Abstract
We have identified a rare (≈0.05–0.1%) population of cells (Thy-1hiCD16+CD44hiCD2−TCRαβ−B220−Mac-1−NK1.1−) in the adult mouse bone marrow that generates CD4+ and CD8+ TCRαβ+ T cells after tissue culture for 48 hr in the presence of Ly5 congenic marrow cells. The essential stages in the maturation of the progenitors were determined; the stages included an early transition from CD2−CD16+CD44hiTCRαβ− to CD2+CD16int/−CD44int/−TCRαβ− cells, and a later transition to CD4+CD8+TCRαβ+ double-positive T cells that rapidly generate the CD4+ and CD8+ single-positive T cells. The maturation of the progenitors is almost completely arrested at the CD2+TCRαβ− stage by the presence of mature T cells at the initiation of cultures. This alternate pathway is supported by the marrow microenvironment; it recapitulates critical intermediary steps in intrathymic T cell maturation.
Although the thymus is the predominant source of T cells in the peripheral lymphoid tissues, extrathymic sources of T cells also contribute to the T cell pool in both lymphoid and nonlymphoid tissues (1–5). CD4+ and CD8+ T cells accumulate in the spleen of congenitally athymic nu/nu mice (1, 6), and in thymectomized, lethally irradiated mice reconstituted with purified hematopoietic stem cells (7). Intestinal intra-epithelial T cells, especially CD8αα T cells, are present in athymic nude mice and thymectomized radiation chimeras (3, 8). Similarly, T cells in the liver, expressing the CD3intIL-2Rβ+ phenotype (IL-2R, IL-2 receptor), are abundant in mice without a functioning thymus (4).
Several sites for the generation of extrathymic T cell maturation have been proposed, including the bone marrow, mesenteric lymph nodes, and the gut (9–14). In the case of the marrow, evidence for in situ generation of T cells includes the presence of deleted circular segments of rearranged T cell receptor (TCR)α chain genes, and transcripts of recombination activating genes (RAG-1, RAG-2) in non-B lineage cells (10). T cell generation from precursors in short-term cultures of dispersed bone-marrow cells (11, 15), and from multilineage colonies grown on methylcellulose-coated plates derived from single pluripotent cells (16) has been reported also.
In a previous study (17), we showed that T cell progenitors, contained in bone-marrow cells depleted of CD4+, CD8+, and TCRαβ+ cells by flow cytometry, give rise to single-positive (CD4+CD8− and CD4−CD8+) T cells in 48-hr cultures through double-positive (CD4+CD8+) intermediary cells. In addition, positive and negative selection of the T cells occurred during the brief (8-hr) period between the transition from double-positive to single-positive T cells (17). Thus, these marrow cultures recapitulated the key elements of intrathymic T cell maturation (18), and demonstrated similar rapid kinetics of the generation of double- and single-positive T cells observed in heterogeneous thymic stromal cell cultures (19, 20). The kinetics of T cell generation in the latter cultures were considerably faster than in whole thymic organ cultures because of the extensive expansion of double-positive T cells in the whole organ (21, 22). Further phenotypic characterization of the newly generated CD4+ and CD8+ T cells in marrow cultures showed that they could not be distinguished from normal splenic T cells, except for a small subset of unusual CD16+CD44hi cells (17).
In a recent study, we identified rare (≈0.05%), clonable committed T cell progenitors in the adult mouse bone marrow with the Thy-1hiCD2−CD16+CD44hiLin− phenotype that had not rearranged the TCR β chain genes (S.D.-J., S.S., M. E. Garcia, and D. Zeng, unpublished data). The latter cells were injected intravenously and reconstituted only T cells in the bone marrow, spleen, lymph nodes, and thymus of adoptive hosts, by using both thymic and extrathymic pathways. In the current study, to determine whether the latter T cell progenitors can give rise to the CD4+ and CD8+ T cells in short-term marrow cultures, we used a progenitor assay system in which congenic Ly5.1 marrow cells support the maturation of purified progenitors from Ly5.2 C57BL/6 mice. We found that the sorted Thy-1hiCD2−CD16+CD44hiLin− cells in the normal adult marrow generated CD4+ and CD8+ T cells through an extrathymic pathway in vitro. These cells recapitulated the main elements reported for intrathymic T cell maturation, including the acquisition of CD2 and down-regulation of CD16 and CD44, before the appearance of the TCRαβ on the intermediary CD4+CD8+ double-positive T cells. The latter cells subsequently matured into single-positive CD4+ and CD8+ T cells.
Materials and Methods
Mice.
Congenic strains of C57BL/6-Thy1.2-Ly5.2 and C57BL/6-Thy1.2-Ly5.1 mice were used for all in vitro experiments. Mice were bred and maintained in the Department of Comparative Medicine animal facility, Stanford University School of Medicine. Male and female mice, 8 to 12 weeks old, were used.
Immunofluorescent Staining and Sorting of Cells.
