Abstract
Members of the polo subfamily of protein kinases play pivotal roles in cell-cycle control and proliferation. In addition to a high degree of sequence similarity in the kinase domain, polo kinases contain a strikingly conserved motif termed “polo-box” in the noncatalytic C-terminal domain. We have previously shown that the mammalian polo-like kinase Plk is a functional homolog of Saccharomyces cerevisiae Cdc5. Here, we show that, in a polo-box- and kinase activity-dependent manner, ectopic expression of Plk in budding yeast can induce a class of cells with abnormally elongated buds. In addition to localization at spindle poles and cytokinetic neck filaments, Plk induces and localizes to ectopic septin ring structures within the elongated buds. In contrast, mutations in the polo-box abolish both localization to, and induction of, septal structures. Consistent with the polo-box-dependent subcellular localization, the C-terminal domain of Plk, but not its polo-box mutant, is sufficient for subcellular localization. Our data suggest that Plk may contribute a signal to initiate or promote cytokinetic event(s) and that an intact polo-box is required for regulation of these cellular processes.
Keywords: septin, mitosis, cytokinesis
Polo kinases play pivotal roles in cell division and proliferation. The polo subfamily members are characterized by the presence of a distinct region of homology in the C-terminal noncatalytic domain, termed the polo-box (amino acids 410–439 in Plk) (1), which appears to be critical for the subcellular localization of polo kinases (2, 3). Members of this subfamily include mammalian Plk (1, 4–7), Snk (8), and Fnk/Prk (9, 10), Xenopus laevis Plx1 (11), Drosophila melanogaster polo (12), Schizosaccharomyces pombe Plo1 (13), and Saccharomyces cerevisiae Cdc5 (14). Studies have shown that polo kinases regulate diverse cellular and biochemical events at various stages of M phase (see reviews, refs. 15 and 16). These include centrosome maturation (17) and bipolar spindle formation (12, 13, 17), activation of Cdc2 through Cdc25C phosphatase (11), DNA damage checkpoint adaptation (18), and regulation of the anaphase-promoting complex (19, 20). In addition to these roles, a growing body of evidence suggests that polo kinases play important role(s) in the regulation of cytokinesis (see review, ref. 21).
Cytokinesis is a highly coordinated cellular process achieved by contractile-ring formation, and subsequent contraction of this ring divides one cell to form two cells. Temporal and spatial regulation of the cytokinetic machinery is pivotal to ensure equal partitioning of genomic and cellular materials into two dividing cells. Studies from various organisms show that polo kinases are widely involved in regulating cytokinetic structures. In fission yeast, loss of plo1+ function leads to mitotic arrest, with failure of both formation of the F-actin ring and deposition of septal components (13). A recent report demonstrates that mutations in plo1 result in defective placement and organization of the medial ring (22), suggestive of a role in the recruitment of medial-ring components and localization of the division plane to the cell center. In contrast, overexpression of plo1+ induces multiple septa at any phase of the cell cycle (13), suggesting that Plo1 is both essential and sufficient for septum- and actin-ring formation. In addition, Plo1 localizes to the spindle poles and spindles of mitotic cells and to the medial ring at the time of its formation (22). It is also shown to interact with Mid1 (22), a protein essential for positioning the division plane to the cell center (23). These data suggest that Plo1 plays a direct role in cytokinesis.
In budding yeast, ectopic expression of a murine polo-like kinase, Plk, a functional homolog of S. cerevisiae Cdc5 (24), can induce a class of cells with unusually elongated buds that possess ectopic septal-ring structures within the abnormally elongated buds (24). Overexpression of Cdc5 also induces elongated cells with ectopic septin-ring structures (3). In addition, both Plk and Cdc5 are shown to localize at spindle poles and cytokinetic neck filaments (2, 3), both of which play important roles in cytokinesis (21).
A role for polo kinases in cytokinesis has been suggested in higher eukaryotic organisms as well. Recent studies (25) in a Drosophila polo mutant suggested that polo is required to form correct midzone and midbody structures during telophase. Furthermore, the polo mutant failed to localize the kinesin-like protein Pavarotti (26) to the midzone and to incorporate actin and the septin Peanut (27) into a contractile ring. In cultured mammalian cells, transient expression of either wild-type or kinase-inactive Plk results in the accumulation of cells with multiple nuclei (28), suggesting that overproduction of Plk functions as dominant-negative and inhibits cytokinesis in a kinase activity-independent manner.
Although it is apparent that polo kinases play multiple roles during M-phase progression and in cytokinesis, the requirement of the polo-box for the proposed functions is not clearly understood. In this communication, we utilized Plk expression in budding yeast to provide evidence that the polo-box is required for the ability of Plk to induce a class of cells with abnormally elongated buds and to induce and localize at ectopic septin-ring structures. In addition, we have also demonstrated that the C-terminal domain of Plk, which contains the polo-box, is sufficient to localize at spindle poles and cytokinetic neck filaments. The data reported here indicate that, in addition to localization of Plk, the polo-box is required for the ability of Plk to induce cytokinetic structures.
