Abstract
Acquisition of planar cell polarity (PCP) in epithelia involves intercellular communication, during which cells align their polarity with that of their neighbors. The transmembrane proteins Frizzled (Fz) and Van Gogh (Vang) are essential components of the intercellular communication mechanism, as loss of either strongly perturbs the polarity of neighboring cells. How Fz and Vang communicate polarity information between neighboring cells is poorly understood. The atypical cadherin, Flamingo (Fmi), is implicated in this process, yet whether Fmi acts permissively as a scaffold, or instructively as a signal is unclear. Here, we provide evidence that Fmi functions instructively to mediate Fz-Vang intercellular signal relay, recruiting Fz and Vang to opposite sides of cell boundaries. We propose that two functional forms of Fmi, one of which is induced by and physically interacts with Fz, form cadherin homodimers that signal bidirectionally and asymmetrically, instructing unequal responses in adjacent cell membranes to establish molecular asymmetry.
Introduction
Polarization of epithelial cells along an axis orthogonal to their apical-basal axes is referred to as planar cell polarity (PCP), or tissue polarity. PCP is essential for a variety of developmental events involving cell fate decisions, morphogenesis and organized cell movements, and the mechanisms controlling PCP are conserved between vertebrates and invertebrates (Wang and Nathans, 2007). To ensure a robust and uniform response to cues that align cell polarity with the tissue axes, current models propose that neighboring cells communicate and coordinate this polarity information by passing it from cell to cell (Adler et al., 1997; Amonlirdviman et al., 2005; Lawrence et al., 2004). Although many genes involved in regulating epithelial PCP have been identified, the molecular mechanism by which epithelial cells communicate and propagate PCP signals is poorly understood.
PCP has been most effectively studied in Drosophila, and is evident in many epithelial structures such as hairs of the wing and abdomen, bristles of the notum and ommatidia of the eye. Genes involved in regulating PCP have been classified as either part of the core set of PCP signaling components that coordinate polarization of neighboring cells, or as part of a module that provides global directional information. Core components include the serpentine receptor Frizzled (Fz), the multi-domain protein Dishevelled (Dsh), the Ankryin repeat protein Diego (Dgo), the 4-pass transmembrane protein Van Gogh (Vang; a.k.a. Strabismus/Stbm), the Lim domain protein Prickle (Pk) and the seven-transmembrane atypical cadherin Flamingo (Fmi; a.k.a. Starry night/Stan)(reviewed in Zallen, 2007). The proposal that these core PCP proteins mediate cell-cell communication rests in large part on the observations that fz and vang mutant clones strongly perturb the polarity of prehairs in adjacent zones of non-mutant tissue, though in opposite directions (Fig. 1C, D; Adler et al., 2000). This phenomenon is called domineering non-autonomy.
Figure 1. Mutual recruitment of Fz and Vang.
(A) Schematic of core PCP protein localization; early (<6 hrs APF), and later stages (> 24 hrs APF).
(B–B″) Prehairs (phalloidin; B′; red in B″) initiate at the distal vertex where Fz is enriched (Fz::GFP; B; green in B″). Distal to the right in this and all subsequent figures.
(C–F) fz, vang, fmi, and vang, fz double mutant clones. Using MARCM, clones are marked by DsRed (blue in [C″–F″]) and Fz::GFP is excluded from the clone ([C–F]; green in [C″–F″]). Prehairs stained with phalloidin ([C′–F′]; red in [C″–F″]). Scale bars in all figures indicate 15 microns.
(C–C″) fzR52 clone showing distal non-autonomy ([C′]; red in [C″]). FzGFP is recruited to the clone border in wildtype neighbors ([C]; green in [C″]; yellow arrowheads).
(D–D″) vangA3clone showing proximal non-autonomy ([D′]; red in [D″]). FzGFP is excluded from the clone border in wildtype neighbors ([D]; green in [D″]; yellow arrowheads).
(E–E″) fmiE45 clone showing cell-autonomous polarity ([E′]; red in [E″]). FzGFP in wildtype neighbors is absent but not repelled from the clone border; note the characteristic accumulation at cell borders perpendicular to the clone border, and contrast with the vangA3clone ([E]; green in [E″]; compare to [D]; yellow arrowheads).
(F–F″) vangA3; fzR52 double mutant clone shows a cell-autonomous phenotype ([F′]; red in [F″]). FzGFP is neither recruited to nor repelled from the clone border ([F]; green in [F″]; yellow arrowheads).
(G–J) Schematics of hair polarity and Fz protein localization (green) in wildtype cells neighboring fzR52 (G), vangA3 (H), fmiE45 (I) and vangA3; fzR52 clones (J).
(K–N) FzΔCRD rescues the fzK21/fzD21 phenotype. fz mutant showing disarrayed polarity in the wing (B) and thorax (D). (A, C) Rescue by the FzΔCRD transgene.
(O) VangΔECDYFP shows asymmetric localization in pupal wing at 30hrs APF.
(P) Schematics of VangΔECDand FzΔCRD. Extracellular loops of Vang are replaced by HA and FLAG tags in VangΔECD, and the CRD domain of Fz is deleted in FzΔCRD.
(Q) Absence of polarity defect in wing clones simultaneously mutant for the five Drosophila Wnts expressed in the wing: Wnt2, Wg, Wnt4, Wnt6 and Wnt10.
The core PCP proteins receive a cue that orients their action with respect to the tissue axes. A controversy exists as to whether the core PCP components receive this global directional cue from a module consisting of the golgi protein Four-jointed (Fj), the atypical cadherins Fat and Dachsous (Ds; Ma et al., 2003; Yang et al., 2002), or from an as yet unidentified “morphogen” gradient (Lawrence et al., 2002). According to the morphogen model, Fj, Ds and Fat function in parallel to the core PCP proteins (Casal et al., 2006).
The distribution of the core PCP proteins is dynamically regulated. Over many hours, these proteins reorganize from an essentially non-polarized distribution to an asymmetric distribution at adherens junctions. In the wing, shortly before the initiation of prehair growth, Fz, Dsh and Dgo are maximally enriched at distal cell borders, while Pk and Vang are enriched at proximal borders (Fig. 1A; Strutt and Strutt, 2005; Zallen, 2007). The asymmetric localization of core PCP components is regulated by a feedback mechanism that signals between neighboring cells. We have proposed that localization of the proximal and distal proteins are mutually antagonistic, thereby forming a bistable switch that coordinates polarization of cells with each other (Amonlirdviman et al., 2005; Tree et al., 2002a). These proteins direct morphological polarization, and at the time at which their maximal asymmetry is observed, actin rich prehairs extend from the distal side of the cell where Fz, Dsh and Dgo colocalize (Fig. 1B).
One of the central challenges in PCP is to understand how cells align their polarity with respect to their neighbors. This alignment, which involves regulated cell-cell communication and is inherently directional, assures the robustness of the polarization response to the global directional cue, and is also responsible for the domineering non-autonomy near fz and vang mutant clones (Ma et al., 2003; Amonlirdviman et al., 2005). Evidence suggests critical functions for Fz and Vang in intercellular PCP signal relay (Adler et al., 2000), but the mechanism by which signals are transmitted, and whether these proteins directly interact remain unclear. We and others have inferred that Fz and Vang recruit each other to opposite sides of intercellular boundaries (Amonlirdviman et al., 2005; Strutt and Strutt, 2007; Tree et al., 2002b), and we hypothesize that this interaction intimately links intercellular communication with the asymmetric subcellular localization of PCP signaling components.