Bone marrow cells were harvested from the femur and tibia as described in detail previously (15). Staining and sorting of cell suspensions with fluorochrome or biotin-conjugated mAbs were also described in detail previously (17). Four-color fluorescence-activated cell sorter (FACS) analysis and sorting were performed with a highly modified dual laser (488-nm argon and 599-nm dye lasers) FACS Vantage (Becton Dickinson) with eight-decade logarithmic amplifiers (17). Two- to three-color sorting was performed on a FACStar (Becton Dickinson). For sorting candidate progenitor cells in the bone marrow, cells were first enriched by incubation with biotin-conjugated anti-Thy1.2 mAb (5a-8, Caltag, South San Francisco, CA), further incubation with streptavidin-conjugated immunomagnetic beads, and positive selection on MACS-MS magnetic separation columns (Miltenyi Biotech, Auburn, CA), according to the manufacturer’s instructions. Stringent T cell depletion of whole bone marrow cells was performed by immunofluorescent staining, and sorting for CD4−CD8−TCRαβ− marrow cells, as described previously (17). The following mAbs were conjugated with fluorochromes as described (20): fluorescein isothiocyanate- (FITC) conjugated anti-Ly5.2 (A20.1.7), biotin-conjugated anti-Ly5.1 (ALI-4A2), and allophycocyanin-conjugated anti-CD44 (IM-781). Additional conjugated mAbs were purchased from Caltag: phycoerythrin- (PE) anti-CD4 (CT-CD4), PE-anti-CD8 (CT-CD8a), PE- and FITC-anti-TCRαβ (H57–597), FITC-anti-CD4 (CT-CD4), FITC- and PE-anti-macrophage (Mac)-1 (M1/70.15). PE- and FITC- anti-B220 (RA3–6B2), PE-anti-Gr-1 (RB6–8C5), PE-anti-NK1.1 (PK136), FITC-anti-CD16/32 (2.4G2), PE- and FITC-anti-CD2 (RM2–5), and Texas red streptavidin were purchased from PharMingen.
In Vitro Assays of Progenitor Maturation.
Short-term (48-hr) cultures of sorted T cell-depleted marrow cells and sorted candidate progenitor cells used RPMI-1640 (Biowhittaker, Walkersville, MA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT), 100 units/ml penicillin/100 μg/ml streptomycin (Biowhittaker), 2 mM glutamine (Biowhittaker), 10 mM Hepes buffer, and 0.1 mM 2-mercaptoethanol (GIBCO/BRL). Approximately 4 × 106 T cell-depleted marrow cells from Ly5.1 mice and 2 × 104 to 5 × 104 sorted candidate progenitor cells from Ly5.2 mice were cultured at 37°C in a humidified atmosphere with 5% CO2 in vented upright T12.5 flasks (Falcon, Franklin Lakes, NJ) at 2 × 106 cells per ml. At the end of the culture period, cells were harvested and washed twice in staining medium before being stained with mAbs. Yields of live nucleated cells were at least 50% in all of the cultures described here (in the figures and legends).
Results
Progenitor Activity of Thy-1hiCD2−Lin− Marrow Cells.
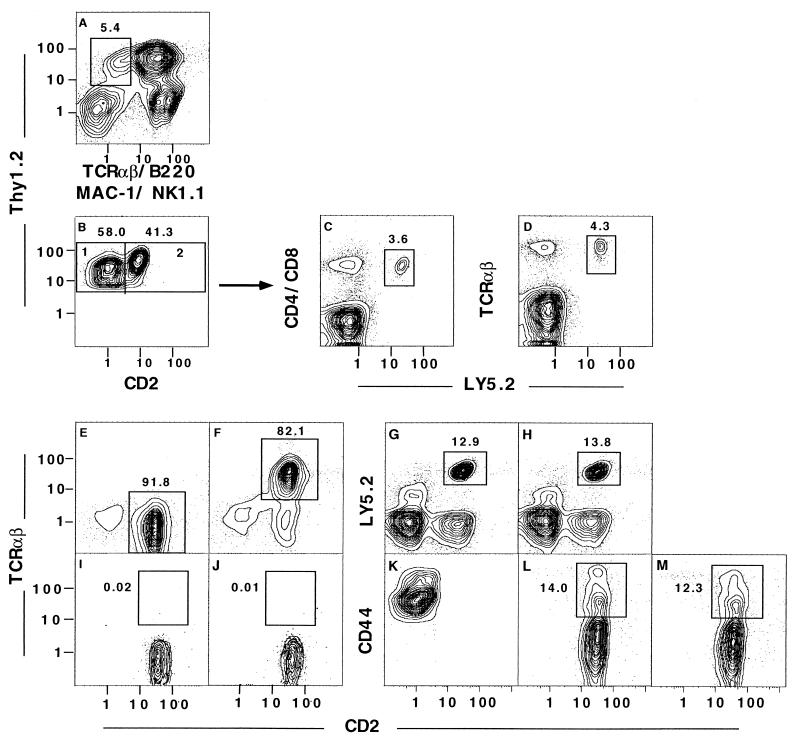
To isolate T cell progenitors in the marrow, we enriched marrow cells for Thy-1+ cells on immunomagnetic bead columns, and stained the enriched cells for surface markers reported to be present or absent on early T cell progenitors. The yield of enriched cells was 1–3% (data not shown). We subdivided Thy-1hiLin− (Thy-1hiTCRαβ−B220−Mac-1−NK1.1−) marrow cells (enclosed within the box in Fig. 1A), into CD2− and CD2+ cells, as shown in boxes 1 and 2, respectively, of Fig. 1B. We tested the progenitor activity of the sorted CD2− cells contained within box 1, because these cells reconstituted only T cells after intravenous injection into lethally irradiated hosts (S.D.