Materials and Methods
Strains, Growth Conditions, and Transformations.
Yeast strains used in this study are 1788 (isogenic diploid of EG123, MATα, leu2–3, 112 ura3–52 trp1-1 his4 can1r) (29). Yeast cells were cultured in YEP (1% yeast extract/2% Bacto-peptone) supplemented with 2% glucose, 2% raffinose (Sigma), or 2% galactose (J. T. Baker) as required. Synthetic minimal medium (30) supplemented with the appropriate nutrients was employed to select for plasmid maintenance. Yeast transformation was carried out by the lithium acetate method (31).
Generation of Plk Mutants and Enhanced Green Fluorescent Protein (EGFP)-Plk Fusion Constructs.
YCplac111-GAL1-HA-Plk, -PlkT210D, and -PlkW414F were described previously (2, 24). YCplac111-GAL1-HA-EGFP-Plk fusion construct was generated by inserting three copies of the 700-bp XhoI fragment of the EGFP coding sequence into the XhoI site present at the N terminus of the PLK coding sequence. Site-directed mutagenesis in a murine PLK cDNA was carried out with the Sculptor in Vitro Mutagenesis System (Amersham Pharmacia). YCplac22-GAL1-HA-EGFP-PlkΔN and -EGFP-PlkΔN/W414F/L427A fusions were generated by replacing a PpuMI–EcoRV fragment within the CDC5 coding sequence in YCplac22-GAL1-HA-EGFP-Cdc5 (3) with PCR fragments (amino acids 322–603 followed by a translational terminal codon) of either wild-type Plk or PlkW414F/L427A. The resulting constructs replace CDC5 coding sequence from amino acids 6 to 678 with that of PLK and possess two copies of EGFP downstream of the hemagglutinin (HA) epitope tag. The EGFP coding sequence was amplified by PCR with the pEGFP-N1 plasmid (CLONTECH) as a template. All the mutants were sequenced to confirm the introduced mutations.
Expression of Plk in S. cerevisiae and Western Analyses.
To express wild-type and mutant forms of Plk in S. cerevisiae under the control of GAL1 promoter, yeast transformants harboring each construct were grown in YEP-raffinose to an OD600 of 0.5 at 30°C. Cells were harvested, resuspended in YEP-galactose, then cultured continuously.
Yeast cells were lysed with an equal volume of glass beads (Sigma) as described previously (24). The obtained lysates were centrifuged at 2,000 × g for 2 min to remove unbroken cells and beads. The resulting supernatants were considered as total cellular lysates. Western analyses were carried out with an anti-GFP Ab (CLONTECH), anti-glutathione S-transferase (GST) Ab (Zymed), and anti-HA Ab at a concentration of 0.5 μg/ml. Proteins that interact with Abs were detected by the enhanced chemiluminescence Western detection system (Amersham Pharmacia).
Cell Staining and Immunofluorescence Microscopy.
Indirect immunofluorescence was performed as described previously (24). Cdc10 was localized by using an affinity-purified rabbit polyclonal anti-Cdc10 Ab (a gift of J. Chant, Harvard University, Cambridge, MA) and rhodamine-conjugated goat anti-rabbit IgG (Zymed). Microtubules were visualized by using YOL1/34 rat anti-tubulin Ab (Accurate Chemical) and goat anti-rat Cy3 Ab (Jackson ImmunoResearch). Actin was localized using rhodamine-conjugated phalloidin (Molecular Probes), and DNA was visualized by staining cells with propidium iodide at 40 μg/ml. Fluorescent images were collected with a Zeiss LSM 410 confocal microscope or a Bio-Rad MRC-1024 confocal scan head mounted on a Nikon Optiphot microscope with a 60× planapochromat lens.
Results
Both the Kinase Domain and the Polo-Box of Plk Are Required for the Induction of Cells with Abnormally Elongated Buds.
We previously observed that ectopic expression of the constitutively active Plk mutant PlkT210D, in an otherwise wild-type genetic background, induces a class of cells with unusually elongated buds (24), indicating that polar bud growth was deregulated in these cells. Because polarized cell growth is initiated through cortical actin or septin cytoskeletal cues (32) and hyperpolarization of the cytoskeleton drives cell-surface expansion, we investigated the role of septins in the induction of elongated buds. Wild-type yeast expressing Plk wild-type under the GAL1 promoter induced cells with abnormally elongated buds only marginally. Similar to an enhanced kinase activity, expression of PlkT210D yielded 3.4% of elongated cells. When GST-fused Cdc10, a component of cytokinetic septin ring structure, was coexpressed with PlkT210D, the elongated bud phenotype was synergistically enhanced from 3.4% to 12.2%, whereas GST-Cdc10 alone did not induce a significant percentage of elongated buds under these conditions (Table1). Cells coexpressing a nonfused form of Cdc10 and PlkT210D showed similar results (data not shown).
