Abstract
Iron (Fe) is a critical element in all aerobic organisms as it participates in a variety of metabolic networks. In this study, aluminum (Al) and gallium (Ga), two Fe mimetics, severely impeded the ability of the soil microbe Pseudomonas fluorescens to perform oxidative phosphorylation. This was achieved by disrupting the activity and expression of complexes I, II, and IV. These toxic metals also inactivated aconitase (ACN) and fumarase A (FUM A), two tricarboxylic acid cycle enzymes dependent on Fe for their catalytic activity, while FUM C, an Fe-independent enzyme, displayed an increase in activity and expression under these stressed situations. Furthermore, in the Al- and Ga-exposed cells, the activity and expression of an H2O-forming NADH oxidase were markedly increased. The incubation of the Al- and Ga-challenged cells in an Fe-containing medium led to the recovery of the affected enzymatic activities. Taken together, these data provide novel insights into how environmental pollutants such as Al and Ga interfere with cellular Fe metabolism and also illustrate the ability of Pseudomonas fluorescens to modulate metabolic networks to combat this situation.
Organisms in O2-enriched environments rely on Fe for the production of ATP via aerobic respiration. In this process, catabolic substrates are systematically oxidized by the concerted action of the tricarboxylic acid (TCA) cycle enzymes to produce NADH and reduced flavin adenine dinucleotide, two electron-rich moieties that drive the synthesis of ATP during oxidative phosphorylation (26). The favorable free-energy change during electron transfer through the respiratory complexes is coupled to the transport of protons across the membrane to produce the necessary proton motive force that powers the synthesis of ATP. This movement of electrons is dependent on several Fe-containing moieties. Thus, aerobic respiration requires a steady supply of Fe. Within the TCA cycle, the activities of aconitase (ACN), fumarase (FUM), and succinate dehydrogenase (SDH) are ineffective without Fe-S clusters (18).
Although Fe is vital for aerobic respiration, it may become toxic if it is not well guarded. Labile Fe is an important generator of reactive oxygen species (ROS). Hence, cells have developed an intricate surveillance system to cope with this Fe dilemma (25). For instance, Fe-deprived conditions are known to trigger the production of siderophores (5). The uptake of the Fe-siderophore complex is achieved by specific receptors which facilitate the transport of the Fe-rich molecules. In order to avoid the toxicity of Fe overload, bacteria are known to express Fe-binding proteins, such as bacterioferritin, that play a pivotal role in modulating the intracellular levels of this transition metal (30). Thus, these regulatory networks enable bacteria to maintain intracellular Fe concentration within a nontoxic range.
It has been documented that ROS readily perturb Fe homeostasis (24). Fe-dependent enzymes which participate in the O2-dependent generation of ATP have been shown to be quite sensitive to oxidative stress. Most notably, the Fe-S clusters in ACN and FUM A have been known to be disrupted quite readily by ROS, a situation that renders the TCA cycle dysfunctional (12). Hence, a vicious cycle may result from the ROS-mediated disruption of Fe-proteins followed by the participation of this redox-active metal in Fenton reactions. Toxic metals such as Ga and Al have also been implicated in the disruption of Fe-proteins (22, 36). Because these two trivalent metals have atomic properties similar to those of Fe, they readily compete for Fe binding sites in proteins (3). Al has been widely regarded as a toxic mimetic of Fe. A variety of Fe-dependent proteins are known to be affected by the presence of Al (18). In fact, the ability of Al to disrupt aerobic metabolism has been suggested as a possible cause of such pathological conditions as Alzheimer's disease and obesity (7, 16).
