Abstract
Objectives: Although selective serotonin reuptake inhibitors (SSRIs) have become the standard of care for the treatment of major depressive disorder (MDD), limited data exist to support their use in MDD with atypical features. The current study investigates the efficacy of the SSRI escitalopram in an 8-week, open-label, flexible-dose, rater-blinded trial.
Method: Seventeen DSM-IV MDD subjects aged from 18 through 65 years completed screening procedures, provided informed consent, and went through a minimum 2-week washout from preexisting antide-pressants except fluoxetine (a minimum 4-week wash-out). They subsequently received escitalopram (10–20 mg/day) for 8 weeks. The primary efficacy measure was a change in score from baseline to end of treatment on the Structured Interview Guide for the Hamilton Rating Scale for Depression-Seasonal Affective Disorder version (SIGH-SAD), which includes a set of items for atypical symptoms. Secondary efficacy measures were defined as changes from baseline to end of treatment in scores on the SIGH-SAD atypical symptoms subscale (consisting of 8 items specific to atypical features), Beck Depression Inventory-II, Beck Anxiety Inventory, Sheehan Disability Scale, and Epworth Sleepiness Questionnaire. The study was conducted from October 2005 to November 2006.
Results: The mean age was 43.9 years, and 70.6% of subjects were women. The dropout rate was 11.8% (N = 2/17). The mean dose of escitalopram was 18.3 mg/day. The total SIGH-SAD score (mean ± SD) reduced by 53.8% from baseline (33.3 ± 8.2) to end of treatment (15.4 ± 9.4) (t = 4.24, p < .001). The atypical symptoms subscale score reduced by 44.5% from baseline (11.0 ± 4.3) to end of treatment (6.1 ± 2.8) (t = 5.26, p = .001). Ten subjects (62.5%) were classified as responders at end of treatment as defined by ≥ 50% reduction in SIGH-SAD total score. Overall, escitalopram was well tolerated.
Conclusions: Our preliminary study indicates that escitalopram may be beneficial in the treatment of MDD with atypical features. Adequately powered, randomized, double-blind, placebo-controlled trials are necessary to determine the efficacy of escitalopram in this disorder.
Trial Registration: clinicaltrials.gov Identifier: NCT00610506
The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)1 added atypical features as a parenthetical modifier of both major depressive disorder (MDD) and dysthymic disorder on the basis of its distinctiveness from other depressive subtypes. Distinguishing factors include treatment response characteristics,2–6 longitudinal course,7 family history,7,8 and physiologic data such as noradrenergic functional markers9 and sleep architecture.10 However, debate continues as to whether MDD with atypical features is a distinct subtype of MDD, one of multiple possible phases during the longitudinal course of MDD, or the idiosyncratic manifestation of depressive symptoms in patients with a personality subtype characterized by “interpersonal rejection sensitivity.”11 The prevalence rate of MDD with atypical features has ranged from 15.7% in community samples12 to 36% among outpatients with MDD, depending upon the criteria employed.13
Several studies, many of which predate the development of selective serotonin reuptake inhibitors (SSRIs), have indicated that patients with MDD with atypical features may respond preferentially to treatment with monoamine oxidase inhibitors ([MAOIs] e.g., phenelzine) when compared to tricyclic antidepressants ([TCAs] e.g., imipramine).2–5 In fact, authors have suggested that the preferential response of atypical depression to MAOIs is a chief factor distinguishing it from melancholic depression.14 Currently, SSRIs have been the mainstay of treatment for MDD for the past 2 decades. However, their role in the treatment of patients with MDD with atypical features needs further clarification. A randomized, double-blind, 6-week study comparing phenelzine (45–90 mg/day) and fluoxetine (20–60 mg/day) for the treatment of patients with MDD with atypical features (N = 40) found both drugs to have comparable efficacy but favored fluoxetine over phenelzine in terms of tolerability and safety.15 A 6-week randomized trial in MDD patients that included a subgroup analysis of patients with atypical features (N = 53) reported superiority of moclo-bemide compared to fluoxetine on some of the efficacy measures used.16 These 2 studies support the use of SSRIs for atypical depression, but conclusions are limited by small sample sizes and lack of placebo arms. A randomized, parallel group, 12-week study comparing moclobemide and sertraline in the treatment of outpatients with MDD with atypical features (N = 197) also demonstrated that both medications produced comparable improvement17; this larger study provides useful data, although it too is limited by the lack of a placebo arm and has been criticized14 due to the suboptimal dose used in the moclobemide arm (mean dose of 410.2 mg/day). A 10-week, randomized, placebo-controlled study of MDD with atypical features (N = 154) showed that fluoxetine was superior to placebo, was comparable to imipramine in terms of efficacy, and was better tolerated than imipramine.18 This article provides the strongest evidence for the use of SSRIs in atypical depression due to the relatively large sample size and utilization of a placebo arm. Overall, more evidence is needed to determine the efficacy of SSRIs in patients with MDD with atypical features.