To investigate whether Plk localization or enzymatic activity is required for this phenotype, the W414F mutation that disrupts Plk localization (2) or the K82M mutation that inactivates the Plk kinase activity (24) was introduced into PlkT210D then coexpressed with GST-Cdc10. Strikingly, introduction of either the W414F or the K82M mutation into PlkT210D completely eliminated the ability of Plk to induce abnormally elongated buds (Table 1). These data indicate that Plk cooperates with the Cdc10 septin to induce this phenotype and that both the polo-box domain and the kinase activity of Plk are required for this event.
Table 1.
Requirement of the polo-box and kinase activity of Plk for the induction of an abnormally elongated bud phenotype
| Plasmid | Cells with abnormally elongated buds, %
|
|||||
|---|---|---|---|---|---|---|
| Vector 1 | K82M | WT | T210D | T210D/W414F | K82M/T210D | |
| Vector 2 | 0.0 | 0.0 | 0.0 | 3.4 | 0.0 | 0.0 |
| GST-Cdc10 | 0.4 | 0.3 | 2.3 | 12.2 | 0.4 | 0.4 |
A diploid wild-type strain, 1788, cotransformed with various YCplac111-GAL1-Plk constructs and YCplac33-GAL1-GST-CDC10, was cultured in YEP-galactose at 30°C for 12 hr. More than 1,500 cells were counted for each transformant. Synergistic induction of cells with an elongated bud by coexpression of PlkT210D and GST-Cdc10 was abolished by either the W414F mutation in the polo-box or the K82M mutation in the ATP-binding site. Data shown represent the average of two independent experiments. Vector 1, YCplac111-GAL1; K82M, YCplac111-GAL1-PlkK82M; WT, YCplac111-GAL1-Plk; T210D, YCplac111-GAL1-PlkT210D; T210D/W414F, YCplac111-GAL1-PlkT210D/W414F; K82M/T210D, YCplac111-GAL1-PlkK82M/T210D; Vector 2, YCplac33-GAL1; GST-Cdc10, YCplac33-GAL1-GST-CDC10.
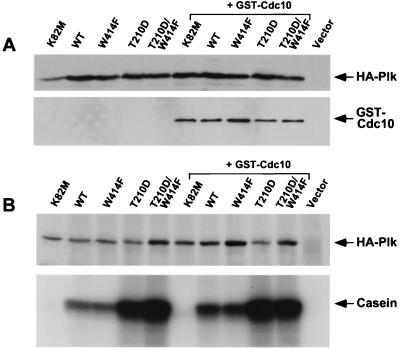
To ensure that this abnormal morphology is not the result of differences in Plk and Cdc10 expression levels or in activities among the different Plk transformants, we carried out Western analysis and in vitro kinase assays. The expression levels of the wild-type and mutant forms of Plk were similar and did not appear to be influenced by the coexpression of GST-Cdc10 (Fig. 1A). Both wild-type Plk and the PlkW414F mutant exhibited similar levels of enzymatic activity, whereas the activity of PlkT210D was severalfold higher, as shown previously (24). Coexpression of GST-Cdc10 did not significantly influence the kinase activities of wild-type or mutant forms of Plk (Fig. 1B). These data indicate that the abrogation of the elongated bud phenotype observed with PlkT210D/W414F expression is due specifically to the impaired function of the polo-box by the W414F mutation.
Figure 1.
Coexpression of wild-type or mutant forms of Plk with GST-Cdc10 in the diploid wild-type S. cerevisiae strain 1788 (isogenic diploid of EG123). (A) Wild-type and mutant forms of Plk were expressed either alone or with GST-Cdc10. Total cellular protein (200 μg of each) was analyzed for HA-Plk expression with anti-Plk Ab (Upper), or for GST-Cdc10 expression with anti-GST Ab (Lower). (B) Total cell extracts were prepared and clarified by centrifugation at 15,000 × g for 30 min. An equal amount (500 μg) of the resulting supernatants (S15) was subjected to immune complex kinase assays with an anti-Plk Ab. Levels of immunoprecipitated HA-Plk were detected with an anti-HA Ab (Upper). HA-Plk kinase activity was measured, using casein as the in vitro substrate (Lower). K82M, YCplac111-GAL1-HA-PlkK82M; WT, YCplac111-GAL1-HA-Plk; W414F, YCplac111-GAL1-HA-PlkW414F; T210D, YCplac111-GAL1-HA-PlkT210D; T210D/W414F, YCplac111-GAL1-HA-PlkT210D/W414F; GST-Cdc10, YCplac33-GAL1-GST-CDC10.