Pollution, industrial activity, and anthropogenic sources have increased the bioavailability of these two trivalent metals, and their impact on the environment is a major concern. As part of our study to identify the molecular mechanisms that enable microorganisms to survive in a toxic environment, we have investigated the metabolic shift triggered by Al and Ga, respectively, in the soil microbe Pseudomonas fluorescens. Here we show that Al and Ga severely alter the activity and expression of several Fe-dependent TCA cycle and electron transport chain (ETC) enzymes. In an attempt to maintain minimal TCA cycle flux, the metal-exposed cells upregulate FUM C, a FUM isozyme which operates in the absence of Fe. In addition, the enhanced activity of an H2O-forming NADH oxidase (NOX) in Al- and Ga-treated cells appeared to be pivotal to the survival of this microbe when subjected to Al and Ga toxicity. Fe appeared to reverse the toxic effects of these metals.
MATERIALS AND METHODS
Microbial growth conditions and isolation of cellular fractions.
The bacterial strain Pseudomonas fluorescens 13525 was obtained from the American Type Culture Collection (ATCC) and grown in a mineral medium containing, per liter of deionized water, Na2HPO4 (6.0 g), KH2PO4 (3.0 g), NH4Cl (0.8 g), MgSO4·7H2O (0.2 g), and citric acid (4 g). Trace elements were present in concentrations as previously described (1). The pH was adjusted to 6.8 with dilute NaOH. The media were dispensed in 200-ml amounts in 500-ml Erlenmeyer flasks. For metal exposure, the test metals (AlCl3, 15 mM; GaNO3, 1 mM; and FeCl3, 1 mM) were complexed with 19 mM citrate and dispensed in 200-ml aliquots. Dose-response assays were performed on cells exposed to 19 mM citrate chelated to 0.1 to 15 mM Al. The media were inoculated with 1 ml of stationary-phase cells grown in a medium unamended with test metals in an aerated gyratory water bath shaker (model 76; New Brunswick Scientific) at 26°C and 140 rpm. The bacterial cells were harvested at 24 h for control cultures, 28 h for Al-stressed cells, and 70 h for Ga-stressed cells and then suspended in a cell storage buffer consisting of 50 mM Tris-HCl, 5 mM MgCl2, and 1 mM phenylmethylsulfonyl fluoride, pH 7.3 (cells were isolated at these times unless otherwise indicated as they correspond to the same growth phase). The cells were disrupted by sonication and centrifuged at 3,000 × g for 30 min at 4°C to remove intact bacteria. Centrifugation at 180,000 × g for 2 h afforded a soluble cell extract (CFE) and a membrane CFE. The soluble fraction was further centrifuged at 180,000 × g for 1 h to afford a membrane-free system. The protein content in the soluble and membrane fractions was determined using the Bradford assay (27). These CFE fractions were kept at 4°C for up to 5 days, and various enzymatic activities were monitored.
Monitoring of Fe-S cluster stability and Fe concentrations.
Fe-S cluster stability was assessed by scanning 5 mg/ml of protein equivalent of the membrane fraction from control, Al-stressed, and Ga-stressed cultures. An absorption band between 395 and 420 nm, attributed to the Fe-S cluster, was monitored (4). The levels of Fe in the membrane and soluble CFE were measured using the ferrozine assay as described previously (33). Four milligrams of protein equivalent of the membrane fractions from control and Al- and Ga-stressed cells was utilized. The Fe complexes were monitored at 562 nm (ɛ = 27, 900 M−1 cm−1). Negative reactions were performed in the absence of substrate or chromophore.
Measurement of H2O2 formation by NOX and activities of ACN.
The production of H2O2 by NOX was ascertained using the method described previously (35), in which 0.2-mg equivalents of the membrane fractions from control and Al- and Ga-stressed cultures were utilized and the oxidation of p-anisidine by H2O2 was monitored at 458 nm (ɛ = 1.173 M−1 cm−1). The consumption of NADH was measured at 340 nm. Control reactions were performed in the absence of NADH. ACN activity was monitored by following the formation of cis-aconitate at 240 nm (ɛ = 3,600 M−1 liter−1) (20). The soluble fractions (400 μg of protein equivalent) were incubated at room temperature in a 2-ml reaction mixture containing reaction buffer (25 mM Tris-HCl [pH 7.4] and 5 mM MgCl2), 10 mM citrate, and 20 mM malonate. Negative reactions were performed in the absence of substrate or chromophore.
