Abstract
Carbapenems are a class of antimicrobials structurally related to penicillin. Doripenem, the newest agent in this class, was recently approved by the Food and Drug Administration for the treatment of complicated intra-abdominal infections and complicated urinary tract infections. Its spectrum of activity is similar to that of meropenem and imipenem/cilastatin. Some studies indicate that approximately 29% of carbapenem-resistant Pseudomonas aeruginosa isolates may remain sensitive to doripenem, although the clinical relevance of that finding has not been determined. Clinical studies, which have been published only in abstract form to date, have found doripenem to be similar to comparator agents. The most common adverse effects related to doripenem therapy were headache, nausea, diarrhea, rash, and phlebitis. Doripenem, like the other carbapenems, may also cause seizures. Because of the lack of published data, the lack of clear advantages over meropenem, and the increased cost compared with meropenem, doripenem will not be available for use at Baylor University Medical Center except by infectious diseases specialists.
Carbapenems are a class of antimicrobials that are structurally related to penicillin. They continue to be one of the most active classes of antibiotics against many resistant pathogens. However, resistance to carbapenems is increasing. Doripenem, the newest addition to the carbapenem class of antibiotics, was recently approved by the Food and Drug Administration to treat complicated intra-abdominal infections and complicated urinary tract infections, including pyelonephritis, caused by susceptible bacteria (1).
Doripenem is a synthetic carbapenem antibiotic that is structurally related to beta-lactam antibiotics (1). The compound is stable in the presence of beta-lactamase and is resistant to inactivation by renal dehydropeptidases (2). Doripenem, like the other carbapenems, inhibits bacterial cell wall synthesis by inactivating essential penicillin-binding proteins (PBPs), ultimately causing cell death (1). It binds to PBP 3 and 4, as well as to PBP 2, which alters the bacterial cell shape in Escherichia coli and Pseudomonas aeruginosa.
PHARMACOKINETICS/PHARMACODYNAMICS
The pharmacokinetic properties of doripenem evaluated after single and multiple doses were given over 1 hour are summarized in Table 1 (1–3). Doripenem exhibits linear pharmacokinetics, meaning that the maximum concentration (Cmax) and area under the curve (AUC) increase in a linear fashion with increasing doses of drug.
Table 1.
Pharmacokinetic properties of doripenem
| Property | Result for doripenem |
| Protein binding | 8.1% |
| Volume of distribution | 16.8 L |
| Metabolism | Via dehydropeptidase-I |
| Elimination half-life | 1 hour |
| Elimination route | Unchanged in the urine |
| Mean Cmax | 23 mcg/mL |
| Mean AUC | 36.3 mcg · h/mL |
max indicates maximum concentration; AUC, area under the curve.
The volume of distribution of doripenem is 16.8 L and approximates that of extracellular fluid (18 L) (1). Protein binding is low and is independent of concentrations of drug in the plasma. Doripenem has good distribution into many body fluids and tissues, including retroperitoneal fluid, peritoneal fluid, gallbladder, bile, and urine (1).
Doripenem is metabolized to an inactive metabolite by dehydropeptidase-I (1). No hepatic metabolism of doripenem was evident in an in vitro evaluation with pooled human liver microsomes. Doripenem is eliminated in the urine as unchanged drug and undergoes glomerular filtration and active tubular secretion (1). One study administered 500 mg of doripenem to healthy adults and found that 70% of the dose was recovered in the urine as unchanged drug and 15% as inactive metabolite.
A single 500-mg dose of doripenem was administered to patients with renal dysfunction, as determined by creatinine clearance, that was mild (50–79 mL/min), moderate (31–50 mL/min), and severe (≤30 mL/min) (1). The AUC was significantly greater in the moderate and severe renal dysfunction groups than in age-matched healthy adult subjects. For this reason, dosing adjustments are recommended for patients with moderate or severe renal impairment. Another study evaluated the AUC of doripenem after a single 500-mg dose was given to patients with end-stage renal disease who were undergoing hemo dialysis (1–3). Patients received doripenem 1 hour before or 1 hour after dialysis. Fifty-two percent of the dose was recovered in the dialysate following a 4-hour hemodialysis session. When doripenem was given after hemodialysis, the AUC was approximately 8 times that of adults with normal renal function. The manufacturer does not make specific recommendations about dosage adjustments for patients with end-stage renal disease who are on hemodialysis.