Ectopically Expressed EGFP-PlkT210D Induces, and Localizes to, Additional Septal Structures and Actin Polarization.
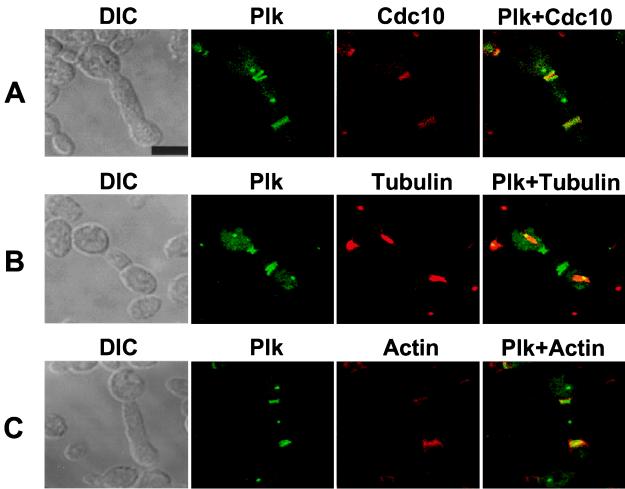
We have previously shown that, in budding yeast, Plk localizes at spindle poles and cytokinetic neck filaments in a polo-box-dependent manner (2). In addition, overexpression of PlkT210D induces additional septal structures (24). Synergistic induction of the elongated bud phenotype observed with Plk and Cdc10 coexpression prompted us to investigate whether Plk has a direct role in the induction of septal structures and an intact polo-box domain is required for these phenomena. Similar to nonfused Plk expression, ectopic expression of EGFP-Plk wild-type, but not the kinase-inactive EGFP-PlkK82M, nor EGFP-PlkW414F, induced cells with elongated buds. Interestingly, in addition to distinct fluorescent dots at spindle poles, as well as one or two bright bands at the mother-bud neck as reported previously (2), overexpression of EGFP-PlkT210D yielded additional distinct fluorescent bands within the abnormally elongated buds after inducing for 10 hr (Fig. 2). To investigate whether the additional fluorescent bands colocalize with the additional septin-ring structures observed previously in cells with abnormally elongated buds (24), we carried out colocalization studies with Cdc10, a component of septin-ring structures (see review, ref. 33). Indirect immunostainings with anti-Cdc10 Ab revealed that EGFP-PlkT210D colocalizes with Cdc10 at both the neck filaments and the additional septal structures (Fig. 2A). The additional septal structures were often seen as single bands; occasionally, distinct double septin-ring structures were also observed (Fig. 2 A–C). This observation, together with the data above, indicates that Plk is also targeted to ectopic, nascent septation sites within the elongated buds.
Figure 2.
Induction of additional septal structures and actin polarization by expression of an activated form of Plk, PlkT210D, in a diploid wild-type strain, 1788. To visualize localization of Plk at additional septal structures, YCplac111-GAL1-HA-EGFP-PlkT210D and YCplac33-GAL1-Cdc10 were cotransformed to increase the population of cells with abnormally elongated buds (see Table 1). Transformants were cultured for subsequent stainings to examine Cdc10, tubulin, and actin localization. (A) Plk (green) and Cdc10 (red) colocalize at the neck filaments and additional septal structures. Septin rings (red) are viewed edge on and therefore appear as lines. (B) Plk (green) localizes at the spindle poles. Spindles were visualized by microtubule staining (red). It is apparent that Plk is present at both ends of spindle structures. (C) Plk (green) induces actin accumulation (red) at the additional septal structures. DIC, differential interference contrast; Plk, EGFP-Plk expression; Cdc10, immunostaining with an anti-Cdc10 Ab. Superimposed images are shown as Plk + Cdc10, Plk + Tubulin, and Plk + Actin. (Bar, 5 μm.)
Because actin and septins are known to polarize independently in response to polarity establishment machinery in budding yeast (see review, ref. 33), we examined whether actin is recruited at the additional septal structures induced by EGFP-PlkT210D. Subsequent staining with rhodamine-conjugated phalloidin revealed accumulation of actin at both the cytokinetic neck filaments and the ectopic septal structures (Fig. 2C). Not all of the additional septal structures accumulated actin, since actin polarization at cytokinetic filaments occurs only during cytokinesis (see review, ref. 34). Furthermore, the presence of actin at the ectopic septal sites indicates that additional cytokinetic components are likely recruited to these structures.
The FA Mutations in the Polo-Box Abolish the Capacity of Plk C-Terminal Domain to Localize at Specific Subcellular Sites.
