Abstract
To quantify the effect of hand-hygiene interventions on rates of gastrointestinal and respiratory illnesses and to identify interventions that provide the greatest efficacy, we searched 4 electronic databases for hand-hygiene trials published from January 1960 through May 2007 and conducted meta-analyses to generate pooled rate ratios across interventions (N=30 studies).
Improvements in hand hygiene resulted in reductions in gastrointestinal illness of 31% (95% confidence intervals [CI]=19%, 42%) and reductions in respiratory illness of 21% (95% CI=5%, 34%). The most beneficial intervention was hand-hygiene education with use of nonantibacterial soap. Use of antibacterial soap showed little added benefit compared with use of nonantibacterial soap.
Hand hygiene is clearly effective against gastrointestinal and, to a lesser extent, respiratory infections. Studies examining hygiene practices during respiratory illness and interventions targeting aerosol transmission are needed.
Many studies have reported an association between improvements in hand hygiene and reductions in rates of infectious illnesses in the community.1 Nevertheless, there are still important questions that must be addressed before guidelines regarding the use of specific interventions for reducing rates of infectious illness in the community can be devised. To our knowledge, a comprehensive meta-analysis comparing the relative effectiveness of specific hand-hygiene interventions used in the community has never been conducted. This makes it difficult to make consistent recommendations to consumers regarding the merit and utility of various hand-hygiene regimens for the prevention of common infectious illnesses.
Analysis of the impact of hand-hygiene interventions for reducing infectious illnesses in the community is important for several reasons. First, there has been an explosion in the options and use of hand-hygiene products in the community.2 Second, hand hygiene is considered an important intervention measure for pandemic public health threats, such as severe acute respiratory syndrome and avian influenza.3–5 Third, research has suggested that there may be risks, including the emergence of antibiotic-resistant bacteria, associated with the use of some hand-hygiene products such as antibacterial soaps.6–9
In this meta-analysis, we assessed the extent to which the published literature has established a benefit of hand-hygiene interventions for the prevention of gastrointestinal and respiratory infectious illnesses. We also identified the specific interventions that provided the greatest potential for reducing these illnesses’ symptoms.
METHODS
Search Strategy
We searched the following databases for articles published in any language from January 1960 through May 2007 by using 241 keyword combinations (search terms are available as a supplement to the online version of this article at http://www.ajph.org): PubMed (1960–2007), EMBASE (1980–2007), Scopus for EMBASE (1974–1980), Science Citation Index (Web of Science; 1960–2007), and Cochrane library (1988–2007), which includes the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, and the United Kingdom National Health Service Database of Abstracts of Review of Effects.
The search results were surveyed for methodological articles and systematic reviews. In addition, the reference lists in all retrieved review papers were searched for additional related articles, and a manual search was performed with A. E. A.’s reference database.
Selection
A. E. A. and R. M. C. independently evaluated selected studies. When consensus was not reached, discussion and further study evaluation with the other authors was used to resolve data extraction discrepancies. Articles were included in the review if the outcome was (1) a reported or diagnosed gastrointestinal illness (such as shigellosis), (2) a reported or diagnosed respiratory illness (such as influenza), (3) a combination of general gastrointestinal or respiratory symptom(s) of infection (such as diarrhea or runny nose), or (4) gastrointestinal or respiratory infectious symptom-related absences (such as school absence for a “cold”), and if the independent variable(s) was a hand-hygiene intervention, such as hand-hygiene education, soap-use intervention (nonanti-bacterial or antibacterial soap), or waterless hand sanitizer.
Articles were restricted to intervention trials conducted in the community and employing a randomized or quasi-experimental study design. Quasi-experimental studies were defined as controlled interventions in which treatment was assigned without the use of a randomized experimental protocol.10,11 These types of studies included crossover studies or interventions with several study arms that were directly assigned by the researcher without randomization.12–20 In some studies it was not possible to ascertain whether randomization was used, and therefore, these studies were classified as nonrandomized.21–30
Articles were excluded if the hand-hygiene intervention was implemented as part of a major public health infrastructure or systems improvement project, such as municipal water supply and waste disposal, or if the setting was a healthcare facility or specialized setting, such as military. Articles that did not provide an effect estimate (such as a rate ratio [RR], odds ratio, etc.) or did not provide enough data to allow calculation of an RR were also excluded (n = 13).31–43
Dave Morrison stands in front of one of his trailers outfitted with rows of sinks that are used by firefighters to wash their hands at fire camps in Dorris, California. Photograph by Lee Juillerat. Used with permission of AP Wide World.
To compare rates of infectious illnesses across studies, we grouped the retrieved articles by specific intervention on the basis of 7 possible categories: (1) hand-hygiene education alone, (2) nonantibacterial soap with hand-hygiene education, (3) antibacterial soap with hand-hygiene education, (4) antibacterial soap alone, (5) alcohol-based hand sanitizer alone, (6) alcohol-based hand sanitizer with hand-hygiene education, and (7) non–alcohol-based hand sanitizer containing benzalkonium chloride. In some instances, both the test and control groups received the same training (e.g., nonantibacterial soap with hand-hygiene education in the test group and hand-hygiene education alone in the control group). In these cases, only the unique intervention for the test group was considered as the tested intervention (e.g., nonantibacterial soap).12,21,44–46
To be classified as having an educational intervention, the study had to state that education was part of the intervention and that there was some systematic provision of hand-hygiene education to the intervention group but not to the control group. Educational interventions were largely determined by setting. For example, interventions in schools were curriculum based, with unit plans and classroom activities. Teachers and students participated in activities together. Educational interventions in day-care centers were largely directed at staff. Most interventions included infection control and hygiene overviews for staff, such as teaching children or assisting with hand-hygiene practices. Educational interventions in lesser-developed regions often included songs, proverbs, games, community-based trainings, and picture stories or posters.
The outcomes were grouped into 3 categories: (1) gastrointestinal symptoms or infection (e.g., diarrhea, dysentery, shigellosis, vomiting), (2) respiratory symptoms or infection (e.g., cold symptoms, influenza virus), and (3) a combination of outcomes (e.g., any combination of gastrointestinal and respiratory symptoms or related absences). If both primary and secondary episodes were presented, we used only primary episode data. There were 2 instances in which this was not possible. The fully adjusted model for 1 study was presented only for secondary illness episodes47 and in another study the effect estimate represented a combination of both primary and secondary episodes.17 The effect estimates used in this meta-analysis applied to episodes of illness rather than duration (e.g., days of illness) with the exception of 1 study that provided only total days with reported symptoms.48
Publication Bias
Publication bias was assessed graphically with funnel plots. In addition, the Begg and Mazumdar rank correlation and the Egger test were used in assessing significant publication bias. For the Begg and Mazumdar rank correlation, a 2-tailed P value of less than .10 was considered evidence of publication bias.49 For the Egger test, a 2-tailed P value of less than .05 was considered evidence of publication bias.49 The Begg test has previously been shown to be slightly less sensitive to publication bias than the Egger test, and therefore, we used a larger P value cut-off.49
Calculation of Effect Estimates
If a study reported RRs and corresponding 95% confidence intervals (CIs), then these estimates were used in the meta-analysis. For studies that did not report RRs or CIs, estimates were calculated with the information provided in the studies. Authors were contacted directly in an attempt to obtain any data that were not reported in their articles.
