Summary
We examined the influence of gender and smoking status on reactivity in two human laboratory stress paradigms. Participants were 46 (21 men, 25 women) healthy individuals who completed the Trier Social Stress Task (i.e., performed a speech and math calculations in front of an audience) and a pharmacological stress provocation (i.e., administration of corticotrophin releasing hormone; CRH) after an overnight hospital stay. Approximately half (53%) of the participants were smokers. Cortisol, adrenocorticotrophin hormone (ACTH), physiologic measures (heart rate, blood pressure), and subjective stress were assessed at baseline and at several time points post-task. Men demonstrated higher baseline ACTH and blood pressure as compared to women, however ACTH and blood pressure responses were more pronounced in women. Women smokers evidenced a more blunted cortisol response as compared to nonsmoking women, whereas smoking status did not affect the cortisol response in men. Finally, there was a more robust cardiovascular and subjective response to the Trier as compared to the CRH. Although preliminary, the findings suggest that women may be more sensitive than men to the impact of cigarette smoking on cortisol response. In addition, there is some evidence for a more robust neuroendocrine and physiologic response to acute laboratory stress in women as compared to men.
Keywords: smoking, nicotine, gender, stress, ACTH, cortisol
Introduction
The hypothalamic-pituitary-adrenal (HPA) axis, one of the primary neuroendocrine stress response systems, is important in helping an organism maintain homeostasis after a stressor, and in supporting normal physiological functioning. In this system, corticotrophin-releasing factor (CRF) is released from the hypothalamus and stimulates the pituitary gland to produce adrenocorticotrophin hormone (ACTH), which triggers the release of other hormones, including cortisol, from the adrenal glands. Cortisol inhibits further release of CRF and ACTH through negative feedback inhibition (Chrousos & Gold, 1992; Munck, Guyre, & Holbrook, 1984). A number of studies suggest gender differences in the HPA axis response to stress that could be related to differences in gonadal hormone levels (e.g., estrogen), protein binding or other factors (for a review see Kudielka & Kirschbaum, 2005). The gender differential in risk for certain disease states may be related to gender differences in HPA axis function. For example, women are more likely than men to suffer from depression and anxiety disorders, both associated with HPA axis dysfunction (Breslau et al., 1995; Cleary, 1987; Kessler 1993; Nolen-Hoeksema, 1994; Seeman, 1997).
While animal studies are relatively consistent in demonstrating higher glucocorticoid levels in females compared to males following HPA stimulation, studies in humans have been less consistent (Kudielka & Kirschbaum, 2005). These inconsistencies may be due to methodological differences (e.g., sample selection, timing of testing, variation in assays used) or to differences in genotype (Bart et al., 2006; Kudielka & Kirschbaum, 2005). It is also likely that some gender differences in the HPA axis response may be stressor-specific. While studies generally show no gender difference in response to physical stressors (Friedmann & Kindermann, 1989), several studies of psychological stress tasks demonstrate higher cortisol and ACTH responses in men as compared to women (Dahl et al., 1992; Kirschbaum, Kudielka, Gaab, Schommer, & Hellhammer, 1999; Kirschbaum, Wust, & Hellhammer, 1992; Kudielka & Kirschbaum, 2005). However, a study by Stroud et al. (Stroud, Salovey, & Epel, 2002) suggested gender differences in response to different types of psychological stressors (social interaction vs. achievement oriented challenge). Because psychological stressors, physical stressors and pharmacologic challenges are likely to impact the HPA axis response through different neural pathways, there may be gender differences in response to these various stimuli.
Smoking and the HPA Axis
Acute administration of nicotine activates the HPA axis, and there are dose-dependent increases in several brain regions (e.g., nucleus accumbens, amygdala, cingulated, frontal lobes), which are involved in both emotion regulation and the HPA axis response to stress (Stein et al., 1998). This increased activity has been correlated with a decreased sensitivity of nicotinic receptors (Semba, Wakuta, Maeda, & Suhara, 2004), but it is not clear how chronic smoking alters these responses or what changes are induced with abstinence or exposure to stress. Cortisol also interacts with neurotransmitters and neuropeptides (i.e., acetylcholine, norepinephrine, dopamine, vasopressin, and beta-endorphin) that mediate the effects of nicotine by attenuating the central nervous system sensitivity, leading to a withdrawal-like state under high stress conditions (Ussher et al., 2006). Since glucocorticoids play a modulator role in CNS activity following stress, HPA axis dysregulation in smokers may contribute to the maintenance of smoking behavior (al'Absi, Hatsukami, & Davis, 2005).
Gender and Smoking
Unlike most substances of abuse, rates of nicotine use and dependence are similar for men and women. Data from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) show relatively equivalent rates for past 12 months tobacco dependence (14.1% men vs. 11.5% women) (Grant, Hasin, Chou, Stinson, & Dawson, 2004). Despite this, gender differences in predictors of use and reasons for relapse exist. For example, cigarette use among women is more strongly associated with weight control and body image concerns, mood and anxiety symptoms, social support, pressure from others to quit, and stressful life events (Berlin et al., 2003; Klesges, Meyers, Klesges, & La Vasque, 1989; McKee, Maciejewski, Falba, & Mazure, 2003; Reynoso, Susabda, & Cepeda-Benito, 2005; Westmaas & Langsam, 2005). In addition, some smoking cessation treatment trials demonstrate nicotine replacement therapies are more efficacious for men than women (Borrelli, Papandonatos, Spring, Hitsman, & Niaura, 2004; Cepeda-Benito, Reynoso, & Erath, 2004; Perkins, Donny, & Caggiula, 1999; Reynoso et al., 2005). Independent of response to nicotine replacement therapy, accumulating evidence indicates that women attempting to quit are more likely than men to lapse (Borrelli et al., 2004), lapse more quickly (Wileyto et al., 2005), and are less likely to successfully quit smoking (Sherman, Fu, Joseph, Lanto, & Yano, 2005). The biologic mechanisms mediating these differential outcomes remain unclear.