-J., S.S., M. E. Garcia, and D. Zeng, unpublished data). Re-analysis of the sorted cells that were used in this experiment and in subsequent experiments showed that more than 99% were contained within the thresholds of box 1 (data not shown). Incubation of 5 × 104 sorted cells from Ly5.2 mice with 4 × 106 T cell-depleted marrow cells from Ly5.1 mice generated CD4 and CD8 Ly5.2+ T cells (Fig. 1 C and D). Ly5.2+ cells, expressing CD4 or CD8 and TCRαβ, accounted for about 3.6–4.3% of cells after culture. Staining of these cultured cells for Ly5.2 vs. Mac-1 (macrophage), B220 (B cell), Gr-1 (granulocyte), and NK1.1 (NK cell) receptors showed that these non-T cells accounted for only 0.1–0.4% of cells (data not shown). Thus, the Thy-1hiCD2−Lin− marrow cells showed only mature T cell progenitor activity in the in vitro assay system. In four experiments, the mean absolute number ± SEM of mature TCRαβ+ T cells generated in the 48-hr cultures was 5.4 ± 0.6 × 105. In further experiments, sorted Thy-1hiCD2+Lin− cells obtained from box 2 of Fig. 1B were tested for progenitor activity; they generated similar numbers of CD4+ and CD8+ TCRαβ+ T cells after 48-hr cultures with T cell-depleted Ly5.1 marrow cells (data not shown).
Figure 1.
Identification of Thy-1hiCD2−Lin− T cell progenitors in the adult bone marrow. C57BL/6 Ly5.2 bone marrow cells were enriched for Thy-1+ cells with immunomagnetic beads, and cells were subsequently stained for Thy-1 with one fluorochrome; TCRαβ, B220, Mac-1, and NK1.1 with a second fluorochrome; and CD2 with a third fluorochrome. Analysis of Thy-1 vs. B220, Mac-1, TCRαβ, and NK1.1 (A); the box encloses Thy-1hiTCRαβ−B220−Mac-1−NK1.1− (Thy-1hiLin−) cells. The latter cells were gated and reanalyzed for Thy-1 vs. CD2 (B); boxes 1 and 2 enclose Thy-1hiCD2−Lin− and Thy-1hiCD2+Lin− cells, respectively. Progenitor activity of sorted Thy-1hiCD2−Lin− Ly5.2 marrow cells was tested by incubating 5 × 104 cells with 4 × 106 Ly5.1 T cell-depleted marrow cells for 48 hr. The cultured cells were analyzed for CD4 and CD8 markers (C) or TCRαβ vs. Ly5.2 markers (D). Boxes enclose Ly5.2+ T cells. In a repeat experiment, the sorted Thy-1hiCD2−Lin− cells were cocultured with T cell-depleted Ly5.1 marrow cells, and cells were harvested at either 24 hr (E) or 48 hr (F). Gated Ly5.2+ cells harvested from the cultures were stained for TCRαβ vs. CD2, and boxes enclose CD2+TCRαβ− (E) or CD2+TCRαβ+ (F) cells. The sorted Thy-1hiCD2−Lin− cells were also cocultured with whole instead of T cell-depleted Ly5.1 marrow cells, and cultures were stained for Ly5.2 vs. CD2 after 24 and 48 hr in G and H, respectively. Gated Ly5.2 cells from the latter cultures were stained for TCRαβ vs. CD2 at 24 and 48 hr (I and J, respectively), and boxes enclose CD2+TCRαβ+ cells. Expression of CD44 on sorted Thy-1hiCD2−Lin− cells before culture (K) is compared with that after coculture with T cell-depleted Ly5.1 marrow cells for 24 (L) or 48 hr (M). Analyses of CD44 vs. CD2 on gated Ly5.2 cells are shown, and boxes enclose CD44hiCD2+ cells. Each pair of profiles is from a separate representative experiment of at least four replicate experiments.
