Abstract
Chemokines comprise a family of low-molecular-weight proteins that elicit a variety of biological responses including chemotaxis, intracellular Ca2+ mobilization, and activation of tyrosine kinase signaling cascades. A subset of chemokines, including regulated upon activation, normal T cell expressed and secreted (RANTES), macrophage inflammatory protein-1α (MIP-1α), and MIP-1β, also suppress infection by HIV-1. All of these activities are contingent on interactions between chemokines and cognate seven-transmembrane spanning, G protein-coupled receptors. However, these activities are strongly inhibited by glycanase treatment of receptor-expressing cells, indicating an additional dependence on surface glycosaminoglycans (GAG). To further investigate this dependence, we examined whether soluble GAG could reconstitute the biological activities of RANTES on glycanase-treated cells. Complexes formed between RANTES and a number of soluble GAG failed to induce intracellular Ca2+ mobilization on either glycanase-treated or untreated peripheral blood mononuclear cells and were unable to stimulate chemotaxis. In contrast, the same complexes demonstrated suppressive activity against macrophage tropic HIV-1. Complexes composed of 125I-labeled RANTES demonstrated saturable binding to glycanase-treated peripheral blood mononuclear cells, and such binding could be reversed partially by an anti-CCR5 antibody. These results suggest that soluble chemokine–GAG complexes represent seven-transmembrane ligands that do not activate receptors yet suppress HIV infection. Such complexes may be considered as therapeutic formulations for the treatment of HIV-1 infection.
Chemokines elicit chemotaxis of susceptible cells through the induction of signaling pathways that involve the mobilization of intracellular Ca2+ (1). These pathways are activated by interactions with seven-transmembrane (7-TM) spanning domain receptors that are coupled to G proteins in the cytoplasm. A number of these receptors also are used by HIV-1 for entry into CD4+ T cells (2–8). This interaction is blocked and infection is suppressed by natural ligands for these receptors (9–11) including the β-chemokines, regulated upon activation, normal T cell expressed and secreted (RANTES), macrophage inflammatory protein-1α (MIP-1α), MIP-1β (12), and macrophage-derived chemokine (11).
It is becoming increasingly apparent that the binding of chemokines to 7-TM receptors also must be accompanied by interactions with glycosaminoglycans (GAG) to achieve full biological activity. The importance of this interaction is illustrated by studies showing that chemokines bound to GAG on endothelial cell surfaces form concentration gradients that direct lymphocyte chemotaxis during inflammation (13–15) and by studies showing that soluble complexes of GAG and IL-8 are more potent chemoattractants than IL-8 alone (16). In the context of HIV-1 infection, it has been shown that RANTES becomes a more potent suppressor of macrophage-tropic (M-tropic) or dual-tropic HIV-1 infection after binding to cell-surface GAG (17, 18) and that the suppression is reversed by antibodies against the GAG-binding site of the chemokine (19). More recently, the ability of RANTES to suppress macrophage infection by HIV was shown to depend on the differential expression of certain cell-surface GAG (20). The importance of GAG in antiviral activity is suggested further by studies showing that RANTES, MIP-1α, and MIP-1β are secreted by cytotoxic T lymphocytes as complexes with GAG and that similar complexes of RANTES and heparan sulfate inhibit infection with M-tropic HIV-1 isolates much more efficiently than the free chemokine (18). In this report, we show that although soluble complexes of RANTES and several GAGs are potent suppressors of M-tropic HIV-1 isolates, they fail to stimulate intracellular Ca2+ mobilization and chemotaxis and, therefore, act as inhibitors of C—C chemokine receptors.
Materials and Methods
Cell Culture.
Peripheral blood mononuclear cells (PBMC) were obtained from healthy donors and collected in EDTA (K3) tubes (Vacutainer, Becton Dickinson). Cells were purified by density centrifugation over Lymphoprep (Becton Dickinson). PBMC then were activated with 5 μg/ml phytohemagglutinin (Sigma) and 20 units/ml recombinant human IL-2 (Boehringer Mannheim) for 72 hr. The cells then were washed and cultured in 20 units/ml IL-2. Medium was replenished every 2–3 days.
Calcium Mobilization.
