Abstract
Objective: This study reports a clinical trial evaluating lamotrigine safety and efficacy as an antidepressant augmentation agent in treatment-resistant depression, therefore adding more empirical evidence to the limited number of studies on the use of lamotrigine.
Method: A double-blind pilot study was conducted between March 2004 and January 2006 with 34 nonbi-polar, nonpsychotic patients who had DSM-IV major depressive disorder and were resistant to at least 2 anti-depressants. The subjects were taking antidepressant therapy and were randomly assigned to receive placebo or lamotrigine as an adjunct therapy for 8 weeks. They were evaluated on a biweekly basis in order to assess the efficacy and the safety of the drug. Efficacy was measured with the Montgomery-Asberg Depression Rating Scale (MADRS) and the Clinical Global Impressions (CGI) scale. Response was defined as a decrease of at least 50% from baseline on the MADRS and a final score ≤ 2 on the CGI. Safety was assessed by keeping record of treatment-emergent adverse events.
Results: The results of the adjunct treatment with lamotrigine did not reveal a significant difference according to the MADRS (p = .45). No differences between the 2 treatment groups were revealed by the repeated-measures analysis of variance or by the analysis based on the CGI (p = .45). More than 50% of the patients had been treated with at least 3 different anti-depressants. The most frequent adverse events were nausea, rash, and dyspepsia in the lamotrigine group and dizziness and headache in the placebo group.
Conclusions: In this study, although it was safe, lamotrigine was not found to be an efficient augmentation agent in treatment-resistant depression. Small sample size, higher chronicity, and refractoriness may be related to treatment failure.
Trial Registration: clinicaltrials.gov Identifier: NCT00652171
Lamotrigine has been investigated as an augmentation agent for patients with treatment-resistant depression. The use of lamotrigine in treatment-resistant depression has been described in a case report1 and evaluated in 3 open trials,2–4 1 double-blind study,5 and 2 retrospective studies.6,7
In a study by Barbosa et al.,5 lamotrigine as augmentation to fluoxetine did not reveal any evidence of efficacy when evaluated according to the 2 major criteria: the Hamilton Rating Scale for Depression8 and the Montgomery-Asberg Depression Rating Scale (MADRS).9 However, lamotrigine was statistically superior to placebo according to a minor criterion, the Clinical Global Impressions (CGI) scale.10 This negative result may be due to some limitations of the study, such as the small and heterogeneous sample (23 unipolar and bipolar II patients) and the lamo-trigine maximum dose of 100 mg/day.
Therefore, lamotrigine effects on treatment-resistant depression are not yet well defined. This study is a pilot study in which we aimed to define key parameters for a subsequent larger trial and to contribute to investigating the efficacy and safety of lamotrigine as an augmentation agent for treatment-resistant patients with depression.
METHOD
Patients
Of 97 depressed outpatients selected between March 2004 and January 2006, 34 met specific inclusion and exclusion criteria for the study. Approximately 20% of the 97 depressed patients were already receiving treatment in our research clinic, 40% were referred by colleagues, and 40% were referred due to public divulgation. They were selected according to a clinical interview based on DSM-IV criteria for major depressive disorder in single or recurrent episodes,11 with moderate to severe intensity. They also had a history of treatment-resistant depression stage II or above according to the criteria by Thase and Rush12: failure to respond to treatment with at least 2 anti-depressants of different classes at the maximum-tolerated dose for at least 6 weeks and absence of psychotic symptoms.
Pregnant or lactating women or those capable of becoming pregnant who were not using contraceptive methods were excluded as well as patients with severe clinical diseases or organic mental disorder. Further exclusion criteria were the presence of acute depression with risk of suicide, psychosis, and bipolar disorder as well as the presence of personality disorders and disorders related to alcohol and other drugs.
Study Design
The study was carried out for a period of 8 weeks. Depressed patients from psychiatry ambulatory services of the Institute of Social Security of the Civil Servants of Minas Gerais, Belo Horizonte, Brazil and from Psychosocial Attention Service of Diamantina, Minas Gerais, Belo Horizonte, Brazil, who agreed to participate in the study were randomly assigned to either the lamotrigine or the placebo group. Random numbers were created using the Epi Info program (Version 3.4.3, Centers for Disease Control and Prevention, Washington, DC). The capsules of lamotrigine and placebo were identical in appearance, and the researcher who accessed the patients was blinded to the given treatments. Another investigator had in a sealed envelope the numbers assigned to patients in each group, which was opened by the time of the analysis of the data. The antidepressants used by the patients were continued at the highest tolerated therapeutic doses throughout the whole study, as were other preexisting medications, such as benzodiazepines, antihypertensives, and contraceptives. Lamotrigine doses were titrated upward, in off-label parameters, from 50 mg/day in the first 2 weeks to 100 mg/day in the third and fourth weeks and 200 mg/day from the fifth week on.