Previous studies reported that CD2− progenitors in the fetal thymus rapidly transform into CD2+TCRαβ− intermediate progeny before giving rise to CD2+TCRαβ+ CD4+ and CD8+ single-positive cells during T cell maturation (23–25). We wished to determine whether a similar transformation occurs in our in vitro marrow progenitor assay system. Accordingly, cultures of the CD2− marrow progenitors were repeated, and cells were harvested at both 24 and 48 hr. Fig. 1 E and F show the TCRαβ vs. CD2 analyses of gated Ly5.2+ cells at each time point, respectively. Whereas more than 99% of the sorted marrow cells from Ly5.2 mice were CD2−TCRαβ− on reanalysis before culture (data not shown), 91.8% were CD2+TCRαβ− at 24 hr (enclosed in box in E). However, at 48 hr, 82.1% of the Ly5.2+ cells were CD2+TCRαβ+ (enclosed in box in F). Because mature T cells have been shown to inhibit the outgrowth of CD4 and CD8 TCRαβ+ single-positive T cells in these in vitro cultures (17), we wanted to know whether mature T cells inhibit the outgrowth of CD2+TCRαβ− and CD2+TCRαβ+ cells from the CD2− progenitors. We cultured the latter cells (5 × 104) with whole Ly5.1 bone marrow cells (4 × 106), instead of with the T cell-depleted Ly5.1 bone marrow cells. As shown in Fig. 1 G and H, 12.9% and 13.8% of cultured cells, at 24 and 48 hr, respectively, were Ly5.2+CD2+. The percentage of Ly5.2+CD2− cells at the initiation of cultures was 1.2%. Thus, the presence of mature T cells did not inhibit the CD2− to CD2+ transition. However, as shown in Fig. 1 I and J, at 24 and 48 hr, respectively, the CD2+ cells did not express TCRαβ (less than 1% enclosed in boxes), and failed to undergo the CD2+TCRαβ− to CD2+TCRαβ+ transition observed with T cell-depleted Ly5.1 marrow cells in Fig. 1 E and F. The failure to express TCRαβ at 48 hr was associated with the failure to express CD4 and CD8 also (data not shown).
Changes in Expression of CD44 on Cultured CD2− Progenitor Cells.
During intrathymic T cell maturation, early CD4−CD8−TCRαβ− precursors express high levels of CD44 on the cell surface, and just before TCRβ chain gene rearrangement, CD44 is down-regulated (26). The changes in the surface expression of CD44 were studied during the transition from CD2− to CD2+ cells in the marrow culture system with T cell-depleted Ly5.1 marrow cells. The freshly sorted Thy-1hiCD2−Lin− marrow cells, obtained from Ly5.2 mice as described above, were stained for CD44; the analysis of CD2 vs. CD44 is shown in Fig. 1K. Almost all of the CD2− sorted cells expressed high levels of CD44 before culture; but after culture, Ly5.2+ cells down-regulated CD44. Only 14.0% of gated Ly5.2 cells harvested at 24 hr were CD44hi (enclosed in box, Fig. 1L) and the rest were CD44lo/−. During the same time, CD2 was up-regulated (Fig. 1L). The surface expression of CD2 and CD44 remained essentially unchanged at 48 hr (Fig. 1M), when the TCRαβ marker was expressed on almost all of the Ly5.2+ gated cells (Fig. 1F).
Changes in Expression of CD16 on Cultured CD2− Progenitor Cells.
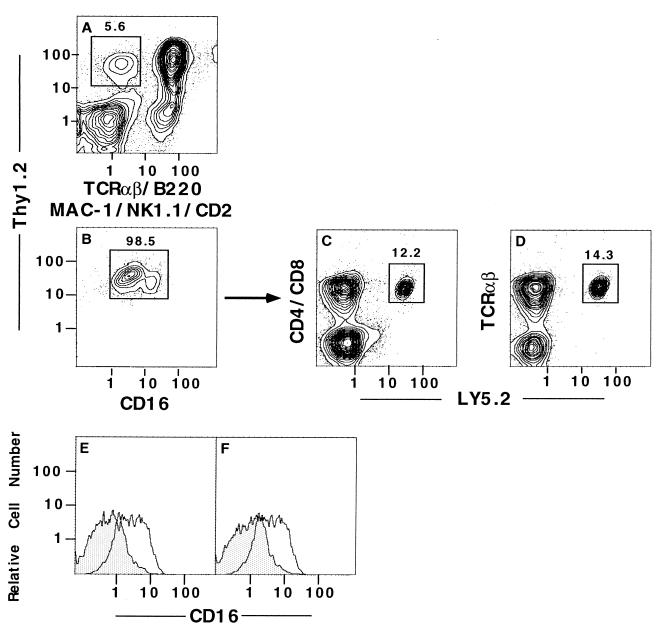
Early T cell progenitors in the fetal blood and fetal and adult thymus express CD16/32 (referred to hereafter as CD16) on the cell surface, and during T cell maturation, expression of CD16 is rapidly down-regulated (24, 27). As shown in Fig. 2 A and B, gated Thy-1hiCD2−Lin− cells from the marrow were stained for CD16. Almost all of the gated cells were CD16+, including a small population of CD16hi and a large population of CD16int cells (enclosed in box in Fig. 2B). The yield of this subset was about 0.05–0.1% of the starting marrow cells. We tested the progenitor activity of this purified population of cells from Ly5.2 mice by sorting cells enclosed in the box in Fig. 2B. The sorted Ly5.2 cells (5 × 104) were incubated for 48 hr with 4 × 106 T cell-depleted marrow cells from Ly5.1 mice, and the staining patterns for CD4, CD8, and TCRαβ markers vs. Ly5.2 after culture are shown in Fig. 2 C and D. A discrete population of CD4+ and CD8+ TCRαβ+ T cells expressing Ly5.2 was observed; they accounted for about 12–13% of the nucleated live cells (enclosed in boxes in C and D). The culture shown in Fig. 2C contained 31% Ly5.2− CD4+ and CD8+ TCRαβ+ T cells, derived from the Ly5.1 T cell-depleted bone marrow cells. The expression of CD16 on the cultured progenitor cells at 24 and 48 hr was studied at both time points by gating on the Ly5.2+ cells. Fig. 2 E and F shows that the brightness of CD16 staining was markedly reduced at 24 and 48 hr (shaded areas), as compared with the freshly sorted cells placed in culture (open areas).