To validate our calculations of RRs and CIs for studies lacking this information, our formulas were applied to studies that provided RRs and CIs as well as the data required, such as the incidence density, to obtain these estimates. Our recalculation of the data and author correspondence resulted in the identification of 1 error published in a study by Butz et al. (CI updated to 0.55 and 0.93 for our review).48 (The formulas50 that were used to calculate RRs and CIs are available as a supplement to the online version of this article at http://www.ajph.org.)
Statistical Analysis
Next, we conducted a meta-analysis of the retrieved studies by using random-effects models with Comprehensive Meta-Analysis Software version 2 (Biostat, Englewood, NJ). At least 2 studies with the same intervention per outcome were required to calculate the meta-analysis pooled estimates. The relative weights of each study were used to compute overall pooled effect estimates:
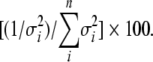 |
(1) |
Forest plots were generated with a mixed-modeling procedure. To assess statistical heterogeneity, we calculated the Cochran Q-statistic and the I2 statistic for each pooled estimate.51,52 An I2 value less than 25% is indicative of homogeneous treatment effects relative to the precision of the individual studies. The Cochran Q-statistic was used to assess significant heterogeneity at P at .05 or below.
To assess potential sources of heterogeneity, we used multilevel random effects models that use the restricted maximum likelihood method (Stata software, version 8.2, StataCorp LP, College Station, TX) to examine age (≤ 5 years vs >5 years), region (developed vs lesser-developed countries as categorized by the World Bank53), study duration, and study design components (masking, randomization, and whether the unit of analysis was the same as the unit of randomization or the study statistically controlled for clustered units).
The age categories were based on the age of the individuals who were included in the outcome assessment. For example, if the intervention was at the household level but illness assessments were conducted only among children younger than 5 years, we categorized the study population as 5 years or younger. If all household members contributed to the illness reports, then the average age of the household was used and the study population was classified as older than 5 years. In 2 studies, the outcome was presented by 2 specific age-group categories and is therefore presented separately in the forest plots.18,54 In addition, we conducted a sensitivity analysis related to study design characteristics by removing 11 studies that lacked masking, lacked randomization, and did not statistically control for clustered units to assess the influence of these studies on the overall effect estimates.12,14,16–20,22,24,28,55
Preventive Fraction for Exposure
To calculate the preventive fraction for exposure (PFE), the RRs and corresponding CIs from all studies were used. We calculated the PFE as
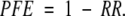 |
(2) |
For example, an RR of 0.80 (95% CI=0.71, 0.90) resulted in a PFE of 1–0.80=0.20. The lower CI for the PFE was 1–0.90=0.10, and the upper CI for the PFE was 1–0.71= 0.29. A PFE was calculated only for the hand-hygiene intervention effect estimates that were statistically significant.
RESULTS
Search Strategy
The initial keyword search provided 5378 articles. After exclusion by screening of titles, 718 studies were initially reviewed by abstract or full article. Of these, 602 studies were retrieved for detailed assessment. In addition to intervention studies, 81 review articles were also retrieved, 8 of which were systematic reviews examining the effectiveness of hand hygiene for reducing infection.1,56–62 (A flowchart of our search process is available as a supplement to the online version of this article at http://www.ajph.org.)
The bibliographies in each of the review articles were searched for pertinent studies that may not have been captured by the initial keyword search, resulting in 1 citation that was not initially identified.24 A total of 572 articles were excluded on the basis of our review criteria, resulting in 30 intervention studies for meta-analyses.
Publication Bias
Publication bias among all studies by each outcome was first assessed graphically with funnel plots (available as a supplement to the online version of this article at http://www.ajph.org). Of these plots, only studies with gastrointestinal illness outcomes showed a clustering suggestive of publication bias.
For gastrointestinal illness outcomes, there was also statistical evidence of publication bias (Begg rank correlation P=.07; Egger regression P = .006). There was no evidence of publication bias in data for respiratory illness outcomes (Begg rank correlation P=.10; Egger regression P = .37). There was no evidence of publication bias among studies examining combined illnesses (Begg rank correlation P=.28; Egger regression P=.48).
Study Characteristics
There were a few differences in the characteristics of the retrieved studies (Table 1 ▶). Overall, a greater proportion of hand-hygiene intervention studies were conducted in developed than in lesser-developed countries (Table 1 ▶). Most of the intervention studies took place in child-care centers or schools rather than in community settings and were conducted among younger age groups (≤ 5 years). A higher proportion of the intervention studies focused on gastrointestinal than on respiratory or combined illness outcomes. (A detailed description of each study and the corresponding study characteristics is available as a supplement to the online version of this article at http://www.ajph.org.)
TABLE 1—
Summary of Hand-Hygiene Intervention Study Characteristics
| Characteristic | % (No.) or Median (Range) | Pa |
| Country | .07 | |
| Developed | 67 (20) | |
| Less developed | 33 (10) | |
| Setting | .14 | |
| Child-care center or school | 63 (19) | |
| Households, village, or community | 37 (11) | |
| Age group,b y | .29 | |
| ≤ 5 | 59 (19) | |
| > 5 | 41 (13) | |
| Illness outcomes | .22 | |
| Only gastrointestinal | 40 (12) | |
| Only respiratory | 17 (5) | |
| Only combined outcomes | 17 (5) | |
| Any combination of outcomesc | 26 (8) | |
| Sample sized | 357.5 (18–6080) |
aP values were calculated with the χ2 test for a difference in proportions.
bUhari et al.54 provided effect estimates for age groups 3 years or younger and older than 3 years. Sircar et al.18 provided effect estimates for age groups under 5 years and 5 years or older.
cSome studies had more than 1 outcome (i.e., gastrointestinal illnesses, respiratory illnesses, or combined illnesses).
dSample size of study population.
