metabolism of lipid substrates observed in this study is likely to arise from multiple protein-mediated binding, transport, and catalytic events, similar to the well-appreciated nonuniformity of cell membrane lipid composition (49). Elucidating the mechanisms that underlie the profound differences in metabolism of AP versus BL lipids could enable the modulation of lipid metabolic fate in the enterocyte, and perhaps in other polarized epithelial cells as well.
Acknowledgments
The authors wish to thank Drs. Charles Mansbach II, R. Ariel Igal, and Malcolm Watford for helpful discussions.
Abbreviations
ACS, acyl CoA synthetase
AP, apical
BL, basolateral
DG, diacylglycerol
DGAT, diacylglycerol acyltransferase
ER, endoplasmic reticulum
FABP, fatty acid binding protein
G3P, glycerol-3-phosphate
IFABP, intestinal FABP
LFABP, liver FABP
MG, monoacylglycerol
PC, phosphatidylcholine
PE, phosphatidylethanolamine
PL, phospholipid
TC, taurocholate
TG, triacylglycerol
Published, JLR Papers in Press, April 17, 2008.
Footnotes
This work was supported by National Institutes of Health Grant DK-38389 and the Busch Biomedical Research Fund.
References
- 1.Clarke S. D. 2001. Polyunsaturated fatty acid regulation of gene transcription: a molecular mechanism to improve the metabolic syndrome. J. Nutr. 131 1129–1132. [DOI] [PubMed] [Google Scholar]
- 2.Dinh T. P., D. Carpenter, F. M. Leslie, T. F. Freund, I. Katona, S. L. Sensi, S. Kathuria, and D. Piomelli. 2002. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl. Acad. Sci. USA. 99 10819–10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gangl A., and R. K. Ockner. 1975. Intestinal metabolism of plasma free fatty acids. Intracellular compartmentation and mechanisms of control. J. Clin. Invest. 55 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gangl A., and F. Renner. 1978. In vivo metabolism of plasma free fatty acids by intestinal mucosa of man. Gastroenterology. 7 847–850. [PubMed] [Google Scholar]
- 5.Mansbach II C. M., and S. Parthasarathy. 1982. A re-examination of the fate of glyceride-glycerol in neutral lipid absorption and transport. J. Lipid Res. 23 1009–1019. [PubMed] [Google Scholar]
- 6.Mansbach C. M., and R. F. Dowell. 1992. Uptake and metabolism of circulating fatty acids by rat intestine. Am. J. Physiol. 263 G927–G933. [DOI] [PubMed] [Google Scholar]
- 7.Trotter P. J., and J. Storch. 1991. Fatty acid uptake and metabolism in a human intestinal cell line (Caco-2): comparison of apical and basolateral incubation. J. Lipid Res. 32 293–304. [PubMed] [Google Scholar]
- 8.Ho S. Y., L. Delgado, and J. Storch. 2002. Monoacylglycerol metabolism in human intestinal Caco-2 cells: evidence for metabolic compartmentation and hydrolysis. J. Biol. Chem. 277 1816–1823. [DOI] [PubMed] [Google Scholar]
- 9.Fielding B. A., S. M. Humphreys, R. F. Allman, and K. F. Frayn. 1993. Mono-, di- and triacylglycerol concentrations in human plasma: effects of heparin injection and of a high-fat meal. Clin. Chim. Acta. 216 167–173. [DOI] [PubMed] [Google Scholar]
- 10.El Maghrabi M. R., M. Waite, L. L. Rudel, and P. Sisson. 1978. Hydrolysis of monoacylglycerol in lipoprotein remnants catalyzed by the liver plasma membrane monoacylglycerol acyltransferase. J. Biol. Chem. 253 974–981. [PubMed] [Google Scholar]
- 11.Thumser A. E. A., A. G. Buckland, and D. C. Wilton. 1998. Monoacylglycerol binding to human serum albumin: evidence that monooleoylglycerol binds at the dansylsarcosine site. J. Lipid Res. 39 1033–1038. [PubMed] [Google Scholar]
- 12.Ho S. Y., and J. Storch. 2001. Common mechanisms of monoacylglycerol and fatty acid uptake by human intestinal Caco-2 cells. Am. J. Physiol. 281 C1106–C1117. [DOI] [PubMed] [Google Scholar]
- 13.Chon S. H., Y. X. Zhou, J. L. Dixon, and J. Storch. 2007. Intestinal monoacylglycerol metabolism: Developmental and nutritional regulation of monoacylglycerol lipase and monoacylglycerol acyltransferase. J. Biol. Chem. 282 33346–33357. [DOI] [PubMed] [Google Scholar]
- 14.Folch J., M. Lees, and G. H. Sloane-Stanley. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226 497–509. [PubMed] [Google Scholar]
- 15.Vaden D. L., V. M. Gohil, Z. Gu, and M. L. Greenberg. 2005. Separation of yeast phospholipids using one-dimensional thin-layer chromatography. Anal. Biochem. 338 162–164. [DOI] [PubMed] [Google Scholar]
- 16.Ontko J. A., and D. Jackson. 1964. Factors affecting the rate of oxidation of fatty acids in animal tissues. Effect of substrate concentration, pH, and coenzyme A in rat liver preparations. J. Biol. Chem. 239 3674–3682. [PubMed] [Google Scholar]
- 17.Kuksis, A., and R. Lehner. 2001. Intestinal synthesis of triacylglycerols. In Intestinal Lipid Metabolism. C. M. Mansbach II, P. Tso, and A. Kuksis, editors. Kluwer Academic/Plenum Publishers, New York. 185–213.