A study conducted by Bhavnani and colleagues found that the pharmacodynamic measure that correlates best with bacterial eradication for doripenem is the time the concentration exceeds the minimum inhibitory concentration (MIC; T > MIC) for Streptococcus pneumoniae, Staphylococcus aureus, and Klebsiella pneumoniae (3). They found that 500 mg administered over 1 hour every 8 hours would achieve appropriate T > MIC against bacterial strains with an MIC <2 mcg/mL. Continuous or extended infusions may be used to reach target concentrations against bacterial strains with an MIC > 2 mcg/mL.
SPECTRUM OF ACTIVITY
Doripenem has a spectrum of activity comparable to that of meropenem and imipenem against gram-positive organisms. Imipenem has more activity than doripenem and meropenem against Enterococcus faecalis, and none of the carbapenems are effective against Enterococcus faecium or methicillin-resistant S. aureus.
Doripenem has in vitro activity almost identical to that of meropenem for most gram-negative organisms. Meropenem has slightly lower MICs than doripenem for Klebsiella species, Proteus mirabilis (both extended-spectrum beta-lactamase [ESBL]– producing strains and non–ESBL-producing strains), Serratia species, Salmonella species, and Shigella species. Imipenem has the lowest MICs against Acinetobacter species, followed by doripenem and meropenem. Meropenem has the lowest MIC for Burkholderia cepacia. None of the carbapenems are active against Stenotrophomonas maltophilia.
Meropenem was more active than doripenem against many anaerobic organisms. Activity against Clostridium species and anaerobic gram-positive organisms was similar for meropenem and doripenem.
Table 2 shows the in vitro activity of doripenem, meropenem, and imipenem (2, 4–7).
Table 2.
In vitro activity of doripenem, meropenem, and imipenem∗
| MIC90 (mcg/mL) | |||
| Organism | Doripenem | Meropenem | Imipenem |
| Gram-positive organisms | |||
| Staphylococcus aureus (methicillin susceptible) | 0.06 | 0.12 | ≤0.5 |
| Staphylococcus aureus (methicillin resistant) | 32 | 32 | 32 |
| Coagulase-negative staphylococci (methicillin susceptible) | 0.06 | 0.12 | ≤0.5 |
| Enterococcus faecalis | 8 | 16 | 4 |
| Enterococcus faecium | >16 | >16 | >8 |
| Streptococcus pneumoniae | 0.008-0.5 | 0.008-0.5 | ≤0.5 |
| Streptococcus pneumoniae (penicillin intermediate) | 0.25 | 0.5 | 0.12 |
| Streptococcus pneumoniae (penicillin resistant) | 1 | 1 | 1 |
| Viridans group streptococci | 0.5 | 0.5 | ≤0.5 |
| Beta-hemolytic streptococci | 0.03 | 0.06 | ≤0.5 |
| Gram-negative organisms | |||
| Escherichia coli | 0.03 | 0.03 | ≤0.5 |
| Escherichia coli (ESBL producing) | 0.06 | 0.06 | ≤0.5 |
| Klebsiella species | 0.06 | 0.03 | ≤0.5 |
| Klebsiella species (ESBL producing) | 0.12 | 0.12 | ≤0.5 |
| Enterobacter species | 0.12 | 0.12 | 1 |
| Enterobacter species (ceftazidime resistant) | 0.12-0.25 | 0.25 | 1 |
| Citrobacter species | 0.06 | 0.06 | 1 |
| Citrobacter species (ceftazidime resistant) | 0.06-0.12 | 0.06-0.12 | 1 |
| Proteus mirabilis | 0.25 | 0.06 | 2 |
| Proteus mirabilis (ESBL producing) | 0.25 | 0.12 | 2 |
| Serratia species | 0.25 | 0.06 | 1 |
| Serratia marcescens (ceftazidime resistant) | 0.25-0.5 | 0.12-0.5 | 1-2 |
| Salmonella species | 0.06 | 0.03 | ≤0.5 |
| Shigella species | 0.06 | 0.03 | ≤0.5 |
| Pseudomonas aeruginosa | 8 | 16 | >8 |
| Pseudomonas aeruginosa (carbapenem resistant) | >32 | >8 | >8 |
| Pseudomonas aeruginosa (metallo-beta-lactamase) | >32 | >8 | >8 |
| Acinetobacter species | 4 | 8 | 2 |
| Burkholderia cepacia | 8 | 4 | 8 |
| Stenotrophomonas maltophilia | >16 | >8 | >16 |
| Aeromonas species | 1 | 1 | 2 |
| Anaerobic organisms | |||
| Bacteroides fragilis | 1 | 0.5 | 0.5 |