BN-PAGE and in-gel activity analysis.
Blue native-polyacrylamide gel electrophoresis (BN-PAGE) was performed as described by Beriault et al. and Schagger and von Jagow with some modifications (2, 29). Cellular fractions isolated from P. fluorescens were prepared in a native buffer (50 mM bis-Tris, 500 mM ɛ-aminocaproic acid, pH 7.0, 4°C) at a final concentration of 4 mg of protein per ml. For the membrane CFE, 1% (vol/vol) β-dodecyl-d-maltoside was included in the preparation in order to solubilize the membrane-bound protein. Once the running front was halfway through the gel, the blue cathode buffer (50 mM Tricine, 15 mM bis-Tris, 0.02% (wt/vol) Coomassie G-250 [pH 7] at 4°C) was changed to a colorless cathode buffer (50 mM Tricine, 15 mM bis-Tris [pH 7] at 4°C). Upon completion, the gel slab was removed from the apparatus and equilibrated for 15 min in a reaction buffer (25 mM Tris-HCl [pH 7.4], 5 mM MgCl2). Enzyme activity was visualized by coupling the enzymatic formation of NAD(P)H to 0.3 mg/ml of phenazine methosulfate (PMS) and 0.5 mg/ml of iodonitrotetrazolium (INT). ACN activity was visualized using a reaction buffer consisting of 5 mM citrate, 0.5 mM NADP, 5 units/ml isocitrate dehydrogenase (Sigma), PMS, and INT. SDH activity was made apparent using 10 mM succinate, 5 mM KCN, PMS, and INT. The in-gel activity of FUM A and C was ascertained in fashion similar to that for ACN. The in-gel detection of FUM A and C was achieved using reaction buffer containing 5 mM fumarate, 5 units/ml malate dehydrogenase, 0.5 mM NAD, PMS, and INT. Since FUM A and C have different mobilities, these two isozymes can be easily identified (10). Complex I and NADH oxidase were detected simultaneously using 0.5 mM NADH, 5 mM KCN, and INT (28). Control reactions for complex I were also performed with 40 μM rotenone. Complex IV was measured by coupling the activity of this respiratory complex to the oxidative polymerization of diaminobenzidene (18). NAD kinase (NADK) activity was measured using an enzyme-coupled assay (19). NADP phosphatase (NADPase) activity was ascertained using a reaction buffer consisting of 5 mM malate, 0.5 mM NADP, 10 units/ml of malate dehydrogenase, PMS, and INT. Reactions were stopped once the bands had achieved their desired intensity using destaining solution (50%, vol/vol, methanol, 10%, vol/vol, acetic acid). Gels were documented and quantified using Scion imaging for Windows. All reactions were performed in triplicate. Proper loading was assured by staining gels with Coomassie R-250. Negative reactions were performed in the absence of substrate. Two-dimensional (2D) BN-PAGE analysis of ACN activity was performed as described above. Bands corresponding to ACN were excised and loaded into the wells of a 10 to 16% native gel. The bands were then electrophoresed using native conditions as described above. Activity was detected as described previously (20).
2D SDS-PAGE and immunoblotting.
Sodium dodecyl sulfate (SDS)-PAGE and 2D SDS-PAGE were performed according to the modified method described previously (2, 13). For SDS-PAGE, 30 μg of protein was solubilized in 62.5 mM Tris-HCl (pH 6.8), 2% SDS, and 2% β-mercaptoethanol at 100°C for 5 min. Following solubilization the protein samples were loaded into a 10% isocratic gel and electrophoresed using a discontinuous buffer system. Following electrophoresis, the proteins were electroblotted to a Hybond-polyvinylidene difluoride membrane for immunoblotting (20). Visualization of the immunoblot was documented via a ChemiDoc XRS system (Bio-Rad Imaging Systems). The ACN antibody was a generous gift from R. A. Eisenstein (University of Wisconsin—Madison). For 2D SDS-PAGE analysis of protein levels, activity bands from native gels were precision cut from the gel and incubated in denaturing buffer (1% β-mercaptoethanol, 5% SDS) for 30 min and then loaded vertically into the SDS gel. Electrophoresis was carried out as described above. Proteins were detected using a silver staining kit purchased from Bio-Rad.