Of note, other antidepressants, such as mirtazapine,19 bupropion,20 and mianserin,21 were found to be potentially beneficial in treating patients with MDD with atypical features in small open-label studies.
Escitalopram is approved by the U.S. Food and Drug Administration (FDA) for the treatment of MDD and generalized anxiety disorder.22 Escitalopram was also found to be superior to placebo in treating anxiety symptoms in MDD patients, including those with a high degree of anxiety.23 Escitalopram has also demonstrated superiority over placebo in MDD patients with severe symptoms24 and chronicity.25 These findings suggest that escitalopram may have a potential for treating MDD with atypical features, since patients with MDD with atypical features are more likely to present with an earlier age at onset, greater comorbidity with anxiety symptoms, greater symptom severity, and longer duration when compared to nonatypical MDD patients.26
There have been no published trials investigating the role of escitalopram in MDD with atypical features. This study was designed as a preliminary trial to investigate the clinical utility and the tolerability of escitalopram in patients with MDD with atypical features. Additionally, given the relative scarcity of published studies of SSRIs in atypical depression, this study aimed to contribute to the growing database regarding the usefulness of SSRIs in this depressive subtype.
METHOD
Study Design
The study's design was a prospective, open-label, rater-blinded, 8-week, flexible dose (10–20 mg per day) trial of escitalopram for patients with MDD with atypical features.
Subjects
The study was approved by the Institutional Review Board of Duke University, Durham, N.C., and granted an Investigational New Drug exemption by the FDA. The eligible subjects were men and women from ages 18 through 65 years who (1) met DSM-IV criteria1 for non-psychotic MDD with atypical features on the Mini International Neuropsychiatric Interview (MINI),27 (2) had an entry score of ≥ 19 on the Structured Interview Guide for the Hamilton Rating Scale for Depression-Seasonal Affective Disorder version (SIGH-SAD),28 and (3) provided informed consent. Approved methods of contraception were required for all women of childbearing age who participated in the study. The study was conducted from October 2005 to November 2006.
Exclusion criteria included subjects with (1) bipolar depression, (2) any Axis I psychotic disorder, (3) suicide risk, (4) history of substance abuse or dependence within 12 months, (5) clinically significant medical diseases, (6) pregnancy or planning pregnancy, (7) history of hypersensitivity to escitalopram or citalopram, (8) treatment with electroconvulsive therapy during the previous 12 weeks, and (9) initiation or termination of psychotherapy during the previous 12 weeks.
A minimum of 2-week washout from existing psychotropics (4 weeks for fluoxetine) was required. No concomitant psychotropic medications were permitted during the trial. Medications for concomitant medical illnesses were permitted; however, the dose had to remain unchanged during the trial.
Study Medication
Escitalopram was initiated at 10 mg/day and titrated to a maximum dose of 20 mg/day on the basis of clinical response and tolerability. After 8 weeks of treatment, escital-opram was tapered for 1 week and discontinued. Subjects were referred to their treating psychiatrist for follow-up. Compliance was measured by pill count.
Efficacy and Safety Measures
Assessments included SIGH-SAD, Clinical Global Impressions-Improvement scale (CGI-I),29 Beck Depression Inventory-II (BDI-II),30 Beck Anxiety Inventory (BAI),31 Sheehan Disability Scale (SDS),32 and Epworth Sleepiness Questionnaire (ESQ).33
The primary efficacy measure was defined as a change in SIGH-SAD total score from baseline to end of treatment. Secondary efficacy measures were defined as changes in scores from baseline to end of treatment on the atypical symptom subscale of the SIGH-SAD (consisting of 8 items specific to atypical symptoms), BDI-II, BAI, SDS, and ESQ. Response was defined as ≥ 50% reduction in SIGH-SAD total score or a CGI score of 1 or 2 at end of treatment.34 Remission was defined as SIGH-SAD total score of ≤ 7 at end of treatment.34
Safety evaluations obtained at screening and end of treatment included vital signs (height, weight, pulse rate, blood pressure, body temperature, and respiratory rate), laboratory testing (complete blood count with differential, liver, kidney, and thyroid function tests as well as serum electrolytes and lipid profile), and electrocardiogram. Adverse effects were determined by the Systematic Assessment for Treatment Emergent Events-General Inquiry.35
Study Visits
Study visits were conducted at screening, at baseline, and during weekly visits from weeks 1 through 8. Assessments were performed at each visit, and study medication was dispensed as per the protocol. The primary endpoint was assessed by a rater blinded to the study protocol and status of subject. The rater was blinded to the study and did not know it was an open-label study. He or she was only involved with using the rating scales.