In the current study, differences in stress response to two different laboratory tasks in men and women who do and do not smoke cigarettes were investigated. Specifically, a 2 (gender) × 2 (smoking status) quasi-experimental design examined differences in neuroendocrine, physiologic, and subjective stress following exposure to a psychological stressor (Trier Social Stress Task: Trier) and a pharmacological stress challenge (corticotrophin releasing hormone: CRH).
Methods
Participants
Participants were 46 individuals (21 men, 25 women) with an average age of 37.1 years (SD = 12.0) who represent the “control” group of a larger study examining the relationship between stress reactivity, HPA axis, and cocaine dependence. Participants were without alcohol or drug use disorders, and other psychiatric conditions that could affect HPA axis functioning (e.g., major depressive disorder, posttraumatic stress disorder, bipolar affective disorder). Individuals were also excluded if they were pregnant, had a body mass index ≥ 35, or had a major medical disorder (e.g., diabetes, HIV) that could affect HPA axis function.
Participants were recruited via newspaper and other media advertisements. Individuals were screened via telephone and if preliminarily eligible, a clinical interview, history and physical examination were conducted. Axis I psychiatric diagnoses were assessed using the Structured Clinical Interview for DSM-IV (First, Spitzer, Gibbon, & Williams, 1994). IRB-approved written informed consent was obtained before any study procedures occurred, and participants were fully informed about all study procedures. Participants were informed that the study aimed to investigate the relationship between stress reactivity, gender and cocaine dependence. The Trier and pharmacologic stress challenge were briefly described to participants as well, and no deception was used. Following baseline assessment, participants were scheduled for two consecutive overnight stays at the Medical University of South Carolina’s (MUSC) General Clinical Research Center (GCRC).
Laboratory Procedures
Participants were required to abstain from alcohol or other substance use (except nicotine and caffeine) for a minimum of two days prior to testing. Abstinence was assessed using self-report, urine drug screen (Roche Diagnostics), and breathalyzer tests (AlcoSensor III, Intoximeters, Inc). In order to control extraneous variables that could affect stress reactivity (e.g., nutrition, caffeine intake, sleep, nicotine use), participants were admitted to the GCRC at approximately 2000h the evening prior to testing. To minimize the impact of nicotine withdrawal, participants who smoked were provided a nicotine patch upon admission and were maintained on nicotine replacement therapy throughout the hospital stay (≥ 20 cigarettes/day = 21 mg patch; 10–19 cigarettes/day = 14mg patch; 5–9 cigarettes/day = 7mg patch).
During the two days of testing in the GCRC, participants completed three laboratory stress tasks: the Trier, administration of CRH, and a cocaine cue exposure. The cocaine cue exposure was included to assess reactivity to cocaine-related cues in cocaine-dependent individuals, but was also administered to individuals in the control group to maintain consistency in study procedures. Only the responses to the Trier and CRH are examined in this paper.
Following the first overnight stay, participants were given a standard breakfast at 0830h. They were allowed to engage in sedentary activities on the unit (e.g., reading magazines, watching TV) until testing. At 1150h, an indwelling intravenous catheter was inserted in the forearm of the non-dominant hand. At 1200h participants were given a standard lunch. Following lunch, participants were connected to electrodes for heart rate and to an intermittently inflatable blood pressure cuff. After instruments were positioned, there was a 30-minute acclimation period.
For the Trier, participants were asked to deliver a 5-minute impromptu job interview speech to an audience of three research staff members unknown to them. Immediately following the 5-minute speech, participants were instructed to complete serial subtractions out loud in front of this same audience for another 5 minutes. No writing instruments or paper were allowed. Audience members were instructed to withhold any verbal or nonverbal reinforcement (e.g., smiling, nodding) during the tasks. The Trier involves elements of uncontrollability and social-evaluative threat, elements found to reliably evoke HPA axis response (Dickerson & Kemeny, 2004). For the pharmacological challenge, participants were administered 1µg/kg ovine CRH supplied by Ferring Pharmaceuticals over a one-minute period through the IV catheter. This provides direct stimulation of the HPA axis. The CRH administration occurred at 1700h on the first day of testing, and the Trier occurred at 1400h on either the first or second day of testing (counterbalanced with the cue exposure paradigm).
For each stress challenge, physiologic indices, blood samples, and subjective stress were measured immediately before, immediately after, and at several time points post-task (i.e., 5, 15, 30 and 60 min for Trier; 10, 25 and 55 min for CRH). Subjective stress was measured using a visual analogue scale derived from the Within Session Rating Scale (Childress, McLellan, & O'Brien, 1986), on the interval 0–10 anchored with adjectival modifiers (“not at all” to “extremely”).
Neuroendocrine Assays
Blood samples were collected in EDTA-prepared tubes and immediately placed on ice. Plasma was obtained by centrifugation under refrigeration, and the serum sample was frozen at 70°C until assayed in duplicate. Allegro HS-ACTH (Nichols Institute Diagnostics) system, which has an intra-assay c.r. of 6% with a sensitivity of 1 pg/ml, was used for ACTH assays. Cortisol was assayed using the Roche Diagnostics Elecsys 2010 immunoassay analyzer and kits based on an electrochemiluminescence competitive immunoassay having a functional sensitivity (lowest reportable concentration) of 8.0 nmol/L (0.29 microgram/dL) and intra-assay reproducibility (coefficient of variation, CV) of less than 2%. GCRC personnel collected all samples, and Rockefeller University personnel analyzed the assays.