Forest Plots
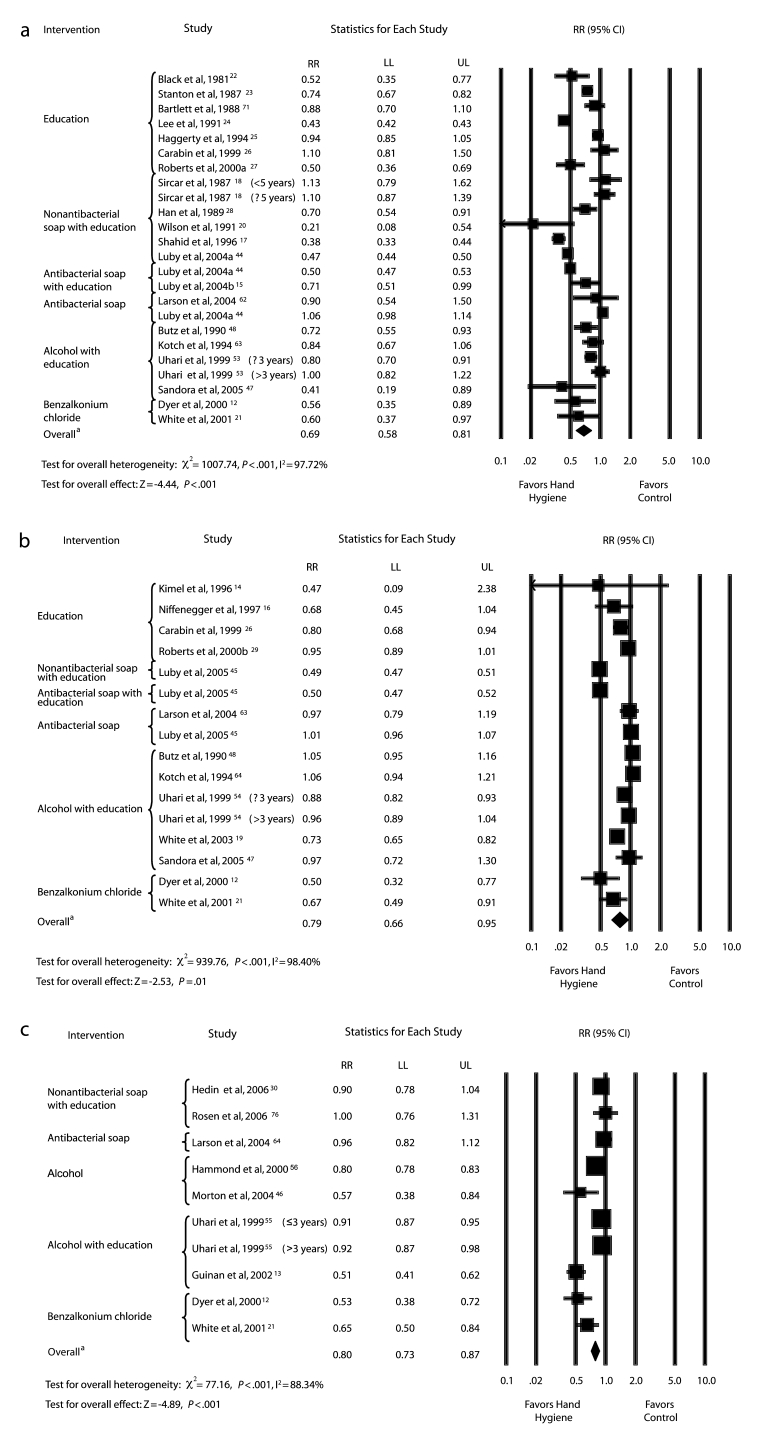
The forest plots and overall summary RRs for each outcome are shown in Figure 1 ▶. There were 24 hand-hygiene intervention effect estimates for gastrointestinal illness outcomes with an overall RR of 0.69 (95% CI=0.58, 0.81; Figure 1a ▶). There were 16 hand-hygiene intervention effect estimates for respiratory illness outcomes with an overall RR of 0.79 (95% CI=0.66, 0.95; Figure 1b ▶). There were 10 hand-hygiene intervention effect estimates for combined illness outcomes with an overall RR of 0.80 (95% CI=0.73, 0.87; Figure 1c ▶).
FIGURE 1—
Rate ratios for the effect of hand-hygiene interventions on gastrointestinal illness (a), respiratory illness (b), and combined illnesses (c).
Note. RR = rate ratio; CI = confidence interval; LL = lower confidence limit; UL = upper confidence limit. Bars with RRs indicate 95% CIs. In studies of antibacterial soap, nonantibacterial soap was provided to the control groups. aOverall weighted summary rate ratio across all studies in the forest plot.
Heterogeneity by Study Characteristics and Design
Heterogeneity in effect estimates by study characteristics and design features were assessed among all studies (Table 2 ▶). Although sources of heterogeneity were not statistically significant for either gastrointestinal or respiratory outcomes, a few of the estimates suggested some influence. For example, there was a larger reduction in gastrointestinal and respiratory illnesses in lesser-developed countries than in more-developed countries and among studies conducted for a shorter duration of time (≤ 100 days vs ≥ 101 days).
TABLE 2—
Heterogeneity in Summary Rate Ratios (RRs) and 95% Confidence Intervals (CIs) Between Study Characteristics
| Gastrointestinal Illness (N = 24) | Respiratory Illness (N = 16) | Combined Illnesses (N = 10) | |||||||
| Characteristic | No. of Studies | RRa (95% CI) | Pb | No. of Studies | RRa (95% CI) | Pb | No. of Studies | RRa (95% CI) | Pb |
| Age, y | .46 | .93 | .03 | ||||||
| ≤ 5 | 16 | 0.72 (0.59, 0.87) | 9 | 0.80 (0.63, 1.01) | 3 | 0.92 (0.77, 1.10) | |||
| > 5 | 8 | 0.62 (0.46, 0.85) | 7 | 0.78 (0.58, 1.05) | 7 | 0.73 (0.65, 0.83) | |||
| Country | .52 | .11 | ≤ .99 | ||||||
| Developed | 12 | 0.73 (0.58, 0.92) | 13 | 0.85 (0.71, 1.01) | 10 | 0.80 (0.73, 0.87) | |||
| Less developed | 12 | 0.66 (0.53, 0.82) | 3 | 0.63 (0.45, 0.87) | NA | NA | |||
| Study duration, days | .40 | .22 | < .001 | ||||||
| 1–100 | 5 | 0.54 (0.36, 0.82) | 5 | 0.64 (0.45, 0.92) | 5 | 0.63 (0.54, 0.72) | |||
| 101–300 | 9 | 0.69 (0.51, 0.92) | 5 | 0.96 (0.71, 1.30) | 2 | 0.83 (0.74, 0.94) | |||
| > 300 | 10 | 0.76 (0.58, 0.99) | 6 | 0.76 (0.58, 1.00) | 3 | 0.92 (0.84, 1.02) | |||
| Clusteringc | .83 | .81 | .70 | ||||||
| No | 14 | 0.68 (0.54, 0.85) | 9 | 0.81 (0.63, 1.03) | 6 | 0.78 (0.70, 0.88) | |||
| Yes | 10 | 0.70 (0.54, 0.92) | 7 | 0.77 (0.60, 0.99) | 4 | 0.81 (0.70, 0.95) | |||
| Randomizationd | .51 | .41 | < .001 | ||||||
| No | 14 | 0.65 (0.52, 0.82) | 7 | 0.72 (0.53, 0.97) | 5 | 0.70 (0.62, 0.80) | |||
| Yes | 10 | 0.74 (0.56, 0.96) | 9 | 0.84 (0.66, 1.07) | 5 | 0.90 (0.80, 1.02) | |||
| Maskinge | .65 | .36 | .74 | ||||||
| No | 19 | 0.68 (0.57, 0.81) | 12 | 0.75 (0.61, 0.93) | 8 | 0.79 (0.71, 0.88) | |||
| Yes | 5 | 0.74 (0.52, 1.06) | 4 | 0.91 (0.64, 1.29) | 2 | 0.82 (0.66, 1.02) | |||
Note. NA = no studies available with the characteristic.
aPooled rate ratios.
bP values calculated for between-study heterogeneity.
cStudies in which the analysis was not conducted at the same unit as the intervention treatment were included in the “No” category.
dStudies that utilized a quasi-experimental design or did not give a description of their randomization procedures were included in the “No” category.
eMasking included either study participants or study participants and the study investigators and staff.