Activated PBMC were analyzed for Ca2+ mobilization as described (19, 21) with the following modifications. Where indicated, PBMC were treated with glycanases to remove cell-surface GAG. Cells were incubated with 1 unit/ml each of heparinase II, heparinase III, and chondroitinase ABC (Sigma) for 1 hr at 37°C. As a control, untreated PBMC were incubated simultaneously in RPMI medium 1640 (Life Technologies, Gaithersburg, MD) supplemented with 10% FBS (Life Technologies) and 50 μg/ml gentamycin (Sigma), denoted hereafter as complete medium. After 1 hr the cells were washed with complete medium and then RPMI 1640 without phenol red or sodium bicarbonate, but with 25 mM Hepes (Life Technologies). Cells then were loaded with Fluo-3 (Molecular Probes) as described (19, 21). RANTES-GAG complexes were analyzed for activity in Ca2+ mobilization assays by using both enzyme-digested and untreated PBMC. The complexes were formed by incubating RANTES (9 μg/ml final concentration) with 1 mg/ml heparin (Sigma) or PBS for 1 hr at 4°C. The formulation then was diluted to bring the concentration of the RANTES component to 3 nM and then analyzed. Data were acquired by a FACSCalibur (Becton Dickinson Immunocytometry Systems) flow cytometer, gating cells by forward- and side-scatter properties. Ca2+ mobilization was determined by analysis in a two-parameter density plot, collecting linear emission at 530 nm in the FL-1 window over time.
Assays for Chemotaxis.
Assays were performed with HL-60 clone 15 cells (American Type Culture Collection) treated with 0.5 μM butyric acid for 48 hr followed by the addition of 10 ng/ml IL-5 to induce differentiation (22). Chemotaxis was measured as described (23, 24) with the following modifications. Six days after the addition of butyric acid and IL-5, cells were harvested and resuspended at a concentration of 1.6 × 106 cells/ml in RPMI medium 1640 containing 25 mM Hepes (Life Technologies) without phenol red or sodium bicarbonate (assay medium). Cells then were loaded with 0.1 μM Calcein AM (Molecular Probes) and an equivalent volume of Pluronic F-127 (Molecular Probes) at 37°C for 30 min. After loading, the cells were washed twice in assay medium and resuspended at a concentration of 4 × 106 cells/ml. Chemotaxis was measured in 96-well ChemoTx disposable chambers with 5-μm pores (Neuroprobe, Cabin John, MD) (25). RANTES, RANTES-GAG complexes, or PBS (total volume, 29 μl) was placed in the lower chamber and covered with a filter. A 25-μl drop containing 1 × 105 cells then was placed on the filter top. The chamber then was placed in a 37°C incubator for 6 hr. The fluorescence emission (26) in the lower chamber was measured at 517 nm by using a Victor fluorescence plate reader (Wallac, Gaithersburg, MD). The fluorescence values were converted to cell number based on a standard curve generated by staining serial concentrations of cells with 0.1 μM Calcein AM as above.
125I-RANTES Binding Assay.
PBMC were cultured as described above and harvested on day 6 for the competitive binding assay and day 10 for the mAb 2D7 blocking assay. Cells were incubated with 1 unit/ml each of heparinase II, heparinase III, and chondroitinase ABC (Sigma) for 1 hr at 37°C. Cells were washed once in complete medium and once in RPMI medium 1640 supplemented with 1% BSA. In the blocking assay, cells were incubated with 100 μg/ml of either mAb 2D7 (PharMingen) (27) or normal mouse polyclonal IgG (Sigma) in PBS supplemented with 0.1% sodium azide at 4°C for 1 hr. Cells then were washed once in RPMI 1640 supplemented with 1% BSA and treated with soluble chemokine–GAG complexes. The complexes were prepared by incubating 125I-RANTES (NEN) (0.5 nM) or nonradioactive RANTES (1 μM) with heparin (83 μM) (Sigma) for 1 hr at 37°C before addition to cells. Binding assays were performed as described (28, 29). Briefly, in saturation-binding assays, 3-fold serial dilutions of 125I-RANTES–heparin complexes (producing 10 nM to 0.03 nM of the RANTES component) were added to 2 × 106 cells for 1 hr at 37°C in triplicate reactions. In competitive binding assays, serial dilutions of either mAb 2D7 or unlabeled RANTES–heparin complexes (producing 100 nM to 0.01 nM of the RANTES component) were mixed with iodinated 125I-RANTES–heparin complexes (RANTES component at 0.3 nM) and then added to the cells. The reaction mixtures then were layered over 20% sucrose in PBS and the cells were pelleted by centrifugation. Cells then were washed once in PBS supplemented with 1% BSA. Heparin–125I-RANTES binding was determined by analyzing the cell pellets in a Wallac gamma counter. Background levels of binding were determined by incubating a 300 molar excess of RANTES-GAG with 125I-RANTES-GAG. Estimations of Kd and Bmax values were calculated by nonlinear regression analysis by using GraphPad prism 2.0b and www.graphpad.com (GraphPad, San Diego), respectively. The total counts added in each assay are as follows: Fig. 3A, 2,270,000 counts at 10 nM concentration; Fig. 3B, 70,000 counts; Fig. 4, 58,000 counts. In each case the specific activity was 2,200 Ci/mmol.
