Abstract
Pax6, a highly conserved member of the paired homeodomain transcription factor family that plays essential roles in ocular, neural, and pancreatic development and effects asymmetric transient dorsal expression during pituitary development, with its expression extinguished before the ventral → dorsal appearance of specific cell types. Analysis of pituitary development in the Small eye and Pax6 −/− mouse mutants reveals that the dorsoventral axis of the pituitary gland becomes ventralized, with dorsal extension of the transcriptional determinants of ventral cell types, particularly PFrk. This ventralization is followed by a marked decrease in terminally differentiated dorsal somatotrope and lactotrope cell types and a marked increase in the expression of markers of the ventral thyrotrope cells and SF-1-expressing cells of gonadotrope lineage. We suggest that the transient dorsal expression of Pax6 is essential for establishing a sharp boundary between dorsal and ventral cell types, based on the inhibition of Shh ventral signals.
As in patterning events and the development of the imaginal disc in Drosophila (1), a spatially specific signaling code sets a pattern of transcription factors that establish specific cell types from common primordium. The pituitary gland has provided a particularly useful model for understanding the signaling transcriptional control of mammalian organogenesis. The mature gland contains six hormone-producing cell types that arise from a common primordium. They are defined by the peptide hormones they release including corticotropes secreting adrenotropin; melanotropes secreting melanocyte-stimulating hormone; thyrotropes secreting thyroid-stimulating hormone (TSH); gonadotropes secreting luteinizing hormone (LH) and follicle-stimulating hormone (FSH); somatotropes secreting growth hormone (GH); lactotropes secreting prolactin (PRL); and a transient embryonic population in the mouse arising rostral tip. LH, FSH, and TSH are heterodimers consisting of a common α-glycoprotein subunit (αGSU) and a distinct β-subunit (2).
The anterior pituitary gland is derived from the anterior neural ridge after the invagination of the oral ectoderm, Rathke’s pouch, and the direct cell contact with the overlying neural epithelium of the ventral diencephalon in mouse embryonic day (e) 8.5 (3, 4). Commitment, determination, and cell differentiation of the pituitary gland are controlled by sequential series of gradients of signaling molecules and tissue-specific transcription factors (5–7). A number of extrinsic and intrinsic signaling molecules regulate initial events in pituitary organogenesis. Shh is uniformly expressed along the oral ectoderm but excluded from the invaginating Rathke’s pouch, creating an ectodermal boundary from which a ventral to dorsal gradient of BMP2 arises. Signaling molecules expressed in the ventral diencephalon, including BMP4, Wnt5a, and Fgf8, control organ commitment and proliferation events. By e10, there are opposing ventral → dorsal BMP and dorsal → ventral Fgf activity gradients in Rathke’s pouch and determine ventral cell phenotypes (gonadotropes, thyrotropes, somatotropes, and lactotropes) and dorsal cell phenotypes (melanotropes and corticotropes; refs. 5–7). These signaling gradients can establish the patterns of transcription factors along the pituitary–hypothalamic axis between e10–e12, including Isl-1 (7), Brn4 (8), PFrk (7), and GATA2 (9) ventrally. Nkx3.1 (10), Six3 (11), Prop-1 (8), and Pax6 (12) were expressed dorsally. The induction of the POU domain transcription factor Pit1 is required for the initial activation of the GH, PRL, and thyrotrope genes, before the appearance of the pituitary cell lineages (13, 14) and after the ventral → dorsal gradient of GATA2 that provides the molecular memory of the control ventral transient signaling events. The orphan nuclear receptor SF-1 is the most ventral in the pituitary gland for the activation of LHβ and FSHβ (15, 16).
