Abstract
Wolbachia pipientis is an endosymbiotic bacterium present in diverse insect species. Although it is well studied for its dramatic effects on host reproductive biology, little is known about its effects on other aspects of host biology, despite its presence in a wide array of host tissues. This study examined the effects of three Wolbachia strains on two different Drosophila species, using a laboratory performance assay for insect locomotion in response to olfactory cues. The results demonstrate that Wolbachia infection can have significant effects on host responsiveness that vary with respect to the Wolbachia strain-host species combination. The wRi strain, native to Drosophila simulans, increases the basal activity level of the host insect as well as its responsiveness to food cues. In contrast, the wMel strain and the virulent wMelPop strain, native to Drosophila melanogaster, cause slight decreases in responsiveness to food cues but do not alter basal activity levels in the host. Surprisingly, the virulent wMelPop strain has very little impact on host responsiveness in D. simulans. This novel strain-host relationship was artificially created previously by transinfection. These findings have implications for understanding the evolution and spread of Wolbachia infections in wild populations and for Wolbachia-based vector-borne disease control strategies currently being developed.
Infections with the endosymbiotic bacterium Wolbachia pipientis are best known for their capacity to cause reproductive manipulations in their insect hosts, including male killing, feminization, parthenogenesis, and most commonly, cytoplasmic incompatibility (CI). Since the microbe is maternally transmitted via the egg, each of these manipulations has the effect of assisting Wolbachia spread through host populations (28). The nature of the symbiotic association has justifiably led to the generation of a large body of knowledge that is gonad centric. Wolbachia bacteria, however, are not only present in the gonads but can be found throughout a diverse array of host organs and tissues, including nerve and muscle (7, 17, 24). The consequences of somatic tissue infection for hosts have not been explored fully (9, 11).
The impact of Wolbachia infection on host longevity represents one of the few nonreproductive phenotypes in the primary model for insects, i.e., Drosophila that have been well studied. In particular, the virulent Wolbachia strain wMelPop has been shown to reduce the life span of both its native host, Drosophila melanogaster, and artificially infected Drosophila simulans (21, 24, 30). This strain is presumed to shorten host life span by overreplicating to the point of host cell rupture in older adult flies. Other strains infecting D. melanogaster have shown milder effects, both positive and negative, on host longevity, but these have tended to be highly dependent on the host genetic background (10). Anecdotally, we have observed in the laboratory that old adults who are consequently heavily infected with wMelPop appear to be more sedentary and erratic in their motion. Reduced locomotory activity as the result of Wolbachia infection has previously been documented for the Drosophila parasitoid Leptopilina heterotoma (9).
The need to understand Wolbachia effects on host locomotion is twofold. First, documented effects of Wolbachia infection on Drosophila fitness are rare (12). The expression of CI is usually relied upon to explain the high infection prevalence of Wolbachia in wild populations (34, 35). This cannot explain the distribution of all strains, though. In species like D. melanogaster, the expression of CI is often weak and decreases with male age (29). The wAu strain present in Australian populations of D. simulans, for example, appears to cause no CI at all (13). With few exceptions (5, 37), standard laboratory and semifield measures of reproductive fitness (productivity, fecundity, etc.), as well as nontraditional measures such as responses to environmental stresses, have not revealed evidence of strong Wolbachia-conferred benefits for hosts (12, 14, 15, 27, 30). It is possible, however, that Wolbachia may alter more complex aspects of host biology in the field, which are not captured by traditional laboratory assays but could benefit infected hosts. Second, wMelPop infection is currently being developed for biological control. The virulence of this strain has placed it at the center of strategies aimed at shortening the life span of insect vectors of human diseases (2, 33). A clear understanding of the complete suite of effects that Wolbachia may have on hosts is necessary to determine if and how the microbe could be utilized for vector control in the field.
This study aimed to determine whether avirulent Wolbachia strains and the virulent, life-shortening wMelPop strain may affect complex behaviors in D. melanogaster and D. simulans. We compared the performance of Wolbachia-infected hosts to that of uninfected controls in a laboratory-based measure of olfactory-cued locomotion for a range of adult ages. Both olfaction and locomotion are critically important for finding food and mates and are therefore of likely ecological significance (38). We predicted that the benign Wolbachia strains native to D. melanogaster and D. simulans might negatively affect host capture performance, given previous evidence from Leptopilina heterotoma (9). We expected that the virulent wMelPop strain would have very detrimental consequences for its hosts, particularly as the insects aged and bacterial densities increased (24). In keeping with novel host theory (1), we also hypothesized that the effects of wMelPop might be more severe in D. simulans, given that this species was artificially infected (21).