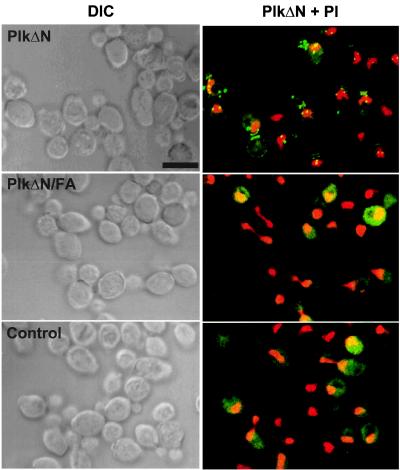
Because the W414F mutation in the polo-box domain abolishes the localization of Plk at specific subcellular locations, we examined whether the C-terminal domain of Plk (PlkΔN; amino acids 322–603) is sufficient for subcellular localization. Expression of the HA-EGFP-fused PlkΔN (HA-EGFP-PlkΔN) yielded distinct fluorescent dots in the cytoplasm as well as two bright bands at the mother-bud neck. Often, more than two dots were also observed in subpopulation of cells (Fig. 3 Upper).
Figure 3.
The C-terminal domain of Plk (PlkΔN) is sufficient to localize at spindle poles and cytokinetic neck filaments. The W414F/L427A (FA) mutations in the polo-box abolish localization of the PlkΔN at these sites. To localize wild-type and mutant forms of the C-terminal domain of Plk in a diploid wild-type strain, 1788, EGFP-PlkΔN and EGFP-PlkΔN/FA fusion constructs were generated and expressed under the control of the GAL1 promoter. Transformants expressing EGFP fusion constructs were stained with propidium iodide to visualize chromosomal DNA and examined by confocal microscopy. PlkΔN, YCplac111-GAL1-HA-EGFP-PlkΔN; PlkΔN/FA, YCplac111-GAL1-HA-EGFP-PlkΔN/W414F/ L427A; Control, YCplac111-GAL1-HA-EGFP. DIC, differential interference contrast; PlkΔN + PI, EGFP-PlkΔN and propidium iodide images superimposed. (Bar, 5 μm.)
Unlike the effect of the W414F mutation in the localization of full-length Plk (2), introduction of the W414F mutation into HA-EGFP-PlkΔN (HA-EGFP-PlkΔN/W414F) did not disrupt its localization completely. In addition to the W414F mutation, the V415A or L427A mutations in the polo-box also influenced the ability of Plk to complement the cdc5–1 defect (2). Thus, an additional mutation, L427A, was introduced into HA-EGFP-PlkΔN/W414F. The resulting HA-EGFP-PlkΔN/W414F/L427A (for simplicity, we will refer to this mutant as PlkΔN/FA) yielded only diffuse signals (Fig. 3 Middle), indicating that, as with full-length Plk, the polo-box domain plays an important role for subcellular localization.
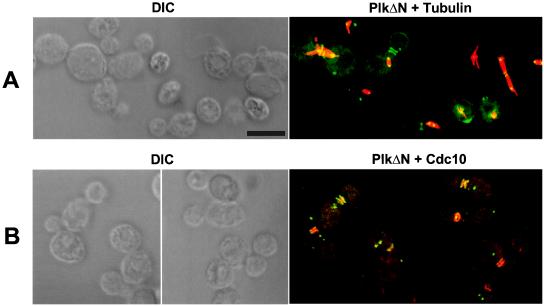
To further characterize the cells expressing HA-EGFP-PlkΔN, subsequent immunostainings were carried out. Stainings with anti-tubulin Ab revealed that two dots were present at each end of spindle (Fig. 4A), suggesting that these signals colocalize with spindle pole bodies. In addition, immunostaining with an anti-Cdc10 Ab revealed that the bands observed colocalize with cytokinetic neck filaments (Fig. 4B). A smaller construct (amino acids 373–603) also localized at spindle poles and neck filaments, whereas the corresponding FA mutant did not (data not shown). This observation indicates that the C-terminal domain of Plk is sufficient to localize at the spindle poles and the neck filaments, and that an intact polo-box is required for this event.
Figure 4.
Localization of the C-terminal domain of Plk at spindle poles and neck filaments. For localization of PlkΔN, transformants were cultured under the induction conditions for 2 hr and prepared for subsequent immunostaining to examine Cdc10 and tubulin. (A) EGFP-PlkΔN localizes at the spindle poles. Spindles were visualized by microtubule staining (red). (B) Plk (green) colocalizes with Cdc10 (red) at the neck filaments. Occasionally EGFP-PlkΔN ring structure was apparent (Right). DIC, differential interference contrast; PlkΔN, EGFP-PlkΔN expression. Superimposed images are shown as PlkΔN + Tubulin and PlkΔN + Cdc10. (Bar, 5 μm.)