Influence of Fe on Al and Ga toxicity.
In order to evaluate the impact of Al and Ga on the activities of FUM A, FUM C, complex I, and H2O-forming NOX, we performed regulation experiments. Ten-milligram protein equivalents from control and Al- and Ga-treated cells were incubated in a phosphate medium containing 1 mM Fe complexed to citrate (final concentration of citrate was 20 mM). Following a 24-h exposure to the Fe-containing medium, the cells were isolated and fractionated as described above. The cell fractions were then treated accordingly for BN-PAGE analysis of enzyme activity. Control and Al- and Ga-stressed cells were isolated at 24 h, 28 h, and 70 h, respectively.
Metabolite analysis.
The levels of fumarate, citrate, ATP, and ADP were ascertained by high-performance liquid chromatography (HPLC) as described previously (15, 16). Two-milligram protein equivalents from the soluble fractions from cells grown in control, Al-stressed (15 mM Al), and Ga-stressed (1 mM) conditions for 24 h, 28 h, and 70 h were treated with 0.5% (vol/vol) perchloric acid to remove the protein. The precipitate was removed, and the supernatant was filtered and injected into an Alliance high-performance liquid chromatograph equipped with a C18 reverse-phase column (Phenomenex).
Statistical analysis.
Data were expressed as means ± standard deviations (SD). Statistical correlations of data were checked for significance using the Student t test. Experiments were performed twice and in triplicate.
RESULTS
Perturbation of Fe-containing enzymes.
P. fluorescens was challenged with Al (15 mM) for 28 h or Ga (1 mM) for 70 h in order to assess the impact of these toxic metals on Fe homeostasis. A three- to fourfold decrease in Fe in the membrane fraction was recorded in cells treated with Al or Ga compared to control (Al, 194.14 ± 91.96 nmol/mg of protein; Ga, 197.13 ± 96.92 nmol/mg of protein; control, 758.66 ± 133.01 nmol/mg of protein). In contrast, the soluble fractions from the Al- and Ga-treated cultures had at least twofold more Fe than the control (Al, 657.11 ± 96.95 nmol/mg of protein; Ga, 558.54 ± 154.51 nmol/mg of protein; control, 328.55 ± 140.06 nmol/mg of protein). The membrane extracts from the Al- and Ga-treated cells also displayed a sharp decrease in the absorbance at 395 to 420 nm, a fingerprint of Fe-S cluster integrity, while the control had a prominent peak in this region.
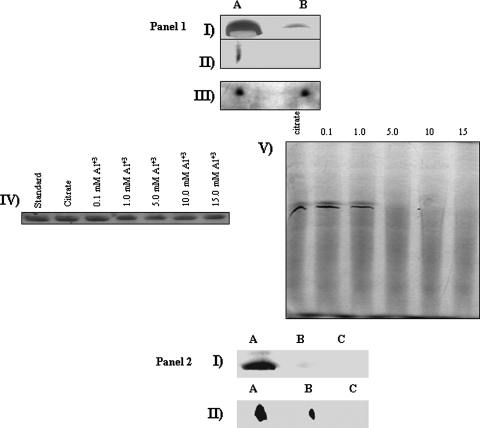
ACN displayed a sharp decrease in activity in the Al- and Ga-stressed medium. However, a strong recovery was observed in the presence of Fe. Specific activities (means ± SD) for growth media containing citrate, Al-citrate, Ga-citrate, and Al-citrate with Fe were 0.124 ± 0.012, 0.018 ± 0.016, 0.038 ± 0.001, and 0.107 ± 0.011 μg/min·mg of protein, respectively (n = 3, P ≤ 0.05). These data are consistent with previous reports showing that toxic trivalent metals such as Al can deactivate ACN by displacing bioavailable Fe (20). The severely diminished activity of ACN was further demonstrated by BN-PAGE (Fig. 1, panel 1, I to V). However, 2D SDS-PAGE analysis revealed that the low ACN activity was not a consequence of decreased protein levels. Immunoblotting for ACN in cells exposed to various concentrations of Al confirmed these observations. Since the presence of Al inhibited the activity of ACN, the sensitivity of this protein to Al was monitored. The exposure to 1 mM Al produced no discernible