Because Pax6 is expressed in such a temporally and spatially restricted fashion in pituitary development, it was of particular interest to investigate its potential role in establishing the dorsal → ventral patterns of cell-type development. Pax6 encodes a transcription factor containing both paired and homeodomain DNA binding motifs and is expressed in the developing eye and nervous system of a wide range of species from nematodes to vertebrates (17), as well as in the olfactory epithelium, pancreas, and pituitary gland in mammals. Studies on Small eye (Sey), a semidominant mouse mutation, and on Pax6 gene-deleted mice (18) have shown that Pax6 is essential for the formation of the eye placode (17), certain structures of the forebrain and diencephalon (19–23), and differentiation of glucagon-producing α-cells in the pancreas (17, 24). In the spinal cord and hindbrain, Pax6 establishes distinct ventral progenitor cell populations and controls the identity of motor neurons and ventral interneurons by mediating graded Shh signaling (5). In the absence of Pax6 functional protein, the gonadotropin-releasing hormone diencephalic neurons do not develop (25). These findings raise the possibility that Pax6 is involved in pituitary gland development by controlling the dorsal → ventral appearance of cell-type specification.
In this article, we show that Pax6 is an early dorsal marker of Rathke’s pouch and early anterior pituitary gland, and its expression controls the established boundaries of somatotrope, lactotrope, and thyrotrope cell types. The absence of Pax6 leads to a marked increase of the thyrotrope cell lineage, whereas the somatotrope and lactotrope cell lineage changes are very much diminished. These data support the hypothesis that Pax6 is an essential component involved in forming the dorsal/ventral boundary in cell-type determination.
Materials and Methods
Pax6 −/− Mice and Sey/Sey Mice.
Embryos were obtained from natural matings of Pax6 +/− (18) and Sey +/− mice, generously provided by M. Goulding (Salk Institute, San Diego). The time point of vaginal plug observation was designated at 0.5 days postcoitus, and embryonic days are listed as days postcoitus. Embryo heads were isolated and fixed in 4% (vol/vol) paraformaldehyde for 3–24 h until analyzed further. Sey/Sey mice were characterized by the absence of the optic vesicle, which made them easy to distinguished from the wild-type litter mates. Both Sey/Sey and Pax6 −/− mice, which we will refer to as mutant, produced identical phenotypes.
In Situ Hybridization and Immunohistochemistry.
Hybridization with 35S-labeled antisense RNA probes was performed as described (13) on 20-μm cryosections of mouse heads. Immunohistochemistry was done on 10-μm paraffin sections. Sections were incubated with primary antibodies in different dilutions in PBS/0.3% Triton/5% (vol/vol) goat serum overnight at 4°C. Detection antibodies were horseradish peroxidase coupled, and staining was visualized by using a diaminobenzidine metal conjugate (Pierce). Sections were counterstained with methyl green. Primary antibodies used were obtained as follows: Pax6 was generously provided by R. Reed (John Hopkins University, Baltimore); αGSU was obtained from the National Hormone and Pituitary Program, (National Institute of Diabetes and Digestive and Kidney Diseases, Rockville, MD); TSH, GH, and PRL were purchased from Dako.
Results
Pax6 Is an Early Dorsal Marker of Pituitary Gland.
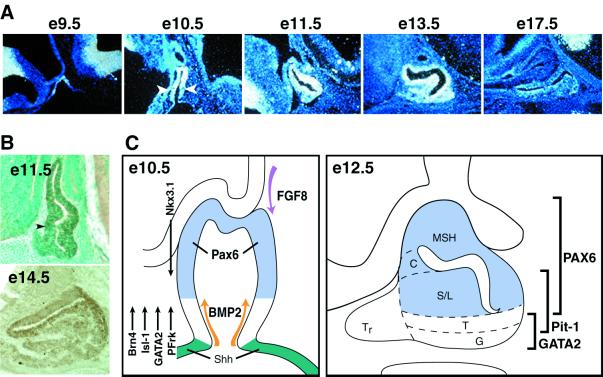
The onset of the pituitary gland is the result of signals from the ventral diencephalon and the oral ectoderm, under Shh exclusion occurring in the invaginating oral ectoderm on budding of Rathke’s pouch at e9 (6, 7). Pax6 transcripts are first detected during this early phase of pituitary gland commitment and remain expressed throughout the onset of positional commitment. Pax6 is expressed in the nascent Rathke’s pouch at e9.0 (Fig. 1) but is excluded from the ventral part of the Shh pouch, creating a ventral zone between the Shh-expressing cells and the Pax6-expressing cells. By e10–e12, as exogenous and endogenous signals have been established, Pax6 expression is monitored at the dorsal Rathke’s pouch, with an apparent dorsal → ventral gradient. Pax6 expression is in a certain zone of exclusion in the rostral part of the pituitary; the ventral gland is separated from the ectoderm, occupying the ventral lip of the pouch (7, 12). When pituitary cell lineage determination is initiated with the appearance of POMC-expressing cells on e12.5, followed by Pit-1 on e13, Pax6 expression is rapidly decreased. By the phase of terminal differentiation of the Pit-1 lineages and gonadotropes (e16–17.5), Pax6 transcripts are no longer detected (Fig. 1).