Physiologic Measurements
Heart rate was collected via three electrodes along the bottom of the participant’s ribcage, bicep, and collar bone. Blood pressure was measured using a Critikon Dinamap automated monitor.
Statistical Analysis
Baseline response was assessed via the regression approach to two-factor (between-subjects factors were smoking status and gender) analysis of variance (ANOVA) and tested using the likelihood ratio methodology and corresponding chi-square test. Where baseline differences were found, the baseline value was considered as a covariate in the model. All physiologic (heart rate, blood pressure) and neuroendocrine (ACTH, cortisol) outcomes were measured across time, and as such, the observations within a given subject are not independent. These outcomes were analyzed using covariance pattern models (Brown and Prescott, 1999), a class of mixed models which accounts for the dependence within a subject by modeling the covariance between repeated observations. In these models, the outcome was modeled as a function of time, smoking status, gender, and baseline response where appropriate. For some outcomes, the variability in the response was found to differ by smoking status or by gender. To account for this nonconstant variability, the estimated covariance matrix was allowed to vary by gender or smoking status, where appropriate. Non-normality of the residuals was addressed via transformation of the appropriate outcome. Unless otherwise stated, all p-values reported represent Bonferroni-adjusted p-values within a class of outcomes (e.g., p-values associated with neuroendocrine outcomes were adjusted to reflect two variables, ACTH and cortisol, within this class). For neuroendocrine outcomes, the peak change, defined as the difference between the maximum response and baseline, were also analyzed using the regression approach to two-factor (smoking status, gender) ANOVA methodology. Where descriptive statistics are presented, they represent the mean ± standard deviation (M ± SD).
Results
Demographic Information
Table 1 presents the demographic characteristics by gender. Most participants were Caucasian, had some college education, and were employed. Women had higher rates of employment than men (p < .05). No other significant differences in demographic characteristics were revealed.
Table 1.
Demographic Characteristics
| Variable | Men | Women | p-value |
|---|---|---|---|
| n = 21 | n = 25 | ||
| Age, M(SD) | 33.9 (12.1) | 39.8 (11.5) | NS |
| Education, % Some College | 81 | 84 | NS |
| Employment, % Employed | 53 | 92 | p<.05 |
| Race, % Caucasian | 56 | 67 | NS |
| Marital Status, % Married | 14 | 28 | NS |
| Smoking Status, % Smokers | 67 | 48 | NS |
Neuroendocrine Measures
ACTH
A main effect of gender was observed for ACTH at baseline, with men demonstrating significantly higher levels of baseline ACTH than women before both the CRH [22.0 ± 8.3 vs. 15.7 ± 5.9; χ2(1) = 6.91, unadjusted p = .009] and the Trier [21.2 ± 5.1 vs. 16.9 ± 5.8; χ2(1) = 5.43, unadjusted p = .02] tasks. As a result, baseline ACTH was included in the model as a covariate. A main effect of Time indicated that ACTH was affected by the administration of CRH [F(3, 31.7) = 36.6, p = .0002] and by the Trier [F(3, 55.4) = 4.2, p = .02].
A trend toward an interaction between baseline ACTH and gender was found, indicating that the effect of baseline ACTH on the ACTH response to CRH differed for men and women, F(1, 26.3) = 4.5, p = .09. Although baseline ACTH levels were lower for women than men, the slope of the ACTH response appeared steeper for women.
Next, peak change in ACTH was analyzed using a two-factor (gender, smoking status) ANOVA. A significant gender × smoking status interaction [χ2(1)=5.09, p = 0.02] was found, indicating that the effect of smoking status on peak change in ACTH in response to CRH administration differed for men and women. Follow-up tests did not reach statistical significance, but the pattern of results show that among women, smokers demonstrated a smaller peak change than nonsmokers. The pattern was opposite for men, with smokers demonstrating a larger peak change than nonsmokers. Analysis of peak change in ACTH in response to the Trier revealed no significant main effects of gender or smoking, or their interaction.
Cortisol
A gender × smoking status interaction was found for baseline cortisol prior to the Trier [χ2(1) = 7.3, unadjusted p = .007], indicating that the effect of smoking status on baseline cortisol was different for men and women (see Figure 2). Among women, there is some evidence to suggest that smokers had lower baseline cortisol levels than nonsmokers (Wilcoxon two sample unadjusted p-value = 0.08); however, no smoking effect was observed among men.
Figure 2.
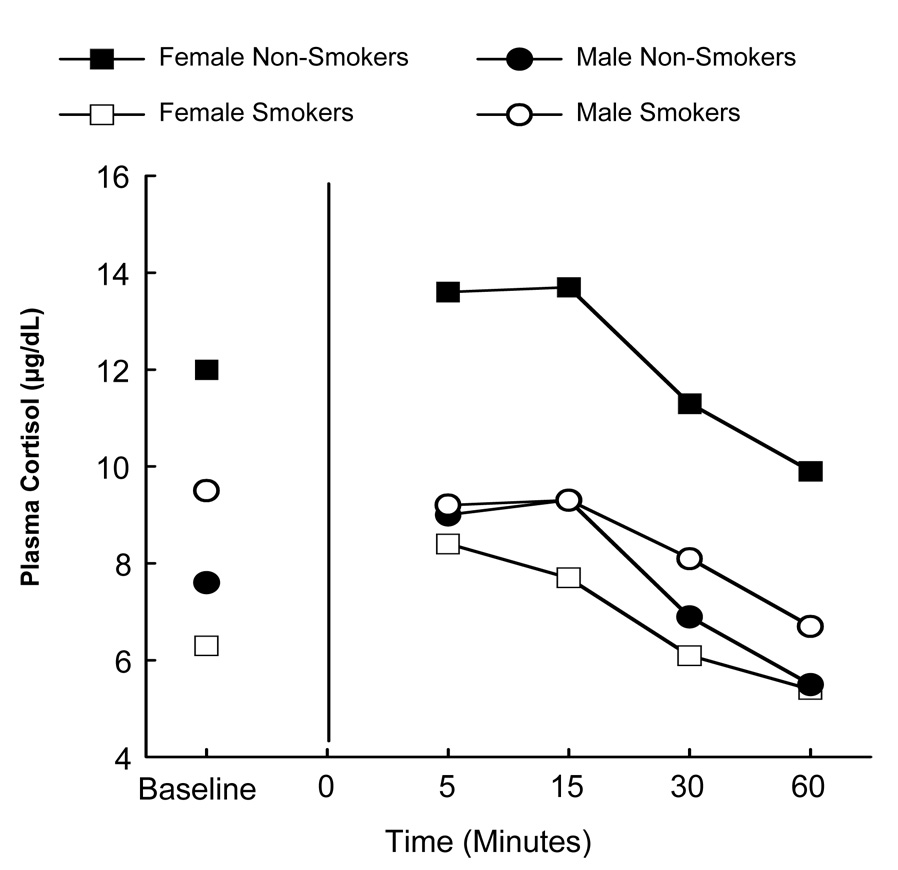
Cortisol response to Trier as a function of gender, smoking status, and time.