Figure 2.
Progenitor activity of Thy-1hiCD16+CD2−Lin− sorted bone marrow cells. Enriched marrow cells from Ly5.2 mice were stained for Thy-1 vs. TCRαβ, B220, Mac-1, NK1.1, and CD2 vs. CD16 with three fluorochromes. The latter cells were analyzed for Thy-1 vs. TCRαβ, B220, Mac-1, NK1.1, and CD2 (A), and the box encloses Thy-1hiCD2−Lin− cells. Cells within the box in A were analyzed for Thy-1 vs. CD16 (B), and the box in B encloses Thy-1hiCD16+ cells. Single-color analysis of the intensity of staining of CD16 is also shown in the open areas of E and F also. The Thy-1hiCD16+ sorted cells (5 × 104) were cocultured with 4 × 106 T cell-depleted Ly5.1 marrow cells for 48 hr, and the newly generated CD4+ or CD8+ TCRαβ+Ly5.2 cells are enclosed in boxes in C and D. Cells were harvested also at 24 and 48 hr, gated on the Ly5.2+ subset, and analyzed for CD16 staining. The intensity of staining of the sorted cells before culture was compared with that after 24 hr (E) or after 48 hr (F). Open area shows staining before culture, and shaded area shows staining after culture.
Changes in Expression of CD4 and CD8 on Cultured CD2− Progenitor Cells.
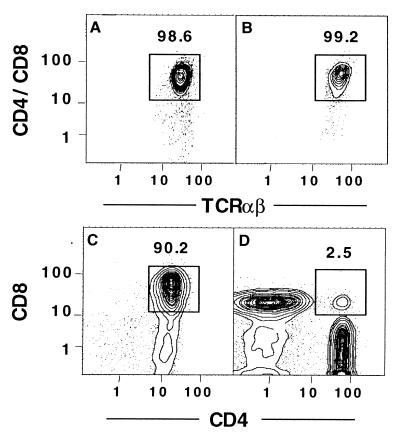
Our previous study of the in vitro generation of CD4+ and CD8+ T cells from stringently T cell-depleted marrow cells showed the development of intermediary double-positive T cells 4 to 8 hr before the appearance of single-positive cells at 48 hr (17). To determine whether the Thy-1hiLin− progenitors mature through the double-positive stage, sorted progenitors were obtained from Ly5.2 donor mice, as shown in the box in Fig. 1A, and 2 × 104 sorted cells were added to the 4 × 106 T cell-depleted Ly5.1 marrow cells; cultures were harvested at 44 and 48 hr. Gated Ly5.2+ cells were analyzed for the expression of CD4 and CD8 vs. TCRαβ markers (Fig. 3 A and B), and for CD4 vs. CD8 markers (C and D) at each time point. Fig. 3 A and B show that almost all the Ly5.2+ progenitor cells expressed CD4 and/or CD8 and TCRαβ at both time points. Fig. 3C shows that almost all Ly5.2+ cells at 44 hr expressed both the CD4 and CD8 markers, but at 48 hr, these cells had matured into either CD4+CD8− or CD4−CD8+ cells, with few residual CD4+CD8+ cells. The rapid transition from double-positive to single-positive T cells over a similar time interval has been reported in heterogeneous thymic stromal cell cultures (19, 20). However, the double-positive cells in the latter cultures persisted during the appearance of the single-positive cells (19, 20).
Figure 3.