Loss of Fmi can disrupt intercellular polarity communication (Lawrence et al., 2004; Strutt and Strutt, 2007). Fmi is an atypical cadherin known to play critical roles in organizing epithelial polarity and patterning neuronal connections (Kimura et al., 2006). In the wing, Fmi can only accumulate at the adherens junction when it is also present in the neighboring cell, consistent with studies showing that Fmi undergoes homophilic interactions in vitro (Usui et al., 1999). Loss of function studies suggest that Fmi homodimers bridging adjacent cells are required for the transmission of PCP signals (Chae et al., 1999; Lawrence et al., 2004; Strutt and Strutt, 2007; Usui et al., 1999). However, the molecular mechanism by which Fmi regulates cell-cell communication is not clear. Morever, in fmi mutant clones, and in wild type neighbors at the clone boundaries, Fmi itself as well as the remaining core PCP components fail to accumulate (Axelrod, 2001; Bastock et al., 2003; Feiguin et al., 2001; Shimada et al., 2001; Strutt and Strutt, 2007; Tree et al., 2002a). It is impossible to distinguish from existing data whether Fmi acts permissively, as a scaffold maintaining other PCP components in place for signaling, or if Fmi can itself transmit an instructive signal that directs intercellular PCP signal relay.
In this study, we show that Fmi acts instructively in recruiting Fz and Vang across cell borders to propagate PCP signals from cell to cell. Fmi homodimers signal bidirectionally and asymmetrically, differentially recruiting Fz and Vang to opposite sides of the intercellular junction, thereby providing a molecular basis for PCP signal transmission across cell borders.
Results
Mutual requirement for Fz and Vang in propagating intercellular PCP signals
The two transmembrane proteins, Fz and Vang, play critical roles in intercellular signaling. Of the core PCP components, only clones mutant for fz or vang demonstrate strong domineering non-autonomy. Near fz clone borders, wildtype cells distal to the clone reverse polarity and point their prehairs toward the clone (Fig. 1C), while near vang clone borders, wildtype cells proximal to the clone reverse polarity and point their prehairs away from the clone (Fig. 1D). We have therefore used non-autonomy as an assay to dissect the molecular mechanism of PCP signal transmission across cell boundaries.
In wildtype cells, Fz and Vang segregate to occupy opposite sides of cell boundaries, and gain- and loss-of-function studies show that Fz accumulates at clone borders where a Vang activity difference exists, and vice versa (Bastock et al., 2003; Strutt, 2001). Mutual recruitment between Fz and Vang on opposite sides of cell boundaries has been hypothesized (Strutt and Strutt, 2007), and we suggest that it begins early, when core PCP proteins are still symmetrically localized. Our feedback amplification model proposes that the balance between recruitment in either orientation is controlled by a bistable feedback mechanism, such that a slight imbalance leads, over time, to a highly polarized distribution of Fz on one side and Vang on the other (Amonlirdviman et al., 2005; Tree et al., 2002a). Clonal absence of either Fz or Vang thus leaves an unbalanced interaction that generates an aberrant signal to the neighboring cells.
To test this hypothesis, we developed an assay to observe how wildtype cells respond at the subcellular level to alterations that affect intercellular signals transmitted by adjacent mutant cells. Mosaic analysis with a repressible cell marker (MARCM; Lee et al., 2000) was used to generate positively marked fz and vang clones, while the localization of FzGFP, as well as prehair location and orientation, were examined in their wildtype neighbors. We observed that fz mutant clones recruit Fz from the neighboring cells to the clone boundary. The recruited Fz localization correlates with reorientation of prehairs, such that they initiate at cell boundaries closest to the clone and point toward the clone (Fig. 1C). In contrast, vang clones repel Fz (Fig. 1D) and recruit Vang (Fig. 5D) in neighboring cells at the clone border, and the prehairs emerge from cell boundaries farthest from the clone border, corresponding to where Fz is enriched.
Figure 5. Fmi exists in two functional forms.
(A–C) vangA3 mutant clone overexpressing Fmi, positively labeled by DsRed (blue), recruits Fz but not Vang across cell boundaries. In the neighboring cells, FzGFP (green in [A]; [B]) is recruited to the Fmi overexpression clone border, but Vang (red in [A]; [C]) is excluded. Arrowhead marks a portion of the clone border.
(D) Vang (visualized with anti-Vang antibody) is recruited to vangA3 mutant clone borders (arrowheads).
(E–G) Negatively marked (red) fmi clones unrescued (E), rescued with FmiYFP (F), or rescued with FmiΔC (G), all stained with anti-Fmi (green) at 28 hrs APF. Note the zigzag pattern of Fmi within the clones.
(E′, F′) fmi clones unrescued (E′) or rescued with FmiYFP (F′), showing phalloidin stained actin at 35 hrs APF.
(H, I, L–N) Simultaneous overexpression of Fz and Fmi in a clone of wildtype cells (H, I) and a clone of vangA3 cells (L–N) can modify the direction of Fmi-Fmi signal relay. Overexpression clones are marked by loss of FzGFP expression ([H, I, M]; green in [L]) and endogenous Vang expression ([N]; red in [L]; MARCM). Though some clone borders still recruit FzGFP ([H, I, M]; green in [L]), many borders lose FzGFP expression (yellow dots in [H, I]) and recruit Vang instead ([N]; red in [L]). Cell by cell variation likely results from the variable over-expression levels and thus varying ratio of Fmi and Fz known to be achieved using the GAL4 system.
(J–K) Schematics showing recruitment of Fz across cell boundaries by Fmi overexpression (J), and Vang recruitment in cells neighboring a Fmi + Fz co-overexpression clone (K). F-Fmi (blue) and V-Fmi (orange). Blue cells overexpress Fmi or Fmi + Fz and lack Vang.
(O, P) While overexpression of FmiΔC recruits Fz to neighboring cell boundaries (O; blue dots mark wildtype neighbors), simultaneous overexpression of FmiΔC and Fz reverses this recruitment (P; yellow dots mark wildtype neighbors with decreased Fz recruitment), as was seen for wildtype Fmi (H, I, L, M).
To determine if unbalanced Vang activity inside fz clones, or Fz inside vang clones, is responsible for generating domineering non-autonomy, we removed them simultaneously. In fz vang double mutant clones, FzGFP is not enriched at the clone border, and domineering non-autonomy is not observed at either the proximal or distal sides of clones (Fig. 1F; Strutt and Strutt, 2007). These data indicate that cells double mutant for fz and vang lose the ability to send intercellular PCP signals, and suggest that it is indeed the unbalanced Fz activity within vang clones, and Vang activity within fz clones, that send mis-polarizing signals to neighboring wildtype cells to cause non-autonomous phenotypes.
We tested directly whether Fz in a vang clone recruits Vang to neighboring cell boundaries by asking whether overexpression of Fz in a clone can recruit Vang from the wildtype neighbors to the clone boundary. Fz was overexpressed in a marked clone using MARCM, and a flip-on cassette simultaneously expressed VangYFP in wildtype neighboring cells (Fig. 2A–B). We observed that in response to Fz overexpression, VangYFP in neighboring cells is indeed recruited to the clone boundary (Fig. 2E–F).