MATERIALS AND METHODS
Fly strains and rearing.
The following three Wolbachia strains were compared in this study: wMel, wMelPop, and wRi. Both wMel (from yw67c23) and wMelPop (from w1118) were backcrossed into the common host genetic background Canton-S for 5 generations prior to experimentation. These host-Wolbachia combinations are denoted Dmel wMel and Dmel wMelPop, respectively. Dsim wRi represents a laboratory stock from the original Riverside, CA, population (16). Dsim wMelPop was generated ∼100 generations previously via transinfection of wMelPop into Dsim wRi hosts that had been tetracycline treated to remove the wRi infection (21, 22). All stocks have been in the laboratory for years and hence were highly inbred and homogeneous in nature. Uninfected controls were created for each of the above lines through standard procedures for tetracycline treatment (16). Large population sizes (>100 females) were treated to reduce drift effects during tetracycline treatment, and lines were reared for >3 generations posttreatment prior to experimentation (10). Introgression of infected and uninfected lines after tetracycline treatment could also have been employed but was not, for two reasons. First, the independently generated uninfected controls for each species did not perform differently from one another. Second, such a regimen dictated by Wolbachia crossing limitations would not homogenize the mitochondrial genome, which is likely to play a role in the phenotypes of interest here (18).
Flies were reared at 25°C with a 12-h light-dark regimen in large bottles containing 100 ml of standard cornmeal diet with active yeast sprinkled on the surface. Bottle populations were maintained at low densities (∼50 females per bottle, with one 8-h day of egg laying) to minimize any possible negative physiological consequences due to crowding. Newly eclosed flies were collected daily and reared to 5, 15, and 35 days of age for testing in capture arenas. Females were isolated from aged bottle populations and placed onto vials 2 days prior to behavioral assessment. Flies were starved for 19 h prior to assessment in 15-ml vials, which contained a layer of cotton wool soaked with distilled water to prevent desiccation.
Olfactory response assays.
We used a modified version of a behavioral response assay, the olfactory trap (6, 39), to score insect responses to an attractive olfactory cue. Traps consisted of a 2-ml glass vial into which we inserted a 0.2-ml Eppendorf pipette tip, with the narrow end trimmed off (Fig. 1). The narrow opening prevented flies from easily exiting the traps once they were captured. Traps were baited with 0.2 ml of Drosophila diet sprinkled with active dry yeast and were placed horizontally in 60-ml plastic wide-mouth jars covered with a square piece of muslin (held in place by a rubber band), which acted as testing arenas. Beginning 1 h after photophase commenced, starved flies were placed individually into arenas via aspiration and assessed for capture every 20 min for a period of 220 min. A total of 150 flies were assessed for each adult age-strain combination. Testing was carried out on three separate days for each treatment (50 flies each). Each fly was used only once to avoid any effects of learning (6, 19). To prevent odor contamination from previously used traps or Drosophila aggregation pheromones (38), arenas and muslin squares were washed and allowed to dry between subsequent experiments, and olfactory traps were discarded after each experiment. A parallel set of experiments (three replicates, with 50 flies each) using unbaited traps was carried out for all strains at 15 days of age to assess baseline activity. Log-rank tests (survival analysis) were used to compare capture performances of infected and uninfected controls for each adult age with the statistics software JMP 7.0 (SAS Institute, Inc.). The strength of this approach was in coding uncaptured flies in both baited and unbaited assays as censored. Individual log-rank comparisons were made between infected and uninfected lines for each host-strain-age combination for diet-baited capture assays. The degrees of freedom are therefore always equal to 1.0 (number of groups − 1). We employed a conservative critical rejection value (α) of 0.01.
FIG. 1.
Drosophila diet-baited trap (a) and capture arena containing a trap (b). After overnight starvation, individual flies representing the different host-strain combinations were placed inside capture arenas with either baited or unbaited traps. The time that individual flies took to enter the trap was recorded during a 220-min assay period.