| Fusobacterium species | 1 | 0.12 | 1 |
| Prevotella species | 0.5 | 0.25 | 0.25 |
| Clostridium species | 2 | 2 | 8 |
| Anaerobic gram-positive cocci | 0.12 | 0.12 | 0.062 |
Doripenem has slightly more activity against wild-type P. aeruginosa than meropenem (2, 8). At a drug concentration of 2 mcg/mL, the rate of susceptible isolates was 92%, 90%, and 89% for doripenem, meropenem, and imipenem, respectively. A study by Jones and colleagues found that carbapenem-resistant isolates of P. aeruginosa were generally resistant to all carbapenems, with 29.4% of isolates sensitive to doripenem and 2.9% sensitive to meropenem (4). However, only 34 carbapenem-resistant isolates were included in this study. Another study by Jones and colleagues evaluated 49 strains of carbapenem-resistant P. aeruginosa and found similar results (22.4% sensitive to doripenem despite MIC > 32 vs 2% sensitive to meropenem) (8). All carbapenems were inactive against the metallo-beta-lactamase–producing strains of P. aeruginosa (4).
Overall, it is hypothesized that doripenem may be useful for highly resistant strains of P. aeruginosa. However, most studies conclude that the spectrum of activity is very similar for doripenem and meropenem and that the diferences in P. aeruginosa susceptibilities may not translate into any significant clinical advantage (9).
MECHANISMS OF RESISTANCE
Alterations in PBPs often lead to beta-lactam resistance—particularly for gram-positive organisms but also for some gram negative organisms (10, 11). However, alterations in PBPs alone will rarely confer a high level of resistance to carbapenems.
There are four molecular classes of beta-lactamases (Ambler class A, B, C, and D) (10, 11). Carbapenems are generally very stable against beta-lactamases compared with other beta-lactam antibiotics. However, they may be hydrolyzed by Ambler class B beta-lactamases such as the IMP, SPM, and VIM enzymes. These are metalloenzymes with a zinc-binding thiol group and are often found in Pseudomonas species, A. baumannii, S. maltophilia, Bacillus species, and other bacteria. A few Ambler class A enzymes have carbapenemase activity as well. AmpC beta-lactamases or ESBLs may hydrolyze carbapenems, but very weakly (9). In a study done by Jones and colleagues, doripenem and meropenem retained activity against ESBL-producing and AmpC beta-lactamase–producing gram-negative organisms (8). Imipenem retained activity against ESBL-producing gram-negative organisms but lost some activity against AmpC beta-lactamase–producing gram-negative organisms.
Carbapenems enter the outer membrane barrier of gram-negative organisms by the OprD outer membrane protein or porin (11). This mechanism for entry applies particularly to imipenem, but to meropenem and doripenem as well. If OprD production is decreased or absent, resistance to imipenem (often in conjunction with beta-lactamase production) occurs. The activity of the other carbapenems is reduced as well, although the MICs for meropenem and doripenem may still be within the susceptible range. This finding suggests that meropenem and doripenem may not enter the cell exclusively via the OprD porin.
Efflux pumps within bacteria can promote resistance by actively pumping the antibiotic out of the bacterial cell (10, 11). P. aeruginosa isolates may have a multidrug efflux system called MexA-MexB-OprM, for which meropenem, penicillin's, cephalosporins, and fuoroquinolones are a substrate. If the expression of this efflux system increases, then the antibiotic is removed from the cell, the MICs are raised, and resistance is conferred. Similar to alterations in the OprD porin, alterations in the efflux pumps alone do not normally confer high-level carbapenem resistance.