- 18.Windmueller H. G., and A. E. Spaeth. 1978. Identification of ketone bodies and glutamine as the major respiratory fuels in vivo for postabsorptive rat small intestine. J. Biol. Chem. 253 69–76. [PubMed] [Google Scholar]
- 19.Porter, K. R., and M. A. Bonneville. 1963. An Introduction to the Fine Structure of Cells and Tissues. Lea & Febiger, Philadelphia.
- 20.Lang I., M. Scholz, and R. Peters. 1986. Molecular mobility and nucleocytoplasmic flux in hepatoma cells. J. Cell Biol. 102 1183–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tso, P., and K. Crissinger. 2006. Digestion and absorption of lipids. In Biochemical and Physiological Aspects of Human Nutrition, 2nd edition. M. H. Stipanuk, editor. W.B. Saunders Company, Philadelphia. 125–141.
- 22.Johnston J. M., F. Paultuf, C. M. Schiller, and L. Schultz. 1970. The utilization of the alpha-glycerophosphate and monoglyceride pathways for phosphatidyl choline biosynthesis in the intestine. Biochim. Biophys. Acta. 218 124–133. [DOI] [PubMed] [Google Scholar]
- 23.Cases S., S. J. Stone, P. Zhou, E. Yen, B. Tow, K. D. Lardizabal, T. Voelker, and R. V. Farese, Jr. 2001. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J. Biol. Chem. 276 38870–38876. [DOI] [PubMed] [Google Scholar]
- 24.Buhman K. K., S. J. Smith, S. J. Stone, J. J. Repa, J. S. Wong, F. F. Knapp, Jr., B. J. Burri, R. L. Hamilton, N. A. Abumrad, and R. V. Farese, Jr. 2002. DGAT1 is not essential for intestinal triacylglycerol absorption or chylomicron synthesis. J. Biol. Chem. 277 25474–25479. [DOI] [PubMed] [Google Scholar]
- 25.Owen M. R., C. C. Corstorphine, V. A. Zammit. 1997. Overt and latent activities of diacylglycerol acytransferase in rat liver microsomes: possible roles in very-low-density lipoprotein triacylglycerol secretion. Biochem. J. 323 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waterman I. J., N. T. Price, and V.A. Zammit. 2002. Distinct ontogenic patterns of overt and latent DGAT activities of rat liver microsomes. J. Lipid Res. 43 1555–1562. [DOI] [PubMed] [Google Scholar]
- 27.Cartwright I. J., D. Plonne, and J. A. Higgins. 2000. Intracellular events in the assembly of chylomicrons in rabbit enterocytes. J. Lipid Res. 41 1728–1739. [PubMed] [Google Scholar]
- 28.Cartwright I. J., J. A. Higgins, J. Wilkinson, S. Bellavia, J. S. Kendrick, and J. M. Graham. 1997. Investigation of the role of lipids in the assembly of very low density lipoproteins in rabbit hepatocytes. J. Lipid Res. 38 531–545. [PubMed] [Google Scholar]
- 29.Mansbach II, C. M. 2001. Triacylglycerol movement in enterocytes. In Intestinal Lipid Metabolism. C. M. Mansbach II, P. Tso, and A. Kuksis, editors. Kluwer Academic/Plenum Publishers, New York. 215–233.