In addition, there was a slightly stronger reduction in both gastrointestinal and respiratory outcomes among studies that did not use masking. For combined illness outcomes, a statistically greater reduction was observed among studies with an older age range (> 5 years vs ≤ 5 years) and shorter duration of study (≤ 100 days vs ≥ 101 days), and studies that did not present randomization procedures.
Next, we conducted a sensitivity analysis related to study design characteristics by removing 11 studies that lacked randomization procedures, did not apply masking, and used a unit of analysis at a different level from that of the unit of randomization (i.e., ignored clustered data structure).12,14,16–20,22,24,28,55 Studies lacking randomization, masking, and adjustment for clustering received a score of zero and those that utilized at least 1 of these methodologies received a score of 1. For gastrointestinal illness outcomes, there was little influence on the overall summary RR (overall RR = 0.69; 95% CI = 0.58, 0.81), versus those with a score of at least 1 (RR = 0.74; 95% CI = 0.62, 0.90).
For respiratory illness outcomes, removal of studies that received a score of zero slightly increased the overall summary RR and the 95% CI contained the null value (all studies RR = 0.79; 95% CI = 0.66, 0.95) versus those with a score of at least 1 (RR = 0.83; 95% CI = 0.68, 1.02). There was little change in combined illness outcomes after we removed studies that received a score of zero (all studies RR = 0.80; 95% CI = 0.73, 0.87) versus those with a score of at least 1 (RR = 0.82; 95% CI = 0.73, 0.91).
Intervention-Specific Rate Ratios for Gastrointestinal Illness
Table 3 ▶ presents the single or pooled RRs (where available) for each outcome by specific intervention measure. Nonantibacterial soap combined with hand-hygiene education showed the strongest protective effect against gastrointestinal illnesses (RR = 0.61; 95% CI = 0.43, 0.88). Similarly, hand-hygiene education showed a strong protective effect against gastrointestinal illnesses (RR = 0.69; 95% CI = 0.50, 0.95). The pooled estimate of the effect of the use of antibacterial soap with hand-hygiene education compared with no intervention in a control group was similar to the summary estimate of the effect of using nonantibacterial soap with hand-hygiene education, but the CI included the null value (RR = 0.59; 95% CI = 0.33, 1.06). Last, the RR was close to null when we compared the effect on gastrointestinal illness rates of using antibacterial soap with using nonantibacterial soap in a control group.
TABLE 3—
Rate Ratios (RRs) and 95% Confidence Intervals (CIs) for the Association Between Specific Hand-Hygiene Interventions and Each Illness Outcome
| Gastrointestinal Illness (N = 24) | Respiratory Illness (N = 16) | Combined Illnesses (N = 10) | ||||
| Intervention | No. of Studies | RRa (95% CI) | No. of Studies | RRa (95% CI) | No. of Studies | RRa (95% CI) |
| Education vs control | 7 | 0.69 (0.50, 0.95) | 4 | 0.86 (0.73, 1.00) | NA | NA |
| Nonantibacterial soap with education vs controlb,c | 6 | 0.61 (0.43, 0.88) | 1 | 0.49 (0.40, 0.61) | 2 | 0.94 (0.74, 1.18) |
| Antibacterial soap with education vs controlb,c | 2 | 0.59 (0.33, 1.06) | 1 | 0.50 (0.40, 0.61) | NA | NA |
| Antibacterial soap vs nonantibacterial soapc | 2 | 0.99 (0.54, 1.83) | 2 | 1.00 (0.84, 1.19) | 1 | 0.96 (0.71, 1.30) |
| Alcohol-based hand sanitizer vs control | NA | NA | NA | NA | 2 | 0.74 (0.59, 0.93) |
| Alcohol-based hand sanitizer with education vs controlb | 5 | 0.77 (0.52, 1.13) | 6 | 0.93 (0.84, 1.03) | 3 | 0.79 (0.67, 0.93) |
| Benzalkonium chloride–based hand sanitizer vs control | 2 | 0.58 (0.30, 1.12) | 2 | 0.60 (0.45, 0.81) | 2 | 0.59 (0.45, 0.78) |
The use of alcohol-based hand sanitizer with a hand-hygiene education intervention was associated with a moderate reduction in gastrointestinal illness rates compared with no intervention in a control group, although the CI included the null value (RR=0.77; 95% CI = 0.52, 1.13). The pooled RR from 2 studies in which the effect of benzalkonium chloride–based hand sanitizer was examined showed a large reduction in gastrointestinal illness rates but the CI included the null value.
Intervention-Specific Rate Ratios for Respiratory Illness
As with gastrointestinal outcomes, the use of nonantibacterial soap combined with hand-hygiene education showed the strongest protective effect on respiratory illness rates (RR = 0.49; 95% CI = 0.40, 0.61), but data were available from only 1 study (Table 3 ▶).45 The same study examined the influence of using antibacterial soap with hand-hygiene education on respiratory illness rates compared with no intervention in a control group, and the RR for this was close to that of using nonantibacterial soap with education.45 The pooled estimate from 4 studies in which hand-hygiene education alone was examined indicated that this intervention was only moderately protective (RR = 0.86; 95% CI = 0.73, 1.00). The use of antibacterial soap compared with the use of nonantibacterial soap had no effect on respiratory illness rates (RR = 1.00; 95% CI = 0.84, 1.19; Table 3 ▶).
The pooled results of 6 studies in which the effect of using alcohol-based hand sanitizer combined with hand-hygiene education was examined showed that this intervention was weak (Table 3 ▶). By contrast, the pooled results of 2 studies in which the effect of using benzalkonium chloride–based hand sanitizer was examined showed a protective effect against respiratory illness outcomes.
Intervention-Specific Rate Ratios for Combined Illness
There were no studies in which the effect of hand-hygiene education alone on combined illness outcomes was examined (Table 3 ▶). The effect of using nonantibacterial soap with hand-hygiene education on combined illness outcomes was weak and not statistically significant (RR = 0.94; 95% CI = 0.74, 1.18). In addition, there was no difference in combined illness outcomes between intervention groups that received antibacterial soap and those that received nonantibacterial soap.
The pooled RR for the use of alcohol-based hand sanitizer with hand-hygiene education showed a significant reduction in combined illnesses (RR=0.79; 95% CI=0.67, 0.93). Similarly, the pooled RR for alcohol-based hand sanitizer use alone showed a significant reduction in combined illness outcomes, as did the pooled RR for using benzalkonium chloride–based hand sanitizer (Table 3 ▶).
Overall Prevention of Illness
For all hand-hygiene interventions combined, the proportion of gastrointestinal illness prevented was 31% (95% CI = 19%, 42%). The use of nonantibacterial soap with education prevented 39% (95% CI = 12%, 57%) of cases compared with no intervention in a control group. The next-greatest impact was the pooled estimate for the effectiveness of hand-hygiene education alone compared with no intervention; the intervention prevented 31% (95% CI = 5%, 50%) of gastrointestinal illnesses.