change in ACN activity in comparison to control. However, the inclusion of 5 mM Al resulted in the complete loss of ACN activity. A similar trend was observed with Ga. No activity was observed at 70 h of incubation (data not shown). These results were confirmed by HPLC analysis, as the Al- and Ga-stressed cultures consumed citrate at a much slower rate than the control (data not shown). SDH, another Fe-S-containing enzyme, experienced a sharp drop in activity when cells were exposed to either Al or Ga. As shown in Fig. 1, panel 2, no activity bands for SDH were recorded in cells exposed to 15 mM Al for 28 h and 1 mM Ga for 70 h. Unlike ACN, SDH displayed a sharp decrease in protein levels following Al or Ga exposure (Fig. 1, panel 2).
FIG. 1.
In-gel detection of ACN and SDH activities in P. fluorescens exposed to control (lanes A) and Al-stressed (lanes B) conditions. (1, I) In-gel detection of ACN in control (lane A) and Al-stressed (lane B) cells. (II) 2D BN-PAGE for ACN activity. Activity bands were excised and electrophoresed under native conditions. (III) 2D SDS-PAGE for ACN expression. (IV) Immunodetection of ACN in P. fluorescens exposed to various concentrations of Al. Standard corresponds to 10 μg of ACN from porcine heart (Sigma). (V) In-gel detection of ACN in P. fluorescens exposed to various concentrations of Al. (2, I) In-gel detection of SDH in P. fluorescens exposed to control (lane A), Al stress (lane B), or Ga stress (lane C) conditions. Control and Al- and Ga-stressed cells were isolated at similar growth phases. Cells were exposed to 15 mM Al or 1 mM Ga unless otherwise indicated. (II) 2D SDS-PAGE analysis of the activity bands from subpanel I. The bands were excised and electrophoresed as described previously. Protein levels were ascertained by silver staining.
Upregulation of FUM C and downregulation of FUM A.
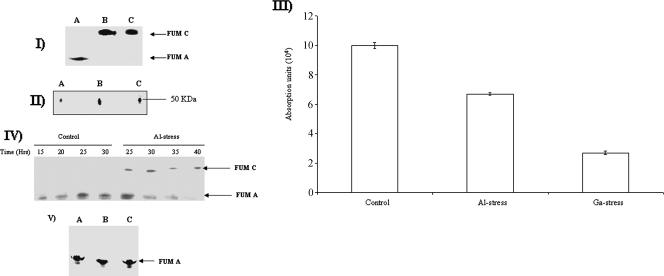
The ability of Al and Ga to disrupt Fe-S cluster-containing enzymes prompted us to monitor the activity and expression of FUM A. This TCA cycle enzyme requires Fe-S clusters for the hydration of fumarate to malate. As shown in Fig. 2I, the presence of 15 mM Al or 1 mM Ga in the culture medium completely abolished the activity of FUM A. Interestingly, a second activity band was detected in the Al- and Ga-treated cells. This band corresponded to FUM C, a FUM isozyme capable of metabolizing fumarate in an Fe-independent fashion. Indeed, the mobility of this band corresponded to that reported in the literature (10) (note that this band was only faintly evident in the control cells after long exposure times). More protein was associated with these activity bands than was seen for the control (Fig. 2II). HPLC analysis revealed that the Al- and Ga-challenged cells metabolized fumarate more readily than the control cells (Fig. 2III). The activity of FUM C was detected in Al-treated cells exposed for a 25-h period. The relative intensity of this activity band remained consistent for the 40-h exposure to Al-citrate (Fig. 2IV). In contrast, the FUM C band was not detected in control cells incubated for as long as 30 h. In addition, the time profile analysis revealed the sensitivity of FUM A to the presence of Al. Indeed, the activity of FUM A was completely abolished following a 30-h exposure to Al (Fig. 2IV). When 1 mM Fe was included in the cultures with Al or Ga, FUM A was fully recovered. No band attributed to FUM C was evident (Fig. 2V).