Note. In comparison to Figure 1, baseline values in Figure 2 are not connected to post-task values because they were used as covariates. It was unnecessary to control for baseline values depicted in Figure 1.
As depicted in Figure 1, a main effect of Time was observed, demonstrating that cortisol levels were affected by the CRH challenge, F(4, 31.3) = 85.9, p = .0002. A main effect of Time was also evidenced for cortisol response to the Trier, F(3, 33) = 47.81, p = .0002.
Figure 1.
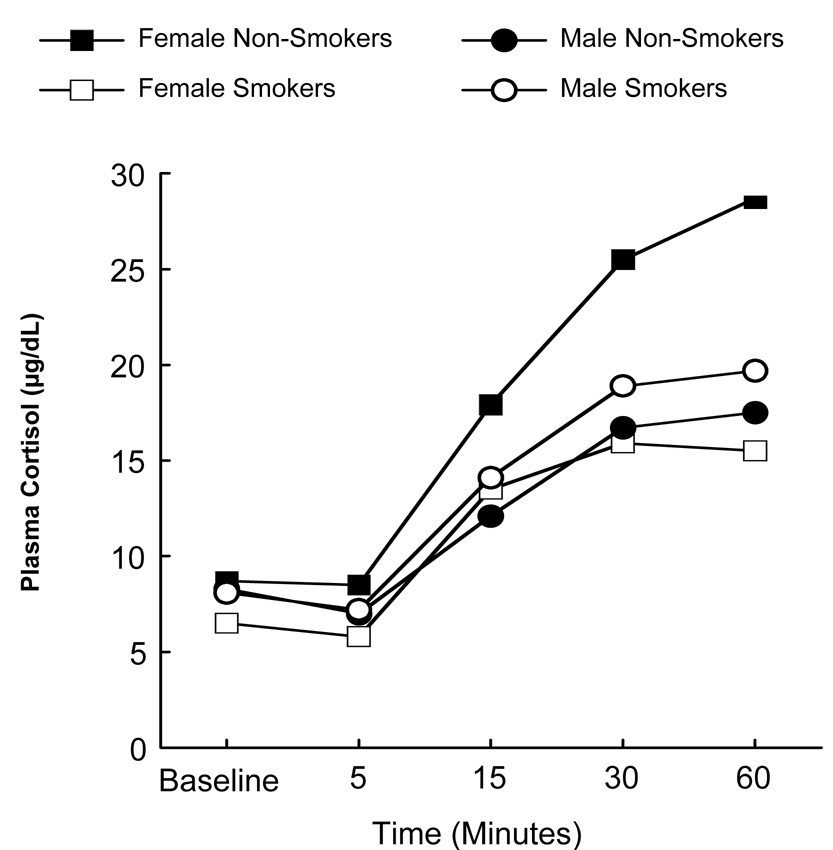
Cortisol response to CRH as a function of gender, smoking status, and time.
Controlling for baseline differences, a significant gender × smoking status interaction was found [F(1, 28.9) = 5.5, p = 0.05], indicating that the effect of gender on cortisol response following the Trier was different for smokers than for nonsmokers. As illustrated in Figure 2, among women, smokers had lower cortisol as compared to nonsmokers, but no difference was observed among smoking and non-smoking men.
The peak change in cortisol in response to the CRH challenge was analyzed and a significant smoking × gender interaction was revealed [χ2(1) = 8.3, unadjusted p = .004]. Follow-up tests revealed that, among women, smokers had a significantly lower cortisol response to the CRH as compared to nonsmokers (Wilcoxon t approximation unadjusted p = 0.02). Among men, no significant difference between nonsmokers and smokers in cortisol response was observed (Wilcoxon t approximation unadjusted p = 0.28).
Analysis of peak change in response to the Trier revealed a trend toward a gender main effect [χ2(1) = 3.10, unadjusted p = .08]. Women demonstrated a larger peak change in cortisol in comparison to men. This is likely due, however, to the larger peak change among nonsmoking women (see Figure 2).
Physiological Measures
Blood Pressure
Systolic (SBP) and diastolic (DBP) blood pressure were assessed. A main effect of gender was observed at baseline, with men demonstrating significantly higher baseline SBP before CRH administration than women [116.0 ± 9.8 vs. 108.5 ± 11.4; χ2(1) = 3.9, unadjusted p = .05] and a trend towards higher baseline SBP before the Trier [118.1 ± 11.1 vs. 111.6 ± 12.6; χ2 = 3.02, unadjusted p = .08]. A trend toward higher baseline SBP among smokers was found before CRH administration [114.6 ± 11.4 vs. 108.2 ± 10.6; χ2(1) = 2.75, unadjusted p = .097]. A main effect of Time was observed for SBP response to the Trier [F(4,128) = 8.15, p = .002], but not the CRH (p > .05).