Data Analysis
The intent-to-treat group (ITT) comprised all subjects who received at least 1 dose of the medication. The changes from baseline to end of treatment in SIGH-SAD total score and scores on the atypical symptom subscale, BDI-II, BAI, SDS, and ESQ were analyzed by 2-tailed paired t test at the .05 level of significance. All analyses employed ITT with last observation carried forward (LOCF) analysis (N = 16). The proportions of a priori–defined responders and remitters during the study were reported in descriptive statistics. Under a 2-tailed α value of .05, the power of our sample to detect an effect size (F = 0.75) was 80%.
RESULTS
Subjects
Thirty-three subjects were screened and 17 eligible subjects were enrolled in the trial. The mean ± SD age was 43.9 ± 11.2 years, 12 subjects (70.6%) were women, and 10 (58.8%) were white. Two subjects (11.8%) dropped out of the study. The mean dose of escitalopram was 18.3 mg/day. Five patients were taking antidepressants (sertraline, N = 2; venlafaxine extended release, N = 2; amitriptyline, N = 1) at the time of study entry.
Primary and Secondary Efficacy Measures
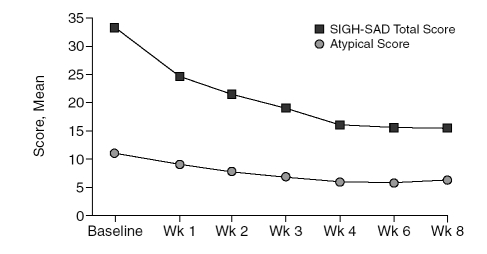
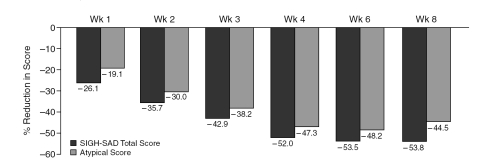
The SIGH-SAD total score (mean ± SD) reduced by 53.8% from baseline (33.3 ± 8.2) to end of treatment (15.4 ± 9.4) (t = 4.24, p < .001). Atypical symptom sub-scale scores also reduced by 44.5% from baseline (11.0 ± 4.3) to end of treatment (6.1 ± 2.8) (t = 5.26, p = .001). Figure 1 summarizes the mean SIGH-SAD total and atypical scores at each week. Figure 2 summarizes the reduction in the SIGH-SAD total and atypical subscale scores during the study.
Figure 1.
Changes in the Structured Interview Guide for the Hamilton Rating Scale for Depression-Seasonal Affective Disorder Version (SIGH-SAD) Total and Atypical Symptom Subscale Scores During the Study (N = 16, intent-to-treat population with last observation carried forward)
Figure 2.
Reduction in the Structured Interview Guide for the Hamilton Rating Scale for Depression-Seasonal Affective Disorder Version (SIGH-SAD) Total and Atypical Subscale Scores From Baseline During the Study (N = 16, intent-to-treat population with last observation carried forward)
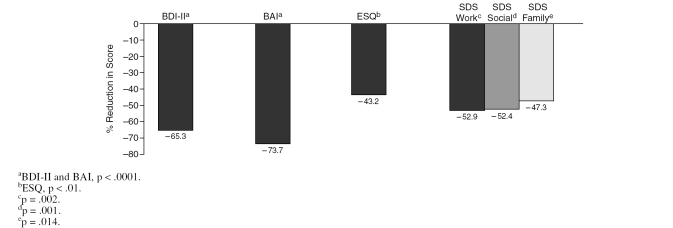
There was also similar trend toward improvement in BDI-II, BAI, SDS, and ESQ scores from baseline to end of treatment, as seen in Figure 3.