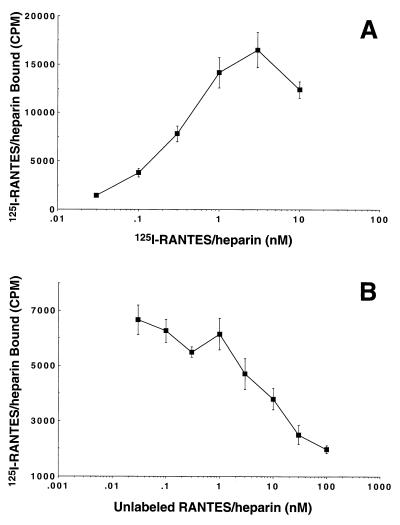
Figure 3.
Binding of 125I-RANTES–heparin complexes to glycanase-treated PBMC. Normal human PBMC were activated, harvested on day 6 of culture, and treated with glycanase mixture as described in Materials and Methods. (A) Binding of serial concentrations of 125I-RANTES complexes (0.03–10 nM of the chemokine component) to treated cells. (B) Competition binding of 0.3 nM 125I-RANTES complexes vs. serial concentrations of unlabeled RANTES complexes (0.01–100 nM chemokine component). Each data point represents the mean value of triplicate assays. SD values are shown with bars.
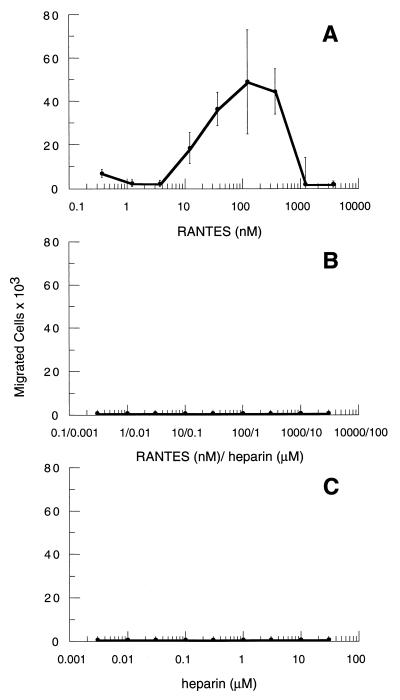
Figure 2.
RANTES–heparin complexes inhibit chemotaxis in differentiated HL-60 cells. HL-60 clone 15 cells were differentiated (see Materials and Methods) by the addition of butyric acid and IL-5. On day 7 of treatment the cells were harvested and assayed for chemotaxis in response to RANTES (A), RANTES–heparin complexes (B), or heparin alone (C). For these experiments, RANTES-GAG complexes were formed as described in the text by mixing RANTES (3 μM) with a 10-fold excess (30 μM) of clinical grade, endotoxin-free heparin (Fujisawa Pharmaceutical, Osaka). Serial concentrations of the complexes were tested in parallel with matching concentrations of either RANTES or heparin alone. Each assay condition was performed in triplicate. Each data point represents the mean value of triplicate assays. SD values are shown with bars.
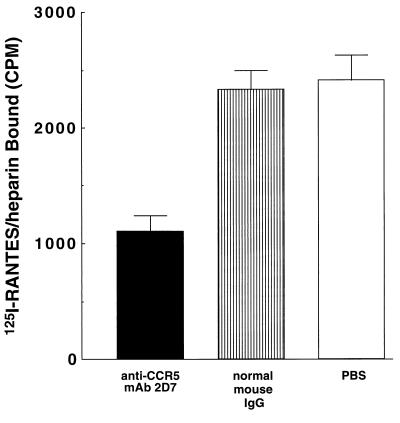
Figure 4.
Inhibition of 125I-RANTES complex binding to glycanase-treated PBMC by anti-CCR5 mAb 2D7. Normal human PBMC were activated as described previously and harvested on day 10 of culture to assay binding. Cells were treated with glycanase mixture for 1 hr. 125I-RANTES was incubated with heparin (1 mg/ml) for 1 hr at 4°C. 125I-RANTES complexes (0.3 nM RANTES component) were added to cells that had been treated with anti-CCR5 mAb 2D7 (10 μg/ml) (solid), normal mouse IgG (10 μg/ml) (striped), or PBS only (open). The values shown reflect the average of triplicate assays. SD values are shown with bars.
Infectivity Assay.