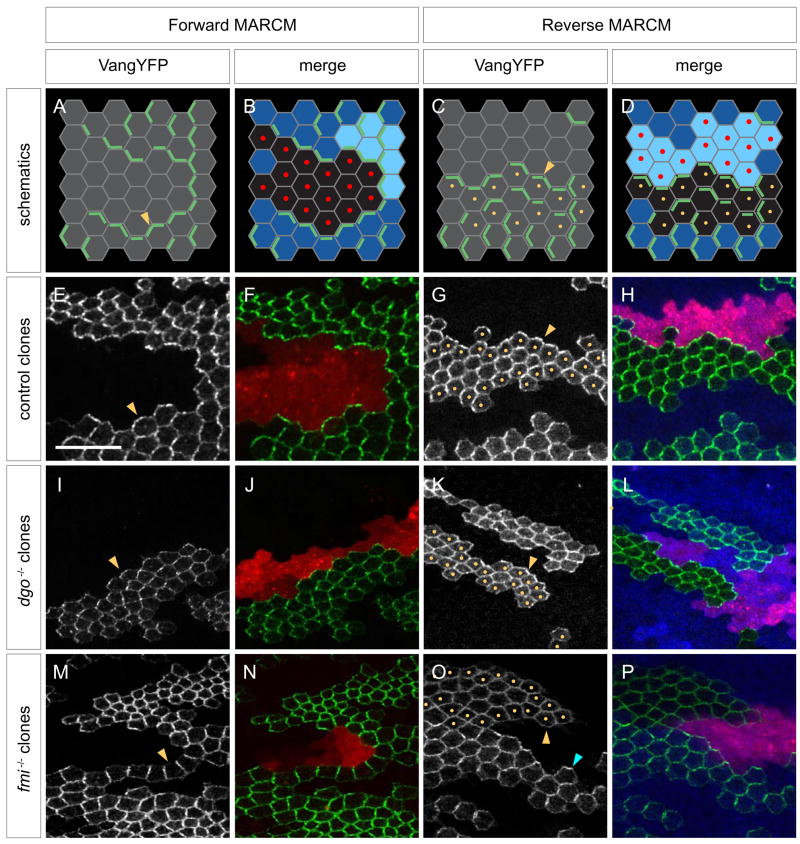
Figure 2. Flamingo is required for Fz-Vang intercellular communication.
(A–B) Paradigm to test functional requirements for individual PCP components in Fz signaling cells. A clone that simultaneously overexpresses Fz while mutant for a PCP gene of interest is generated using forward MARCM (positively labeled by DsRed [red dots]). Flip-on induced expression of VangYFP (green) in neighboring wildtype cells assays recruitment of VangYFP.
(C–D) Paradigm to test functional requirements for individual PCP components in responding cells. Reverse MARCM generates a clone of cells mutant for PCP gene of interest (marked by loss of LacZ [blue; yellow dots]) and a twin spot that overexpresses Fz (bright blue; marked by DsRed [red dots]). Simultaneous flip-on induced expression of VangYFP (green) in the mutant cells assays recruitment of VangYFP.
(E–F) Forward MARCM control. VangYFP from wildtype cells is recruited to the border of a Fz overexpressing LacZ clone (arrowhead in [E]; green in [F]). Fz overexpressing clone is positively marked by co-expression of DsRed (red).
(G–H) Reverse MARCM control. VangYFP inside a LacZ clone (yellow dots [G]; absence of blue [H]) is recruited toward the Fz overexpressing clone (arrowhead in [G]; green in [H]).
(I–J) VangYFP in cells neighboring a Fz overexpressing clone that is mutant for dgo380. VangYFP is recruited to the clone border (arrowhead in [I]; green in [J]).
(K–L) VangYFP inside a dgo380 clone (yellow dots and absence of blue) is recruited toward the Fz overexpressing clone (arrowhead in [K]; green in [L]).
(M–N) VangYFP in cells neighboring a Fz overexpressing clone that is mutant for fmiE45. VangYFP is not recruited to the clone border (arrowhead in [M]; green in [N]).
(O–P) VangYFP inside a fmiE45 (yellow dots and absence of blue) clone is not recruited toward the Fz overexpressing clone (yellow arrow in [O]; green in [P]). Recruitment is seen near non-mutant cells (blue arrow).
The inability of fz vang clones to send intracellular PCP signals or to reverse the polarity of neighboring wildtype cell is reminiscent of fmi clones (Fig. 1E). Loss of Fmi appears to block cell-cell PCP communication (Lawrence et al., 2004; Strutt and Strutt, 2007), and fmi clones do not produce domineering non-autonomy. At both sides of fmi clone borders, the entire core PCP protein complex fails to accumulate. The similar phenotypes of fmi and fz vang double mutant clones suggest that loss of Fmi may block cell-cell communication by preventing the accumulation of Fz and Vang at cell junctions.
Fz-Vang intercellular signal relay does not depend on direct Fz-Vang interactions
We next sought to determine whether the interaction between Fz and Vang in mediating intercellular PCP signaling is direct, or mediated though other PCP components. Though direct interaction between these two transmembrane proteins is possible, the small size of the Vang extracellular loops and the tightly packed Fz CRD domain make a proposed physical bridge between Fz and Vang across the intercellular gap at the adherens junction (estimated > 250Å; Pokutta and Weis, 2007) unlikely. Nevertheless, to address the possibility of a direct interaction, we generated transgenes of Fz and Vang with mutated extracellular domains. The Fz transgene deletes the entire CRD domain (FzΔCRD), and the Vang transgene replaces the two extracellular loops with tags (VangΔECD) (Fig. 1P). We reasoned that if either transgene is capable of localizing asymmetrically or rescuing null mutant phenotypes, its activity would be independent of the deleted domains, and a direct Fz-Vang interaction in propagating PCP signals would therefore be unlikely.
The FzΔCRD transgene appreciably rescued the fz loss-of-function phenotype in adult wings and thorax (Fig. 1K–N; Fig. S1). We observed better rescue than did other investigators using a similar construct (Chen et al., 2004). It is exceedingly unlikely that the small extracellular domains remaining in the FzΔCRDconstruct could contact Vang across the intercellular gap. FzΔCRD does not carry tags to allow for assessment of its subcellular localization, and available anti-Fz antibodies are inadequate for immunofluorescence. However, VangΔECDcan be visualized by its fused YFP, and we observed that VangΔECDshows asymmetric localization along proximal-distal (P-D) cell boundaries (Fig. 1O). Vang−ECDlocalizes weakly to adherens junctions and appears to be too weakly expressed to achieve phenotypic rescue. While not formally ruled out by these results, extracellular contact between Fz and Vang seems unlikely to be necessary for Vang localization. S2 cell aggregation assays also failed to reveal any interaction between Fz and Vang expressing cells (data not shown). Taken together, these observations suggest that intercellular PCP signaling is unlikely mediated by direct Fz-Vang interaction.
Wnt ligands are unlikely to mediate Fz-Vang intercellular signaling
It has been speculated that a Wnt ligand mediates intercellular PCP signaling in Drosophila (Adler et al., 1997; Strutt and Strutt, 2005), and Wnt5a, Wnt7a and Wnt11 are known to control planar polarity in specific vertebrate tissues (Seifert and Mlodzik, 2007). We therefore considered the possibility that a Wnt either directly or indirectly mediates interactions between Fz and Vang on adjacent cells. Rescue of fz mutant polarity by FzΔCRD, which lacks the high affinity Wnt binding domain, not only favors indirect Fz-Vang interactions, but also suggests that Wnts are unlikely to be involved in PCP signaling. In Drosophila, loss-of-function studies involving several Wnts have so far failed to demonstrate any PCP defects (Lawrence et al., 2002), but, a requirement for Wnts in Drosophila PCP signaling has not been ruled out due to possible redundancy between multiple Wnts and potential binding of Wnts to the extracellular loops of Fz. To test this further, we first characterized expression of Wnt family members in the wing disc and pupal wing (Fig. S2). WntD and DWnt5 are not expressed, nor do they bind the Fz CRD (Wu and Nusse, 2002). We therefore generated clones of cells simultaneously mutant for the remaining five Wnts: wingless, DWnt-2, DWnt-4, D-Wnt-6, and D-Wnt-10. Absence of all 5 Wnts failed to reveal polarity defects (Fig. 1Q). In support of this finding, clones mutant for porcupine, which is required for lipid modification of the Wnt family (Willert et al., 2003), also failed to perturb hair polarity (not shown). It is thus unlikely that Wnts are ligands for Fz during PCP signaling.