FIG. 2.
In-gel detection of FUM A and C activity in P. fluorescens exposed to control (lanes A), Al stress (lanes B), or Ga stress (lanes C) conditions. The concentrations of Al and Ga used were 15 mM and 1 mM. (I) In-gel detection of FUM A and C activity. (II) 2D BN-PAGE analysis of the FUM C activity bands from panel I. Proteins were electrophoresed and detected by silver staining. Control and Al- and Ga-stressed cells were isolated at similar growth phases. (III) HPLC analysis of the fumarate levels. Cells were isolated at 24 h for control, 28 h for Al stress, and 70 h for Ga stress and subsequently treated with 0.5% perchloric acid for HPLC injection. The peak corresponding to fumarate was quantified using EMPOWER software. Injections were performed in triplicate (n = 3). Values are means ± SD (P ≤ 0.05). (IV) Time-dependent profile of FUM A and FUM C activity in cells exposed to 15 mM Al. (V) Activity staining for FUM C in control (lane A), Al-stressed (lane B), and Ga-stressed (lane C) cells exposed to a culture medium containing 1 mM Fe. Ten-milligram equivalents of protein from control, Al-stressed, and Ga-stressed cells were exposed to an Fe-citrate medium for 24 h and then isolated for analysis.
Aerobic respiration during Al and Ga toxicity.
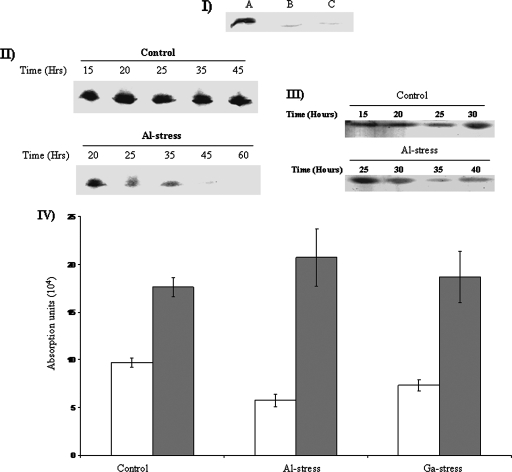
Cells exposed to 15 mM Al or 1 mM Ga for 28 h and 70 h, respectively, displayed a severe loss in complex I activity (Fig. 3I). Time profile experiments revealed a decline in complex I after 25 h of incubation in Al-stressed cells (Fig. 3II). A maximal deficiency was observed in cells exposed to 10 to 15 mM Al (data not shown). Complex IV was also perturbed. Exposure to Al for more than 30 h severely limited the activity of complex IV (Fig. 3III). The drastic changes in these complexes had a negative influence on the aerobic production of ATP. Marked reductions in ATP production were observed in the Al- and Ga-treated cells (Fig. 3IV).
FIG. 3.
(I) In-gel detection of complex I activity in P. fluorescens exposed to control (lane A), Al-stressed (lane B), and Ga-stressed (lane C) conditions. Control, Al-stressed, and Ga-stressed cells were isolated at similar growth phases. Cells were exposed to 15 mM Al or 1 mM Ga. (II) Time-dependent profile of complex I activity in cells exposed to 15 mM Al. (III) Time-dependent profile of complex IV activity in cells exposed to 15 mM Al. The activity of complex IV was visualized with the aid of diaminobenzidene. (IV) HPLC analysis of the nucleotide levels. Cells were isolated at 24 h for control, 28 h for Al stress, and 70 h for Ga stress and subsequently treated with 0.5% perchloric acid for HPLC injection. The peaks corresponding to ATP (white bars) and ADP (gray bars) were quantified using EMPOWER software. Injections were performed in triplicate (n = 3). Values are means ± SD (P ≤ 0.05).