Infectivity assays were performed as described (19) with the following modifications. Activated PBMC were infected for 2 hr at 37°C with a primary, macrophage-tropic HIV-1 isolate, NSI.03 (30) at a ratio of 2 × 106 cells to 500 TCID50 (tissue culture 50% infective dose) in 5 ml of culture medium. Cells then were washed to remove virus and placed in tissue culture wells at a density of 2 × 105 cells in 250 μl. Complexes were formed by incubating RANTES (5 μg/ml final concentration) with 1 mg/ml of either heparin, heparan sulfate, chondroitin sulfate, or dermatan sulfate for 1 hr at 4°C to produce complex formulations containing 641 nM chemokine and 83 μM GAG. The resulting complexes then were serially diluted, and 250 μl was added to culture wells to achieve a total final assay volume of 500 μl. Control assays were carried out in parallel with sham formulations containing either RANTES or GAG alone at concentrations equal to the amounts present in the RANTES-GAG complex formulations. The cells were fed 3 days postinfection by removing 250 μl of medium and replacing with an equal volume of fresh medium containing the appropriate concentrations of RANTES, GAG, or RANTES-GAG complexes. Additional control assays were carried out with medium alone. Levels of infection were determined 6 days postinfection by measuring HIV-1 p24 levels by antigen-capture ELISA (Beckman Coulter).
Results
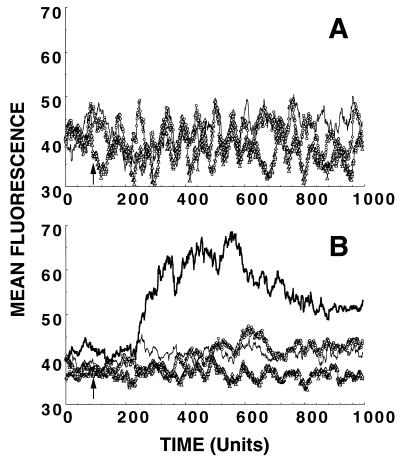
In previous studies (19) we found that the removal of GAG from cell surfaces by glycanase treatment abrogated the ability of RANTES to stimulate intracellular Ca2+ mobilization in phytohemagglutinin-activated PBMC. In the experiment shown in Fig. 1, we attempted to restore the signaling response in glycanase-treated cells by mixing chemokine with soluble GAG. RANTES (1 μM) was incubated with an excess of heparin (83 μM) for 1 hr at 4°C, thus forming RANTES-GAG complexes. The complexes then were assayed for the ability to stimulate intracellular Ca2+ mobilization (11, 19, 21) in PBMC previously treated with heparinase II, heparinase III, and chondroitinase ABC (Sigma) as described (19) to remove surface GAG. Control experiments also were carried out in parallel with untreated PBMC. As shown in Fig. 1A, no increase in intracellular Ca2+ was detected in the presence of the soluble RANTES–heparin complexes, demonstrating that they could not compensate for the absence of cell-surface GAG on the glycanase-treated cells. Moreover, the complexes also failed to stimulate Ca2+ mobilization in the control assays with untreated PBMC (Fig. 1B). Similar experiments carried out with complexes formed between RANTES and heparan sulfate, chondroitin sulfate, or dermatan sulfate produced the same results (data not shown); therefore, the lack of activity was not specific to heparin binding.
Figure 1.
RANTES–heparin complexes do not induce cytoplasmic Ca2+ mobilization in glycanase-treated or untreated PBMC. Normal PBMC were activated, as described in Materials and Methods, and harvested after 11 days in culture to measure the intracellular Ca2+ responses. RANTES-GAG complexes (○), then were analyzed with glycanase-treated (A) or untreated (B) PBMC. In control assays, glycanase-treated PBMC (A) were stimulated with heparin alone (▵) or with PBS (thin line). Untreated PBMC (B) were stimulated with RANTES–heparin complexes (○), heparin alone (▵), PBS (thin line), or RANTES (thick line). Fluorescence changes over time were measured by flow cytometry in the FL-1 window. One time unit is equal to 0.2 sec.
The chemotactic response to the RANTES–heparin complexes also was examined by using the promyelocytic cell line, HL-60 clone 15, after inducing expression of CCR1 and CCR3 by treatment with butyric acid and IL-5 (22). Experiments carried out with 125I-RANTES–heparin complexes demonstrated binding of the complexes to the treated HL-60 cells in a manner that was competitive with nonradioactive RANTES–heparin complexes (data not shown). As shown in Fig. 2, uncomplexed RANTES induced chemotaxis in the treated cells at concentrations between 1 μM and 10 nM. In contrast, the RANTES–heparin complexes failed to elicit chemotaxis at any concentration tested in spite of their ability to bind the cells. As expected, heparin alone also failed to stimulate chemotaxis.
Because the soluble RANTES-GAG complexes were unable to elicit intracellular Ca2+ signaling or chemotaxis, additional experiments were carried out to characterize in greater detail their interactions with the cell surface. As shown in Fig. 3, 125I-RANTES–heparin complexes demonstrated dose-dependent binding to glycanase-treated PBMC that was saturating at 1 nM (Fig. 3A) and was competitive with respect to nonradioactive RANTES–heparin complexes (Fig. 3B). Notably, 100% of the cells used in these experiments expressed CCR5 as determined by flow cytometric analyses with the anti-CCR5 mAb 2D7 (27) (data not shown). Based on these data, a Kd of 0.87 nM and a Bmax of approximately 560 binding sites per cell were calculated by nonlinear curve fitting after subtracting nonspecific binding. This Kd is in good agreement with values reported for the binding of RANTES to 7-TM receptors (31, 32) and suggested that RANTES-GAG complexes retain their ability to bind natural receptors on glycanase-treated PBMC surfaces. Additional competitive binding experiments carried out with L1.2 cells expressing recombinant human CCR5 (33) produced Kd values of 0.67 and 0.95 (data not shown) in accordance with the values obtained with PBMC.