As can be seen in Figure 3, a significant gender × time interaction in the DBP response to the Trier was found, indicating a gender difference in the time course of the response, F(5, 33.4) = 3.52, p = .05. Similar to the ACTH response described earlier, women’s DBP was lower than men’s at baseline, but the slope of the DBP response was steeper for women than for men. No gender × smoking interactions were revealed.
Figure 3.
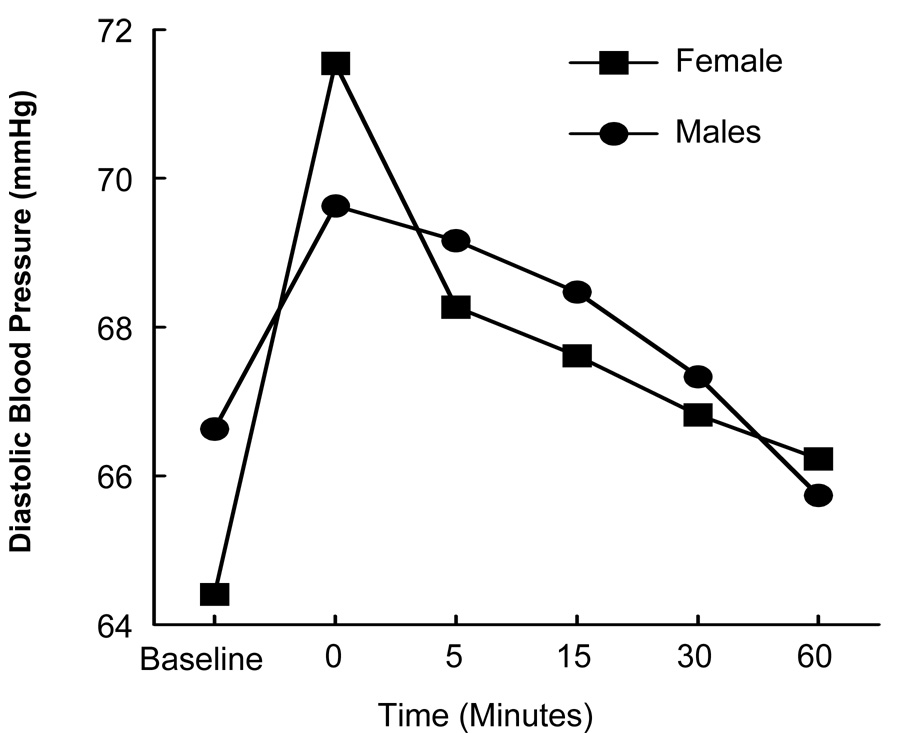
Diastolic blood pressure response to the Trier as a function of gender and time.
Heart Rate
A main effect of Time indicated that heart rate was affected by the Trier [F(5, 164) = 6.89, p = 0.0004], but not the CRH (p > .05). A main effect of gender was observed with women having significantly higher baseline heart rate before CRH administration as compared to men [70.4 ± 9.3 vs. 63.7 ± 8.4; χ2(1) = 4.99, unadjusted p = .03]. A trend toward a main effect of smoking status was also observed with smokers demonstrating higher heart rate response to CRH as compared to nonsmokers, F(1, 31.5) = 3.93, unadjusted p = .06, adjusted p = .2. No gender × smoking interactions were revealed.
Subjective Stress
The majority of individuals (70%) did not report increased subjective distress in response to CRH. Because of this, the subjective stress response to CRH was analyzed using the nonparametric signed rank test, separately for gender and smoking status. No main effects of gender or smoking status, or their interaction, were observed.
In contrast to the response to CRH, the majority of participants (85%) did report increased subjective distress in response to the Trier (signed rank test S = 214, unadjusted p = .0001). Similar to the response to CRH, no main effects of gender or smoking status, or their interaction were observed.
Discussion
In the current study, the impact of gender and smoking status on neuroendocrine, physiologic and subjective response to a psychosocial stressor (i.e., the Trier) and a pharmacological stress challenge (i.e., administration of CRH) was examined. As expected, both laboratory stress tasks were associated with HPA axis response, as evidenced by an increase in ACTH and cortisol. There was a more robust cardiovascular and subjective response to the Trier as compared to CRH. The majority of participants (85%) reported an increase in subjective ratings of stress following the Trier and a significant increase in heart rate and blood pressure was observed. In contrast, the majority (70%) did not report increased subjective stress following CRH, and no significant increases in heart rate or blood pressure were observed in response to CRH. The fact that the Trier evoked higher subjective stress and cardiovascular response as compared to CRH stimulation is likely related to the uncontrollability and social evaluative threat of the task, evoking a limbic system response (Dickerson & Kemeny, 2004) and activating a complex set of responses including autonomic nervous system response. On the other hand, the CRH infusion appears to be functioning primarily as a pharmacologic probe of the HPA axis without activation of other limbic system circuitry in this paradigm. As such, these tasks might be used in conjunction to explore the relationship between various components of the stress response.