RESULTS
Capture performance. (i) Baited traps.
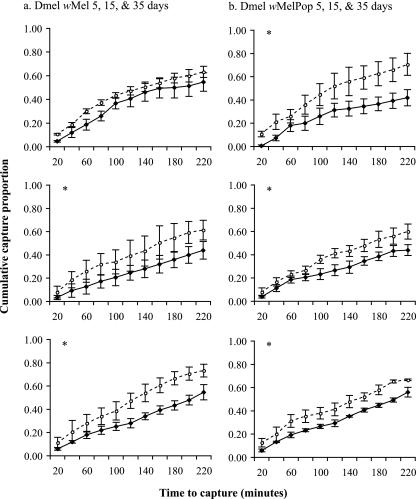
D. melanogaster flies infected with wMel performed slightly worse than uninfected controls at 15 and 35 days of age (for day 5, χ2 = 2.6 and P = 0.10; for day 15, χ2 = 9.6 and P = 0.0019*; and for day 35, χ2 = 12.2 and P = 0.0005*) (asterisks indicate significant differences) (Fig. 2a). The wMelPop strain decreased host performance at all three ages (for day 5, χ2 = 24.1 and P < 0.0001*; for day 15, χ2 = 7.8 and P = 0.0052*; and for day 35, χ2 = 8.0 and P = 0.0045*) (Fig. 2b). The largely parallel slopes of the cumulative capture curves also indicate that most of the differences in host performance can be attributed to the earliest time points (20 and 40 min). Additional direct comparisons between wMel- and wMelPop-infected flies for each age (for day 5, χ2 = 5.08 and P = 0.024; for day 15, χ2 = 0.017 and P = 0.89; and for day 35, χ2 = 0.61 and P = 0.43) indicated that the two strains did not differ with respect to their effects on the host. Comparisons between the two uninfected lines at each age also showed no differences (for day 5, χ2 = 0.91 and P = 0.33; for day 15, χ2 = 0.25 and P = 0.61; and for day 35, χ2 = 0.33 and P = 0.56), which was not surprising given the homogenization of host genetic background via backcrossing just prior to tetracycline treatment.
FIG. 2.
Cumulative mean proportion of D. melanogaster flies captured ± standard error of the mean (SEM) per 20-min period in baited traps. Means represent three replicate assay dates each, based on the performance of 50 individual flies. Infected (solid lines) and uninfected (dashed lines) flies were tested at each of three adult ages (5, 15, and 35 days). Significant differences between the performances of infected and uninfected flies for each adult age were determined by the log-rank test. *, P ≤ 0.01.
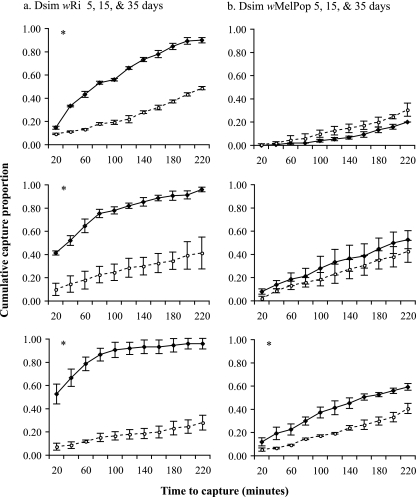
In contrast, the wRi strain in D. simulans dramatically increased the performance of flies at all adult ages (for day 5, χ2 = 177.1 and P < 0.0001*; for day 15, χ2 = 135.9 and P < 0.0001*; and for day 35, χ2 = 212.4 and P < 0.0001*) (Fig. 3a). The wMelPop strain did not alter the behavior of 5- and 15-day-old flies relative to that of controls (for day 5, χ2 = 5.2 and P = 0.021; and for day 15, χ2 = 4.06 and P = 0.043), but it did marginally improve the performance of 35-day-old flies (χ2 = 14.5 and P = 0.0001*) (Fig. 3b). Total capture of wRi-infected D. simulans ranged from 90 to 96% for the three adult ages, which was substantially greater than that for uninfected control flies, which ranged from 28 to 48%. Direct comparisons, as described above, between wRi- and wMelPop-infected D. simulans flies not surprisingly revealed significant differences at all adult ages (for day 5, χ2 = 167.7 and P < 0.0001*; for day 15, χ2 = 110.9 and P < 0.0001*: and for day 35, χ2 = 115.2 and P < 0.0001*). The two uninfected control D. simulans lines differed from one another only at 5 days of age (for day 5, χ2 = 15.6 and P < 0.0001*; for day 15, χ2 = 0.0064 and P = 0.93; and for day 35, χ2 = 4.8 and P = 0.027). These two host lines have a less recent shared origin (∼100 generations previous) than that of the D. melanogaster uninfected controls.