CD4+CD8+ progeny of cultured T cell progenitors. Sorted Thy-1hiLin− Ly5.2 marrow cells (2 × 104) were cultured with T cell-depleted Ly5.1 marrow cells (4 × 106), and cells were harvested at 44 and 48 hr (A, C, and B, D, respectively). At each time, Ly5.2+ cells were gated and analyzed for CD4/CD8 vs. TCRαβ (A and B) or CD4 vs. CD8 markers (C and D). In A and B, boxes enclose CD4+ and/or CD8+ TCRαβ+ T cells. In C and D, boxes enclose double-positive (CD4+CD8+) T cells.
Discussion
The object of the current study was to determine whether sorted T cell progenitors in the marrow can mature into CD4+ and CD8+ T cells in vitro, supported by the marrow microenvironment in the absence of thymic stromal cells or exogenous cytokines. Previous studies of early T cell progenitors in the fetal thymus showed that the progenitor cells were Thy-1+CD16+CD2−TCRαβ− (24). In the adult thymus, the earliest progenitors are not committed to the T cell lineage, and are able to generate T, B, NK, and dendritic cells (28, 29). In the fetal blood, Thy-1hiCD3− progenitors were reported to be committed to the T cell lineage (30), but a recent report indicated that most of these cells express NK1.1 on the cell surface, and are capable of generating both NK and T cells (23). Our recent study showed that Thy-1hiCD2−CD16+CD44hiLin− cells in the adult bone marrow reconstitute only T cells in the lymphoid tissues of adoptive hosts after intravenous injection (S.D.-J., S.S., M. E. Garcia, and D. Zeng, unpublished data).
To isolate T cell progenitors in the marrow, we enriched marrow cells for Thy-1+ cells on immunomagnetic bead columns. A discrete population of sorted Thy-1hiCD2−Lin− cells was capable of maturing into CD4+ and CD8+ single-positive T cells in vitro within 48 hr, as judged by a progenitor assay in which candidate progenitors were incubated with Ly5 congenic T cell-depleted marrow cells. The sorted progenitor cells accounted for approximately 1 in 2,000 of the starting nucleated marrow cells, and have not yet rearranged the Vβ gene segments (S.D.-J., S.S., M. E. Garcia, and D. Zeng, unpublished data). We were unable to identify a discrete population of the Thy-1hiCD2−Lin− progenitors in the adult thymus, because after depletion of Lin− cells, including CD4+ and CD8+ cells, residual thymocytes were almost all Thy-1int/loCD2−Lin− cells (data not shown).
The Thy-1hiLin− marrow progenitor cells generated CD4+ and CD8+ single-positive T cells at 48 hr through CD4+CD8+ double-positive intermediary cells, which were identified at 44 hr. The rapid transition of double-positive to single-positive T cells in a matter of hours has been observed also during T cell maturation in heterogeneous thymic stromal cell cultures (19, 20). However, in the latter cultures, the double-positive cells persist, along with the newly generated single-positive T cells, whereas the double-positive cells disappear in the bone marrow cultures. The current results are consistent with our previous report of the transient appearance of double-positive T cells at 40 and 44 hr after culture of T cell-depleted bone marrow (17). Positive selection of single-positive T cells was documented previously by the marked increase in Vβ17+ cells among the single-positive CD4+ T cells, as compared with CD4+CD8+ T cells generated in cultures with SWR (H-2q) bone marrow cells, but not with C57BL/6 (H-2b) marrow cells (17). Negative selection was documented by the deletion of Vβ3, Vβ5.1, Vβ11, and Vβ12 receptors among the single-positive CD4+ and CD8+ T cells generated with BALB/c (H-2d) bone marrow cell cultures (17).
Almost all of the Thy-1hiCD2−Lin− progenitor cells acquired surface CD2, but not TCRαβ, within 24 hr after culture, and acquired both markers by 48 hr. The transition from TCRαβ−CD2+ at 24 hr to TCRαβ+CD2+ at 48 hr was inhibited by the presence of mature T cells in the co-cultured Ly5.1 marrow cells. This result is consistent with our previous report that mature T cells, added to T cell-depleted marrow cultures, inhibit the outgrowth of CD4+ and CD8+ T cells from the marrow progenitors (17). In concert with the previously reported CD2−CD16+ phenotype of fetal T cell precursors (24), almost all of the CD2− progenitors in the marrow expressed surface CD16. The appearance of CD16 on progenitor cells in the fetus is associated with the loss of the potential to mature into B lymphocytes and myeloid cells, and with restriction toward T cell maturation (26). During the first 24 hr of the sorted Thy-1hiCD16+CD2−Lin− cells in culture, the CD16 surface expression was down-regulated, in association with the up-regulation of CD2. A similar reciprocal change in CD2 and CD16 occurred during T cell maturation from early progenitors in the fetal thymus (24). During the first 24 hr in marrow culture, the CD2− sorted cells also down-regulated surface expression of CD44. Down-regulation of both CD16 and CD44 appears to be closely linked in time to the rearrangement and subsequent expression of the TCR β chain genes (21, 24). The appearance of the TCRαβ on the surface of the cultured Thy-1hiCD2− cells within 24 hr after down-regulation of CD16 and CD44 recapitulates this intrathymic transition in the marrow culture system.