Consistent with prior research, women in this study had significantly higher heart rates as compared to men at baseline (Back, Brady, Jackson, Salstrom, & Zinzow, 2005; Fox et al., 2006; Kajantie & Phillips, 2006), whereas men demonstrated significantly higher baseline ACTH and blood pressure than women (al'Absi, 2006; Brady et al., 2006; Fox et al., 2006; Kudielka & Kirschbaum, 2005). While ACTH and blood pressure levels were lower at baseline for women, the slope of the response to CRH was steeper for women as compared to men. This finding is congruent with animal data which show greater response in females as compared to males after HPA axis stimulation (Handa, Burgess, Kerr, & O'Keefe, 1994; Kitay, 1961; Kudielka & Kirschbaum, 2005; Louvart et al., 2006) and with some clinic data showing steeper rates of change in ACTH or cortisol following interpersonal stress among women as compared to men (Kietcolt-Glaser et al., 1997; Stroud et al., 2002). However, the current findings are in contrast to some findings previously reported in the literature (Kajantie & Phillips, 2006; Kudielka & Kirschbaum, 2005; Uhart et al., 2006). For example, Fox et al. (2006) also reported higher baseline levels of ACTH and blood pressure among men, but found higher responses in ACTH and blood pressure following exposure to a stressful imagery task among men as compared to women. Several important differences may help explain the discrepancies. In their study, a different stress challenge was used, patients were cocaine-dependent, smoking patients were not on nicotine replacement therapy and were allowed to smoke a cigarette in the morning before testing, and smoking was not an independent variable in the model. The type of stressor used may be particularly important. As mentioned previously, Stroud et al. (2002) found higher cortisol stress responses in men following an achievement-based stressor that involved math calculations, but higher cortisol stress responses in women following an interpersonal stressor that involved social rejection. The Trier task employed in the current study is unique in that it may be considered an amalgam of both achievement-based and interpersonal stress (i.e., math calculations and a speech in front of a non-supportive audience). This and other methodological differences (e.g., age of testing, menstrual cycle phase, duration of stressor, accounting for smoking status, genetic factors, clinical or healthy population) may help explain some of the discrepant findings in gender differences. Clearly, more systematic and controlled research in this area is needed to help reconcile these differences found in the literature.
The impact of smoking status on HPA axis function and stress response are of interest. In an important series of studies, al’Absi and colleagues have demonstrated a blunted cortisol response to a laboratory stressor in smokers during early abstinence as compared to a non-smoking control group (al'Absi, Wittmers, Erickson, Hatsukami, & Crouse, 2003). A recent follow-up study (al’Absi et al., 2006) found that for men who smoked, attenuated cortisol and ACTH responses to an acute laboratory stressor administered during early abstinence were associated with early relapse. However, for women who smoked, relapse was more strongly predicted by the intensity of withdrawal symptoms and negative affect (al’Absi et al., 2006). Importantly, in this trial (al'Absi, 2006) the stress response was investigated during nicotine withdrawal. In the present study, important gender differences in the HPA axis response to both human laboratory paradigms were also seen. The cortisol response to both CRH and Trier were blunted for women smokers as compared to non-smokers. In contrast, the cortisol response was not affected by smoking status in men. In the present study, all smokers were maintained on nicotine replacement during testing. As such, the present study may provide more information about the impact of chronic nicotine use on HPA axis function, while the studies of al’Absi and colleagues inform about the impact of nicotine withdrawal on HPA axis function. However, significant gender differences were found across studies indicating a gender-specific impact of nicotine use and withdrawal on HPA axis function which could be important in understanding gender differences in nicotine dependence.
As mentioned previously, there is accumulating evidence that women have more difficulty with smoking cessation as compared to men (Borrelli et al., 2004; Sherman et al., 2005; Wileyto et al., 2005). The biologic mechanisms mediating these differential outcomes remain unclear, but further investigation of gender differences in HPA axis disruption with chronic nicotine use and nicotine withdrawal may provide valuable information to inform gender-specific treatments. There is also some evidence that mu opioid receptor genes, which modulate the HPA axis and are associated with the development of substance use disorders, are influenced by gender. In a recent study of healthy subjects, Bart et al. (2006) found that women with at least one copy of the 118G allele evidenced significantly greater basal cortisol levels as compared to women with the prototypical A118A genotype. This difference was more pronounced among women than men. Further work is needed to help understand how these genotype differences may impact vulnerability to developing psychiatric disorders and treatment response in men and women.
The results of the current study suggest that the HPA axis is more sensitive to the impact of chronic nicotine use in women as compared to men. Several studies have found greater dysregulation of the HPA axis with chronic alcohol use in women as compared to men (Brady et al., 2006; Gianoulakis, Dai, & Brown, 2003). Other studies have demonstrated an increased vulnerability of women to adverse medical and psychosocial consequences of substance use in general (Chatham, Hiller, Rowan-Szal, Joe, & Simpson, 1999; Gentilello et al., 2000; Henskens, Garretsen, Mulder, Bongers, & Kroon, 2005; Hernandez-Avila, Rounsaville, & Kranzler, 2004; Mann et al., 2005). Furthermore, women have been shown to advance more rapidly than men from initial to regular alcohol or drug use, and to first treatment episode (Hernandez-Avila et al., 2004; Piazza, Vrbka, & Yeager, 1989; Randall et al., 1999; Wagner & Anthony, 2007). Psychiatric comorbidity, such as co-occurring mood and anxiety disorders, is associated with poorer outcomes for individuals with substance use disorders (George & Krystal, 2000; Grella, Hser, Joshi, & Rounds-Bryant, 2001; Ouimette, Brown, & Najavits, 1998). Of interest, mood and anxiety disorders, which are also characterized by HPA axis dysfunction, are approximately twice as common in women as compared to men. In one study investigating the HPA axis response in alcohol dependence and PTSD (Brady et al., 2006), women with either alcohol dependence, PTSD, or both disorders had more profound HPA dysregulation as compared to men with similar psychopathology. Investigation of relationships between stress response, HPA axis dysregulation and mood/anxiety disorders in individuals with substance use disorders may provide important insights into mechanisms underlying gender differences in addiction.