To further evaluate the binding of the chemokine–GAG complexes to cell-surface receptors, glycanase-treated PBMC were incubated with an anti-CCR5 mAb 2D7, known to block RANTES interactions (27), and then assayed for binding to soluble 125I-RANTES–heparin complexes. As shown in Fig. 4, incubation of cells with mAb 2D7 reduced 125I-RANTES binding by approximately 40% relative to experiments carried out with an isotype control, suggesting that a portion of these complexes interact with cell-surface CCR5.
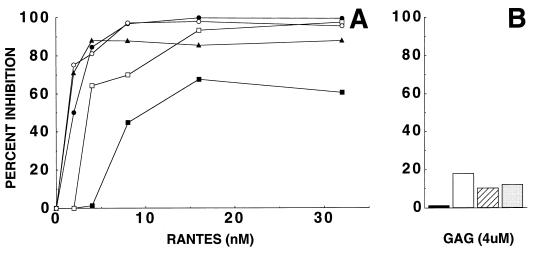
The ability of the complexes to bind HIV coreceptors also was tested in cell-free infectivity assays with PBMC and a primary M-tropic HIV-1 isolate, NSI.03, demonstrated previously to use CCR5 for viral entry (30). As shown in Fig. 5A, RANTES-GAG complexes suppressed infection by this isolate in accordance with the cell-surface binding data (Figs. 3 and 4). In contrast, sham formulations containing only GAG at the highest final concentration used to produce the complexes (4 μM) exhibited much lower levels (less than 20%) of virus inhibition (Fig. 5B), consistent with previous findings (34, 35). In additional experiments carried out with a panel of the primary macrophage-tropic isolates (BaL, NSI.03, and ADA-M), the antiviral activity of RANTES was consistently retained after complexing with GAG. Sensitivities to uncomplexed GAG alone was minor and variable (no more than 36% inhibition) at the highest concentration (250 nM heparan sulfate) used to prepare the complexes (data not shown).
Figure 5.
Inhibition of HIV-1 replication by RANTES-GAG complexes. PBMC were infected with HIV-1 NSI.03 and treated with RANTES, GAG, or RANTES-GAG complexes as described in Materials and Methods. (A) RANTES was tested after incubation with heparin (▴), heparan sulfate (●), chondroitin sulfate (□), or dermatan sulfate (○). Untreated RANTES was tested as control (■). The plots reflect the amount of the RANTES component present in each assay. (B) Results obtained with sham formulations containing only heparin (solid), heparan sulfate (open), chondroitin sulfate (striped), or dermatan sulfate (shaded) at a concentration matching the highest amount tested in the RANTES-GAG preparations (4 μM) are shown for comparison. Levels of infection determined 6 days postinfection by HIV-1 p24 antigen-capture ELISA are shown. Percent inhibition was determined for each assay relative to the control assays carried out in medium alone. The results obtained with RANTES, heparin, and RANTES plus heparin represent the mean of duplicate assays, whereas all other results represent the mean of triplicate assays.
Discussion
In a previous report we demonstrated that receptor activation by RANTES depends on cell-surface interactions between the C-terminal portion of the molecule and cell-surface GAG (19). Studies performed elsewhere (17) showed that GAG interactions are also necessary for the antiviral activity of this chemokine. The results presented here suggest a higher level of complexity in RANTES-GAG interactions, in that the form of GAG being engaged determines the activities of the chemokine. Whereas cell-surface GAGs appear to facilitate receptor activation, soluble GAGs do not (Fig. 1). The reason for this difference apparently is not related to a gross absence of 7-TM binding by the soluble RANTES-GAG complexes, because they suppressed infection by macrophage-tropic HIV-1 (Fig. 5) and exhibited binding that was competitive with respect to an anti-CCR5 mAb (Fig. 4). Because chemokines are thought to interact with coreceptors through multiple contact sites (36–42), it is possible that soluble GAG induce structural changes in RANTES that allow binding to the HIV-1 entry domain of CCR5 but prevent interactions with determinants for signaling activity. The antiviral activity of the RANTES-GAG complexes suggests that this putative structure either competes efficiently with the HIV envelope (33, 43) for coreceptor attachment or induces coreceptor down-regulation, which can occur in the absence of receptor activation (44, 45). In comparison, cell-surface GAGs may induce a different RANTES structure that facilitates binding to both antiviral and signaling domains. Although most of our data primarily address CCR5 interactions, the residual binding observed with glycanase-treated cells in the presence of mAb 2D7 (Fig. 4) suggests that the RANTES-GAG complexes also bind other RANTES receptors, such as CCR1, CCR3, and CCR4 (46–48), without stimulating Ca2+ mobilization. However, antibodies to these receptors that also compete for RANTES binding are not available currently, and so we could not accurately determine at this time whether the complexes engaged these receptors on PBMC.