Fmi is required on both sides of intercellular boundaries
Because it appears Fz and Vang do not directly interact to transmit intercellular signals, we used the Fz overexpression paradigm to test which of the core PCP components are required in the signal-sending cells for the recruitment of Vang by Fz. We refer to Fz overexpressing cells as signal-sending cells, and cells abutting these clones as signal-receiving cells. By placing large amounts of Fz in the membranes of signal sending cells, this overexpression system is expected to eliminate the requirement for the potentially confounding feedback amplification that operates in wildtype cells, and that occurs at the boundaries of loss-of-function clones. Using MARCM, we made the signal-sending cell mutant for other PCP genes. We found that Fz does not require Dsh, Dgo and Pk in the signal-sending cells to recruit Vang, as mutations in these genes do not affect VangYFP recruitment outside of the clone (Fig. 2 I–J and Fig. S3). By contrast, overexpression of Fz in fmi mutant cells fails to recruit VangYFP across cell boundaries (Fig. 2M–N).
We used reverse MARCM to perform the complementary experiment and test which of the core PCP components are required in the signal-receiving cells to recruit Vang to the clone borders. Using this approach (Fig. 2C–D, G–H), we found that Dsh, Dgo and Pk are not required in the signal-receiving cells (Fig. 2K–L and Fig. S3), while Fmi is required for recruitment (Fig. 2O–P).
Therefore, although all core PCP components can mutually affect each others’ sub-cellular localization, of the known core PCP components, only Fmi is required for Fz-Vang intercellular signal transmission when feedback amplification is not occurring. Other investigators reached a similar conclusion using different experimental paradigms (Lawrence et al., 2004; Strutt and Strutt, 2007). Pk, Dsh and Dgo are not essential for intercellular signal relay, and are most likely required for the amplification of asymmetry. Furthermore, we show that in the absence of Pk, Dsh and Dgo, individual cells can not only send and receive intercellular signals, but also generate PCP protein asymmetry.
Because Fmi protein fails to accumulate at the border of fmi mutant clones, and is thought to act as a homodimer, it has been proposed that Fmi is present on both sides of intercellular boundaries (Usui et al., 1999), consistent with our finding that it is required on both sides. We formally tested this by expressing UAS-FmiYFP at moderate levels in a mosaic pattern, and found that in single cells expressing the UAS-FmiYFP transgene, FmiYFP is expressed at both the proximal and distal edges of cells, with no reproducible difference in either the quantity or quality of the signal on either side of the cell border (Fig. S4).
Fmi plays an instructive role in generating intercellular PCP signaling
Because wildtype cells abutting fmi mutant clones can not localize any core PCP components, including Fmi itself, at clone borders (Bastock et al., 2003; Feiguin et al., 2001), loss-of-function experiments do not allow one to distinguish whether Fmi functions permissively, perhaps as a scaffold, or whether Fmi itself provides an instructive signal in Fz-Vang intercellular signaling. We therefore turned to gain-of-function experiments. Overexpression of Fmi in clones or in the patched domain non-autonomously affects the polarity of wildtype neighbors, causing hairs to point toward the overexpression domain (Tree et al., 2002a; Usui et al., 1999). This phenotype resembles fz loss-of-function and vang gain-of-function clones. At least two possible explanations can account for this result. First, Fmi overexpression might cell autonomously potentiate Vang activity or inhibit Fz activity to generate non-autonomy. Second, Fmi may provide an instructive intercellular PCP signal to neighboring cells.
To distinguish between these two possibilities, we asked whether Fmi overexpressed in clones requires either Fz or Vang to produce an intercellular signal. If Fmi does not require Fz or Vang to produce a signal, then it must act instructively. Fmi was overexpressed in either wildtype, fz, vang, or fz vang double mutant clones, and repolarization of wildtype neighbors was assayed by hair polarity as well as by the localization of FzGFP. In the control, we found that overexpression of Fmi recruits FzGFP from wildtype neighbors and causes prehairs to point toward the clones (Fig. 3A). Using MARCM to overexpress Fmi in vang, fz or fz, vang double mutant cells, we found that Fmi can still recruit FzGFP across clone borders and cause prehairs to point toward the clone (Fig. 3B–D), indicating that Fmi does not require Vang or Fz to recruit Fz from the neighboring cells and repolarize them. We conclude that Fmi has an instructive function in intercellular signal relay. This result also affirms our conclusion that intercellular PCP signaling does not require direct Fz-Vang interactions. Remarkably, since Fmi repolarizes neighbors and recruits Fz to clone boundaries in the absence of both Fz and Vang in the signaling cells, these results also indicate that the intercellular signal transmitted by Fmi has an intrinsic bi-directional asymmetry.
Figure 3. Fmi generates an instructive signal.
(A–D) Fmi overexpression in LacZ (A), fzR52 (B), vangA3 (C) or vangA3; fzR52 (D) clones non-autonomously recruits Fz from neighboring wildtype cells to the clone border (arrowheads). Clones overexpressing Fmi are positively marked by DsRed (blue in [A″–D″]) and exclude FzGFP expression ([A–D]; green in [A″–D″]). Prehairs stained with phalloidin ([A′–D′]; red in [A″–D″]). Hairs are repolarized to point toward the clone.
Fmi acts homophilically to transmit intercellular PCP signals
Previous studies showed that Fmi can mediate homophilic adhesive interactions in vitro (Usui et al., 1999). We therefore asked whether Fmi signals homophilically in vivo via its ectodomain, or whether it might signal through an as yet unidentified pathway to recruit Fz across cell borders. To do so, we first tested if Fmi in the neighboring cell is required for the overexpressed Fmi to recruit Fz to the clone boundaries. In fmi mutant cells abutting Fmi overexpressing cells, FzGFP failed to localize to the overexpression clone border (Fig. 4A–B). Therefore, overexpressed Fmi requires Fmi in the responding cell to recruit Fz and repolarize the cell.
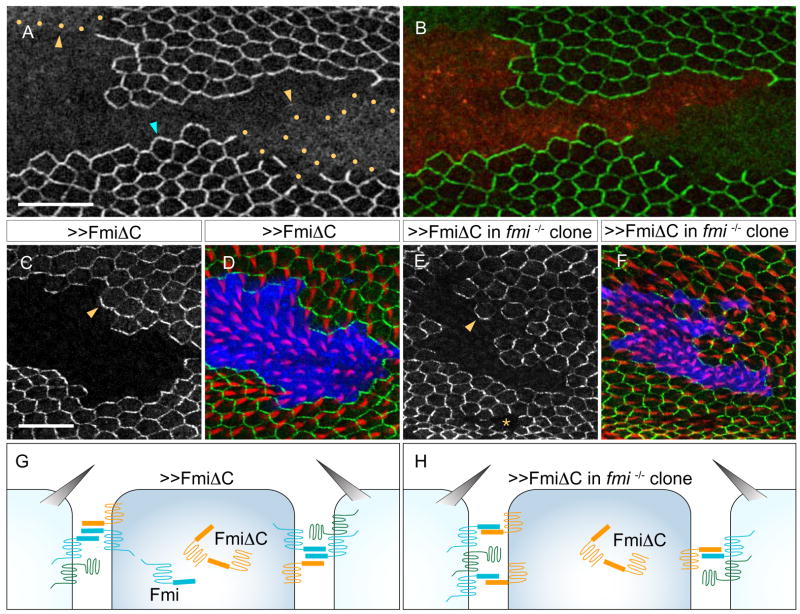
Figure 4. Flamingo acts homophilically.
(A–B) A Fmi overexpression clone and its twin-spot that is mutant for fmiE45 (MARCM). Fmi overexpressing clone positively marked by DsRed (red in [B]) and excluding FzGFP expression. FzGFP in the fmiE45 clone ([A]; green in [B]; yellow dots outline the fmi clone) fails to localize to the cell membrane and remains largely intracellular (yellow arrowheads), whereas it is recruited from wildtype neighbors (blue arrowhead).