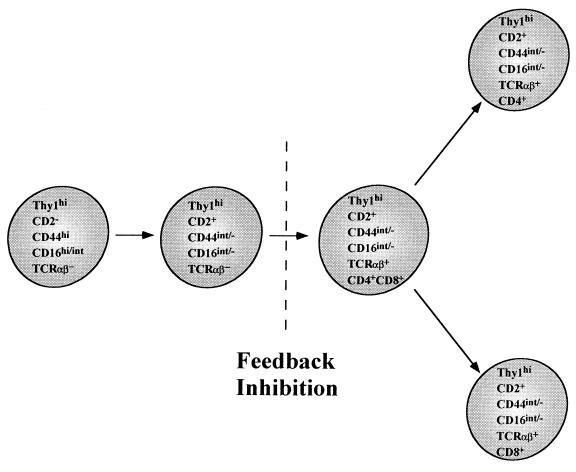
Fig. 4 summarizes the T cell maturation pathway that occurs during the 48-hr bone marrow cultures. Thy-1hiCD2−CD16hi/intCD44hiTCRαβ− progenitors rapidly mature into Thy-1hiCD2+CD16int/−CD44int/−TCRαβ− cells during the first 24 hr. During the subsequent 24-hr interval, the latter cells acquire TCRαβ and develop into CD4+CD8+ intermediate cells that go on to generate single-positive CD4+ and CD8+ T cells. The ability of the bone marrow cultures to recapitulate the essential elements of maturation of thymic T cells indicates that the microenvironment of the thymus is not unique in its capacity to support T cell maturation. However, the alternate T cell developmental pathway in the marrow appears to be under tight regulatory control, and is almost completely inhibited in vitro at the Thy-1hiCD2+TCRαβ− to CD4+CD8+TCRαβ+ cell transition by the presence of mature T cells. In the fresh marrow or marrow cultures, the CD4+CD8+ cells that do not mature rapidly into single-positive cells appear to die rapidly, probably because of lack of positive selection; they are not renewed from progenitors because of feedback inhibition, mediated by double-positive or single-positive T cells. On the other hand, single-positive T cells that are positively selected in the marrow may not require continuous renewal because of their longer life span. In thymic stromal cell cultures, it is likely that the CD4+CD8+ T cells also die rapidly because of the lack of positive selection, but they are renewed from progenitor cells that are not sensitive to feedback inhibition by mature T cells. The contribution of the intramarrow pathway to the total T cell pool of normal mice is not yet clear. However, Thy-1hiCD16hiCD2−Lin− marrow progenitors generate only T cells in vivo by both extrathymic and intrathymic pathways within 4 weeks after intravenous injection into irradiated congenic hosts (S.D.-J., S.S., M. E. Garcia, and D. Zeng, unpublished data).
Figure 4.
Diagram of proposed alternative pathway of T cell maturation in the adult bone marrow. Thy-1hiTCRαβ−CD2−CD16hi/intCD44hi progenitors rapidly mature into Thy-1hiTCRαβ−CD2+CD16int/−CD44int/− cells in association with TCR β chain gene rearrangement. Subsequently, TCR α chain gene rearrangement results in the development of CD4+CD8+ (double-positive) intermediary cells. Single-positive CD4+CD8− and CD4−CD8+ T cells are derived from the double-positive cells during processes of positive and negative selection. The transition from Thy-1hiTCRαβ−CD2+ to CD4+CD8+ intermediary cells is arrested in the presence of mature T cells (feedback inhibition).
Acknowledgments
We thank Dr. I. L. Weissman for help in the design and analysis of experiments, A. Mukhopadhyay for technical assistance, and V. Cleaver for preparation of the manuscript. The studies were supported by grants AI-43013, HL-58250, and HL-57443 from the National Institutes of Health.