NOX was upregulated in the Al- and Ga-treated cells.
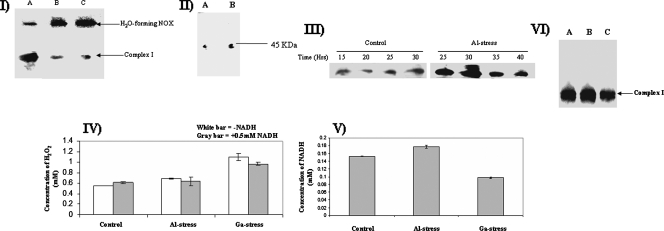
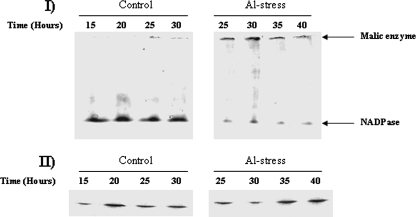
As the ETC was severely inhibited by the presence of Al or Ga, it became important to evaluate if any other strategy was initiated to oxidize NADH in the metal-stressed cells. During the detection of complex I activity, a higher-activity band was observed due to the oxidation of NADH. This band appeared to be more prominent in the metal-stressed cells. We hypothesized that the higher activity might be an alternative oxidase. The membrane fractions isolated from P. fluorescens exposed to 15 mM Al or 1 mM Ga displayed an activity band at 180 kDa more intense that that displayed by the control (Fig. 4I). To confirm that the higher-activity band was a NOX, 2D SDS-PAGE and silver staining were performed on the higher bands. Indeed, 45-kDa subunits were observed following 2D SDS-PAGE analysis (Fig. 4II). NOX has been shown previously to be a homotetramer comprising 45-kDa subunits (11). The activity of NOX was also not sensitive to rotenone (data not shown). Time profile analysis of NOX activity revealed that this alternative oxidase was immediately induced following Al exposure (Fig. 4III). In order to determine if the NOX observed in the metal-exposed cells was an H2O-forming enzyme, we evaluated the ability of these cells to produce H2O2 from NADH. Although the Ga-treated cells produced more H2O2 than the control, there was no significant increase in H2O2 production when membrane extracts were incubated in the absence or presence of NADH (Fig. 4IV). In addition, NADH was readily consumed by the membrane fractions from the control and Al- and Ga-treated cells despite sharp decreases in complex I activity (Fig. 4V). Thus, these data indicate that an H2O-forming NOX plays an important role in the adaptation of P. fluorescens to Al and Ga toxicity. When Fe was included in the metal-challenged cultures, the activity of the H2O-forming NOX was abolished (Fig. 4VI). Furthermore, Fe supplementation recovered the activity of complex I in the Al- and Ga-exposed cells. As NAD and NADP homeostasis is key to the survival of P. fluorescens in Al- and Ga-treated cultures, the activities of NADK and NADPase were monitored. A dearth of NADPase activity was recorded in the Al-stressed cultures (Fig. 5I). In sharp contrast, NADK was sharply increased (Fig. 5II).
FIG. 4.