The overall proportion of respiratory illness prevented by all hand-hygiene interventions combined was 21% (95% CI=5%, 34%). The use of nonantibacterial soap with hand-hygiene education prevented 51% (95% CI=39%, 60%) of respiratory illnesses compared with no intervention in a control group. This estimate was based on a single study by Luby et al. because there were no other intervention studies that assessed the effect of nonantibacterial soap on respiratory illnesses.45
The next-greatest impact was the effectiveness of antibacterial soap with hand-hygiene education compared with no intervention in a control group from the same study; this intervention prevented 50% (95% CI = 39%, 60%) of cases. Pooled data from 2 studies showed that benzalkonium chloride–based hand sanitizer prevented 40% (95% CI=19%, 55%) of respiratory illnesses. None of the other pooled estimates for interventions against respiratory illnesses were associated with strong protective effects (i.e., antibacterial soap compared with nonantibacterial soap; alcohol-based hand sanitizer compared with hand-hygiene education).
For all hand-hygiene interventions combined, the proportion of combined illness prevented was 20% (95% CI=13%, 27%). The use of benzalkonium chloride–based hand sanitizer prevented 41% (95% CI=22%, 55%) of illnesses. Alcohol-based hand sanitizer alone prevented 26% (95% CI = 7%, 41%) of illnesses. The proportion of combined illnesses prevented by the use of alcohol-based hand sanitizer combined with hand-hygiene education was 21% (95% CI=7%, 33%). None of the other interventions were associated with significant prevention of combined illness outcomes (i.e., antibacterial soap compared with nonantibacterial soap; nonantibacterial soap compared with hand-hygiene education).
DISCUSSION
This is the first meta-analysis to show that the effectiveness of hand-hygiene procedures varies depending on both the hygiene intervention method and infectious illness symptoms. The results of our study suggest that the use of nonantibacterial soap with hand-hygiene education interventions is efficacious for preventing both gastrointestinal and respiratory illnesses.
Our review follows several earlier systematic reviews of hand-hygiene interventions, of which only 3 were meta-analyses.1,56–62 Two of the earlier meta-analyses focused solely on the effect of hand washing with soap and water on gastrointestinal illness in lesser-developed regions of the world.57,61 The third meta-analysis, by Rabie et al.,56 examined the effect of various hand-hygiene interventions on respiratory illnesses only.
The percentage reduction in respiratory illnesses associated with the pooled effects of hand hygiene that we observed was similar to the reduction reported by Rabie et al.56 (21% vs 16%, respectively). Our meta-analysis included several studies that were not included in their study,14,26,45,47,48,54,55,63,64 and excluded 3 studies included in their study that did not meet our study criteria.42,65,66 Unlike our study, this earlier meta-analysis provided no information on hygiene intervention–specific pooled estimates and it included a total of only 8 studies, compared with 30 in our study.56
Our review indicated that some hand-hygiene interventions were not efficacious against respiratory illnesses, including educational interventions and the use of alcohol-based hand sanitizers. The consistent application of hand hygiene during critical points in the chain of transmission is likely to play a major role in shaping the relative effectiveness of hand-hygiene interventions by disease outcome.
Differences in the frequency and timing of hand-hygiene episodes may account for the stronger reductions in rates of gastrointestinal illnesses than rates of respiratory illnesses. For example, even with consistent education messages that advocate hand hygiene directly after coughing or sneezing, such practices may not be as consistent or as frequent as hand-hygiene practices directly after defecation.
Very few studies in this review rigorously assessed hand-hygiene practices during the intervention period or monitored the use of products. Future hand-hygiene interventions should seek to incorporate information on the frequency, duration, and triggers for hand-hygiene episodes.
Surprisingly, the use of alcohol-based hand sanitizers combined with hand-hygiene education was not strongly associated with reduced rates of gastrointestinal illnesses or respiratory illnesses. This was unexpected given that alcohol-based antiseptics containing 60% to 80% weight per volume have been shown to be effective against a range of viruses and bacteria, including agents that cause diarrhea or respiratory infections.67
The use of benzalkonium chloride, a less-commonly used hand sanitizer, did show significant reductions in respiratory and combined illness outcomes. However, these data were from only 2 studies, 1 of which had several design flaws.12 Findings from the clinical setting have supported the effectiveness of alcohol-based hand sanitizer for preventing healthcare-associated infections,68 but it is likely that individuals living in the community have very different hand-hygiene habits from those of staff in the healthcare setting.
Although population-based estimates are not available, a large observational survey sponsored by the American Society for Microbiology has suggested that hand hygiene in the United States is suboptimal.69 Results from their study of 7836 individuals in 5 major US cities showed that only 67% of participants washed their hands after using a public restroom.69 Overall, more women (75%) than men (58%) washed their hands, suggesting gender differences in practices.69 Clearly, consistent and targeted hand hygiene should be advocated in the United States to increase the frequency of use.
Antibacterial Soaps
The reviewed studies provided no evidence to support the use of antibacterial soap as a more effective alternative to nonantibacterial soap for prevention of either gastrointestinal or respiratory illnesses. By contrast, the use of antibacterial soap with hand-hygiene education did show some efficacy against both gastrointestinal and respiratory illnesses when compared with no intervention in a control group.15,44,45 These studies were conducted in lesser-developed countries where the control groups had limited access to basic necessities such as clean water and soap.15,44,45
On the other hand, intervention studies that enabled a comparison of the use of antibacterial soap with the use of nonantibacterial soap in a control group were conducted in both lesser-developed and developed regions of the world.44,45,63 The pooled estimates of these studies clearly show that there were no differences in the efficacy of antibacterial versus nonantibacterial soap for reducing gastrointestinal and respiratory illnesses. This is consistent with an earlier qualitative review of some of these studies.9
It could be argued that antibacterial soap is targeted at bacteria and that the symptoms assessed in these studies may have been viral rather than bacterial in origin. However, the outcomes assessed in this meta-analysis are the most common infectious illnesses affecting younger children globally.70 Moreover, antibacterial soaps have been implicated in the laboratory in the emergence of antibiotic-resistant bacteria.7,9,71 Thus, the ineffectiveness of antibacterial soap compared with nonantibacterial soap observed in this study is concerning.
The Non-Prescription Drug Advisory Committee of the US Food and Drug Administration was convened in October 2005 to discuss the benefits and risks associated with antiseptic products marketed for consumer use such as “antibacterial” hand soap. This meeting resulted in a call for further research regarding the risks and benefits of specific consumer antiseptic products used in the community.