FIG. 3.
Cumulative mean proportion of D. simulans flies captured ± SEM per 20-min period in baited traps. Means represent three replicate assay dates, each based on the performance of 50 individual flies. Infected (solid lines) and uninfected (dashed lines) flies were tested at each of three adult ages (5, 15, and 35 days). Significant differences between performances of infected and uninfected flies for each adult age were determined by the log-rank test. *, P ≤ 0.01.
(ii) Unbaited traps.
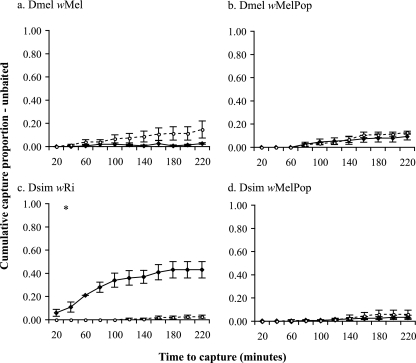
A set of assays (three replicates, with 50 flies each) were carried out at 15 days of age with unbaited traps to assess how much of the capture success was due to baseline activity of the flies rather than to olfactory-cued locomotion. Capture percentages were generally very low for all host-strain combinations (Fig. 4). D. melanogaster flies infected with wMel showed no difference in activity (χ2 = 0.55; P = 0.45) relative to controls (Fig. 4a). The activity levels of D. melanogaster (χ2 = 1.03; P = 0.30) and D. simulans (χ2 = 1.60; P = 0.20) were not altered by the wMelPop strain (Fig. 4b and d). Most surprisingly, the wRi strain significantly increased the D. simulans activity level (χ2 = 212.4; P < 0.0001*), with up to 48% of flies being captured, compared to only 2.7% of uninfected controls (Fig. 4c). This represents roughly half of the capture percentage obtained when traps were baited (Fig. 3a).
FIG. 4.
Cumulative mean proportion of D. melanogaster and D. simulans flies captured ± SEM per 20-min period in unbaited traps. Means represent three replicate assay dates, each based on 50 individual flies. Infected (solid lines) and uninfected (dashed lines) flies were tested at a single adult age (15 days). Significant differences between performances of infected and uninfected flies were determined by the log-rank test. *, P ≤ 0.01.
DISCUSSION
Our results show that Wolbachia infection may induce strain- and host species-specific changes in olfactory-cued locomotion in adult Drosophila flies that could influence the host's behavior in nature. The patterns of insect performance across our study system revealed several key points. In D. melanogaster, both wMel and wMelPop decrease host performance. More surprisingly, wMelPop-induced changes are not more severe than those induced by wMel, nor do they worsen with increasing host age. In D. simulans, the wRi strain enhances host performance. This increase includes a component of heightened baseline activity as well as greater responsiveness to food cues. The wMelPop strain has no effect in young flies of this species but improves host performance in old age.
While the direction and magnitude of Wolbachia's effects on responsiveness vary quite substantially between the two host species, such differences are not without precedent in these associations. D. simulans and D. melanogaster have already been shown to exhibit different levels of CI expression (23) and to harbor quite specific Wolbachia infection densities (22). Direct relationships between whole-insect Wolbachia levels and various infection-induced phenotypes, while satisfying in their simplicity, seem increasingly inadequate (3, 4, 25, 26, 36). Pilot measurements of bacterial density on whole flies did not reveal clear relationships with capture performance (unpublished data) for any of the host-strain combinations. Also, the capture performance of wMelPop-infected D. melanogaster flies did not worsen with age as predicted with the overreplication model (24). The traditionally higher densities of Wolbachia bacteria associated with D. simulans infections (22), if beneficial in terms of capture performance, could explain the trends for wRi and possibly for older flies infected with wMelPop. Targeted examination of densities in relevant tissues for the different species may reveal a more complex set of density models that are in fact predictive of host phenotypes.