Sakyo and colleagues conducted a study to evaluate the potency of carbapenems for the prevention of carbapenem-resistant mutants of P. aeruginosa (12). The study was conducted in Japan on 144 clinical P. aeruginosa isolates. The results showed that mutants did develop because of a reduced expression of OprD and that the mutants had a reduced susceptibility to doripenem, meropenem, and imipenem. The authors indicated that merope-nem and imipenem might select for these carbapenem-resistant mutants. They also stated that a mutant must lack OprD porin and have increased expression of the MexA-MexB-OprM efflux pump to become highly resistant to meropenem or doripenem and that this type of double mutant would not generally occur during carbapenem therapy. The MICs for doripenem were approximately one dilution lower than those for meropenem and eight dilutions lower than for imipenem. The clinical relevance of this study is unknown.
Another study by Mushtaq and colleagues found that doripe-nem resembled meropenem in activity and behavior, with identical MICs or with doripenem having MICs one dilution lower (9). Both drugs had reduced activity against intrinsically resistant P. aeruginosa, indicating that doripenem and meropenem are both affected by efflux mechanisms of resistance. Alterations in multiple mechanisms were found to be necessary to develop clear resistance to doripenem or meropenem, and these combinations are less likely to be selected in vivo. Additionally, the loss of OprD increased doripenem MICs, indicating that doripenem enters the cell through this porin. Doripenem also lost activity against organisms with the IMP and VIM beta-lactamase enzymes. The study also found that in vitro resistance to doripenem could be selected out, although it was lower for doripenem than for meropenem and imipenem, most likely because doripenem is new and organisms have not been exposed to it long enough to develop resistance.
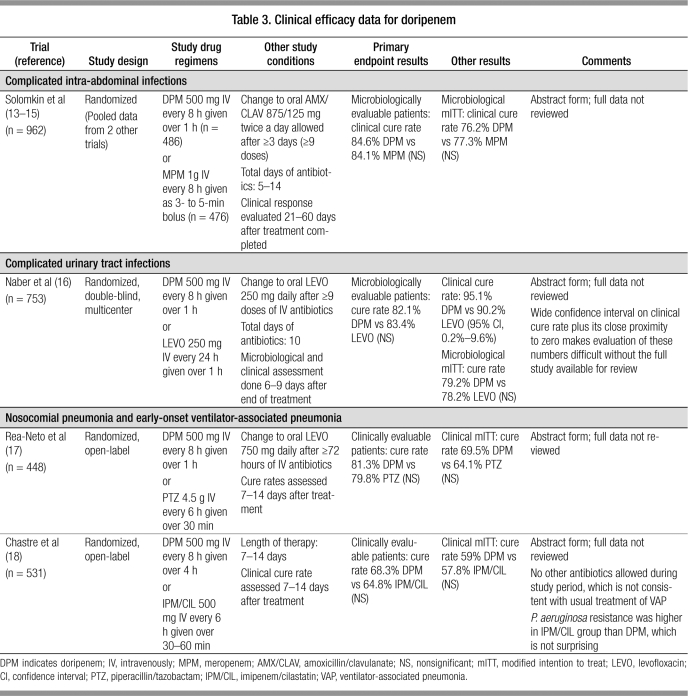
CLINICAL EFFICACY
Currently, all clinical studies evaluating the efficacy of doripe-nem have been published only in abstract form (13–18). A summary of available data is presented in Table 3. Overall, doripenem was found to be similar to comparator agents for each type of infection studied, with no statistical differences found in the primary endpoints.
Table 3.
Clinical efficacy data for doripenem
ADVERSE EFFECTS
The most common adverse reactions to doripenem in phase III clinical trials were headache, nausea, diarrhea, rash, and phlebitis (all >5%) (1). Other adverse events included anemia, increased hepatic enzymes, oral candidiasis, and vulvomycotic infections.
Serious and fatal anaphylactic hypersensitivity reactions have occurred in patients receiving beta-lactam antibiotics, especially when a prior history of sensitivity to multiple allergens is present. The overall incidence of hypersensitivity, including anaphylaxis, with carbapenems is low (approximately 3%) (19). However, due to structural similarities, there a risk of cross-reactivity when carbapenems are administered to patients allergic to penicillin (approximately 10%). Thus, if a patient has a history of anaphylaxis when receiving penicillins, cephalosporins, or other carbapenems, the patient should not receive doripenem. Cases of anaphylaxis with doripenem have been noted in postmarketing reports.
Postmarketing reports have also identified the following adverse events in patients receiving doripenem: Stevens-Johnson syndrome, toxic epidermal necrolysis, interstitial pneumonia, and seizures. Clostridium difficile–associated diarrhea may be associated with antimicrobial use, including use of doripenem.