Our findings suggest that there is a need for policy decisions that address the continued use of antimicrobial soaps in the community. This is particularly the case for those products containing triclosan and triclocarban, the ingredients in the antibacterial soaps reviewed in this study that are found in many hand and body soaps.2
Sources of Heterogenity
Assessments of heterogeneity indicated that studies of shorter duration and those conducted in lesser-developed countries were more likely to report a greater reduction in rates of both gastrointestinal and respiratory illness. It is possible that participants in studies of shorter duration are more likely to adhere to protocols or enhance normal hand hygiene in response to being a participant than participants in studies of longer duration in which participants may be more likely to revert to their normal hand-hygiene habits. The finding that participants in lesser-developed areas showed a stronger effect against gastrointestinal and respiratory illnesses is not surprising given the differences in the prevalence of common infectious illnesses in developed versus lesser-developed regions.70
In terms of heterogeneity stemming from study design, studies that lacked randomization and masking appeared to show a slightly stronger reduction in illnesses. Nevertheless, these differences were not statistically significant for all outcomes, suggesting that these factors did not contribute to a great deal of heterogeneity across studies. A lack of accounting for clustering had no effect on heterogeneity. This might be attributed to the fact that “gastrointestinal illnesses” and “respiratory illnesses” do not represent a single pathogen that could cluster in time and space, but more likely have multiple etiologies.
Use of an outcome that captures a mixture of different pathogen-specific diseases is therefore unlikely to show significant clustering of cases. For example, if some gastrointestinal cases were associated with eating food contaminated with Shigella species and other cases were related to fecal–oral spread of Escherichia coli, any clustering effects would be reduced by the inclusion of 2 different disease-causing agents in the outcome definition.
There were methodological differences across studies. Often, the definition of symptoms or illnesses varied. Consistent definitions of respiratory infections are needed to further facilitate comparisons across studies. This was of less concern with gastrointestinal illnesses, because most studies used diarrhea as an outcome. Only 3 studies of gastrointestinal illnesses used microbiological analyses to identify the agent associated with symptoms.17,22,72
In addition, very few studies directly examined the microflora of the hands. This is important given the complex biology of skin bacteria and the potential importance of this transmission route in the studied disease outcomes.73,74 None of the respiratory illness studies used microbiological assessments of respiratory pathogens. Clearly, further research on hand hygiene and communicable illnesses should employ an assessment of the microbiological characteristics of the infecting agent.
More-recent studies scored higher on methodological quality.25,27,29,44,45,47,63,64,75 Additional studies that use formal randomization procedures, masking, and clustering such as the procedures of Larson et al.63 are needed. However, such studies are extremely costly; it is often difficult to conduct masked studies in many settings; and it may not be logistically feasible to randomize.
Many of the earlier studies did not control for clustered study units, such as schools or classrooms, in which the likelihood of individual infectious outcomes are considered dependent. This could have led to overly narrow CIs and a higher type-1 error rate, but as mentioned previously, this did not have a significant influence on heterogeneity or overall pooled estimates.76 Nevertheless, future studies should consider more-specific measurement of the outcome and the use of analytic strategies for clustered data such as generalized estimating equations and mixed modeling techniques where appropriate.76
Limitations
As with all meta-analytic procedures, we had to make informed decisions on the classification of study interventions and outcome measures. In some cases, classification of the intervention was not clear because of multiple components. Nevertheless, there were very few studies that combined other hygiene-related interventions in addition to the hand-hygiene measures of interest.
For some studies we had to perform calculations to obtain the RRs and 95% CIs using the available data presented. These calculations may not have been as precise as calculations using the actual raw data. We applied our mathematical formulas to studies that provided an RR, 95% CI, and all components required for deriving these effect estimates; our calculated RRs and 95% CIs using the raw data components were consistent with the reported results of these studies.
For some interventions, such as the use of nonantibacterial and antibacterial soaps, only single studies were available, and therefore, we were unable to generate a pooled estimate for these interventions. In addition, some interventions had only 2 studies available for calculation of pooled estimates. Therefore, intervention-specific single estimates and summary estimates utilizing 2 studies should be interpreted with caution until further research can corroborate these findings.
Heterogeneity was significant in pooled estimates across all studies. We assessed factors such as age, region, and study design characteristics that accounted for some of the heterogeneity. In addition, our intervention-specific pooled estimates provided an assessment across more-similar studies. Head-to-head assessments of more than 1 intervention were conducted in a few studies, but not all, and therefore, conclusions regarding relative efficacy should be made judiciously.
Last, there was evidence of publication bias for gastrointestinal illness outcomes. Therefore, the pooled estimates generated by our meta-analysis of published studies may be exaggerated for this outcome.
Conclusions
The results of our meta-analyses provide the needed data synthesis for formulating consistent community-based hand-hygiene guidelines. First, we confirmed that hand-hygiene interventions are efficacious for preventing gastrointestinal illnesses, in both developed and lesser-developed countries. However, the overall impact of hand hygiene was less efficacious for respiratory illnesses. Overall, there was little evidence for an additional impact of new products, such as alcohol-based hand sanitizers or antibacterial soaps compared with nonantibacterial soaps, for reducing either gastrointestinal or respiratory infectious illness symptoms. Last, there is a need to include microbiological assessments of the agents that may be associated with clinical symptoms of infection so that agent-specific targeted hand-hygiene practices can be evaluated.
Acknowledgments
We acknowledge Ananda Sen at the Center for Statistical Consultation and Research at the University of Michigan for helpful consultation regarding various aspects of the statistical analysis.
Human Participant Protection No approval was required.
Peer Reviewed
Contributors A. E. Aiello and E. L. Larson originated the study. A. E. Aiello, R. M. Coulborn, and V. Perez carried out the statistical analysis and interpretation of the data. A. E. Aiello drafted the initial article. All authors participated in critical revisions of the article.
References
- 1.Aiello AE, Larson EL. What is the evidence for a causal link between hygiene and infections? Lancet Infect Dis. 2002;2:103–110. [DOI] [PubMed] [Google Scholar]
- 2.Perencevich EN, Wong MT, Harris AD. National and regional assessment of the antibacterial soap market: a step toward determining the impact of prevalent antibacterial soaps. Am J Infect Control. 2001;29: 281–283. [DOI] [PubMed] [Google Scholar]
- 3.Lau JT, Tsui H, Lau M, Yang X. SARS transmission, risk factors, and prevention in Hong Kong. Emerg Infect Dis. 2004;10:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muller MP, McGeer A. Febrile respiratory illness in the intensive care unit setting: an infection control perspective. Curr Opin Crit Care. 2006;12:37–42. [DOI] [PubMed] [Google Scholar]
- 5.Rothman RE, Irvin CB, Moran GJ, et al. Respiratory hygiene in the emergency department. Ann Emerg Med. 2006;48:570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhargava HN, Leonard PA. Triclosan: applications and safety. Am J Infect Control. 1996;24:209–218. [DOI] [PubMed] [Google Scholar]
- 7.Aiello AE, Larson EL. Antibacterial cleaning and hygiene products as an emerging risk factor for antibiotic resistance in the community. Lancet Infect Dis. 2003;3:501–506. [DOI] [PubMed] [Google Scholar]
- 8.Levy SB. Antibacterial household products: cause for concern. Emerg Infect Dis. 2001;7(3 suppl): 512–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aiello AE, Larson EL, Levy SB. Consumer antibacterial soaps: effective or just risky? Clin Infect Dis. 2007;45(suppl 2):S137–S147. [DOI] [PubMed] [Google Scholar]
- 10.Cook T, Campbell, D. Quasi-Experimentation: Design and Analysis Issues for Field Settings. Boston, MA: Houghton Mifflin Co; 1979.