The decreased responsiveness of D. melanogaster flies infected with Wolbachia could be due to greater energetic demands incurred by harboring the infection. A “cost” of Wolbachia has been documented for a few non-Drosophila species (5, 8, 9, 31). Sequencing of the wMel genome revealed that the microbe is well suited to direct uptake of amino acids from its environment and is incapable of synthesizing a number of metabolic intermediates (40). Both point to specific examples where the microbe is utilizing host resources. D. melanogaster may cope with such demands by limiting physical activity. Alternatively, the presence of Wolbachia in nervous or muscle tissue could have direct effects on the functioning of olfaction or locomotion (7, 24). The latter scenario could also explain the results for D. simulans if the two hosts possess different patterns of Wolbachia tissue tropism. Previous work with Drosophila has demonstrated that damage to the mushroom bodies can actually increase the insect's activity level (20). Heightened activity in D. simulans could also reflect a species-specific response to meet greater infection-associated energetic demands by seeking food more often.
The comparative effects of wMelPop on the different Drosophila species are of particular interest given current strategies being developed to use the strain to shorten the life span of the dengue fever vector Aedes aegypti (2, 32, 33). Surprisingly, the effects of wMelPop on D. melanogaster were no different from those of wMel. Additionally, wMelPop-transinfected D. simulans flies performed no differently from uninfected counterparts until 35 days of age. The wMelPop strain still confers shortened life spans in both of these host species (unpublished data) and hence confers some level of virulence. Since the popcorn effect is temperature dependent (24), however, wMelpop-induced behavioral changes should also be examined with higher insect-rearing temperatures. For transinfected mosquitoes, understanding the effects of wMelPop on insect locomotion and olfaction will take on particular importance with respect to vertebrate host seeking/blood feeding.
We do not wish to overinterpret the meaning of our results for insects in the field. The magnitude and repeatability of the capture performance phenotypes, at the very least, however, provide strong evidence for Wolbachia's ability to alter complex phenotypes beyond reproduction in Drosophila. The findings highlight a need to more closely examine Wolbachia's role in an expanded set of host behaviors, under both laboratory and field conditions, and to try to develop working mechanistic models for Wolbachia's local impact on host tissues and physiological processes.
Acknowledgments
We thank Sara Tromp for early assistance in developing methods, Myron Zalucki for helpful discussions regarding interpretation, and Scott O'Neill and several reviewers for comments on the manuscript.
This work was supported by Australian Research Council Discovery grant DP0557987 to E. A. McGraw.
Footnotes
Published ahead of print on 2 May 2008.
REFERENCES
- 1.Anderson, R. M., and R. M. May. 1982. Coevolution of hosts and parasites. Parasitology 85:411-426. [DOI] [PubMed] [Google Scholar]
- 2.Brownstein, J. S., E. Hett, and S. L. O'Neill. 2003. The potential of virulent Wolbachia to modulate disease transmission by insects. J. Invertebr. Pathol. 84:24-29. [DOI] [PubMed] [Google Scholar]
- 3.Clark, M. E., Z. Veneti, K. Bourtzis, and T. L. Karr. 2002. The distribution and proliferation of the intracellular bacteria Wolbachia during spermatogenesis in Drosophila. Mech. Dev. 111:3-15. [DOI] [PubMed] [Google Scholar]
- 4.Clark, M. E., Z. Veneti, K. Bourtzis, and T. L. Karr. 2003. Wolbachia distribution and cytoplasmic incompatibility during sperm development: the cyst as the basic cellular unit of CI expression. Mech. Dev. 120:185-198. [DOI] [PubMed] [Google Scholar]
- 5.de Crespigny, F. E., T. D. Pitt, and N. Wedell. 2006. Increased male mating rate in Drosophila is associated with Wolbachia infection. J. Evol. Biol. 19:1964-1972. [DOI] [PubMed] [Google Scholar]
- 6.Devaud, J. M. 2003. Experimental studies of adult Drosophila chemosensory behaviour. Behav. Processes 64:177-196. [DOI] [PubMed] [Google Scholar]
- 7.Dobson, S. L., K. Bourtzis, H. R. Braig, B. F. Jones, W. Zhou, F. Rousset, and S. L. O'Neill. 1999. Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem. Mol. Biol. 29:153-160. [DOI] [PubMed] [Google Scholar]