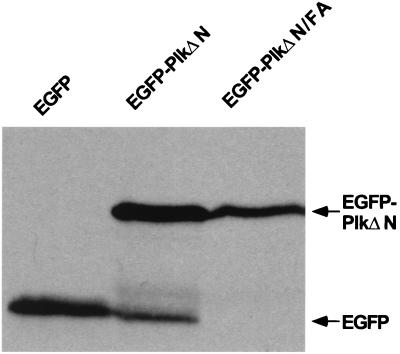
It is possible that the inability of EGFP-PlkΔN/FA to localize at the cytokinetic septal structures could be because of differences in the levels of protein expression. To exclude this possibility, we examined the levels of wild-type and mutant forms of EGFP- PlkΔN after culturing cells under the induction conditions. In total cellular lysates, PlkΔN was about 3-fold more abundant than the corresponding FA mutant after inducing for 2 hr (Fig. 5). However, even though the PlkΔN/FA mutant becomes more abundant after 10-hr induction than PlkΔN induced for 2 hr, the FA mutant still failed to localize at spindle poles and neck filaments (data not shown). Thus, it is unlikely that the differences in the expression levels have contributed to the apparent lack of localization. Taken together, the inability of the FA mutant to localize at specific subcellular structures is apparently because of the lack of an intact polo-box.
Figure 5.
The FA mutations in the polo-box do not significantly influence the expression level of PlkΔN. The wild-type 1788 cells bearing various YCplac22-GAL1-HA-EGFP-PlkΔN constructs were cultured under inducing conditions for 2 hr and then harvested. An equal amount (200 μg each) of total cellular lysates prepared from various transformants was loaded onto each lane. After the proteins were transferred onto a poly(vinylidene difluoride) membrane, proteins interacting with the anti-GFP Ab were detected by immunoblotting. EGFP, YCplac22-GAL1-HA-EGFP; EGFP-PlkΔN, YCplac22-GAL1-HA-EGFP-PlkΔN; EGFP-PlkΔN/FA, YCplac22-GAL1-HA-EGFP-PlkΔN/W414F/L427A. Although the expression level of the FA mutant induced for 10 hr becomes greater than that of wild type induced for 2 hr, it failed to yield distinctly localized signals (see text).
Discussion
We have utilized budding yeast as a system to shed light on the function of the polo-box of mammalian Plk. We have previously shown that either a conservative mutation in the polo-box, W414F, or a mutation inactivating the kinase activity of Plk, K82M, abolishes the capacity of Plk to functionally complement the cdc5–1 defect. In addition, the W414F mutation disrupts Plk localization at spindle poles and cytokinetic neck filaments (2). Here, we have shown that both an intact polo-box and the kinase activity of Plk are required for the capacity of Plk to induce abnormally elongated buds, and that Plk induces to and localizes at the ectopically induced septal structures in the abnormally elongated buds. However, the kinase activity is not required for Plk localization because EGFP-PlkK82M localizes normally at spindle poles and cytokinetic neck filaments (S.S. and K.S.L., unpublished data). Consistent with this observation, the kinase domain-deficient, C-terminal domain of Plk, but not the corresponding FA mutant, is sufficient to localize at spindle poles and cytokinetic neck filaments. Thus, it appears that the polo-box-dependent localization at specific subcellular sites is a prerequisite for Plk kinase activity-dependent induction of elongated buds and ectopic septal structures. Taken together, these data suggest that an intact polo-box is required for Plk to localize at specific subcellular structures, and that it may function as an interaction domain to target the catalytic activity of the enzyme to these locations. Our data further suggest that the polo-box domain is required for the function of polo kinases in cytokinesis.
It is widely accepted that the initiation of cytokinesis in various organisms correlates with, and normally requires, the inactivation of the kinase activity of Cdk1. Although we cannot rule out the possibility that overexpression of Plk may indirectly allow cytokinetic events to occur simply by inactivating Cdc28 through anaphase promoting complex activation, Plk localization at the ectopic septal structures suggests a more direct role for polo kinases in cytokinesis. Data obtained from studies in Schizosaccharomyces pombe (13, 22), Drosophila (25), and human cells (28) further support a role for polo kinases in cytokinesis. Bahler et al. (22) suggested that S. pombe Plo1 plays an important role in the positioning of division sites by regulating the Mid1 protein (23). In addition, Pom1 is also shown to be required for the placement of the Mid1 ring to the cell center (35). However, budding yeast does not appear to have homologs of Mid1 or Pom1, likely because the future cleavage plane is already defined by the presence of septin-ring structures early in the cell cycle. Although the fundamental mechanisms of cytokinesis are likely to be conserved in a wide range of organisms, this observation suggests that budding yeast may utilize distinct molecular events to specify the site of cell division.