The patients were examined on a biweekly basis. The MADRS9 and the CGI10 scale were used. Patients were asked about adverse events during each evaluation interview before they were randomly assigned.
Ethical Aspects
This study was approved by the research ethics committee of Israel Pinheiro Hospital, Institute of Social Security of the Civil Servants of Minas Gerais, Belo Horizonte, Brazil. A written informed consent form was signed by the patients.
Statistical Analysis
The homogeneity of the 2 treatment groups was evaluated on the first day by Mann-Whitney test for quantitative variables and by χ2 for qualitative variables.12
Efficacy was estimated by comparing the mean score obtained on the MADRS and CGI scales. A decrease of at least 50% in the mean MADRS score and a final CGI score ≤ 2 was considered a positive therapeutic response.12 The evolution was compared using a 2-way analysis of variance (group × time) with repeated measures for time. The last-observation-carried-forward technique was used for the missing evaluations.12
Safety was assessed by keeping record of the treatment-emergent adverse events and determining changes in body weight, pulse frequency, and blood pressure.
RESULTS
Patient Characteristics
Seven of the 34 selected patients did not conclude the study: 3 in the lamotrigine group and 4 in the placebo group. The reasons for premature withdrawal were incidence of rash (N = 2) and noncompliance with treatment (N = 1) in the lamotrigine group and worsening of depressive symptoms (N = 2), manic episode (N = 1), and moving to another city (N = 1) in the placebo group. Two patients (1 who had a manic episode and 1 who was non-compliant) were excluded from statistical analysis.
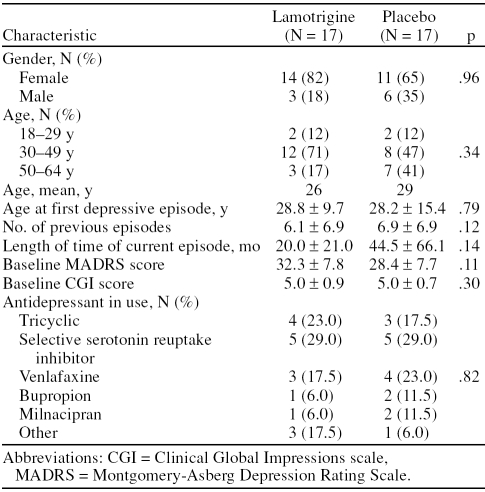
Demographic and clinical characteristics of the 2 groups were comparable (Table 1). Approximately 76% of the study participants were women. The mean ± SD age of the patients was 38.2 ± 8.7 and 42.6 ± 11.7 years in the lamotrigine and placebo groups, respectively (p = .34). The mean ± SD duration of the clinical condition was 9.4 ± 6.7 and 15.5 ± 12.2 years in the lamotrigine and placebo groups, respectively (p = .11). The mean ± SD duration of the current episode was 20.0 ± 21.0 and 44.5 ± 66.1 months in the lamotrigine and placebo groups, respectively (p = .14).
Table 1.
Demographic and Clinical Characteristics
The mean ± SD degrees of refractoriness were 4.41 ± 2.38 and 3.8 ± 2.05 in the lamotrigine and placebo groups, respectively, according to the criteria of Massachusetts General Hospital.13 More than half of the patients in both groups had tried at least 3 different antidepressants. No significant difference was observed between the study groups. Also, at the baseline visit, no significant difference was observed in respect to the score obtained for symptom intensity according to the MADRS and CGI scale. The mean MADRS scores were 32 and 28 for the lamotrigine and placebo groups, respectively (p = .12). The mean CGI score was 5 for both groups.
Efficacy
MADRS. The global MADRS score decreased in both study groups during the study. The total score (mean ± SD) decreased from 32.3 ± 7.8 at the baseline visit to 21.8 ± 10.6 on day 56 in the lamotrigine group and from 28.4 ± 7.7 to 21.2 ± 11.3 in the placebo group. No significant difference was observed between the groups with regard to the final mean MADRS score (p = .45).
If we consider a 50% reduction or higher in the initial MADRS score as a response criterion, it can be stated that at the end of the study, 26.7% of the patients in the lamo-trigine group and 35.7% in the placebo group responded to the treatment. No significant difference was observed between the study groups (p = .6).