Figure 3.
Reduction in the Scores on Beck Depression Inventory-II (BDI-II), Beck Anxiety Inventory (BAI), Epworth Sleepiness Questionnaire (ESQ), and Sheehan Disability Scale ([SDS] work, social, and family domains) From Baseline to End of Treatment (N = 16, intent-to-treat population with last observation carried forward)
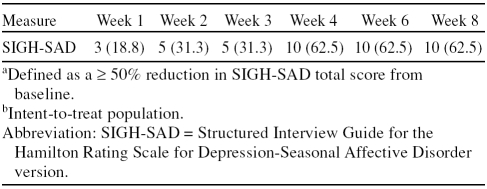
At end of treatment, 62.5% (N = 10) and 75.0% (N = 12) of the subjects were classified as responders as defined by ≥ 50% reduction in SIGH-SAD total score and by a CGI-I score of 1 or 2, respectively. As shown in Table 1, approximately one third (31.3%) of patients had shown a response by week 3. At end of treatment, 12.5% (N = 2) of subjects had remitted as defined by SIGH-SAD total score of ≤ 7.
Table 1.
Number (%) of Patients Respondinga at Each Week During the Study (N = 16)b
Completer analyses (N = 15) and ITT population analyses showed similar results in the mean changes on the primary and secondary efficacy measures, and they showed the same rates of responder and remitter. From baseline to end of treatment, the SIGH-SAD total score and atypical symptom subscores significantly reduced by 45.3% (p < .001) and 49.6% (p = .001), respectively. The changes in scores from baseline to end of treatment on the BDI-II, BAI, and ESQ were also significant by 78.8% (p < .0001), 80.7% (p < .0001), and 50.0% (p = .002), respectively, as well as on the SDS work, social, and family domains by 55.1% (p = .002), 53.1% (p = .001), and 45.8% (p = .014), respectively.
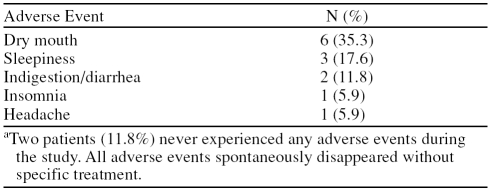
Adverse Events
Table 2 shows the adverse events reported by patients. Most of the treatment-emergent adverse events were mild to moderate in severity. Two subjects withdrew due to side effects (1 due to dry mouth and 1 due to diarrhea). No patients experienced any serious adverse events. Patients did not significantly gain or lose weight during the study (baseline, mean ± SD, 212.5 ± 85.0 lb; end of treatment, mean ± SD, 207.2 ± 77.6 lb; p = .880). Similarly, there were no significant differences in vital signs or laboratory parameters from baseline to end of treatment.
Table 2.
Treatment-Emergent Adverse Events During the Study (N = 17)a
DISCUSSION
This open-label study examined the clinical utility of escitalopram in the treatment of MDD with atypical features. On the primary endpoint of SIGH-SAD total score, there was an 18-point (53.8%) reduction from baseline to end of treatment. Furthermore, the atypical symptom subscale score showed a 4.9-point (44.5%) reduction, indicating that there was improvement in atypical as well as nonatypical depressive symptoms with escitalopram. There was a parallel improvement seen on the secondary efficacy measures that reflected self-reports of depression and anxiety, clinician ratings of improvement, and measures of sleepiness and functioning. This broad and consistent improvement across most measures indicates that escitalopram may have a potential role in treating people with MDD.
Of note, 62.5% of the subjects were classified as responders at end of treatment as defined by ≥ 50% reduction in SIGH-SAD total score, and 75.0% were considered responders based on CGI-I ratings. The CGI-based response rate in our study is comparable to the CGI-based response rates seen in randomized controlled clinical trials with fluoxetine (51%), imipramine (53%), phenelzine (61%–85%), sertraline (77.5%), and moclobemide (67.5%) in MDD with atypical features.3,15–18 Interestingly, the magnitude of improvement in primary outcome measure and responder rate was consistent with the results from registration clinical trials of escitalopram for MDD.36–38 In addition, our responders continued to maintain their response until the end of study, without fluctuation of the response. This finding may suggest that the effectiveness of escitalopram might be potentially consistent, although clear-cut conclusion could not be established since the duration of the study was only a short term. Hence, we need long-term trials of escitalopram for treating atypical depression.