Abbreviations
- PE
phycoerythrin
- TCR
T cell receptor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.MacDonald H R, Lees R K, Bron C, Sordat B, Miescher G. J Exp Med. 1987;166:195–209. doi: 10.1084/jem.166.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benveniste P, Chadwick B S, Miller R G, Reimann J. J Immunol. 1990;144:411–419. [PubMed] [Google Scholar]
- 3.Rocha B, Vassalli P, Guy-Grand D. J Exp Med. 1994;180:681–686. doi: 10.1084/jem.180.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato K, Ohtsuka K, Hasegawa K, Yamagiwa S, Watanabe H, Asakura H, Abo T. J Exp Med. 1995;182:759–767. doi: 10.1084/jem.182.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leclercq G, Plum J. Leukemia. 1996;10:1853–1859. [PubMed] [Google Scholar]
- 6.Lawetzky A, Hunig T. Eur J Immunol. 1988;18:409–416. doi: 10.1002/eji.1830180314. [DOI] [PubMed] [Google Scholar]
- 7.Dejbakhsh-Jones S, Jerabek L, Weissman I L, Strober S. J Immunol. 1995;155:3338–3344. [PubMed] [Google Scholar]
- 8.Guy-Grand D, Cerf-Bensussan N, Malissen B, Malassis-Seris M, Briottet C, Vassalli P. J Exp Med. 1991;173:471–481. doi: 10.1084/jem.173.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guy-Grand D, Vanden Broecke C, Briottet C, Malassis-Seris M, Selz F, Vassalli P. Eur J Immunol. 1992;22:505–510. doi: 10.1002/eji.1830220232. [DOI] [PubMed] [Google Scholar]
- 10.Makino Y, Koseki H, Adachi Y, Akasaka T, Tsuchida K, Taniguchi M. Int Rev Immunol. 1994;11:31–46. doi: 10.3109/08830189409061715. [DOI] [PubMed] [Google Scholar]
- 11.Dejbakhsh-Jones S, Okazaki H, Strober S. J Exp Med. 1995;181:2201–2211. doi: 10.1084/jem.181.6.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenai H, Matsuzaki G, Lin T, Yoshida H, Nomoto K. Eur J Immunol. 1995;25:3365–3369. doi: 10.1002/eji.1830251224. [DOI] [PubMed] [Google Scholar]
- 13.Clegg C H, Rulffes J T, Wallace P M, Haugen H S. Nature (London) 1996;384:261–263. doi: 10.1038/384261a0. [DOI] [PubMed] [Google Scholar]
- 14.Freedman A R, Zhu H, Levine J D, Kalams S, Scadden D T. Nat Med. 1996;2:46–51. doi: 10.1038/nm0196-46. [DOI] [PubMed] [Google Scholar]
- 15.Palathumpat V, Dejbakhsh-Jones S, Holm B, Wang H, Liang O, Strober S. J Immunol. 1992;148:373–380. [PubMed] [Google Scholar]
- 16.Hattori M, Sudo T, Izawa H, Kano S, Minato N. Int Immunol. 1989;1:151–159. doi: 10.1093/intimm/1.2.151. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Ojeda M E, Dejbakhsh-Jones S, Weissman I L, Strober S. J Exp Med. 1998;187:1813–1823. doi: 10.1084/jem.187.11.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adkins B, Mueller C, Okada C Y, Reichert R A, Weissman I L, Spangrude G J. Annu Rev Immunol. 1987;5:325–365. doi: 10.1146/annurev.iy.05.040187.001545. [DOI] [PubMed] [Google Scholar]
- 19.Sen-Majumdar A, Lieberman M, Alpert S, Wiessman I L, Small M. J Exp Med. 1992;176:543–551. doi: 10.1084/jem.176.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akashi K, Weissman I L. Immunity. 1996;5:147–161. doi: 10.1016/s1074-7613(00)80491-4. [DOI] [PubMed] [Google Scholar]
- 21.Godfrey D I, Kennedy J, Suda T, Zlotnik A. J Immunol. 1993;150:4244–4252. [PubMed] [Google Scholar]
- 22.Kydd R, Lundberg K, Vremec D, Harris A W, Shortman K. J Immunol. 1995;155:3806–3814. [PubMed] [Google Scholar]
- 23.Carlyle J R, Zuniga-Pflucker J C. Immunity. 1998;9:187–197. doi: 10.1016/s1074-7613(00)80601-9. [DOI] [PubMed] [Google Scholar]
- 24.Rodewald H R, Awad K, Moingeon P, D’Adamio L, Rabinowitz D, Shinkai Y, Alt F W, Reinherz E L. J Exp Med. 1993;177:1079–1092. doi: 10.1084/jem.177.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yagita H, Asakawa J, Tansyo S, Nakamura T, Habu S, Okumura K. Eur J Immunol. 1989;19:2211–2217. doi: 10.1002/eji.1830191206. [DOI] [PubMed] [Google Scholar]
- 26.Hattori N, Kawamoto H, Katsura Y. J Exp Med. 1996;184:1901–1908. doi: 10.1084/jem.184.5.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodewald H R, Moingeon P, Lucich J L, Dosiou C, Lopez P, Reinherz E L. Cell. 1992;69:139–150. doi: 10.1016/0092-8674(92)90125-v. [DOI] [PubMed] [Google Scholar]
- 28.Wu L, Antica M, Johnson G R, Scollay R, Shortman K. J Exp Med. 1991;174:1617–1627. doi: 10.1084/jem.174.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ardavin C, Wu L, Li C L, Shortman K. Nature (London) 1993;362:761–763. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- 30.Rodewald H R, Kretzschmar K, Takeda S, Hohl C, Dessing M. EMBO J. 1994;13:4229–4240. doi: 10.1002/j.1460-2075.1994.tb06743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]