The inability of the soluble complexes to activate receptors is particularly significant in light of evidence that RANTES is secreted from activated CD8+ T cells in the form of a large complex with other β chemokines and Granzyme A (18). Our results predict that, in this form, RANTES retains its antiviral activity but is unable to elicit signaling. The soluble chemokine–GAG complexes released by activated cytotoxic T lymphocytes therefore may comprise a natural family of chemokine inhibitors. One possible role of such complexes in normal immunological processes might be to limit the migration of cells toward effector sites after the local cytolytic killing of infected targets has begun. It is notable that the N-terminal proteolysis of chemokines by the dipeptidyl peptidase, CD26 (49–57), also generates receptor antagonists and, in the case of RANTES, results in the enhancement of antiviral activity (49, 51, 55, 57). These findings and our data collectively suggest that chemokine activities may be naturally regulated by several overlapping mechanisms of posttranslational modification.
Our results also suggest that GAG–chemokine complexes might be useful in the treatment or prevention of HIV-1 infection. This possibility is supported by three arguments. First, it is likely that the GAG–chemokine complexes can be administered with minimal risk of side effects because they are unable to signal via 7-TM receptors. Second, because they do not trigger cytoplasmic Ca2+ mobilization, the GAG–chemokine complexes should reduce the chances of chemokine-mediated enhancement of HIV-1 replication, which is primarily caused by receptor activation (58–62). Third, preformed GAG–chemokine complexes are less likely than unmodified chemokines to be deposited on extracellular matrices composed of GAG and, therefore, have a greater access to the cellular targets of HIV-1. Thus, chemokine–GAG complexes warrant examination as the basis for therapeutic formulations to treat HIV-1 infection.
Acknowledgments
We thank Dr. R. I. Connor (The Aaron Diamond AIDS Research Center, New York) for providing the viral isolate, NSI.03, Dr. L. Wu (Leukocyte, Inc., Cambridge, MA) for providing L1.2 CCR5 cells, and Mike Merges for performance of antiviral assays. This work was supported by National Institutes of Health Grant 1RO1 AI38192 (to G.K.L.) and by the Institute of Human Virology. J.M.B. is supported by National Institutes of Health Predoctoral Training Grant T32 AI-07540.
Abbreviations
- GAG
glycosaminoglycans
- 7-TM
seven-transmembrane
- RANTES
regulated upon activation, normal T cell expressed and secreted
- MIP-1α and MIP-1β
macrophage inflammatory protein-1α and -1β
- PBMC
peripheral blood mononuclear cells
References
- 1.Baggiolini M. Nature (London) 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 2.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, et al. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 4.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, et al. Nature (London) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 5.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 6.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y X, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, et al. Nature (London) 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 7.Feng Y, Broder C C, Kennedy P E, Berger E A. Science. 1996;272:872–877. [Google Scholar]
- 8.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, et al. Nature (London) 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 9.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. Nature (London) 1996;382:829–832. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 10.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J L, Arenzana-Seisdedos F, Schwartz O, Heard J M, Clark-Lewis I, Legler D F, et al. Nature (London) 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 11.Pal R, Garzino-Demo A, Markham P D, Burns J, Brown M, Gallo R C, DeVico A L. Science. 1997;278:695–698. doi: 10.1126/science.278.5338.695. [DOI] [PubMed] [Google Scholar]