- 8.Duron, O., P. Labbe, C. Berticat, F. Rousset, S. Guillot, M. Raymond, and M. Weill. 2006. High Wolbachia density correlates with cost of infection for insecticide resistant Culex pipiens mosquitoes. Evolution 60:303-314. [PubMed] [Google Scholar]
- 9.Fleury, F., F. Vavre, N. Ris, P. Fouillet, and M. Bouletreau. 2000. Physiological cost induced by the maternally-transmitted endosymbiont Wolbachia in the Drosophila parasitoid Leptopilina heterotoma. Parasitology 121:493-500. [DOI] [PubMed] [Google Scholar]
- 10.Fry, A. J., M. R. Palmer, and D. M. Rand. 2004. Variable fitness effects of Wolbachia infection in Drosophila melanogaster. Heredity 93:379-389. [DOI] [PubMed] [Google Scholar]
- 11.Fytrou, A., P. G. Schofield, A. R. Kraaijeveld, and S. F. Hubbard. 2006. Wolbachia infection suppresses both host defence and parasitoid counter-defence. Proc. Biol. Sci. 273:791-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harcombe, W., and A. A. Hoffmann. 2004. Wolbachia effects in Drosophila melanogaster: in search of fitness benefits. J. Invertebr. Pathol. 87:45-50. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann, A. A., D. Clancy, and J. Duncan. 1996. Naturally-occurring Wolbachia infection in Drosophila simulans that does not cause cytoplasmic incompatibility. Heredity 76:1-8. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann, A. A., D. J. Clancy, and E. Merton. 1994. Cytoplasmic incompatibility in Australian populations of Drosophila melanogaster. Genetics 136:993-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann, A. A., M. Turelli, and L. G. Harshman. 1990. Factors affecting the distribution of cytoplasmic incompatibility in Drosophila simulans. Genetics 126:933-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann, A. A., M. Turelli, and G. M. Simmons. 1986. Unidirectional incompatibility between populations of Drosophila simulans. Evolution 40:692-701. [DOI] [PubMed] [Google Scholar]
- 17.Ijichi, N., N. Kondo, R. Matsumoto, M. Shimada, H. Ishikawa, and T. Fukatsu. 2002. Internal spatiotemporal population dynamics of infection with three Wolbachia strains in the adzuki bean beetle, Callosobruchus chinensis (Coleoptera: Bruchidae). Appl. Environ. Microbiol. 68:4074-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katewa, S. D., and J. W. Ballard. 2007. Sympatric Drosophila simulans flies with distinct mtDNA show difference in mitochondrial respiration and electron transport. Insect Biochem. Mol. Biol. 37:213-222. [DOI] [PubMed] [Google Scholar]
- 19.Kim, Y. C., H. G. Lee, and K. A. Han. 2007. Classical reward conditioning in Drosophila melanogaster. Genes Brain Behav. 6:201-207. [DOI] [PubMed] [Google Scholar]
- 20.Martin, J. R., R. Ernst, and M. Heisenberg. 1998. Mushroom bodies suppress locomotor activity in Drosophila melanogaster. Learn. Mem. 5:179-191. [PMC free article] [PubMed] [Google Scholar]
- 21.McGraw, E. A., D. J. Merritt, J. N. Droller, and S. L. O'Neill. 2002. Wolbachia density and virulence attenuation after transfer into a novel host. Proc. Natl. Acad. Sci. USA 99:2918-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGraw, E. A., D. J. Merritt, J. N. Droller, and S. L. O'Neill. 2001. Wolbachia-mediated sperm modification is dependent on the host genotype in Drosophila. Proc. R. Soc. Lond. B 268:2565-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mercot, H., and S. Charlat. 2004. Wolbachia infections in Drosophila melanogaster and D. simulans: polymorphism and levels of cytoplasmic incompatibility. Genetica 120:51-59. [DOI] [PubMed] [Google Scholar]
- 24.Min, K., and S. Benzer. 1997. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc. Natl. Acad. Sci. USA 94:10792-10796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mouton, L., F. Dedeine, H. Henri, M. Bouletreau, N. Profizi, and F. Vavre. 2004. Virulence, multiple infections and regulation of symbiotic population in the Wolbachia-Asobara tabida symbiosis. Genetics 168:181-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mouton, L., H. Henri, M. Bouletreau, and F. Vavre. 2005. Multiple infections and diversity of cytoplasmic incompatibility in a haplodiploid species. Heredity 94:187-192. [DOI] [PubMed] [Google Scholar]
- 27.Olsen, K., K. T. Reynolds, and A. A. Hoffmann. 2001. A field cage test of the effects of the endosymbiont Wolbachia on Drosophila melanogaster. Heredity 86:731-737. [DOI] [PubMed] [Google Scholar]
- 28.O'Neill, S. L., A. A. Hoffmann, and J. H. Werren (ed.). 1997. Influential passengers: inherited microorganisms and arthropod reproduction. Oxford University Press, Oxford, United Kingdom.