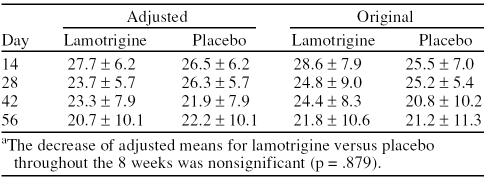
The mean ± SD values adjusted by the repeated-measures model for time reveal that the MADRS score of the patients in the lamotrigine group decreased throughout the 8 weeks, although this decrease was not significantly higher than that observed in the placebo group (p = .879) (Table 2). The analysis of variance reveals a linear relation between the initial and final MADRS values (p < .0001) for both groups. No significant interaction was observed between the study groups and time (p = .761).
Table 2.
Montgomery-Asberg Depression Rating Scale Adjusted Versus Original Mean ± SD Valuesa
No decrease in score or significant difference was observed between the groups when the items of the MADRS scale were observed separately.
CGI. If we consider a CGI score ≤ 2 at the last visit as a response criterion, it can be stated that 23.5% of the patients in the lamotrigine group and 35.7% in the placebo group responded to the treatment. No statistically significant difference was observed between the study groups (p = .45).
Safety
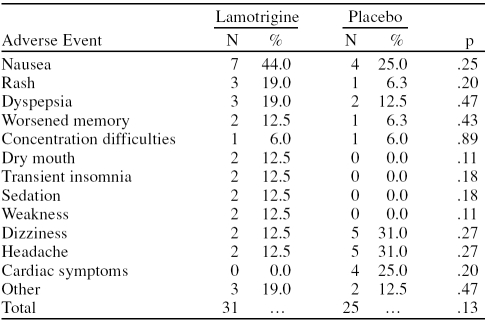
Despite the fact that none of the patients presented serious symptoms, most of them—93% and 76% of the patients in the lamotrigine and placebo groups, respectively—experienced some kind of adverse event (p = .28). The most frequent adverse event observed in the lamotri-gine group was nausea in 44% (7 of 16) of the patients, followed by rash and dyspepsia in 19.0% (3 of 16) of the patients. The most frequent adverse events observed in the placebo group were headache and dizziness in 31% (5 of 16) of the patients, followed by nausea and cardiac symptoms in 25% (4 of 16), dyspepsia and other symptoms in 12.5% (2 of 16), and rash, memory problems, and concentration difficulties in 6% (1 of 16). No significant difference in occurrence and frequency of adverse events was observed between the study groups (p = .13) (Table 3).
Table 3.
Adverse Events
DISCUSSION
The results of this trial did not reveal a therapeutic efficacy based on the evaluation criteria used (reduction of at least 50% in the mean MADRS score and final CGI score ≤ 2). According to the analysis of variance, the efficacy of the 2 treatments was equivalent, since no significant interaction was found between the groups and the MADRS scores throughout the 8 weeks of the study. The results of this analysis reveal a linear dependence between the MADRS initial and final values for both groups. Patients with higher initial MADRS scores had higher scores at the end of the experiment, remained depressed, and showed a lesser response to the treatments.
The present trial is in accordance with the one by Barbosa et al.,5 which found no significant difference between lamotrigine and placebo as fluoxetine augmentation agent. The other studies were open2–4 or retrospective6,7 and prone to bias.
Intending to reach therapeutic doses as fast as possible, we used an off-label titration of lamotrigine. None of the participants reported serious adverse events. The side effects observed were statistically similar to those reported in the literature—nausea, dizziness, and sedation. 14,15 Lamotrigine was generally well tolerated by most of the patients in this study.
This experiment had several limitations that may have had an influence on the results. The protocol stipulated that the 200-mg lamotrigine dose should be administered only from day 28 onward. Therefore, the maximum dose was administered for only 4 weeks. A longer follow-up period may have been necessary.
The small sample size reduced the power of the study.16 The small number of patients in the sample was due to the difficulty in obtaining treatment-resistant depression patients who met the inclusion and exclusion criteria of the research protocols. A possible solution for this problem would be to carry out multicenter trials.