We observed that, only in the presence of kinase activity and an intact polo-box domain, Plk cooperates with Cdc10 to induce ectopic septal structures within the elongated buds. This observation suggests that Plk may provide a signal to induce septal structures in a polo-box-dependent manner. During the normal cell cycle of budding yeast, however, Cdc5 becomes abundant in late S phase (19), and its kinase activity peaks in mitosis (36, 37), whereas the septin ring is formed prior to bud emergence. It is also apparent that septin rings relocalize to the new bud sites following cell separation (38), indicating that septin rings, or at least polarized septins, are present at budding sites throughout the cell cycle. In addition, when cdc5–1 cells are cultured at the restrictive temperature, septal structures are weakened but not completely disassembled (39). Thus, although we cannot completely rule out the possibility that Cdc5 initiates septin deposition at future cytokinetic sites, rather it may be important for septin-ring organization or assembly processes. At present, how ectopic septin rings are induced by Plk overexpression is not clear. It is possible that Plk overexpression may have provided additional signals that can reinforce the stimuli leading to the septin-ring formation. Many components are likely to be involved in relaying signals to the cell cortex to induce and organize septin-ring structures. Recent reports show that the Gin4 kinase plays an important role in septin organization, apparently through direct interactions with septins (40, 41). Whether Plk directly interacts with septin components or other proteins important for regulating septin-ring structures awaits further investigation.
In this communication, we have demonstrated that ectopically expressed Plk, but not the polo-box mutant, induces and localizes to ectopic septin-ring structures within the abnormally elongated buds. Our data suggest that polo kinases may contribute a signal to initiate or promote cytokinetic events in a polo-box-dependent manner. In addition, the polo-box appears to play a crucial role in targeting the catalytic activity of Plk to the spindle poles and cytokinetic neck filaments. Identification of polo-box-interacting proteins may provide a key step toward understanding the roles of polo kinases in regulating important events in cytokinesis.
Acknowledgments
We thank Michael Lichten for critical reading of the manuscript, Susan Garfield for assisting confocal microscopy, and John Chant for the provision of an anti-Cdc10 Ab. The early part of this work was supported by a Postdoctoral/Postresidency Fellowship from the American Cancer Society, Massachusetts Division (to K.S.L.), and National Institutes of Health Grant CA42580 (to R.L.E.). R.L.E. is the John F. Drum American Cancer Society Research Professor.
Abbreviations
- EGFP
enhanced green fluorescent protein
- HA
hemagglutinin
- GST
glutathione S-transferase
References
- 1.Clay F J, McEwen S J, Bertoncello I, Wilks A F, Dunn A R. Proc Natl Acad Sci USA. 1993;90:4882–4886. doi: 10.1073/pnas.90.11.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee K S, Grenfell T Z, Yarm F R, Erikson R L. Proc Natl Acad Sci USA. 1998;95:9301–9306. doi: 10.1073/pnas.95.16.9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song, S., Grenfell, T., Garfield, S., Erikson, R. & Lee, K. (1999) Mol. Cell. Biol., in press. [DOI] [PMC free article] [PubMed]
- 4.Golsteyn R M, Schultz S J, Bartek J, Ziemiecki A, Ried T, Nigg E A. J Cell Sci. 1994;107:1509–1517. doi: 10.1242/jcs.107.6.1509. [DOI] [PubMed] [Google Scholar]
- 5.Hamanaka R, Maloid S, Smith M R, O’Connell C D, Longo D L, Ferris D K. Cell Growth Differ. 1994;5:249–257. [PubMed] [Google Scholar]
- 6.Holtrich U, Wolf G, Bräuninger A, Karn T, Böhme B, Rübsamenwaigmann H, Strebhardt K. Proc Natl Acad Sci USA. 1994;91:1736–1740. doi: 10.1073/pnas.91.5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lake R J, Jelinek W R. Mol Cell Biol. 1993;13:7793–7801. doi: 10.1128/mcb.13.12.7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simmons D L, Neel B G, Stevens R, Evett G, Erikson R L. Mol Cell Biol. 1992;12:4164–4169. doi: 10.1128/mcb.12.9.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donohue P J, Alberts G F, Guo Y, Winkles J A. J Biol Chem. 1995;270:10351–10357. doi: 10.1074/jbc.270.17.10351. [DOI] [PubMed] [Google Scholar]