(C–D) FmiΔC overexpression clone, positively marked by DsRed (blue in [D]), and excluding FzGFP expression ([C]; green in [D]), repolarizes neighboring wildtype cells and recruits FzGFP to the clone border (arrowhead). Prehairs stained with phalloidin (red in [D]).
(E–F) FmiΔC overexpression in fmiE45 mutant clone, positively marked by DsRed (blue in [F]), and excluding FzGFP expression ([E]; green in [F]), repolarizes neighboring wildtype cells (arrowhead in [E]). Prehairs stained with phalloidin (red in [F]). Cells homozygous for fmiE45 fail to recruit Fz from neighbors (asterisk in [E]).
(G–H) Schematic of FmiΔC overexpression clone in wildtype (G) and in fmi mutant cells (H).
To more directly test whether Fmi-Fmi homophilic interaction can send signals to repolarize neighboring cells, we used a Fmi construct that lacks its intracellular C-terminal tail (FmiΔC; Kimura et al., 2006). This transgene retains the ability to mediate homotypic binding in vitro, but it fails to rescue Fmi’s function in regulating dendritic morphogenesis, and fails to rescue to viability, indicating that truncation of the C-terminus eliminates an essential function of Fmi. We found that overexpression of FmiΔC in a wildtype clone recruits FzGFP to the clone border in neighboring cells and repolarizes their prehairs (Figure 4C–D and G). To rule out the possibility that FmiΔC cis-dimerizes with or otherwise requires endogenous, intact Fmi for this recruitment, we overexpressed Fmi-ΔC in fmi mutant clones, and observed a similar result (Fig. 4E–F and H). Taken together, our results are consistent with Fmi actively transmitting intercellular PCP signals through ectodomain-dependent homodimerization.
Fmi homodimers signal asymmetrically by selectively recruiting Fz and Vang to opposite sides of the cell boundary
If Fmi homodimers span the intercellular gap and provide instructive signals that are required for Fz and Vang to mutually recruit each other to opposing boundaries, then the homodimers must somehow function asymmetrically to assure that Fz selectively recruits Vang and that Vang selectively recruits Fz. Earlier, we showed that overexpressing Fmi in cells lacking Fz, Vang or both, recruits Fz from the responding cells, suggesting that Fmi by itself behaves as though it is on the proximal side of wildtype cells, where Vang accumulates. To determine whether this recruitment is indeed selective, we asked whether overexpressing Fmi, which recruits Fz from responding cells, simultaneously recruits Vang. To visualize the Vang protein selectively in responding cells, we removed it from the signaling cells. Remarkably, although Vang protein from wildtype cells accumulates at vang mutant clone borders (Fig. 5D; Bastock et al., 2003), Fmi overexpression in vang mutant cells recruits Fz, yet selectively repels Vang from responding cells at the clone borders (Fig. 5A–C). We conclude that Fmi in the signaling cell provides an instructive intercellular signal that is selective for recruitment of Fz in the responding cell.
Two functional forms of Fmi
Upon Fz overexpression, Vang, but not Fz, is recruited to the neighboring cell boundary (Fig. 2 and data not shown). Since the signal passes through Fmi homodimers, this suggests that Fmi in the responding cell selectively recruits Vang. In contrast, upon Fmi overexpression, Fmi in the responding cell recruits Fz. Therefore, depending on the signal in the neighboring cell, Fmi in the responding cell can autonomously recruit either Fz or Vang. These data lead us to propose a model in which Fmi exists in two functional forms: a form that associates with Fz (F-Fmi; on the distal side of wildtype cells), and a form that associates with Vang (V-Fmi; on the proximal side of wildtype cells). By preferentially interacting with each other, rather than with their like forms, V-Fmi and F-Fmi interactions would favor the asymmetric assembly of complexes with Fz on one side and Vang on the other side of the intercellular boundary (Fig. 7A).
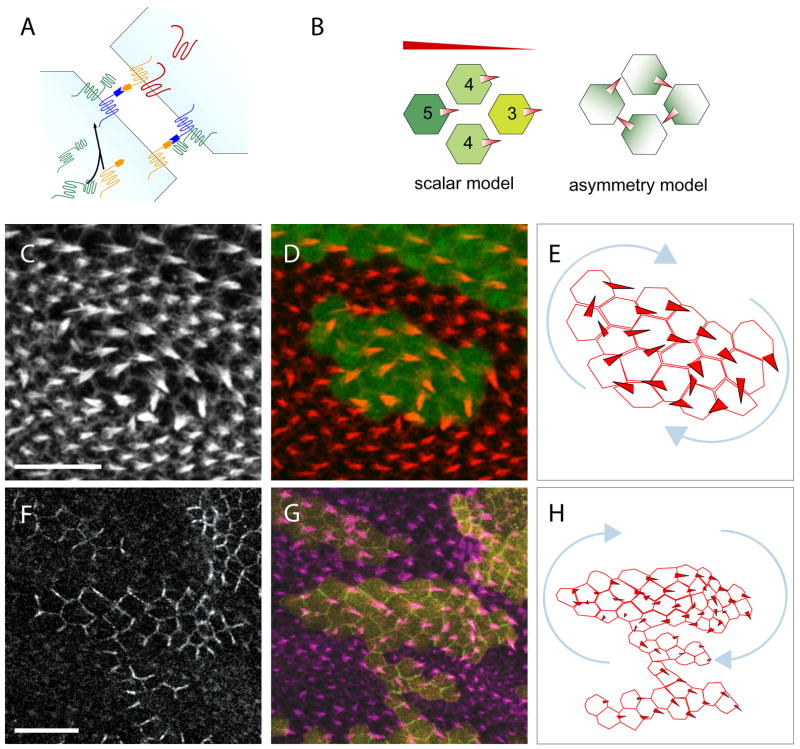
Figure 7. Model.
(A) Fmi exists in two functional forms: a Fz-associated form (F-Fmi; dark blue) that interacts with and is induced by Fz in the same cell, and a Vang-associated form (V-Fmi, orange) that interacts with Vang in the same cell. The two functional forms preferentially interact with each other, rather than with their like forms.
(B) The Fz scalar slope and the PCP protein asymmetry models in clones surrounded by fmi mutant cells. Shading represents Fz activity and/or localization. Cells in the Fz scalar model cannot be arranged to allow a polarity swirl with hairs pointing from high toward low Fz activity levels.
(C–E) Wildtype cells (positively marked by DsRed; green in [D]) surrounded by fmiE45 mutant cells shows a swirling polarity defect. Prehairs stained with phalloidin ([C]; red in [D]). (E) Schematic of polarity pattern.
(F–H) Wildtype cells (positively marked by DsRed; yellow in [G]) surrounded by fmiE45 mutant cells shows another swirl. Prehairs stained with phalloidin (magenta in [G]). FzGFP ([F]; green in [G]).
Two forms of Fmi, alternatively spliced to encode unique 6 or 11 amino acid sequences at the C-terminus, are expressed in the fly wing (Wasserscheid et al., 2007). It is conceivable that the two splice forms represent the two functional forms of Fmi, and are selectively recruited or retained on opposite sides of intercellular boundaries. However, in Drosophila, a single Fmi isoform can at least partially rescue fmi null mutant polarity (Strutt and Strutt, 2007, Supplementary data). Furthermore, by demonstrating asymmetric localization and correction of the hair polarity defect, we show that a single Fmi isoform (Fmi-YFP) can rescue fmi mutant clones (Fig. 5E–F′). Indeed, the truncated FmiΔC also rescues polarity in fmi mutant clones (Fig. 5G). These data show that isoform diversity does not account for Fmi’s asymmetric function and suggest that the two forms of Fmi must result from post-translational regulation.