- 12.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Science. 1996;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 13.Butcher E C. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka Y, Adams D H, Hubscher S, Hirano H, Siebenlist U, Shaw S. Nature (London) 1993;361:79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka Y, Kimata K, Wake A, Mine S, Morimoto I, Yamakawa N, Habuchi H, Ashikari S, Yamamoto H, Sakurai K, et al. J Exp Med. 1996;184:1987–1997. doi: 10.1084/jem.184.5.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webb L M, Ehrengruber M U, Clark-Lewis I, Baggiolini M, Rot A. Proc Natl Acad Sci USA. 1993;90:7158–7162. doi: 10.1073/pnas.90.15.7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oravecz T, Pall M, Wang J, Roderiquez G, Ditto M, Norcross M A. J Immunol. 1997;159:4587–4592. [PubMed] [Google Scholar]
- 18.Wagner L, Yang O O, Garcia-Zepeda E A, Ge Y, Kalams S A, Walker B D, Pasternack M S, Luster A D. Nature (London) 1998;391:908–911. doi: 10.1038/36129. [DOI] [PubMed] [Google Scholar]
- 19.Burns J M, Gallo R C, DeVico A L, Lewis G K. J Exp Med. 1998;188:1917–1927. doi: 10.1084/jem.188.10.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amzazi S, Ylisastigui L, Bakri Y, Rabehi L, Gattegno L, Parmentier M, Gluckman J C, Benjouad A. Virology. 1998;252:96–105. doi: 10.1006/viro.1998.9452. [DOI] [PubMed] [Google Scholar]
- 21.Burns J M, Lewis G K. BioTechniques. 1997;23:1022–1026. doi: 10.2144/97236bm11. [DOI] [PubMed] [Google Scholar]
- 22.Tiffany H L, Alkhatib G, Combadiere C, Berger E A, Murphy P M. J Immunol. 1998;160:1385–1392. [PubMed] [Google Scholar]
- 23.Martinet Y, Martinet N, Vignaud J M, Plenat F. J Immunol Methods. 1994;174:209–214. doi: 10.1016/0022-1759(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 24.Zaitseva M B, Lee S, Rabin R L, Tiffany H L, Farber J M, Peden K W, Murphy P M, Golding H. J Immunol. 1998;161:3103–3113. [PubMed] [Google Scholar]
- 25.Fevert C W, Wong V A, Goodman R B, Goodwin R, Martin T R. J Immunol Methods. 1998;213:41–52. doi: 10.1016/s0022-1759(98)00016-7. [DOI] [PubMed] [Google Scholar]
- 26.Denholm E M, Stankus G P. Cytometry. 1995;19:366–369. doi: 10.1002/cyto.990190412. [DOI] [PubMed] [Google Scholar]
- 27.Wu L, LaRosa G, Kassam N, Gordon C J, Heath H, Ruffing N, Chen H, Humblias J, Samson M, Parmentier M, et al. J Exp Med. 1997;186:1373–1381. doi: 10.1084/jem.186.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhmann S E, Platt E J, Kozak S L, Kabat D. J Virol. 1997;71:8642–8656. doi: 10.1128/jvi.71.11.8642-8656.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Platt E J, Wehrly K, Kuhmann S E, Chesebro B, Kabat D. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J M, McVicar D W, Oppenheim J J, Kelvin D J. J Exp Med. 1993;177:699–705. doi: 10.1084/jem.177.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Riper G, Siciliano S, Fischer P A, Meurer R, Springer M S, Rosen H. J Exp Med. 1993;177:851–856. doi: 10.1084/jem.177.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, et al. Nature (London) 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 34.Callahan L N, Phelan M, Mallinson M, Norcross M A. J Virol. 1991;65:1543–1550. doi: 10.1128/jvi.65.3.1543-1550.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roderiquez G, Oravecz T, Yanagishita M, Bou-Habib D C, Mostowski H, Norcross M A. J Virol. 1995;69:2233–2239. doi: 10.1128/jvi.69.4.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gayle R B, III, Sleath P R, Srinivason S, Birks S W, Weerawarna K S, Cerretti D P, Kozlosky C J, Nelson N, Vanden Bos T, Beckmann M P. J Biol Chem. 1993;268:7283–7289. [PubMed] [Google Scholar]
- 37.Siciliano S J, Rollins T E, DeMartine J, Konteatis Z, Malkowitz L, Van Riper G, Bondy S, Rosen H, Springer M S. Proc Natl Acad Sci USA. 1994;91:1214–1218. doi: 10.1073/pnas.91.4.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wells T N C, Proudfoot A E I, Power C A, Lusti-Narasimhan M, Alouani S, Hoogewerf A J, Peitsch M C. Methods Companion Methods Enzymol. 1996;10:126–134. doi: 10.1006/meth.1996.0086. [DOI] [PubMed] [Google Scholar]
- 39.Wu L, Ruffing N, Shi X, Newman W, Soler D, Mackay C R, Qin S. J Biol Chem. 1996;271:31202–31209. doi: 10.1074/jbc.271.49.31202. [DOI] [PubMed] [Google Scholar]