Limitations
The current study has several important limitations. Although the sample size is comparable to prior investigations, replication with a larger sample will be important to ascertain generalizability. Menstrual cycle phase, which can impact the HPA axis (Kajantie & Phillips, 2006), was uncontrolled in this study and may have influenced the findings. Despite these limitations, the current study builds on earlier investigations in documenting differences in the stress response based on gender and smoking status. Furthermore, this study is strengthened by the assessment of a triad of indices of stress reactivity (neuroendocrine, physiologic, and subjective) and a well-controlled research environment (the GCRC). In addition, unlike previous studies that examined response to stress among smokers during early abstinence when smokers were likely to be in nicotine withdrawal, the current study examined smokers on nicotine replacement therapy. Thus, the current findings expand upon earlier findings by helping to elucidate questions regarding HPA axis “tone” and stress response system in smokers and non-smokers exposed to acute stress. In addition, the current study reports on the response to both a pharmacological challenge and a psychosocial stressor.
Conclusions
In summary, women smokers evidenced a diminished cortisol response to the pharmacological challenge and psychosocial stress task. In contrast, smoking status did not affect cortisol response among men. In addition, some measures of stress reactivity (e.g., ACTH and BP) to acute laboratory stress tasks were more pronounced in women than men. The findings also showed that the Trier had a more robust effect on cardiovascular and subjective stress as compared to the CRH. Although the findings are preliminary in nature, they suggest that women may be more sensitive than men to the impact of chronic nicotine on cortisol response to stress. Further investigations are needed to replicate the current findings and to help understand possible treatment implications.
Acknowledgements
Supported by National Institute on Drug Abuse grants P50 AR049551 and K25 DA00435 (Brady), and National Institute of Health grant 5 M01 RR001070 (Reves).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al'Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol. 2006;59(3):218–227. doi: 10.1016/j.ijpsycho.2005.10.010. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Hatsukami D, Davis GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology. 2005;181(1):107–117. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Wittmers LE, Erickson J, Hatsukami D, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacol Biochem Behav. 2003;74(2):401–410. doi: 10.1016/s0091-3057(02)01011-0. [DOI] [PubMed] [Google Scholar]
- Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology (Berl) 2005;180(1):169–176. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- Bart G, LaForge KS, Borg L, Lilly C, Ho A, Kreek MJ. Altered levels of basal cortisol in healthy subjects with a 118G allele in exon 1 of the mu opioid receptor gene. Neuropsychopharmacology. 2006;31:2313–2317. doi: 10.1038/sj.npp.1301128. [DOI] [PubMed] [Google Scholar]
- Berlin I, Singleton EG, Pedarriosse AM, Lancrenon S, Rames A, Aubin HJ, et al. The Modified Reasons for Smoking Scale: factorial structure, gender effects and relationship with nicotine dependence and smoking cessation in French smokers. Addiction. 2003;98(11):1575–1583. doi: 10.1046/j.1360-0443.2003.00523.x. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Papandonatos G, Spring B, Hitsman B, Niaura R. Experimenter-defined quit dates for smoking cessation: adherence improves outcomes for women but not for men. Addiction. 2004;99(3):378–385. doi: 10.1111/j.1360-0443.2004.00648.x. [DOI] [PubMed] [Google Scholar]
- Brady KT, Waldrop AE, McRae AL, Back SE, Saladin ME, Upadhyaya HP, Anton RF, Randall PK. The impact of alcohol dependence and posttraumatic stress disorder on cold pressor task response. J Stud Alcohol. 2006;67(5):700–706. doi: 10.15288/jsa.2006.67.700. [DOI] [PubMed] [Google Scholar]
- Breslau N, Schultz L, Peterson E. Sex differences in depression: a role for preexisting anxiety. Psychiatry Res. 1995;58(1):1–12. doi: 10.1016/0165-1781(95)02765-o. [DOI] [PubMed] [Google Scholar]
- Cepeda-Benito A, Reynoso JT, Erath S. Meta-analysis of the efficacy of nicotine replacement therapy for smoking cessation: differences between men and women. J Consult Clin Psychol. 2004;72(4):712–722. doi: 10.1037/0022-006X.72.4.712. [DOI] [PubMed] [Google Scholar]
- Chatham LR, Hiller ML, Rowan-Szal GA, Joe GW, Simpson DD. Gender differences at admission and follow-up in a sample of methadone maintenance clients. Subst Use Misuse. 1999;34(8):1137–1165. doi: 10.3109/10826089909039401. [DOI] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O'Brien CP. Abstinent opiate abusers exhibit conditioned craving, conditioned withdrawal and reductions in both through extinction. Br J Addict. 1986;81(5):655–660. doi: 10.1111/j.1360-0443.1986.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267(9):1244–1252. [PubMed] [Google Scholar]
- Cleary PD. Gender differences in stress-related disorders. In: Barnett RS, Biener L, Baruch GK, editors. Gender and Stress. New York: Free Press; 1987. pp. 39–72. [Google Scholar]
- Dahl RE, Siegel SF, Williamson DE, Lee PA, Perel J, Birmaher B, et al. Corticotropin releasing hormone stimulation test and nocturnal cortisol levels in normal children. Pediatr Res. 1992;32(1):64–68. doi: 10.1203/00006450-199207000-00012. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer R, Gibbon M, Williams J. Patient Edition (SCID-I/P, vs 2.0) 1994. Structured clinical interview for Axis I DSM-IV disorders. [Google Scholar]
- Fox H, Garcia M, Kemp K, Milivojevic V, Kreek M, Sinha R. Gender differences in cardiovascular and corticoadrenal response to stress and drug cues in cocaine dependent individuals. Psychopharmacology. 2006;185:348–357. doi: 10.1007/s00213-005-0303-1. [DOI] [PubMed] [Google Scholar]