Figure 1.
Pax6 expression pattern during pituitary gland development. (A) Pax6 transcripts are detected very early in oral ectoderm at e9.5 as they occupy the Shh-nonexpressing area. Later, at e10–e12, Pax6 is characterized by a dorsalhigh to ventrallow expression pattern along the Rathke’s pouch. Pax6 transcripts are diminished during embryonic development, and no signal is detected before birth. (B) Pax6 protein is detected at the dorsal pituitary gland at e11.5. Pax6-positive cells are located only in the lumen at e14.5. (C) Schematic representation of Pax6 expression in combination with the ventral and dorsal signaling molecules and transcription factors. By e12.5, Pax6 expression is excluded from the most ventral cell types, the gonadotropes and thyrotropes. MSH, melanocyte-stimulating hormone; C, corticotropes; S/L, somatotropes/lactotropes; T, thyrotropes; G, gonadotropes; Tr, rostral tip thyrotropes.
Thus, both the early Pax6 expression in the pouch with exclusion from the most rostral and the caudal cells adjacent to the Shh-expressing cells at the ectodermal boundary suggest that it might be the component of the transcriptional apparatus that establishes a sharp boundary, modulating the transcriptional response required for positional determination of the cell types of the three Pit-1 lineages, although they will not appear until 4–5 days later in pituitary organogenesis.
Pax6 Is Essential in Patterning the Developing Pituitary.
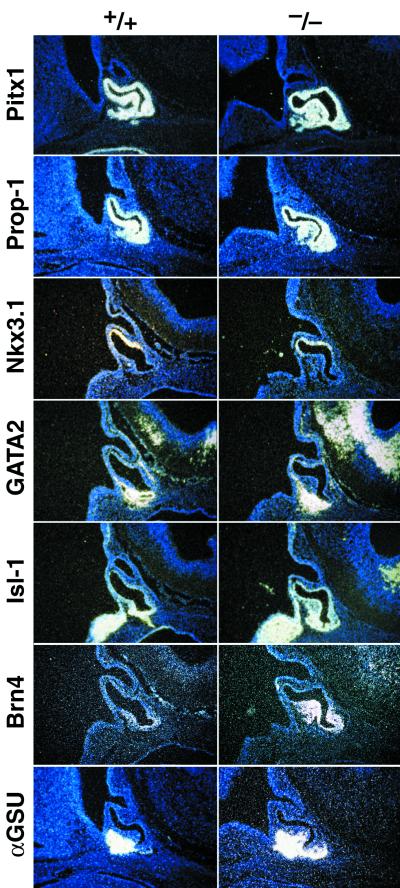
To begin to test this model of Pax6 function in pituitary development, we examined pituitary development in mice by controlling these types by disruption of the Pax6 gene. The first is the Sey/Sey mutation (26); the second is the targeted deletion of the Pax6 locus (18). First, we examined the appearance of transcripts of signaling molecules and transcription factors as modulators of early pituitary cell-type commitment (7, 9), including the ventral expression of Brn4, PFrk, Isl-1, and GATA2. Their differential critical expression has been proposed to be mechanistically linked to ventral cell development programs (9). Therefore, we examined the expression patterns in the Sey/Sey embryos and Pax6 gene-deleted mice to identify potential alteration patterns in their temporal and spatial expression. Indeed, by e12.5, the αGSU normally expressed in a rostral/ventral pattern and became widely expressed throughout the ventral anterior part of the gland (Fig. 2), and this expanded expression pattern remained until birth (see Fig. 4). In contrast, there was no difference of the dorsal → ventral gradient with the Nkx-related homeobox gene Nkx3.1, the most dorsally expressed marker (Fig. 2; ref. 7). The expression pattern of the ventral markers was next assayed, including the POU domain factor Brn4; the wind-helix transcription factor PFrk; the GATA2, a zinc finger protein that has ventral induction in the pituitary gland in respond to BMP2 (8); and Isl-1, encoding a LIM-homeodomain gene, which becomes restricted to a ventral expression pattern. We found a uniform dorsalization in the expression pattern of these four factors exemplified by the expression of PFrk and GATA2 at e15.5 (Fig. 3). There was also an increase in the Pit-1 expressing cells, which led to the prediction that there would be an increase in the thyrotrope population, because it requires both Pitx1 and GATA2 for determination (9).