The starting dose of escitalopram was 10 mg/day and the mean dose was 18 mg/day, similar to the doses reported in registration clinical trials for MDD.36–38 Currently available data do not support the benefit of 20 mg/day over 10 mg/day for patients with MDD, as noted in manufacturer's product information.39 Since our protocol did not employ fixed doses of escitalopram, we cannot comment whether lower doses than the mean dose (18 mg/day) may be equally efficacious. However, it may be clinically prudent to start with 10 mg/day of escitalo-pram and titrate the dose between 10 to 20 mg/day based on response and tolerability.
There were no major safety concerns in the present study. Overall, the drug was well tolerated. There were no serious adverse events, and 2 patients (11.8%) dropped out due to adverse events. It is worth noting that dry mouth was the most common adverse event in this trial, while nausea and insomnia were common adverse events in the pooled data of registration clinical trials of escitalopram for MDD. Most of the treatment-emergent adverse events were mild to moderate in severity and did not require any clinical intervention, a result that further supports the tolerability of escitalopram in this population.
The principal limitations of the present study were the small sample size, open-label design, and lack of placebo arm. We tried to alleviate the inherent limitation of open-label design by having a blinded interviewer rate the primary outcome measure (SIGH-SAD). However, without a placebo arm, the improvement seen in the present study cannot be definitively attributed to the study drug. Yet, given the robust and consistent response, the results support the need for a larger randomized placebo-controlled trial.
In conclusion, the present study indicates the potential benefit and tolerability of escitalopram in the treatment of MDD with atypical features and adds to the growing literature supporting the use of SSRIs in this depressive subtype. Adequately powered, randomized, double-blind, placebo-controlled trials are necessary to fully evaluate the efficacy and safety of escitalopram in this patient population. As described, the field is in need of such trials to evaluate the usefulness of SSRIs in atypical depression.
Drug names: bupropion (Wellbutrin and others), citalopram (Celexa and others), escitalopram (Lexapro and others), fluoxetine (Prozac and others), imipramine (Tofranil and others), mirtazapine (Remeron and others), phenelzine (Nardil), sertraline (Zoloft and others), venlafaxine (Effexor and others).
Financial disclosure: Dr. Pae has received research support and/or honoraria from GlaxoSmithKline Korea, GlaxoSmithKline, AstraZeneca Korea, Janssen Korea, Eli Lilly Korea, Korean Research Foundation, Otsuka Korea, Wyeth Korea, McNeil Consumer and Specialty, and Korean Institute of Science and Technology Evaluation and Planning and is on the speakers bureaus for GlaxoSmithKline Korea, Lundbeck Korea, AstraZeneca Korea, Janssen Korea, Eli Lilly Korea, and Otsuka Korea. Dr. Masand is a consultant to Bristol-Myers Squibb, Cephalon, Eli Lilly, Forest, GlaxoSmithKline, i3CME, Janssen, Jazz Pharmaceuticals, Organon, Pfizer, Targacept, and Wyeth; is on the speakers bureau for AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Forest, GlaxoSmithKline, Janssen, Pfizer, and Wyeth; and has received research support from AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, Forest, GlaxoSmithKline, Ortho-McNeil, Janssen, and Wyeth. Dr. Mannelli has received research support from National Institutes of Health, AstraZeneca, Bristol-Myers Squibb, Forest, GlaxoSmithKline, Janssen, McNeil Consumer and Specialty Inc., Organon, Jazz Pharmaceuticals, Reckitt Benckiser, and Pfizer. Dr. Han has received research support from Korea Research Foundation Grant (MOEHRD) (KRF-2007-013-E00033) and from Korea University Neuropsychiatric Alumni Grant. Dr. Marks is a consultant for Bristol-Myers Squibb; is on the speakers bureau of Eli Lilly; and has received research support from DOV Pharmaceutical, Endo, Janssen, GlaxoSmithKline, Pfizer, Bristol-Myers Squibb, Johnson & Johnson, Eli Lilly, Saegis Pharmaceuticals, Sepracor, and Somaxon. Dr. Patkar is an employee of Duke University; is a consultant to Bristol-Myers Squibb, Forest, GlaxoSmithKline, and Reckitt Benckiser; is on the speakers bureau for Bristol-Myers Squibb, Pfizer, Cephalon, GlaxoSmithKline, and Reckitt Benckiser; and has received research support from National Institutes of Health, AstraZeneca, Bristol-Myers Squibb, Forest, GlaxoSmithKline, Janssen, McNeil Consumer and Specialty, Organon, Jazz Pharmaceuticals, Titan, and Pfizer. Dr. Peindl reports no financial or other relationships related to the subject of this article.