- Friedmann B, Kindermann W. Energy metabolism and regulatory hormones in women and men during endurance exercise. Eur J Appl Physiol Occup Physiol. 1989;59(1–2):1–9. doi: 10.1007/BF02396572. [DOI] [PubMed] [Google Scholar]
- Gentilello LM, Rivara FP, Donovan DM, Villaveces A, Daranciang E, Dunn CW, et al. Alcohol problems in women admitted to a level I trauma center: a gender-based comparison. J Trauma. 2000;48(1):108–114. doi: 10.1097/00005373-200001000-00018. [DOI] [PubMed] [Google Scholar]
- George TP, Krystal JH. Comorbidity of psychiatric and substance abuse disorders. Current Opinion in Psychiatry. 2000;13(3):327–331. [Google Scholar]
- Gianoulakis C, Dai X, Brown T. Effect of chronic alcohol consumption on the activity of the hypothalamic-pituitary-adrenal axis and pituitary beta-endorphin as a function of alcohol intake, age, and gender. Alcohol Clin Exp Res. 2003;27(3):410–423. doi: 10.1097/01.ALC.0000056614.96137.B8. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11):1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Grella CE, Hser YI, Joshi V, Rounds-Bryant J. Drug treatment outcomes for adolescents with comorbid mental and substance use disorders. J Nerv Ment Dis. 2001;189(6):384–392. doi: 10.1097/00005053-200106000-00006. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28(4):464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Henskens R, Garretsen H, Mulder CL, Bongers I, Kroon H. Fidelity of an outreach treatment program for chronic crack abusers in the Netherlands to the ACT model. Psychiatr Serv. 2005;56(11):1451–1454. doi: 10.1176/appi.ps.56.11.1451. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74(3):265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29(2–3):85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31(2):151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61(2):154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom Med. 1992;54(6):648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Kitay JI. Sex differences in adrenal cortical secretion in the rat. Endocrinology. 1961;68:818–824. doi: 10.1210/endo-68-5-818. [DOI] [PubMed] [Google Scholar]
- Klesges RC, Meyers AW, Klesges LM, La Vasque ME. Smoking, body weight, and their effects on smoking behavior: a comprehensive review of the literature. Psychol Bull. 1989;106(2):204–230. doi: 10.1037/0033-2909.106.2.204. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69(1):113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Louvart H, Maccari S, Lesage J, Leonhardt M, Dickes-Coopman A, Darnaudery M. Effects of a single footshock followed by situational reminders on HPA axis and behaviour in the aversive context in male and female rats. Psychoneuroendocrinology. 2006;31(1):92–99. doi: 10.1016/j.psyneuen.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A. Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcohol Clin Exp Res. 2005;29(5):896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- McKee SA, Maciejewski PK, Falba T, Mazure CM. Sex differences in the effects of stressful life events on changes in smoking status. Addiction. 2003;98(6):847–855. doi: 10.1046/j.1360-0443.2003.00408.x. [DOI] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5(1):25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychol Bull. 1994;115(3):424–443. doi: 10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- Ouimette PC, Brown PJ, Najavits LM. Course and treatment of patients with both substance use and posttraumatic stress disorders. Addict Behav. 1998;23(6):785–795. doi: 10.1016/s0306-4603(98)00064-1. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1(4):301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- Piazza NJ, Vrbka JL, Yeager RD. Telescoping of alcoholism in women alcoholics. Int J Addict. 1989;24(1):19–28. doi: 10.3109/10826088909047272. [DOI] [PubMed] [Google Scholar]
- Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME. Telescoping of landmark events associated with drinking: a gender comparison. J Stud Alcohol. 1999;60(2):252–260. doi: 10.15288/jsa.1999.60.252. [DOI] [PubMed] [Google Scholar]
- Reynoso J, Susabda A, Cepeda-Benito A. Gender differences in smoking cessation. Journal of Psychopathology and Behavioral Assessment. 2005;27(3):227–234. [Google Scholar]
- Seeman MV. Psychopathology in women and men: focus on female hormones. Am J Psychiatry. 1997;154(12):1641–1647. doi: 10.1176/ajp.154.12.1641. [DOI] [PubMed] [Google Scholar]
- Semba J, Wakuta M, Maeda J, Suhara T. Nicotine withdrawal induces subsensitivity of hypothalamic-pituitary-adrenal axis to stress in rats: implications for precipitation of depression during smoking cessation. Psychoneuroendocrinology. 2004;29(2):215–226. doi: 10.1016/s0306-4530(03)00024-6. [DOI] [PubMed] [Google Scholar]
- Sherman SE, Fu SS, Joseph AM, Lanto AB, Yano EM. Gender differences in smoking cessation services received among veterans. Womens Health Issues. 2005;15(3):126–133. doi: 10.1016/j.whi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, et al. Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry. 1998;155(8):1009–1015. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biol Psychiatry. 2002;52(4):318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Ussher M, West R, Evans P, Steptoe A, McEwen A, Clow A, et al. Reduction in cortisol after smoking cessation among users of nicotine patches. Psychosom Med. 2006;68(2):299–306. doi: 10.1097/01.psy.0000204926.27215.a1. [DOI] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. Male-female differences in the risk of progression from first use to dependence upon cannabis, cocaine, and alcohol. Drug Alcohol Depend. 2007;86(2–3):191–198. doi: 10.1016/j.drugalcdep.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Westmaas JL, Langsam K. Unaided smoking cessation and predictors of failure to quit in a community sample: effects of gender. Addict Behav. 2005;30(7):1405–1424. doi: 10.1016/j.addbeh.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Wileyto EP, Patterson F, Niaura R, Epstein LH, Brown RA, Audrain-McGovern J, et al. Recurrent event analysis of lapse and recovery in a smoking cessation clinical trial using bupropion. Nicotine Tob Res. 2005;7(2):257–268. doi: 10.1080/14622200500055673. [DOI] [PubMed] [Google Scholar]