Figure 2.
Ventral markers are overexpressed in the absence of Pax6 in the early stage of pituitary development. At e12 in Rathke’s pouch, the ventral markers αGSU, GATA2, Brn4, Isl-1, and Prop-1 are overexpressed in the Pax6 mutant mice. No difference has been observed for the POMC and Nkx3.1 levels. Pitx1, a uniformly expressed pituitary marker, showed an up-regulation at the ventral site of the pouch.
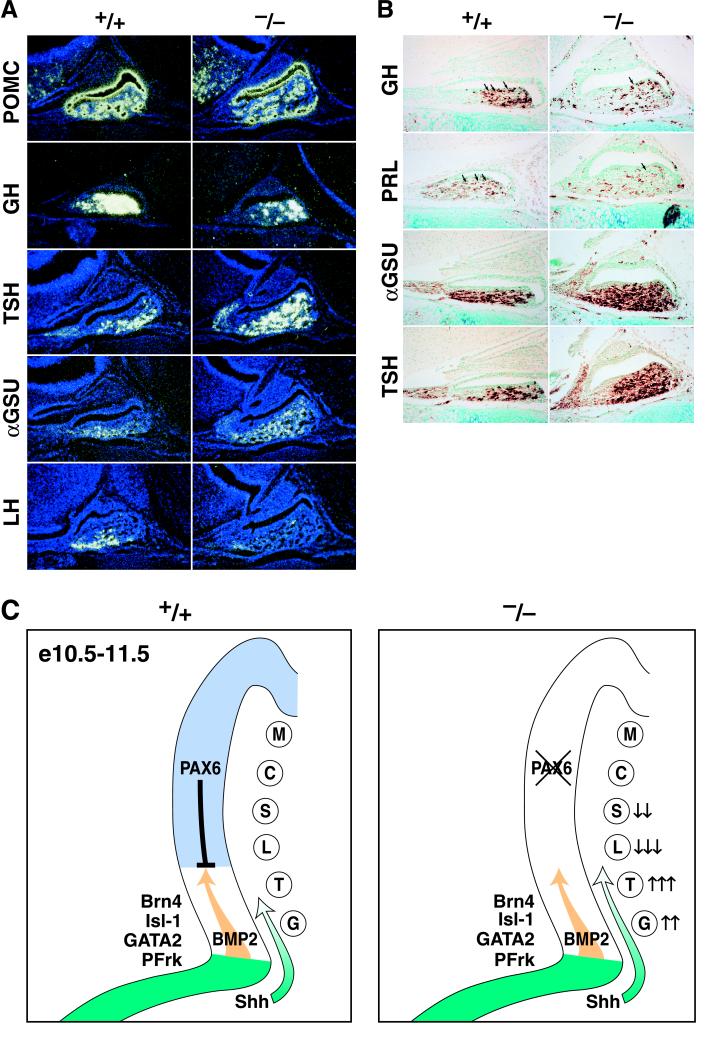
Figure 4.
Ventral pituitary cell types are increased in the absence of Pax6. (A) Newborn Pax6 mutant mice are characterized by increased GH, TSH, and αGSU as well as by down-regulation of LH but not by a difference in POMC levels. (B) Very few dorsal GH- and PRL-positive cells are detected in mutant mice. (C) Pax6 establishes the boundary along the dorsoventral pituitary axis as early as e10 in response to the inhibitory Shh gradient and maybe the BMP2 from the ventral Rathke’s pouch and oral ectoderm. In the absence of Pax6, there is a dorsal shift of the boundary, with up-regulation of the transcription factors determining the most ventral cell types, the gonadotropes and thyrotropes. M, melanotropes; other abbreviations are defined in the legend to Fig. 1.