Postmarketing reports from other countries have identified seizures as a potential adverse event in patients who receive doripenem (1). Horiuchi and colleagues conducted an animal experiment to evaluate the seizure potential of doripenem vs other beta-lactam antibiotics, including meropenem and imipenem/cilastatin (20). In this experiment, imipenem/cilastatin caused seizure activity, as seen on electroencephalogram (EEG) in rats, and obvious clonic convulsions at a dose of 400 mg/kg. Meropenem did not affect the EEG but did produce wet dog shaking behavior at 200 and 400 mg/kg; doripenem did not cause any EEG or behavior changes in the rats at 400 mg/kg. Doripenem also did not cause seizure activity in experimental dog models. It appears from these experiments that doripenem may have a lower potential to cause seizures than mipenem/cilastatin or meropenem. However, all carbapenems have been noted to cause seizures, and the incidence of seizures cannot be determined until the agent is evaluated in the postmarketing setting in the USA.
DRUG INTERACTIONS
Carbapenems can cause a significant decrease in serum val-proic acid levels, which could result in a loss of seizure control (1). This decrease may be caused by inhibition of valproic acid glucuronide hydrolysis, but the precise mechanism is unknown. Thus, caution should be used when initiating doripenem therapy in a patient already stabilized on valproic acid, and levels should be monitored more frequently.
Doripenem is eliminated via active tubular secretion (1). Probenecid interferes with the secretion, resulting in increased doripenem concentrations.
DOSE/ADMINISTRATION
The recommended dose of doripenem for complicated intra-abdominal infections and complicated urinary tract infections, including pyelonephritis, is 500 mg intravenously every 8 hours. The following dosage adjustments for doripenem are required for patients with renal dysfunction:
If creatinine clearance is ≥30 to ≤50 mL/min, the dose is 250 mg intravenously every 8 hours
If creatinine clearance is > 10 to <30 mL/min, the dose is 250 mg intravenously every 12 hours
Doripenem is stable at room temperature for up to 12 hours, while meropenem and imipenem/cilastatin are stable at room temperature for only 1 to 4 hours (19). Both meropenem and doripenem are stable for up to 24 hours when refrigerated, which may allow doripenem to be more easily used in an extended infusion for patients with resistant organisms.
At Baylor University Medical Center, the acquisition cost of doripenem is approximately $50 more than that of meropenem 500 mg intravenously every 6 hours and $20 more than that of meropenem 1 g intravenously every 8 hours.
Meropenem is the current carbapenem of choice at Baylor as of June 2007. Because of the recent conversion to meropenem, the similar spectrum of activity of meropenem and doripenem, the lack of published data, the similarity in cure rates between doripenem and comparator agents (according to published abstracts), and the increased cost of doripenem, meropenem will continue to be the carbapenem of choice at Baylor. However, infectious diseases specialists may order doripenem for select patients who have multidrug-resistant infections that are sensitive to this agent.
References
- 1.Doribax™ (doripenem for injection) [package insert] Raritan, NJ: Ortho-McNeil Pharmaceutical Inc, October 2007. Available at http://www.doribax.com/doribax/interactive_pi.html; accessed April 8, 2008.