Footnotes
This article was supported by Forest Laboratories, New York, New York, through an Investigator-Initiated Award.
Poster presented at the 160th annual meeting of the American Psychiatric Association; May 19–24, 2007; San Diego, Calif.
Financial disclosure appears at the end of this article.
REFERENCES
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association. 1994 [Google Scholar]
- Liebowitz MR, Quitkin FM, and Stewart JW. et al. Antidepressant specificity in atypical depression. Arch Gen Psychiatry. 1988 45:129–137. [DOI] [PubMed] [Google Scholar]
- Quitkin FM, McGrath PJ, and Stewart JW. et al. Atypical depression, panic attacks, and response to imipramine and phenelzine: a replication. Arch Gen Psychiatry. 1990 47:935–941. [DOI] [PubMed] [Google Scholar]
- Quitkin FM, Stewart JW, and McGrath PJ. et al. Columbia atypical depression. A subgroup of depressives with better response to MAOI than to tricyclic antidepressants or placebo. Br J Psychiatry. 1993 163suppl 21. 30–34. [PubMed] [Google Scholar]
- Stewart JW, Tricamo E, and McGrath PJ. et al. Prophylactic efficacy of phenelzine and imipramine in chronic atypical depression: likelihood of recurrence on discontinuation after 6 months' remission. Am J Psychiatry. 1997 154:31–36. [DOI] [PubMed] [Google Scholar]
- Quitkin FM, Harrison W, and Stewart JW. et al. Response to phenelzine and imipramine in placebo nonresponders with atypical depression: a new application of the crossover design. Arch Gen Psychiatry. 1991 48:319–323. [DOI] [PubMed] [Google Scholar]
- Stewart JW, McGrath PJ, and Rabkin JG. et al. Atypical depression: a valid clinical entity? Psychiatr Clin North Am. 1993 16:479–495. [PubMed] [Google Scholar]
- Lam RW, Stewart JN.. The validity of atypical depression in DSM-IV. Compr Psychiatry. 1996;37:375–383. doi: 10.1016/s0010-440x(96)90020-6. [DOI] [PubMed] [Google Scholar]
- Asnis GM, McGinn LK, Sanderson WC.. Atypical depression: clinical aspects and noradrenergic function. Am J Psychiatry. 1995;152:31–36. doi: 10.1176/ajp.152.1.31. [DOI] [PubMed] [Google Scholar]
- Quitkin FM, Rabkin JG, and Stewart JW. et al. Sleep of atypical depressives. J Affect Disord. 1985 8:61–67. [DOI] [PubMed] [Google Scholar]
- Parker GB. Atypical depression: a valid subtype? J Clin Psychiatry. 2007 68suppl 3. 18–22. [PubMed] [Google Scholar]
- Eaton WW, Kessler LG. Epidemiological Field Methods in Psychiatry: the NIMH Epidemiological Catchment Area Program. Orlando, Fla: Academic Press. 1985 [Google Scholar]
- Zisook S, Shuchter SR, and Gallagher T. et al. Atypical depression in an outpatient psychiatric population. Depression. 1993 1:268–274. [Google Scholar]
- Stewart JW, Thase ME.. Treating DSM-IV depression with atypical features. J Clin Psychiatry. 2007;68:e10. doi: 10.4088/jcp.0407e10. [DOI] [PubMed] [Google Scholar]
- Pande AC, Birkett M, and Fechner-Bates S. et al. Fluoxetine versus phenelzine in atypical depression. Biol Psychiatry. 1996 40:1017–1020. [DOI] [PubMed] [Google Scholar]
- Lonnqvist J, Sihvo S, and Syvalahti E. et al. Moclobemide and fluoxetine in atypical depression: a double-blind trial. J Affect Disord. 1994 32:169–177. [DOI] [PubMed] [Google Scholar]
- Søgaard J, Lane R, and Latimer P. et al. A 12-week study comparing moclobemide and sertraline in the treatment of outpatients with atypical depression. J Psychopharmacol. 1999 13:406–414. [DOI] [PubMed] [Google Scholar]
- McGrath PJ, Stewart JW, and Janal MN. et al. A placebo-controlled study of fluoxetine versus imipramine in the acute treatment of atypical depression. Am J Psychiatry. 2000 157:344–350. [DOI] [PubMed] [Google Scholar]
- Falkai P. Mirtazapine: other indications. J Clin Psychiatry. 1999 60suppl 17. 36–40.discussion 46–38. [PubMed] [Google Scholar]