- 30.Mansbach II C. M., and A. Arnold. 1986. Steady-state kinetic analysis of triacylglycerol delivery into mesenteric lymph. Am. J. Physiol. 251 G263–G269. [DOI] [PubMed] [Google Scholar]
- 31.Storch J., and A. E. A. Thumser. 2000. The fatty acid transport function of fatty acid-binding proteins. Biochim. Biophys. Acta. 1486 28–44. [DOI] [PubMed] [Google Scholar]
- 32.Shields H. M., M. L. Bates, N. M. Bass, C. J. Best, D. H. Alpers, and R. K. Ockner. 1986. Light microscopic immunocytochemical localization of hepatic and intestinal types of fatty acid-binding proteins in rat small intestine. J. Lipid Res. 27 549–557. [PubMed] [Google Scholar]
- 33.Alpers D. H., N. M. Bass, M. J. Engle, K. De, and K. Schryver-Kecskemeti. 2000. Intestinal fatty acid binding protein may favor differential apical fatty acid binding in the intestine. Biochim. Biophys. Acta. 1483 352–362. [DOI] [PubMed] [Google Scholar]
- 34.Oikawa E., H. Iijima, T. Suzuki, H. Sasano, H. Sato, A. Kamataki, H. Nagura, M-J. Kang, T. Fujino, H. Suzuki, and T.T. Yamamoto. 1998. A novel acyl-CoA synthetase, ACS5, expressed in intestinal epithelial cells and proliferating preadipocytes. J. Biochem. 124 679–685. [DOI] [PubMed] [Google Scholar]
- 35.Fujino T., M-J. Kang, H. Suzuki, H. Iijima, and T. Yamamoto. 1996. Molecular characterization and expression of rat acyl-CoA synthetase 3. J. Biol. Chem. 271 16748–16752. [DOI] [PubMed] [Google Scholar]
- 36.Smith, P. J. 2001. Acyl coenzyme A synthetases. In Nutrient-Gene Interactions in Health and Disease. N. Moustaid-Moussa and C. D. Berdainer, editors. CRC Press, Boca Raton, FL. 77–100.
- 37.Kim J-H., T. M. Lewin, and R. A. Coleman. 2001. Expression and characterization of recombinant rat Acyl-CoA synthetases 1, 4, and 5. Selective inhibition by triacsin C and thiazolidinediones. J. Biol. Chem. 276 24667–24673. [DOI] [PubMed] [Google Scholar]
- 38.Lewin T. M., J-H. Kim, D. A. Granger, J. E. Vance, and R. A. Coleman. 2001. Acyl-CoA synthetase isoforms 1, 4, and 5 are present in different subcellular membranes in rat liver and can be inhibited independently. J. Biol. Chem. 276 24674–24679. [DOI] [PubMed] [Google Scholar]
- 39.Igal R. A., P. Wang, and R. A. Coleman. 1997. Triacsin C blocks de novo synthesis of glycerolipids and cholesterol esters but not recycling of fatty acid into phospholipid: evidence for functionally separate pools of acyl-CoA. Biochem. J. 324 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McManus J. P., and K. J. Isselbacher. 1970. Effect of fasting versus feeding on the rat small intestine. Morphological, biochemical, and functional differences. Gastroenterology. 59 214–221. [PubMed] [Google Scholar]
- 41.Nervi F. 2000. Significance of biliary phospholipids for maintenance of the gastrointestinal mucosal barrier and hepatocellular integrity. Gastroenterology. 118 1265–1267. [DOI] [PubMed] [Google Scholar]
- 42.Vance, D. E. 2002. Phospholipid synthesis in eukaryotes. In Biochemistry of Lipids, Lipoproteins, and Membranes. 4th edition. D. E. Vance and J. E. Vance, editors. Elsevier, Amsterdam. 205–232.
- 43.Gurr M. I., D. N. Brindley, and G. Hubscher. 1965. Metabolism of phospholipids. 8. Biosynthesis of phosphatidylcholine in the intestinal mucosa. Biochim. Biophys. Acta. 98 486–501. [DOI] [PubMed] [Google Scholar]
- 44.Cui Z., J. E. Vance, M. H. Chen, D. R. Voelker, and D. E. Vance. 1993. Cloning and expression of a novel phosphatidylethanolamine N-methyltransferase. A specific biochemical and cytological marker for a unique membrane fraction in rat liver. J. Biol. Chem. 268 16655–16663. [PubMed] [Google Scholar]
- 45.Redgrave T. G. 1971. Association of Golgi membranes with lipid droplets (pre-chylomicrons) from within intestinal epithelial cells during absorption of fat. Aust. J. Exp. Biol. Med. Sci. 49 209–224. [DOI] [PubMed] [Google Scholar]
- 46.Siddiqi S. A., S. Siddiqi, J. Mahan, K. Peggs, F. S. Gorelick, and C. M. Mansbach II. 2006. The identification of a novel endoplasmic reticulum to Golgi SNARE complex used by the prechylomicron transport vesicle. J. Biol. Chem. 281 20974–20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dowhan, W., and M. Bogdanov. 2002. Functional roles of lipids in membranes. In Biochemistry of Lipids, Lipoproteins, and Membranes. 4th edition. D. E. Vance and J. E. Vance, editors. Elsevier, Amsterdam. 1–36.
- 48.Gibbons G. F., K. Islam, and R. J. Pease. 2000. Mobilisation of triacylglycerol stores. Biochim. Biophys. Acta. 1483 37–57. [DOI] [PubMed] [Google Scholar]
- 49.Holthuis J. C., G. van Meer, and K. Huitema. 2003. Lipid microdomains, lipid translocation and the organization of intracellular membrane transport. Mol. Membr. Biol. 20 231–241. [PubMed] [Google Scholar]