- 11.Rothman KJ, Greenland S. Modern Epidemiology. 2nd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 1998.
- 12.Dyer DL, Shinder A, Shinder F. Alcohol-free instant hand sanitizer reduces elementary school illness absenteeism. Fam Med. 2000;32:633–638. [PubMed] [Google Scholar]
- 13.Guinan M, McGuckin M, Ali Y. The effect of a comprehensive handwashing program on absenteeism in elementary schools. Am J Infect Control. 2002;30: 217–220. [DOI] [PubMed] [Google Scholar]
- 14.Kimel LS. Handwashing education can decrease illness absenteeism. J Sch Nurs. 1996;12:14–16,18. [DOI] [PubMed] [Google Scholar]
- 15.Luby SP, Agboatwalla M, Hoekstra RM, Rahbar MH, Billhimer W, Keswick BH. Delayed effectiveness of home-based interventions in reducing childhood diarrhea, Karachi, Pakistan. Am J Trop Med Hyg. 2004;71: 420–427. [PubMed] [Google Scholar]
- 16.Niffenegger JP. Proper handwashing promotes wellness in child care. J Pediatr Health Care. 1997;11: 26–31. [DOI] [PubMed] [Google Scholar]
- 17.Shahid NS, Greenough WB III, Samadi AR, Huq MI, Rahman N. Hand washing with soap reduces diarrhoea and spread of bacterial pathogens in a Bangladesh village. J Diarrhoeal Dis Res. 1996;14: 85–89. [PubMed] [Google Scholar]
- 18.Sircar BK, Sengupta PG, Mondal SK, et al. Effect of handwashing on the incidence of diarrhoea in a Calcutta slum. J Diarrhoeal Dis Res. 1987;5:112–114. [PubMed] [Google Scholar]
- 19.White C, Kolble R, Carlson R, et al. The effect of hand hygiene on illness rate among students in university residence halls. Am J Infect Control. 2003;31: 364–370. [DOI] [PubMed] [Google Scholar]
- 20.Wilson JM, Chandler GN, Muslihatun J. Hand-washing reduces diarrhoea episodes: a study in Lombok, Indonesia. Trans R Soc Trop Med Hyg. 1991;85: 819–821. [DOI] [PubMed] [Google Scholar]
- 21.White CG, Shinder FS, Shinder AL, Dyer DL. Reduction of illness absenteeism in elementary schools using an alcohol-free instant hand sanitizer. J Sch Nurs. 2001;17:258–265. [PubMed] [Google Scholar]
- 22.Black RE, Dykes AC, Anderson KE, et al. Hand-washing to prevent diarrhea in day-care centers. Am J Epidemiol. 1981;113:445–451. [DOI] [PubMed] [Google Scholar]
- 23.Stanton BF, Clemens JD. An educational intervention for altering water-sanitation behaviors to reduce childhood diarrhea in urban Bangladesh. II. A randomized trial to assess the impact of the intervention on hygienic behaviors and rates of diarrhea. Am J Epidemiol. 1987;125:292–301. [DOI] [PubMed] [Google Scholar]
- 24.Lee W, Stoeckel J, Jintaganont P, Romanarak T, Kullavanijaya S. The impact of a community based health education program on the incidence of diarrheal disease in southern Thailand. Southeast Asian J Trop Med Public Health. 1991;22:548–556. [PubMed] [Google Scholar]
- 25.Haggerty PA, Muladi K, Kirkwood BR, Ashworth A, Manunebo M. Community-based hygiene education to reduce diarrhoeal disease in rural Zaire: impact of the intervention on diarrhoeal morbidity. Int J Epidemiol. 1994;23:1050–1059. [DOI] [PubMed] [Google Scholar]
- 26.Carabin H, Gyorkos TW, Soto JC, Joseph L, Payment P, Collet JP. Effectiveness of a training program in reducing infections in toddlers attending day care centers. Epidemiology. 1999;10:219–227. [PubMed] [Google Scholar]
- 27.Roberts L, Jorm L, Patel M, Smith W, Douglas RM, McGilchrist C. Effect of infection control measures on the frequency of diarrheal episodes in child care: a randomized, controlled trial. Pediatrics. 2000;105(4 pt 1): 743–746. [DOI] [PubMed] [Google Scholar]
- 28.Han AM, Hlaing T. Prevention of diarrhoea and dysentery by hand washing. Trans R Soc Trop Med Hyg. 1989;83:128–131. [DOI] [PubMed] [Google Scholar]
- 29.Roberts L, Smith W, Jorm L, Patel M, Douglas RM, McGilchrist C. Effect of infection control measures on the frequency of upper respiratory infection in child care: a randomized, controlled trial. Pediatrics. 2000; 105(4 pt 1):738–742. [DOI] [PubMed] [Google Scholar]
- 30.Hedin K, Petersson C, Cars H, Beckman A, Håkansson A. Infection prevention at day-care centres: feasibility and possible effects of intervention. Scand J Prim Health Care. 2006;24:44–49. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed NU, Zeitlin MF, Beiser AS, Super CM, Gershoff SN. A longitudinal study of the impact of behavioural change intervention on cleanliness, diarrhoeal morbidity and growth of children in rural Bangladesh. Soc Sci Med. 1993;37:159–171. [DOI] [PubMed] [Google Scholar]
- 32.Monsma M, Day R, St Arnaud S. Handwashing makes a difference. J Sch Health. 1992;62:109–111. [DOI] [PubMed] [Google Scholar]
- 33.Ulione MS. Effectiveness of a health promotion program in Head Start. Mo Nurse. 1996;65:16. [PubMed] [Google Scholar]
- 34.Ulione MS. Health promotion and injury prevention in a child development center. J Pediatr Nurs. 1997;12:148–154. [DOI] [PubMed] [Google Scholar]
- 35.Sheth M, Obrah M. Diarrhea prevention through food safety education. Indian J Pediatr. 2004;71: 879–882. [DOI] [PubMed] [Google Scholar]
- 36.Lee GM, Salomon JA, Friedman JF, et al. Illness transmission in the home: a possible role for alcohol-based hand gels. Pediatrics. 2005;115:852–860. [DOI] [PubMed] [Google Scholar]
- 37.Pinfold JV, Horan NJ. Measuring the effect of a hygiene behaviour intervention by indicators of behaviour and diarrhoeal disease. Trans R Soc Trop Med Hyg. 1996;90:366–371. [DOI] [PubMed] [Google Scholar]
- 38.Luby SP, Agboatwalla M, Painter J, et al. Combining drinking water treatment and hand washing for diarrhoea prevention, a cluster randomised controlled trial. Trop Med Int Health. 2006;11:479–489. [DOI] [PubMed] [Google Scholar]
- 39.Pönkä A, Poussa T, Laosmaa M. The effect of enhanced hygiene practices on absences due to infectious diseases among children in day care centers in Helsinki. Infection. 2004;32:2–7. [DOI] [PubMed] [Google Scholar]
- 40.Krilov LR, Barone SR, Mandel FS, Cusack TM, Gaber DJ, Rubino JR. Impact of an infection control program in a specialized preschool. Am J Infect Control. 1996;24:167–173. [DOI] [PubMed] [Google Scholar]
- 41.Khan MU. Interruption of shigellosis by hand washing. Trans R Soc Trop Med Hyg. 1982;76: 164–168. [DOI] [PubMed] [Google Scholar]