- 29.Reynolds, K. T., and A. A. Hoffmann. 2002. Male age, host effects and the weak expression or non-expression of cytoplasmic incompatibility in Drosophila strains infected by maternally transmitted Wolbachia. Genet. Res. 80:79-87. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds, K. T., L. J. Thomson, and A. A. Hoffmann. 2003. The effects of host age, host nuclear background and temperature on phenotypic effects of the virulent Wolbachia strain popcorn in Drosophila melanogaster. Genetics 164:1027-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rigaud, T., and J. Moreau. 2004. A cost of Wolbachia-induced sex reversal and female-biased sex ratios: decrease in female fertility after sperm depletion in a terrestrial isopod. Proc. Biol. Sci. 271:1941-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinkins, S. P., and F. Gould. 2006. Gene drive systems for insect disease vectors. Nat. Rev. Genet. 7:427-435. [DOI] [PubMed] [Google Scholar]
- 33.Sinkins, S. P., and S. L. O'Neill. 2000. Wolbachia as a vehicle to modify insect populations, p. 271-288. In A. M. Handler and A. A. James (ed.), Insect transgenesis methods and applications. CRC Press, New York, NY.
- 34.Turelli, M., and A. A. Hoffmann. 1995. Cytoplasmic incompatibility in Drosophila simulans: dynamics and parameter estimates from natural populations. Genetics 140:1319-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turelli, M., and A. A. Hoffmann. 1991. Rapid spread of an inherited incompatibility factor in California Drosophila. Nature 353:440-442. [DOI] [PubMed] [Google Scholar]
- 36.Veneti, Z., M. E. Clark, S. Zabalou, T. L. Karr, C. Savakis, and K. Bourtzis. 2003. Cytoplasmic incompatibility and sperm cyst infection in different Drosophila-Wolbachia associations. Genetics 164:545-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weeks, A. R., M. Turelli, W. R. Harcombe, K. T. Reynolds, and A. A. Hoffmann. 2007. From parasite to mutualist: rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biol. 5:e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wertheim, B., R. Allemand, L. E. Vet, and M. Dicke. 2006. Effects of aggregation pheromone on individual behaviour and food web interactions: a field study on Drosophila. Ecol. Entomol. 31:216-226. [Google Scholar]
- 39.Woodard, C., T. Huang, H. Sun, S. L. Helfand, and J. Carlson. 1989. Genetic analysis of olfactory behavior in Drosophila: a new screen yields the ota mutants. Genetics 123:315-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu, M., L. V. Sun, J. Vamathevan, M. Riegler, R. Deboy, J. C. Brownlie, E. A. McGraw, W. Martin, C. Esser, N. Ahmadinejad, C. Wiegand, R. Madupu, M. J. Beanan, L. M. Brinkac, S. C. Daugherty, A. S. Durkin, J. F. Kolonay, W. C. Nelson, Y. Mohamoud, P. Lee, K. Berry, M. B. Young, T. Utterback, J. Weidman, W. C. Nierman, I. T. Paulsen, K. E. Nelson, H. Tettelin, S. L. O'Neill, and J. A. Eisen. 2004. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biol. 2:e69. [DOI] [PMC free article] [PubMed] [Google Scholar]