- 2.Fritsche TR, Stilwell MG, Jones RN. Antimicrobial activity of doripe-nem (S-4661): a global surveillance report (2003) Clin Microbiol Infect. 2005;11(12):974–984. doi: 10.1111/j.1469-0691.2005.01271.x. [DOI] [PubMed] [Google Scholar]
- 3.Bhavnani SM, Hammel JP, Cirincione BB, Wikler MA, Ambrose PG. Use of pharmacokinetic-pharmacodynamic target attainment analyses to support phase 2 and 3 dosing strategies for doripenem. Antimicrob Agents Chemother. 2005;49(9):3944–3947. doi: 10.1128/AAC.49.9.3944-3947.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones RN, Huynh HK, Biedenbach DJ. Activities of doripenem (S-4661) against drug-resistant clinical pathogens. Antimicrob Agents Chemother. 2004;48(8):3136–3140. doi: 10.1128/AAC.48.8.3136-3140.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones RN, Huynh HK, Biedenback DJ, Fritsche TR, Sader HS. Doripe-nem (S-4661), a novel carbapenem: comparative activity against contemporary pathogens including bactericidal action and preliminary in vitro methods evaluations. J Antimicrob Chemother. 2004;54(1):144–154. doi: 10.1093/jac/dkh298. [DOI] [PubMed] [Google Scholar]
- 6.Ge Y, Wikler MA, Sahm DF, Blosser-Middleton RS, Karlowsky JA. In vitro antimicrobial activity of doripenem, a new carbapenem. Antimicrob Agents Chemother. 2004;48(4):1384–1396. doi: 10.1128/AAC.48.4.1384-1396.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wexler HM, Engel AE, Glass D, Li C. In vitro activities of doripenem and comparator agents against 364 anaerobic clinical isolates. Antimicrob Agents Chemother. 2005;49(10):4413–4417. doi: 10.1128/AAC.49.10.4413-4417.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones RN, Sader HS, Fritsche TR. Comparative activity of doripenem and three other carbapenems tested against gram-negative bacilli with various beta-lactamase resistance mechanisms. Diagn Microbiol Infect Dis. 2005;52(1):71–74. doi: 10.1016/j.diagmicrobio.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Mushtaq S, Ge Y, Livermore DM. Doripenem versus Pseudomonas ae-ruginosa in vitro: activity against characterized isolates, mutants, and transconjugants and resistance selection potential. Antimicrob Agents Chemother. 2004;48(8):3086–3092. doi: 10.1128/AAC.48.8.3086-3092.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Opal SM. Molecular mechanisms of antibiotic resistance in bacteria. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 6th ed. New York: Elsevier/Churchill Livingstone; 2005. [Google Scholar]
- 11.Chambers HF. Carbapenems. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 6th ed. New York: Elsevier/Churchill Livingstone; 2005. [Google Scholar]
- 12.Sakyo S, Tomita H, Tanimoto K, Fujimoto S, Ike Y. Potency of carbapenems for the prevention of carbapenem-resistant mutants of Pseudomonas aeruginosa: the high potency of a new carbapenem doripenem. J Antibiot (Tokyo) 2006;59(4):220–228. doi: 10.1038/ja.2006.31. [DOI] [PubMed] [Google Scholar]
- 13.Solomkin J, Umeh O, Jiang J, Kaniga K, Friedland I. Doripenem versus meropenem with an option for oral step-down therapy in the treatment of complicated intra-abdominal infections Presented at the 47th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), September 17–20, 2007, Chicago, IL.
- 14.Lucasti C, Jasovich A, Umeh O, Jiang J, Kaniga K. Treatment of complicated intra-abdominal infections: doripenem versus meropenem. Int J Antimicrob Agents. 2007;29(Suppl 2):S212. [Google Scholar]
- 15.Malafaia O, Umeh O, Jiang J. Doripenem versus meropenem for the treatment of complicated intra-abdominal infections Presented at the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), September 27–30, 2006, San Francisco, CA.
- 16.Naber K, Redman R, Kotey P, Llorens L, Kaniga K. Intravenous therapy with doripenem versus levofloxacin with an option for oral step-down therapy in the treatment of complicated urinary tract infections and pyelonephritis. Int J Antimicrob Agents. 2007;29(Suppl 2):S212. doi: 10.1128/AAC.00837-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rea-Neto A, Niederman M, Lee M, Kaniga K, Prokocimer P, Friedland I. Efficacy and safety of intravenous doripenem vs. piperacillin/tazobactam in nosocomial pneumonia Presented at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), September 17–20, 2007, Chicago, IL.
- 18.Chastre J, Wunderink R, Prokocimer P, Lee M, Kaniga K, Friedland I. Efcacy and safety of doripenem versus imipenem for ventilator-associated pneumonia Presented at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), September 17–20, 2007, Chicago, IL.
- 19.Lister PD. Carbapenems in the USA: focus on doripenem. Expert Rev Anti Infect Ter. 2007;5(5):793–809. doi: 10.1586/14787210.5.5.793. [DOI] [PubMed] [Google Scholar]
- 20.Horiuchi M, Kimura M, Tokumura M, Hasebe N, Arai T, Abe K. Absence of convulsive liability of doripenem, a new carbapenem antibiotic, in comparison with beta-lactam antibiotics. Toxicology. 2006;222(1–2):114–124. doi: 10.1016/j.tox.2006.02.004. [DOI] [PubMed] [Google Scholar]