The model in which Fmi can adopt two functional forms makes several predictions. First, endogenous Fmi, in the absence of Fz and Vang, should have V-Fmi activity. Consistent with this, adjacent to fmi mutant clones, we observe no accumulation of FzGFP from neighboring cells. By contrast, near fz vang double mutant clones, which contain endogenous Fmi protein, we consistently observe a modest amount of FzGFP recruited from neighboring cells (compare Fig. 1E–F). This suggests that the endogenous Fmi behaves as V-Fmi, interacting with F-Fmi across the cell border to recruit a small amount of Fz in the neighbor.
The second prediction is that while endogenous or overexpressed Fmi in the absence of Fz or Vang exists in the V-Fmi form, V-Fmi should be converted to F-Fmi by the presence of Fz. To test this, we overexpressed Fz and Fmi together. Remarkably, while overexpression of Fmi recruits Fz from neighboring cells, and therefore behaves as V-Fmi (Fig. 5A–C), simultaneous overexpression of Fz and Fmi recruits Vang instead, while FzGFP accumulation is diminished, over substantial portions of clone borders (Fig. 5H–N). Furthermore, the amounts of FzGFP and Vang protein at a given location on the clone border appear to be inversely proportional (Fig. 5L–N). We conclude that Fmi alone is in the V-Fmi form, but the presence of Fz converts Fmi from V-Fmi into F-Fmi, consequently recruiting V-Fmi and Vang to the opposite side of the cell border (Fig. 7A).
Fz physically interacts with Fmi
To address how Fz modifies Fmi function, we asked if the two transmembrane proteins physically interact. Fmi was transfected into S2 cells that inducibly express Fz, and was immunoprecipitated using anti-Fmi antibody. Fmi co-immunoprecipitates Fz when Fz expression is induced, indicating a specific interaction (Fig. 6A). We more precisely defined the interaction domain within Fmi by transfecting FmiΔC and FmiΔN (Fig. 6A–B; Kimura et al., 2006), and found that both specifically co-immunoprecipitate Fz. Therefore, Fz interacts with the central portion of Fmi containing the transmembrane and HRM domains but lacking the extracellular cadherin, laminin G and EGF-like repeats and the intracellular C-terminal tail. Consistent with this mapping, we found that, like full length Fmi, the ability of overexpressed FmiΔC to recruit Fz from neighboring cells is abrogated by simultaneous expression of Fz (compare Figs. 4C–F and Fig. 5O–P), and FmiΔC can rescue polarity in fmi mutant clones (Fig. 5E and G). To confirm that this interaction occurs in vivo, we immunoprecipitated Fmi from 24 hour APF pupae, and found that Fz indeed co-immunoprecipitates with Fmi (Fig. 6C). Therefore, Fz physically interacts with the central portion of Fmi, and this interaction can be detected in vivo, providing a route for Fz to induce the F-form of Fmi.
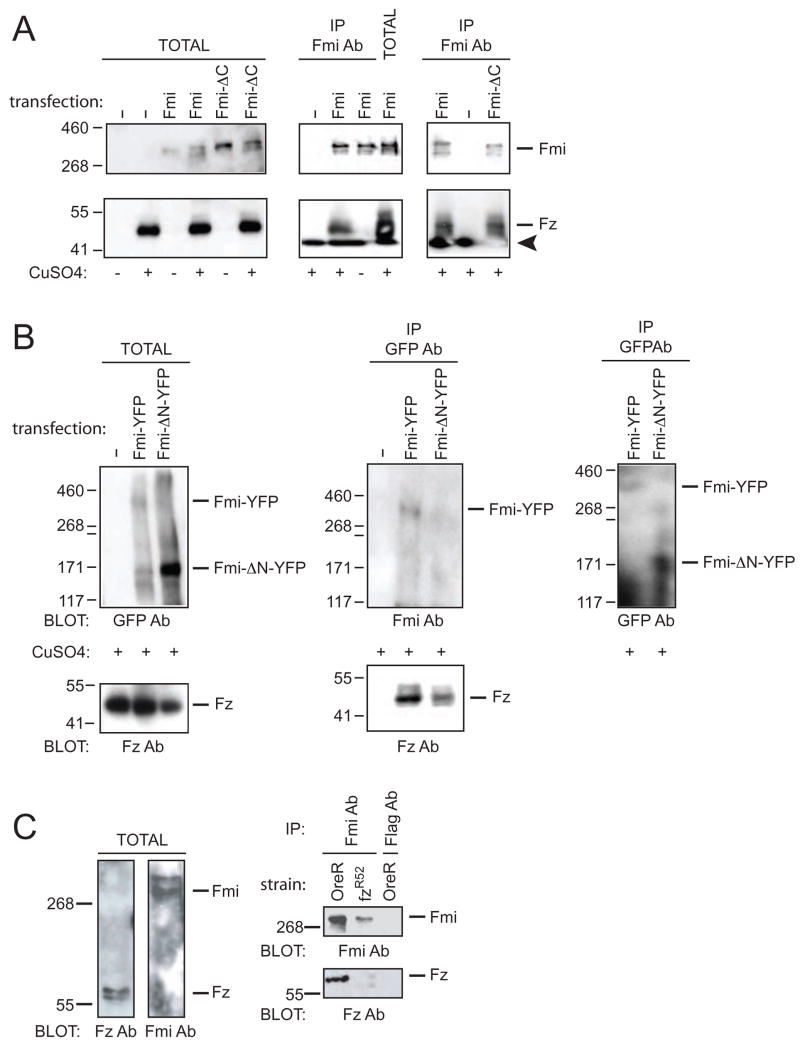
Figure 6. Fz and Fmi physically interact.
(A) Fmi co-immunoprecipitates Fz from inducible S2 cells. Fmi or FmiΔC transfected into S2 cells with or without CuSO4 induction of Fz expression. Immunoblots of total extract (left). Fz is co-immunoprecipitated by Fmi (center) or FmiΔC (right) only when Fmi or FmiΔC is transfected and Fz expression is induced. Upper panels probed for Fmi and lower panels probed for Fz. Arrowhead marks the dye front.
(B) FmiΔNYFP co-immunoprecipitates Fz. FmiYFP or FmiΔNYFP transfected into S2 cells with Fz expression induced. Immunoblots of total extract (left). Fz is co-immunoprecipitated by FmiYFP or FmiΔNYFP. Anti-Fmi detects FmiYFP but not FmiΔNYFP (center), while anti-GFP detects both (right). Some degradation of Fmi is observed.
(C) Fmi co-immunoprecipitates Fz from pupae. Total extracts from wildtype (OreR) probed for Fmi and Fz (left). Fmi co-immunoprecipitates Fz from OreR but only trace amounts from fzR52 pupae. Control anti-Flag does not precipitate either Fmi or Fz. Note that Fz runs at approximately 65 Kd in these gels, whereas in the S2 cell experiments, the majority runs at approximately 50 Kd, with a minor band at 65 Kd (not shown). This difference likely depends on different denaturing conditions, as samples cannot be boiled without losing all Fz signal.
Discussion
Asymmetric bidirectional signaling through Fmi homodimers
Non-classical cadherins generally exhibit weak homophilic binding in vitro (Chen and Gumbiner, 2006), raising the possibility that they regulate signaling rather than adhesion. Moreover, after cell-cell recognition, cadherins are thought to function either homophilically and symmetrically, or heterophilically and asymmetrically between cells (Yamada and Nelson, 2007). Here, we show that the atypical cadherin Fmi acts homophilically to communicate PCP signals between neighboring cells, yet its action is asymmetric, serving to link the accumulation of Fz on one cell boundary with Vang on the adjacent cell boundary, and vice versa. Our data lead us to propose a model in which Fmi exists in two functional forms on opposite sides of intercellular borders, one of which selectively and cell-autonomously interacts with Fz (F-Fmi), and the other with Vang (V-Fmi; Fig. 7A). The native form of Fmi is V-Fmi, but upon interaction with Fz, V-Fmi is converted to F-Fmi. We infer that Fmi homodimers consist preferentially of opposite forms, thereby producing asymmetric function of the complex. By virtue of this mechanism, Fmi mediated intercellular signaling communicates information about PCP protein asymmetry between neighboring cells.