The patients in this study had long-term depression (mean ± SD, 9.4 ± 6.7 and 15.5 ± 12.2 years in the lamotrigine and placebo groups, respectively), and the length of time of current episode was also long (20.0 ± 21.0 and 44.5 ± 66.1 months in the lamotrigine and placebo groups, respectively). The duration of the present episode tended to be longer in the placebo group—not statistically significant. It would be expected that more patients in the lamotrigine than in the placebo group would achieve response. More than half of the patients in both groups had already tried at least 3 therapeutic strategies. Higher chronicity17 and refractoriness18,19 are associated with poor response to antidepressant augmentation therapy and may have contributed to the therapeutic failure in this study of lamotrigine as an augmentation agent. The level of placebo response was high (35.7%), which may also have contributed to the negative result of the present study.
Further limitations of this study include the diversity of antidepressants used by the patients enrolled in the study, the fact that the plasmatic levels of antidepressants and lamotrigine were not measured, and information about previous treatment was obtained only from the reports of the patients.
In conclusion, although it was proven that lamotrigine can be safely used as an antidepressant augmentation agent, it was not proven that it is effective for treating treatment-resistant depression.
Drug names: bupropion (Wellbutrin and others), fluoxetine (Prozac and others), lamotrigine (Lamictal and others), venlafaxine (Effexor and others).
Footnotes
The authors report no financial or other relationships relevant to the subject of this article.
REFERENCES
- Maltese TM.. Adjunctive lamotrigine treatment for major depression [letter] Am J Psychiatry. 1999 Nov;156(11):1833. doi: 10.1176/ajp.156.11.1833. [DOI] [PubMed] [Google Scholar]
- Gabriel A.. Lamotrigine adjunctive treatment in resistant unipolar depression: an open, descriptive study. Depress Anxiety. 2006;23(8):485–488. doi: 10.1002/da.20211. [DOI] [PubMed] [Google Scholar]
- Schindler F, Anghelescu IG.. Lithium versus lamotrigine augmentation in treatment resistant unipolar depression: a randomized, open-label study. Int Clin Psychopharmacol. 2007 May;22(3):179–182. doi: 10.1097/YIC.0b013e328014823d. [DOI] [PubMed] [Google Scholar]
- Manning JS, Haykal RF, and Connor PD. et al. Sustained remission with lamotrigine augmentation or monotherapy in female resistant depressives with mixed cyclothymic-dysthymic temperament. J Affect Disord. February2005 84(2–3):259–266. [DOI] [PubMed] [Google Scholar]
- Barbosa L, Berk M, Vorster M.. A double-blind, randomized, placebo-controlled trial of augmentation with lamotrigine or placebo in patients concomitantly treated with fluoxetine for resistant major depressive episodes. J Clin Psychiatry. 2003 Apr;64(4):403–407. doi: 10.4088/jcp.v64n0407. [DOI] [PubMed] [Google Scholar]
- Barbee JG, Jamhour NJ.. Lamotrigine as an augmentation agent in treatment-resistant depression. J Clin Psychiatry. 2002 Aug;63(8):737–741. doi: 10.4088/jcp.v63n0813. [DOI] [PubMed] [Google Scholar]
- Rocha FL, Hara C.. Lamotrigine augmentation in unipolar depression. Int Clin Psychopharmacol. 2003;18:97–99. doi: 10.1097/00004850-200303000-00006. [DOI] [PubMed] [Google Scholar]
- Hamilton M.. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M.. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology. US Dept Health, Education, and Welfare publication (ADM) 76–338. Rockville, Md: National Institute of Mental Health. 1976 218–222. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association. 1994 [Google Scholar]
- Thase ME, Rush AJ. When at first you don't succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997 58suppl 13. 23–29. [PubMed] [Google Scholar]
- Fava M.. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649–659. doi: 10.1016/s0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- Normann C, Hummel B, and Scharer LO. et al. Lamotrigine as adjunct to paroxetine in acute depression: a placebo-controlled, double-blind study. J Clin Psychiatry. 2002 63(4):337–344. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Bowden CL, and Sachs GS. et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry. February1999 60(2):79–88. [DOI] [PubMed] [Google Scholar]
- Rocha FL.. Ensaios clínicos com antidepressivos. J Bras Psiq. 1997;46:309–318. [Google Scholar]
- Rybakowski JK, Suwalska A, Chlopocka-Wozniak M.. Potentiation of antidepressants with lithium or carbamazepine in treatment-resistant depression. Neuropsychobiology. 1999 Sep;40(3):134–139. doi: 10.1159/000026610. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Papakostas GI, and Petersen T. et al. Lithium augmentation of nortriptyline for subjects resistant to multiple antidepressants. J Clin Psychopharmacol. 2003 23:92–95. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, and Wisniewski SR. et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. November2006 163(11):1905–1917. [DOI] [PubMed] [Google Scholar]