- Rye DB, Dihenia B, Bliwise DL.. Reversal of atypical depression, sleepiness, and REM-sleep propensity in narcolepsy with bupropion. Depress Anxiety. 1998;7:92–95. [PubMed] [Google Scholar]
- McGrath PJ, Robinson D, Stewart JW.. Atypical panic attacks in major depression. Am J Psychiatry. 1985;142:1224. doi: 10.1176/ajp.142.10.1224a. [DOI] [PubMed] [Google Scholar]
- Forest Pharmaceuticals I. Prescribing information of escitalopram. Available at http://www.frx.com/pi/lexapro_pi.pdf. Accessed Dec 20, 2007. [Google Scholar]
- Bandelow B, Andersen HF, Dolberg OT.. Escitalopram in the treatment of anxiety symptoms associated with depression. Depress Anxiety. 2007;24:53–61. doi: 10.1002/da.20141. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Andersen HF, Lam RW.. Efficacy of escitalopram in the treatment of major depressive disorder compared with conventional selective serotonin reuptake inhibitors and venlafaxine XR: a meta-analysis. J Psychiatry Neurosci. 2006;31:122–131. [PMC free article] [PubMed] [Google Scholar]
- Kornstein SG, Bose A, and Li D. et al. Escitalopram maintenance treatment for prevention of recurrent depression: a randomized, placebo-controlled trial. J Clin Psychiatry. 2006 67:1767–1775. [DOI] [PubMed] [Google Scholar]
- Novick JS, Stewart JW, and Wisniewski SR. et al. Clinical and demographic features of atypical depression in outpatients with major depressive disorder: preliminary findings from STAR*D. J Clin Psychiatry. 2005 66:1002–1011. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, and Sheehan KH. et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998 59suppl 20. 22–33. [PubMed] [Google Scholar]
- Williams M, Link N, and Rosenthal L. et al. Structured Interview Guide for the Hamilton Depression Rating Scale-Seasonal Affective Disorder Version.Wilsonville, Ore. Society for Light Treatment and Biological Rhythms. 1988 [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology. US Dept Health, Education, and Welfare publication (ADM) 76–338. Rockville, Md: National Institute of Mental Health. 1976 [Google Scholar]
- Beck A, Steer R, and Brown G. Manual for Beck Depression Inventory II (BDI-II). San Antonio, Tex: Psychology Corporation. 1996 [Google Scholar]
- Beck AT, Epstein N, and Brown G. et al. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988 56:893–897. [DOI] [PubMed] [Google Scholar]
- Sheehan D. The Anxiety Disease. New York, NY: Scribner and Sons. 1983 [Google Scholar]
- Johns MW.. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Martiny K, Lunde M, and Simonsen C. et al. Relapse prevention by citalopram in SAD patients responding to 1 week of light therapy: a placebo-controlled study. Acta Psychiatr Scand. 2004 109:230–234. [DOI] [PubMed] [Google Scholar]
- Levine J, Schooler NR.. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull. 1986;22:343–381. [PubMed] [Google Scholar]
- Burke WJ, Gergel I, Bose A.. Fixed-dose trial of the single isomer SSRI escitalopram in depressed outpatients. J Clin Psychiatry. 2002;63:331–336. doi: 10.4088/jcp.v63n0410. [DOI] [PubMed] [Google Scholar]
- Wade A, Michael Lemming O, Bang Hedegaard K.. Escitalopram 10 mg/day is effective and well tolerated in a placebo-controlled study in depression in primary care. Int Clin Psychopharmacol. 2002;17:95–102. doi: 10.1097/00004850-200205000-00001. [DOI] [PubMed] [Google Scholar]
- Lepola UM, Loft H, Reines EH.. Escitalopram (10–20 mg/day) is effective and well tolerated in a placebo-controlled study in depression in primary care. Int Clin Psychopharmacol. 2003;18:211–217. doi: 10.1097/00004850-200307000-00003. [DOI] [PubMed] [Google Scholar]
- Lexapro [package, insert]. St. Louis, Mo: Forest Pharmaceuticals, Inc. 2002 [Google Scholar]