How might Fmi achieve its homophilic, yet asymmetric function? Although two splice forms exist, we have demonstrated that a single form can fulfill both V- and F-Fmi functions. A second possibility is that different stoichiometries of Fmi interact on opposite sides of the boundary – for example, cis-dimers of Fmi might behave as V-Fmi while monomers function as F-Fmi. However, we have been unable to detect a reproducible proximal-distal difference in levels of tagged Fmi when expressed in a mosaic pattern. A third possibility is that posttranslational regulation results in two distinct forms of Fmi that are selectively recruited or retained, directly or indirectly, by Fz or Vang. A fourth model is that V-Fmi and F-Fmi are alternate conformers or modified forms of Fmi where conversion of V-Fmi to F-Fmi depends on interaction with Fz. While we cannot distinguish between the latter three possibilities, our finding that Fz and Fmi directly interact favors models in which Fz physically alters the properties of V-Fmi, thereby inducing the F-Fmi form. Extensive evidence shows that interacting proteins can modify the activity of cadherins (Halbleib and Nelson, 2006). Of note, Xenopus Fz7, which mediates convergent extension during gastrulation, has been reported to directly bind a protocadherin through its extracellular domain (Medina et al., 2004). Detailed molecular and structural studies will be required to determine the precise nature of V-Fmi and F-Fmi, and how they interact with Fz and Vang.
Fmi signaling and generation of planar cell polarity
During PCP signaling, cells each receive a signal that orients polarization. Cells then consolidate this information by amplifying the asymmetry in a process that involves communicating and aligning polarity with surrounding cells. By signaling to a neighbor that a given cell boundary is enriched for either Fz or Vang, asymmetric Fmi homodimers transmit this information bidirectionally between cells. In wildtype, amplification through feedback control is required to produce sharp differences between Fz and Vang levels on adjacent cell surfaces (Axelrod, 2001; Strutt, 2001; Tree et al., 2002a). As shown here, and in another recent report (Strutt and Strutt, 2007), Pk, Dgo and Dsh are required for this amplification, though they are not required for intercellular signaling per se. The mechanism by which this amplification occurs is unknown, but the result is a mutual exclusion of Fz and Vang from a given region of the cell surface. Fmi therefore serves to link the action of feedback loops in neighboring cells, assuring a coordinated polarization.
Asymmetric PCP protein localization
We and others have proposed that asymmetric placement of core PCP proteins is itself the signal that controls morphological polarization (Amonlirdviman et al., 2005; Axelrod, 2001; Strutt, 2001). However, an alternative model has been proposed in which the absolute, scalar value of Fz activity within each cell varies in a gradient across the tissue in response to an unidentified ligand. Scalar Fz levels are proposed to be refined by an averaging process between neighboring cells. According to this view, asymmetric PCP protein localization is only an epiphenomenon, and is not required for function. We suggest that several observations are inconsistent with this model. First, the model predicts that the extent of domineering non-autonomy near fz mutant clones should vary according to position in the Fz activity gradient. However, its extent was reported to be equal throughout the abdomen (Casal et al., 2006), and no proximal-distal difference is evident in the fly wing (Adler et al., 2000). Second, FzΔCRD rescues polarity in fz null mutant flies despite deletion of the CRD, leaving little protein to which an extracellular ligand might bind.
In essence, the scalar Fz model argues for a Fz gradient across the tissue, whereas the asymmetric protein localization model invokes gradients of core PCP protein localization or activity within each cell, but not across the tissue. As an additional test to distinguish between these two models, we examined small wildtype islands of ~20 cells in size surrounded by fmi mutant cells. These wildtype cells are prevented from communicating with and receive no repolarizing signal from the surrounding fmi mutant cells (Lawrence et al., 2004; Strutt and Strutt, 2007; Usui et al., 1999). The scalar Fz model predicts that these small wildtype islands should still be directly responsive to the proposed morphogen gradient and should therefore generate a normal Fz activity slope, resulting in normal polarity (Fig. 7B). In contrast, because the asymmetric PCP protein model posits only subcellular gradients of PCP protein activity, but no tissue level gradient of Fz or other core PCP protein activity (Amonlirdviman et al., 2005), each cell’s tendency to align polarity with its neighbors could lead to other patterns of local alignment (Fig. 7B). Consistent with this latter possibility, prehairs in many of these islands exhibited PCP defects and formed swirling patterns (Fig. 7C–H). Because there is no discontinuity as one follows the polarity of cells in these islands, the scalar Fz activity model cannot accommodate this result without invoking an Escher’s staircase of infinitely rising Fz levels. In contrast, the asymmetric protein model easily explains this result by organizing proximal and distal PCP protein domains in a spoke-like pattern between the cells of the swirl, as is indeed observed (Fig. 7B, F–H). In light of the evidence presented here that Fmi homodimers can instructively generate asymmetric Fz and Vang localization and locally align the polarity of neighboring cells, we favor a model in which instructive protein localization mediated by Fmi homodimers is itself the signal that transmits PCP information between cells. We believe this is the only known example of a cadherin homodimer providing dissimilar signals across intercellular boundaries.
Experimental Procedures
Molecular cloning
FzΔCRD was described previously (Povelones and Nusse, 2005). VangΔECD replaces segments of the two extracellular loops (amino acid residues 176–189 and 258–275) with two FLAG tags and two HA tags and is expressed in pTub (Povelones and Nusse, 2005).
Fly strains and clonal analyses
The following fly stocks and mutant alleles were used: FRT42D, tubP-Gal80, armP-Fz::GFP and actinP-Fz::GFP, fzR52 FRT2A, FRT42D vangA3, FRT42D fmiE45, FRT42DubiP-NLS::mRFP (gift from J. Lipsick), tubP-VangΔECD::EYFP, tubP-FzΔCRD, y w f por2E FRT19A, pi-myc FRT40A wnt2EMSO, Df(2L)RF FRT40A wnt2EMSO, D174Gal4 (a pan-wing driver), T155Gal4, actinP-Gal4, actinP>stop>Vang::EYFP TM2, UAS-Fz, UAS-myr-mRFP, FRT42D dgo380, FRT42D pk-sple13, armP-LacZ FRT19A, dsh3 FRT19A, UAS-Fmi::EYFP, actinP>y+>Gal4-PR, UAS-Fmi and UAS-FmiΔC. Rescue experiments were in a fzK21/fzJW background. For MARCM and reverse MARCM experiments, early 3rd instar larvae were incubated at 37°C for 2 hours, and pupae with appropriate clones were selected for analysis at 34 hrs after puparium formation (APF). For flip-on clones using the actinP>y+>Gal4-PR construct, early 3rd instar larvae were incubated at 37 C for 30 minutes. For rescue of fmi clones using Gal4-PR, flies were grown on medium containing 200μg/ml RU486, and pupae were selected for analysis at 28 and 34 hrs APF. A complete list of genotypes is provided in Supplementary Methods.