- 10.Li B, Ouyang B, Pan H, Reissmann P T, Slamon D J, Arceci R, Lu L, Dai W. J Biol Chem. 1996;271:19402–19408. doi: 10.1074/jbc.271.32.19402. [DOI] [PubMed] [Google Scholar]
- 11.Kumagai A, Dunphy W G. Science. 1996;273:1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- 12.Llamazares S, Moreira A, Tavares A, Girdham C, Spruce B A, Gonzalez C, Karess R E, Glover D M, Sunkel C E. Genes Dev. 1991;5:2153–2165. doi: 10.1101/gad.5.12a.2153. [DOI] [PubMed] [Google Scholar]
- 13.Ohkura H, Hagan I M, Glover D M. Genes Dev. 1995;9:1059–1073. doi: 10.1101/gad.9.9.1059. [DOI] [PubMed] [Google Scholar]
- 14.Kitada K, Johnson A L, Johnston L H, Sugino A. Mol Cell Biol. 1993;13:4445–4457. doi: 10.1128/mcb.13.7.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glover D M, Hagan I M, Tavares A A M. Genes Dev. 1998;12:3777–3787. doi: 10.1101/gad.12.24.3777. [DOI] [PubMed] [Google Scholar]
- 16.Lane H, Nigg E A. Trends Cell Biol. 1997;7:63–68. doi: 10.1016/S0962-8924(96)10051-9. [DOI] [PubMed] [Google Scholar]
- 17.Lane H A, Nigg E A. J Cell Biol. 1996;135:1701–1713. doi: 10.1083/jcb.135.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toczyski D P, Galgoczy D J, Hartwell L H. Cell. 1997;90:1097–1106. doi: 10.1016/s0092-8674(00)80375-x. [DOI] [PubMed] [Google Scholar]
- 19.Shirayama M, Zachariae W, Ciosk R, Nasmyth K. EMBO J. 1998;17:1336–1349. doi: 10.1093/emboj/17.5.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Descombes P, Nigg E A. EMBO J. 1998;17:1328–1335. doi: 10.1093/emboj/17.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Field C, Li R, Oegema K. Curr Opin Cell Biol. 1999;11:68–80. doi: 10.1016/s0955-0674(99)80009-x. [DOI] [PubMed] [Google Scholar]
- 22.Bahler J, Steever A B, Wheatley S, Wang Y I, Pringle J R, Gould K L, McCollum D. J Cell Biol. 1998;143:1603–1616. doi: 10.1083/jcb.143.6.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohrmann M, Fankhauser C, Brodbeck C, Simanis V. Genes Dev. 1996;10:2707–2719. doi: 10.1101/gad.10.21.2707. [DOI] [PubMed] [Google Scholar]
- 24.Lee K S, Erikson R L. Mol Cell Biol. 1997;17:3408–3417. doi: 10.1128/mcb.17.6.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmena M, Riparbelli M G, Minestrini G, Tavares A M, Adams R, Callaini G, Glover D M. J Cell Biol. 1998;143:659–671. doi: 10.1083/jcb.143.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams R, Tavares A, Salzberg A, Bellen H, Glover D. Genes Dev. 1998;12:1483–1494. doi: 10.1101/gad.12.10.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neufeld T P, Rubin G M. Cell. 1994;77:371–379. doi: 10.1016/0092-8674(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 28.Mundt K E, Golsteyn R M, Lane H A, Nigg E A. Biochem Biophys Res Commun. 1997;239:377–385. doi: 10.1006/bbrc.1997.7378. [DOI] [PubMed] [Google Scholar]
- 29.Siliciano P G, Tatchell K. Cell. 1984;37:969–978. doi: 10.1016/0092-8674(84)90431-8. [DOI] [PubMed] [Google Scholar]
- 30.Sherman F, Fink G R, Hicks J B. Methods in Yeast Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1986. [Google Scholar]
- 31.Ito H, Fukuda Y, Murata K, Kimura A. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drubin D G, Nelson W J. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- 33.Chant J. Cell. 1996;84:187–190. doi: 10.1016/s0092-8674(00)80972-1. [DOI] [PubMed] [Google Scholar]
- 34.Lew D J, Reed S I. Curr Opin Genet Dev. 1995;5:17–23. doi: 10.1016/s0959-437x(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 35.Bahler J, Pringle J. Genes Dev. 1998;12:1356–1370. doi: 10.1101/gad.12.9.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fesquet D, Fitzpatrick P J, Johnson A L, Kramer K M, Toyn J H, Johnston L H. EMBO J. 1999;18:2424–2434. doi: 10.1093/emboj/18.9.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng L, Hunke L, Hardy C F J. Mol Cell Biol. 1998;18:7360–7370. doi: 10.1128/mcb.18.12.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lippincott J, Li R. J Cell Biol. 1998;143:1947–1960. doi: 10.1083/jcb.143.7.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim H B, Haarer B K, Pringle J R. J Cell Biol. 1991;112:535–544. doi: 10.1083/jcb.112.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carroll C W, Altman R, Schieltz D, Yates J R, Kellogg D. J Cell Biol. 1998;143:709–717. doi: 10.1083/jcb.143.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Longtine M S, Fares H, Pringle J R. J Cell Biol. 1998;143:719–736. doi: 10.1083/jcb.143.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]