- 40.Proudfoot A E I, Power C A, Hoogewerf A J, Montjovent M O, Borlat F, Offord R E, Wells T N C. J Biol Chem. 1996;271:2599–2603. doi: 10.1074/jbc.271.5.2599. [DOI] [PubMed] [Google Scholar]
- 41.Pakianathan D R, Kuta E G, Artis D R, Skelton N J, Hebert C A. Biochemistry. 1997;36:9642–9648. doi: 10.1021/bi970593z. [DOI] [PubMed] [Google Scholar]
- 42.Simmons G, Clapham P R, Picard L, Offord R E, Posenkilde M M, Schwartz T W, Buser R, Wells T N C, Proudfoot A E I. Science. 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- 43.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. Nature (London) 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 44.Amara A, Gall S L, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier J L, Arenzana-Seisdedos F. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alkhatib G, Locati M, Kennedy P E, Murphy P M, Berger R A. Virology. 1997;234:340–348. doi: 10.1006/viro.1997.8673. [DOI] [PubMed] [Google Scholar]
- 46.Neote K, DiGregorio D, Mak J Y, Horuk R, Schall T J. Cell. 1993;72:415–425. doi: 10.1016/0092-8674(93)90118-a. [DOI] [PubMed] [Google Scholar]
- 47.Ponath P D, Qin S, Post T W, Wang J, Wu L, Gerard N P, Newman W, Gerard C, Mackay C R. J Exp Med. 1996;183:2437–2448. doi: 10.1084/jem.183.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Power C A, Meyer A, Nemeth K, Bacon K B, Hoogewerf A J, Proudfoot A E, Wells T N. J Biol Chem. 1995;270:19495–19500. doi: 10.1074/jbc.270.33.19495. [DOI] [PubMed] [Google Scholar]
- 49.Oravecz T, Pall M, Roderiquez G, Gorrel M D, Ditto M, Nguyen N Y, Boykins R, Unsworth E, Norcross M A. J Exp Med. 1997;186:1865–1872. doi: 10.1084/jem.186.11.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohtsuki T, Hosono O, Kobayashi H, Munakata Y, Souta A, Shioda T, Morimoto C. FEBS Lett. 1998;431:236–240. doi: 10.1016/s0014-5793(98)00763-7. [DOI] [PubMed] [Google Scholar]
- 51.Proost P, DeMeester I, Schols D, Struyf S, Lambeir A M, Wuyts A, Opdenakker G, DeClercq E, Scharpe S, VanDamme J. J Biol Chem. 1998;273:7222–7227. doi: 10.1074/jbc.273.13.7222. [DOI] [PubMed] [Google Scholar]
- 52.Proost P, Struyf S, Couvreur M, Lenaerts J P, Conings R, Menten P, Verhaert P, Wuyts A, Van Damme J. J Immunol. 1998;160:4034–4041. [PubMed] [Google Scholar]
- 53.Proost P, Struyf S, Schols D, Durinx C, Wuyts A, Lenaerts J P, De Clercq E, De Meester I, Van Damme J. FEBS Lett. 1998;432:73–76. doi: 10.1016/s0014-5793(98)00830-8. [DOI] [PubMed] [Google Scholar]
- 54.Shioda T, Kato H, Ohnishi Y, Tashiro K, Ikegawa M, Nakayama E E, Hu H, Kato A, Sakai Y, Liu H, et al. Proc Natl Acad Sci USA. 1998;95:6331–6336. doi: 10.1073/pnas.95.11.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Struyf S, De Meester I, Scharpe S, Lenaerts J P, Menten P, Wang J M, Proost P, Van Damme J. Eur J Immunol. 1997;28:1262–1271. doi: 10.1002/(SICI)1521-4141(199804)28:04<1262::AID-IMMU1262>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 56.Van Coillie E, Proost P, Van Aelst I, Struyf S, Polfliet M, De Meester I, Harvey D J, Van Damme J, Opdenakker G. Biochemistry. 1998;37:12672–12680. doi: 10.1021/bi980497d. [DOI] [PubMed] [Google Scholar]
- 57.Schols D, Proost P, Struyf S, Wuyts A, De Meester I, Scharpe S, Van Damme J, De Clercq E. Antiviral Res. 1998;39:175–187. doi: 10.1016/s0166-3542(98)00039-4. [DOI] [PubMed] [Google Scholar]
- 58.Kelley M D, Naif H M, Adams S L, Cunningham A L, Lloyd A R. J Immunol. 1998;160:3091–3095. [PubMed] [Google Scholar]
- 59.Schmidtmayerova H, Sherry B, Bukrinsky M. Nature (London) 1996;382:767. doi: 10.1038/382767a0. [DOI] [PubMed] [Google Scholar]
- 60.Kinter A, Catanzaro A, Monaco J, Ruiz M, Justement J, Moir S, Arthos J, Oliva A, Ehler L, Mizell S, et al. Proc Natl Acad Sci USA. 1998;95:11880–11885. doi: 10.1073/pnas.95.20.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dolei A, Biolchini A, Serra C, Curreli S, Gomes E, Dianzani F. AIDS. 1998;12:183–190. doi: 10.1097/00002030-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 62.Gordon C J, Muesing M A, Proudfoot A E, Power C A, Moore J P, Trkola A. J Virol. 1999;73:684–694. doi: 10.1128/jvi.73.1.684-694.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]