Immunohistochemistry
Primary antibodies: mouse anti-LacZ (1:500 dilution, Promega), rabbit anti-Vang (1:1000; (Wolff and Rubin, 1998), monoclonal anti-myc (clone 9E10). Secondary antibodies: AlexaFluor 633-goat anti-rabbit (1:200, Invitrogen), Cy3-anti-mouse, AlexaFluor 633-goat-anti-mouse (1:200, Invitrogen). Phalloidin: Alexa 660 and Alexa 488 conjugated phalloidin (Molecular Probes)
Imaging
Images were obtained using a Leica TCS SP5 AOBS confocal microscope and processed with LAS AF (Leica), Adobe Photoshop and Illustrator. Adult wings were imaged using a Spot camera attached to a Ziess Axioplan2.
In situ Analysis
Hybridizations were performed on wildtype Canton-S third instar larval and pupal wings. Details are provided in Supplementary Methods.
Immunoprecipitations
Co-immunoprecipitations were performed according to standard methods. A detailed description is provided in Supplementary Methods.
Supplementary Material
Acknowledgments
This work was supported by grants from NIH (to J.D.A.), a Stanford Graduate Fellowship (W.-S. C.) and HHMI (R.N.). We thank the following for reagents: C. Fuerer, J. Lipsick, M. Mlodzik, D. Strutt, T. Uemura, T. Wolff. We thank M. Simon, C. Tomlin, W. Nelson, W. Weiss, T. Clandinin and the Axelrod lab for discussions and comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler PN, Krasnow RE, Liu J. Tissue polarity points from cells that have higher Frizzled levels towards cells that have lower Frizzled levels. Curr Biol. 1997;7:940–949. doi: 10.1016/s0960-9822(06)00413-1. [DOI] [PubMed] [Google Scholar]
- Adler PN, Taylor J, Charlton J. The domineering non-autonomy of frizzled and van Gogh clones in the Drosophila wing is a consequence of a disruption in local signaling. Mech Dev. 2000;96:197–207. doi: 10.1016/s0925-4773(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Amonlirdviman K, Khare NA, Tree DR, Chen WS, Axelrod JD, Tomlin CJ. Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science. 2005;307:423–426. doi: 10.1126/science.1105471. [DOI] [PubMed] [Google Scholar]
- Axelrod JD. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 2001;15:1182–1187. doi: 10.1101/gad.890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130:3007–3014. doi: 10.1242/dev.00526. [DOI] [PubMed] [Google Scholar]
- Casal J, Lawrence PA, Struhl G. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development. 2006;133:4561–4572. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae J, Kim MJ, Goo JH, Collier S, Gubb D, Charlton J, Adler PN, Park WJ. The Drosophila tissue polarity gene starry night encodes a member of the protocadherin family. Development. 1999;126:5421–5429. doi: 10.1242/dev.126.23.5421. [DOI] [PubMed] [Google Scholar]
- Chen CM, Strapps W, Tomlinson A, Struhl G. Evidence that the cysteine-rich domain of Drosophila Frizzled family receptors is dispensable for transducing Wingless. Proc Natl Acad Sci U S A. 2004;101:15961–15966. doi: 10.1073/pnas.0407103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Gumbiner BM. Crosstalk between different adhesion molecules. Curr Opin Cell Biol. 2006;18:572–578. doi: 10.1016/j.ceb.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Feiguin F, Hannus M, Mlodzik M, Eaton S. The ankyrin repeat protein Diego mediates Frizzled-dependent planar polarization. Dev Cell. 2001;1:93–101. doi: 10.1016/s1534-5807(01)00010-7. [DOI] [PubMed] [Google Scholar]
- Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- Kimura H, Usui T, Tsubouchi A, Uemura T. Potential dual molecular interaction of the Drosophila 7-pass transmembrane cadherin Flamingo in dendritic morphogenesis. J Cell Sci. 2006;119:1118–1129. doi: 10.1242/jcs.02832. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Casal J, Struhl G. Towards a model of the organisation of planar polarity and pattern in the Drosophila abdomen. Development. 2002;129:2749–2760. doi: 10.1242/dev.129.11.2749. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Casal J, Struhl G. Cell interactions and planar polarity in the abdominal epidermis of Drosophila. Development. 2004;131:4651–4664. doi: 10.1242/dev.01351. [DOI] [PubMed] [Google Scholar]
- Lee T, Winter C, Marticke SS, Lee A, Luo L. Essential roles of Drosophila RhoA in the regulation of neuroblast proliferation and dendritic but not axonal morphogenesis. Neuron. 2000;25:307–316. doi: 10.1016/s0896-6273(00)80896-x. [DOI] [PubMed] [Google Scholar]
- Ma D, Yang CH, McNeill H, Simon MA, Axelrod JD. Fidelity in planar cell polarity signalling. Nature. 2003;421:543–547. doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]
- Medina A, Swain RK, Kuerner KM, Steinbeisser H. Xenopus paraxial protocadherin has signaling functions and is involved in tissue separation. Embo J. 2004;23:3249–3258. doi: 10.1038/sj.emboj.7600329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokutta S, Weis WI. Structure and Mechanism of Cadherins and Catenins in Cell-Cell Contacts. Annu Rev Cell Dev Biol. 2007 doi: 10.1146/annurev.cellbio.22.010305.104241. [DOI] [PubMed] [Google Scholar]
- Povelones M, Nusse R. The role of the cysteine-rich domain of Frizzled in Wingless-Armadillo signaling. Embo J. 2005;24:3493–3503. doi: 10.1038/sj.emboj.7600817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8:126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Usui T, Yanagawa S, Takeichi M, Uemura T. Asymmetric colocalization of Flamingo, a seven-pass transmembrane cadherin, and Dishevelled in planar cell polarization. Curr Biol. 2001;11:859–863. doi: 10.1016/s0960-9822(01)00233-0. [DOI] [PubMed] [Google Scholar]
- Strutt D, Strutt H. Differential activities of the core planar polarity proteins during Drosophila wing patterning. Dev Biol. 2007;302:181–194. doi: 10.1016/j.ydbio.2006.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt DI. Asymmetric localization of frizzled and the establishment of cell polarity in the Drosophila wing. Mol Cell. 2001;7:367–375. doi: 10.1016/s1097-2765(01)00184-8. [DOI] [PubMed] [Google Scholar]
- Strutt H, Strutt D. Long-range coordination of planar polarity in Drosophila. Bioessays. 2005;27:1218–1227. doi: 10.1002/bies.20318. [DOI] [PubMed] [Google Scholar]
- Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002a;109:371–381. doi: 10.1016/s0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- Tree DRP, Shulman JM, Rousset R, Scott M, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002b;109:371–381. doi: 10.1016/s0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, Schwarz TL, Takeichi M, Uemura T. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–595. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- Wasserscheid I, Thomas U, Knust E. Isoform-specific interaction of Flamingo/Starry Night with excess Bazooka affects planar cell polarity in the Drosophila wing. Dev Dyn. 2007;236:1064–1071. doi: 10.1002/dvdy.21089. [DOI] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Wolff T, Rubin GM. Strabismus, a novel gene that regulates tissue polarity and cell fate decisions in Drosophila. Development. 1998;125:1149–1159. doi: 10.1242/dev.125.6.1149. [DOI] [PubMed] [Google Scholar]
- Wu CH, Nusse R. Ligand receptor interactions in the Wnt signaling pathway in Drosophila. J Biol Chem. 2002;277:41762–41769. doi: 10.1074/jbc.M207850200. [DOI] [PubMed] [Google Scholar]
- Yamada S, Nelson WJ. Synapses: Sites of Cell Recognition, Adhesion, and Functional Specification. Annu Rev Biochem. 2007;76:267–294. doi: 10.1146/annurev.biochem.75.103004.142811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Axelrod JD, Simon MA. Regulation of Frizzled by Fat-like Cadherins during Planar Polarity Signaling in the Drosophila Compound Eye. Cell. 2002;108:675–688. doi: 10.1016/s0092-8674(02)00658-x. [DOI] [PubMed] [Google Scholar]
- Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–1063. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.