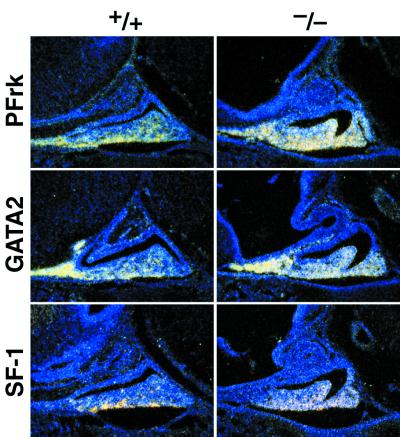
Figure 3.
Ventral markers are overexpressed in the absence of Pax6 in the determined pituitary gland. At e15, the PFrk, GATA2, and SF-1 (a gonadotrope-specific marker) expression levels are increased in the absence of Pax6.
Absence of Pax6 Leads to an Increase in Ventral Cell Types and Decrease in Somatotropes/Lactotropes.
Examination of the appearance of fully differentiated cell types revealed an increase in cells with the most ventral phenotypes. Thus, there was an increase rostrally in expression of both αGSU and TSHβ, (Fig. 4A). Ultimately, GH was ventrally diminished (Fig. 4 A and B). However, POMC-expressing cells, the most dorsal cell type, remained equivalent in wild-type and Pax6 mutant mice (Fig. 4 A and B). The same results were obtained when expressing protein levels of αGSU and TSHβ, both of which were expanded in the mutant pituitary gland at postnatal day 0 and which were accompanied by the harbored down-regulation of GH and PRL (Fig. 4B). Similar observations for GH and PRL immunoreactivity have been described in another Pax6 point mutation, Pax6neu (27). LHβ-expressing cells are not detected, consistent with the published effects of Pax6 on gonadotropin-releasing hormone neurons (25), but the SF-1 expression presumptive gonadotropes also increase expression in Pax6 gene-deleted mice, suggesting an expansion of determined gonadotropes incapable of terminal differentiation based on the absence of the gonadotropin-releasing hormone (25). Together, these results show that the absence of Pax6 results in an increased thyrotrope population but decreased numbers of lactotropes and somatotropes derived from the Pit-1 lineage. Thus, there may be a dorsal extension of ventral cell type determination of Pax6 expression in response to Shh ventrally. There is no evidence that Pax6 can affect the BMP signaling response directly (7). Thus, we hypothesize that Pax6 serves as a transcriptional regulator that opposes the effects of ventral signaling molecules, critical in causing determination of gonadotrope and thyrotrope cell determination events, thereby sharpening the delineation of dorsal cells in this differentiation phase of pituitary development.
Discussion
The critical events in the appearance of specific cell phenotypes from a common primordium seem to be the establishment of opposing dorsal → ventral Fgf and ventral → dorsal Shh and BMP2 gradients. In turn, these events cause induction of a series of critical transcription factors expressed dorsally or ventrally. In this article, we have provided evidence that there are additional mechanisms to focus the boundaries at which specific cell types will arise. Thus, in early pituitary organogenesis, there is a dorsal expression of Pax6 possibly induced by dorsal signals, and this expression remains throughout the period of determination of pituitary cell types and is extinguished by e14.5–e16.
Based on analysis of two different Pax6 gene-deleted or mutant mice (18, 26), the role of Pax6 seems to be to impose a sharp boundary of attenuation of the ventral signals that dictate thyrotrope and gonadotrope cell lineages. In the absence of Pax6, the ventral lineages, particularly thyrotropes, become dorsally extended at the expression of somatotropes and lactotrope cell types.