- 42.Master D, Hess Longe SH, Dickson H. Scheduled hand washing in an elementary school population. Fam Med. 1997;29:336–339. [PubMed] [Google Scholar]
- 43.Hill JM, Woods ME, Dorsey SD. A human development intervention in the Philippines: effect on child morbidity. Soc Sci Med. 1988;27:1183–1188. [DOI] [PubMed] [Google Scholar]
- 44.Luby SP, Agboatwalla M, Painter J, Altaf A, Billhimer WL, Hoekstra RM. Effect of intensive hand-washing promotion on childhood diarrhea in high-risk communities in Pakistan: a randomized controlled trial. JAMA. 2004;291:2547–2554. [DOI] [PubMed] [Google Scholar]
- 45.Luby SP, Agboatwalla M, Feikin DR, et al. Effect of handwashing on child health: a randomised controlled trial. Lancet. 2005;366:225–233. [DOI] [PubMed] [Google Scholar]
- 46.Morton JL, Schultz AA. Healthy Hands: use of alcohol gel as an adjunct to handwashing in elementary school children. J Sch Nurs. 2004;20:161–167. [DOI] [PubMed] [Google Scholar]
- 47.Sandora T, Taveras EM, Shih MC, et al. A randomized controlled trial of a multifaceted intervention including alcohol-based hand sanitizer and hand-hygiene education to reduce illness transmission in the home. Pediatrics. 2005;116:587–594. [DOI] [PubMed] [Google Scholar]
- 48.Butz AM, Larson E, Fosarelli P, Yolken R. Occurrence of infectious symptoms in children in day care homes. Am J Infect Control. 1990;18:347–353. [DOI] [PubMed] [Google Scholar]
- 49.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–1129. [DOI] [PubMed] [Google Scholar]
- 50.Morgenstern H. Encyclopedia of Epidemiology. Thousand Oaks, CA: Sage Publications; 2007.
- 51.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 52.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.World Bank. Data and statistics. Country classification. Available at: http://web.worldbank.org/WBSITE/EXTERNAL/DATASTATISTICS. Accessed July 1, 2007.
- 54.Uhari M, Möttönen M. An open randomized controlled trial of infection prevention in child day-care centers. Pediatr Infect Dis J. 1999;18:672–677. [DOI] [PubMed] [Google Scholar]
- 55.Hammond B, Ali Y, Fendler E, Dolan M, Donovan S. Effect of hand sanitizer use on elementary school absenteeism. Am J Infect Control. 2000;28:340–346. [DOI] [PubMed] [Google Scholar]
- 56.Rabie T, Curtis V. Handwashing and risk of respiratory infections: a quantitative systematic review. Trop Med Int Health. 2006;11:258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Curtis V, Cairncross S. Effect of washing hands with soap on diarrhoea risk in the community: a systematic review. Lancet Infect Dis. 2003;3:275–281. [DOI] [PubMed] [Google Scholar]
- 58.Meadows E, Le Saux N. A systematic review of the effectiveness of antimicrobial rinse-free hand sanitizers for prevention of illness-related absenteeism in elementary school children. BMC Public Health. 2004; 4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee T, Jordan NN, Sanchez JL, Gaydos JC. Selected nonvaccine interventions to prevent infectious acute respiratory disease. Am J Prev Med. 2005;28: 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCutcheon H, Fitzgerald M. The public health problem of acute respiratory illness in childcare. J Clin Nurs. 2001;10:305–310. [DOI] [PubMed] [Google Scholar]
- 61.Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JM Jr. Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis. 2005;5:42–52. [DOI] [PubMed] [Google Scholar]
- 62.Fung IC, Cairncross S. Effectiveness of handwashing in preventing SARS: a review. Trop Med Int Health. 2006;11:1749–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Larson EL, Lin SX, Gomez-Pichardo C, Della-Latta P. Effect of antibacterial home cleaning and handwashing products on infectious disease symptoms: a randomized, double-blind trial. Ann Intern Med. 2004;140:321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kotch JB, Weigle KA, Weber DJ, et al. Evaluation of an hygienic intervention in child day-care centers. Pediatrics. 1994;94(6 pt 2):991–994. [PubMed] [Google Scholar]
- 65.Ryan MA, Christian RS, Wohlrabe J. Handwashing and respiratory illness among young adults in military training. Am J Prev Med. 2001;21:79–83. [DOI] [PubMed] [Google Scholar]
- 66.Ladegaard MB, Stage V. Hand-hygiene and sickness among small children attending day care centers. An intervention study [in Danish]. Ugeskr Laeger. 1999;161:4396–4400. [PubMed] [Google Scholar]
- 67.Ali Y, Dolan, MJ, Fendler EJ, Larson EL. Alcohols. In: Disinfection, Sterilization and Preservation. 5th ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 2001.
- 68.Boyce JM, Pittet D. Guideline for Hand Hygiene in Health-Care Settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Infect Control Hosp Epidemiol. 2002;23(12 suppl):S3–S40. [DOI] [PubMed] [Google Scholar]
- 69.American Society for Microbiology. America’s Dirty Little Secret—Our Hands. Clean Hands Campaign [Web site]. Available at: http://www.washup.org/page03.htm. Accessed June 5, 2008.
- 70.Global Health Council. Leading Causes of Death Due to Infectious Diseases, 2002 [table]. Available at: http://www.globalhealth.org/view_top.php3?id=228. Accessed July 1, 2007.
- 71.Levy SB. Antimicrobial consumer products: where’s the benefit? What’s the risk? Arch Dermatol. 2002;138:1087–1088. [DOI] [PubMed] [Google Scholar]
- 72.Bartlett AV, Jarvis BA, Ross V, et al. Diarrheal illness among infants and toddlers in day care centers: effects of active surveillance and staff training without subsequent monitoring. Am J Epidemiol. 1988;127: 808–817. [DOI] [PubMed] [Google Scholar]
- 73.Maibach HI, Hildick-Smith G, eds. Skin Bacteria and Their Role in Infection. New York, NY: McGraw-Hill; 1965.
- 74.Maibach HI, Aly R, eds. Skin Microbiology, Relevance to Clinical Infection. New York, NY: Springer-Verlag; 1981.
- 75.Rosen L, Manor O, Engelhard D, et al. Can a handwashing intervention make a difference? Results from a randomized controlled trial in Jerusalem preschools. Prev Med. 2006;42:27–32. [DOI] [PubMed] [Google Scholar]
- 76.Campbell MK, Mollison J, Steen N, Grimshaw JM, Eccles M. Analysis of cluster randomized trials in primary care: a practical approach. Fam Pract. 2000;17: 192–196. [DOI] [PubMed] [Google Scholar]