In the chicken and mouse neural tube, Pax6 has similar spatial and temporal expression patterns. Pax6 is expressed by cells at all dorsal → ventral positions, with an extended ventrallow → dorsalhigh gradient, and its expression is extinguished soon after the differentiation of postnitotic motor neurons. Pax6 controls the ventral progenitor cells, because no Pax6 can be detected in Sey/Sey cellular populations. These ventral progenitor cells respond to Shh signal in a graded manner. The ventrallow → dorsalhigh Pax6 gradient is reconstructed in vitro by exposure of neural cells to differential Shh concentrations. Repression of Pax6 requires high Shh concentrations, and Pax6 expression remains sensitive to ambient Shh concentration during neurogenesis (28). In the pituitary gland, the ventralhigh → dorsallow gradient of Pax6 might be a combinatorial effect of the graded Shh signal from the oral ectoderm and the hypothalamus. Sey/Sey mice also fail to establish boundaries that specify longitudinal domains in the forebrain, such as the hypothalamus–telencephalic transition zone, ventral thalamus, and a number of forebrain nuclei (19).
A defect in the hypothalamus of the Sey/Sey mice, which have an abnormally large third ventricle, affected formation of the transition zone between the telencephalic vesicle and hypothalamus (20). In the neural tube, Pax6 (28) and Nkx2.2 (29) control the ventral progenitor cell identity with respect in motor neurons or interneuron patterning in response to graded Shh signals (28). There is no delay or failure of Rathke’s pouch formation in the Pax6 mutants, because Pitx1 (Fig. 2) and Lxh3 (data not shown) are normally expressed in the e12.5 gland. Therefore, the most likely hypothesis is that the transient dorsal expression of Pax6 in a cell-autonomous fashion permits lower Shh concentrations from inducing transcription factor expression to the ventral cell-type programs, thereby exerting a physiological role in creating a sharp boundary for somatotrope/lactotrope vs. thyrotrope/gonadotrope cell-type determination.
Acknowledgments
The authors thank all former and current members of the laboratories for their data contributions and P. Myers for preparing the illustrations. C.K. is supported by National Institutes of Health Grant 5R01 DK 18477. M.G.R. is an Investigator with the Howard Hughes Medical Institute. These studies were performed under a grant from National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases).
Abbreviations
- αGSU
common α-glycoprotein subunit
- TSH
thyroid-stimulating hormone
- LH
luteinizing hormone
- FSH
follicle-stimulating hormone
- GH
growth hormone
- PRL
prolactin
- en
embryonic day n
References
- 1.Lawrence P A, Struhl G. Cell. 1996;85:951–961. doi: 10.1016/s0092-8674(00)81297-0. [DOI] [PubMed] [Google Scholar]
- 2.Voss J W, Rosenfeld M G. Cell. 1992;70:527–530. doi: 10.1016/0092-8674(92)90422-9. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson E M, Li P, Leon-del-Rio A, Rosenfeld M G, Aggarwal A K. Genes Dev. 1997;11:198–212. doi: 10.1101/gad.11.2.198. [DOI] [PubMed] [Google Scholar]
- 4.Couly G F, Le Douarin N M. Dev Biol. 1985;110:422–439. doi: 10.1016/0012-1606(85)90101-0. [DOI] [PubMed] [Google Scholar]
- 5.Ericson J, Norlin S, Jessell T M, Edlund T. Development (Cambridge, UK) 1998;125:1005–1015. doi: 10.1242/dev.125.6.1005. [DOI] [PubMed] [Google Scholar]
- 6.Takuma N, Sheng H Z, Furuta Y, Ward J M, Sharma K, Hogan B L, Pfaff S L, Westphal H, Kimura S, Mahon K A. Development (Cambridge, UK) 1998;125:4835–4840. doi: 10.1242/dev.125.23.4835. [DOI] [PubMed] [Google Scholar]
- 7.Treier M, Gleiberman A S, O’Connell S M, Szeto D P, McMahon J A, McMahon A P, Rosenfeld M G. Genes Dev. 1998;12:1691–1704. doi: 10.1101/gad.12.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sornson M W, Wu W, Dasen J S, Flynn S E, Norman D J, O’Connell S M, Gukovsky I, Carriere C, Ryan A K, Miller A P, et al. Nature (London) 1996;384:327–333. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- 9.Dasen J S, O’Connell S M, Flynn S E, Treier M, Gleiberman A S, Szeto D P, Hooshmand F, Aggarwal A K, Rosenfeld M G. Cell. 1999;97:587–598. doi: 10.1016/s0092-8674(00)80770-9. [DOI] [PubMed] [Google Scholar]
- 10.Bieberich C J, Fujita K, He W W, Jay G. J Biol Chem. 1996;271:31779–31782. doi: 10.1074/jbc.271.50.31779. [DOI] [PubMed] [Google Scholar]
- 11.Oliver G, Mailhos A, Wehr R, Copeland N G, Jenkins N A, Gruss P. Development (Cambridge, UK) 1995;121:4045–4055. doi: 10.1242/dev.121.12.4045. [DOI] [PubMed] [Google Scholar]
- 12.Kioussi C, Carriere C, Rosenfeld M G. Mech Dev. 1999;81:23–35. doi: 10.1016/s0925-4773(98)00229-9. [DOI] [PubMed] [Google Scholar]
- 13.Simmons D M, Voss J W, Ingraham H A, Holloway J M, Broide R S, Rosenfeld M G, Swanson L W. Genes Dev. 1990;4:695–711. doi: 10.1101/gad.4.5.695. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Crenshaw E B, III, Rawson E J, Simmons D M, Swanson L W, Rosenfeld M G. Nature (London) 1990;347:528–533. doi: 10.1038/347528a0. [DOI] [PubMed] [Google Scholar]
- 15.Ingraham H A, Lala D S, Ikeda Y, Luo X, Shen W H, Nachtigal M W, Abbud R, Nilson J H, Parker K L. Genes Dev. 1994;8:2302–2312. doi: 10.1101/gad.8.19.2302. [DOI] [PubMed] [Google Scholar]
- 16.Luo X, Ikeda Y, Parker K L. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 17.Walther C, Gruss P. Development (Cambridge, UK) 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- 18.St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Nature (London) 1997;387:406–409. doi: 10.1038/387406a0. [DOI] [PubMed] [Google Scholar]
- 19.Hill R E, Davidson D R. Curr Biol. 1994;4:1155–1157. doi: 10.1016/s0960-9822(00)00262-1. [DOI] [PubMed] [Google Scholar]
- 20.Stoykova A, Fritsch R, Walther C, Gruss P. Development (Cambridge, UK) 1996;122:3453–3465. doi: 10.1242/dev.122.11.3453. [DOI] [PubMed] [Google Scholar]
- 21.Warren N, Price D J. Development (Cambridge, UK) 1997;124:1573–1582. doi: 10.1242/dev.124.8.1573. [DOI] [PubMed] [Google Scholar]
- 22.Mastick G S, Davis N M, Andrew G L, Easter S S., Jr Development (Cambridge, UK) 1997;124:1985–1997. doi: 10.1242/dev.124.10.1985. [DOI] [PubMed] [Google Scholar]
- 23.Grindley J C, Hargett L K, Hill R E, Ross A, Hogan B L. Mech Dev. 1997;64:111–126. doi: 10.1016/s0925-4773(97)00055-5. [DOI] [PubMed] [Google Scholar]
- 24.Sander M, Neubuser A, Kalamaras J, Ee H C, Martin G R, German M S. Genes Dev. 1997;11:1662–1673. doi: 10.1101/gad.11.13.1662. [DOI] [PubMed] [Google Scholar]
- 25.Dellovade T L, Pfaff D W, Schwanzel-Fukuda M. Brain Res Dev Brain Res. 1998;107:233–240. doi: 10.1016/s0165-3806(98)00007-8. [DOI] [PubMed] [Google Scholar]
- 26.Hill R E, Favor J, Hogan B L, Ton C C, Saunders G F, Hanson I M, Prosser J, Jordan T, Hastie N D, van Heyningen V. Nature (London) 1991;354:522–525. doi: 10.1038/354522a0. , and erratum (1992) 355, 750. [DOI] [PubMed] [Google Scholar]
- 27.Bentley C A, Zidehsarai M P, Grindley J C, Parlow A F, Barth-Hall S, Roberts V J. Endocrine. 1999;10:171–177. doi: 10.1385/ENDO:10:2:171. [DOI] [PubMed] [Google Scholar]
- 28.Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, van Heyningen V, Jessell T M, Briscoe J. Cell. 1997;90:169–180. doi: 10.1016/s0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- 29.Briscoe J, Sussel L, Serup P, Hartigan O C D, Jessell T M, Rubenstein J L, Ericson J. Nature (London) 1999;398:622–627. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]