Analysis of NOX activity. (I) In-gel detection of NOX activity in P. fluorescens exposed to control (lane A), Al-stressed (lane B), or Ga-stressed (lane C) conditions. (II) 2D SDS-PAGE analysis of NOX expression. Activity bands from panel I were excised (control and Al stress bands) and electrophoresed under denaturing conditions. Protein levels were detected by silver staining. Cells were isolated at similar growth phases for NOX analysis. Control, Al-stressed, and Ga-stressed cells were isolated at similar growth phases. Cells were exposed to 15 mM Al or 1 mM Ga. (III) Time-dependent profile of NOX activity in cells exposed to 15 mM Al. (IV) Measurement of H2O2 formation by NOX. Protein (0.2 mg) from membrane CFE isolated from control, Al-stressed, Ga-stressed cells was incubated for 15 min in a reaction buffer in the presence or absence of 0.5 mM NADH. Peroxide formation was detected using p-anisidine. -NADH, no NADH. Values are means ± SD (n = 3; P ≤ 0.05). (V) Measurement of NADH levels. Protein (0.2 mg) from membrane CFE from control, Al-stressed, and Ga-stressed cells was incubated for 15 min in a reaction buffer containing 0.5 mM NADH (n = 3). Values are means ± SD (P ≤ 0.05). (VI) Activity staining for NOX and complex I in control (lane A), Al-stressed (lane B), and Ga-stressed (lane C) cells exposed to a culture medium containing 1 mM Fe. Ten-milligram equivalents of protein from control, Al-stressed, and Ga-stressed cells were exposed to an Fe-citrate medium for 24 h and then isolated for analysis.
FIG. 5.
Analysis of NADPase and NADK activity. (I) Time-dependent profile of NADPase activity. (II) Time-dependent profile of NADK activity.
DISCUSSION
The foregoing data show the pivotal role that FUM C and H2O-forming NOX play in the adaptation of Pseudomonas fluorescens to the toxicity of Al and Ga. The toxic impact of both Al and Ga was demonstrated by the ability of these two metals to perturb several Fe-dependent enzymes in the TCA cycle and the ETC. In the present study, the inhibitory influence of Al and Ga on aerobic energy metabolism was evident in the activity of ACN, an enzyme which requires an Fe-S cluster to isomerize citrate to isocitrate. The negative impact of Al and Ga on ACN activity was further illustrated by the slower metabolism of citrate. Since the structural properties of Al and Ga are similar to those of Fe, these toxic metals can readily compete for Fe binding sites in enzymes. Indeed, the exposure of P. fluorescens to these two toxic metals severely reduced the amount of bioavailable Fe in the cell. Furthermore, other toxic metals such as Ni and Zn have been shown to interfere with ACN activity by perturbing the Fe-S cluster (6, 14). The ability of Al and Ga to perturb ACN activity may also be due to their pro-oxidant capabilities. ROS molecules have been implicated in disrupting the activity of ACN by dismantling the Fe-S cluster (8). In addition, we have recently shown that both Al and Ga can promote oxidative stress in P. fluorescens (3, 31). Thus, Al and Ga may produce an oxidative and Fe-starved environment, leading to a decrease in ACN activity. In the present study, Al and Ga perturbed the activity but not the expression of ACN. This may be due to the fact that ACN may have other functions in the cell (9).
FUM A and SDH, two TCA cycle enzymes that depend on Fe-S clusters for their enzymatic activity, were also affected by these toxic metals. In contrast to ACN, both Al and Ga severely reduced the biosyntheses of these two enzymes. Indeed, the expression of both SDH and FUM A has been shown to be markedly diminished in Fe-deprived environments (21). The negative influence of Al and Ga on aerobic metabolism was demonstrated further by the reduced levels of ATP in the metal-exposed cells. We have previously demonstrated that Al interferes with the aerobic ATP-producing machinery, resulting in a severe reduction in cellular energy levels (16). Furthermore, oxidative stress has been implicated in damaging the respiratory complexes, rendering the O2-dependent ATP-producing machinery ineffective.
Recent work has shown that, although Al and Ga impede the action of several TCA cycle enzymes such as NAD-isocitrate dehydrogenase and α-ketoglutarate dehydrogenase, P. fluorescens survives by counteracting the toxic effects of these metals. Indeed, this microbe increases the activity of the glyoxylate shunt enzyme isocitrate lyase in order to detoxify Al and maintain TCA cycle flux (20). In the present study, Al- and Ga-treated cells resorted to FUM C, a FUM isozyme capable of hydrating fumarate without the aid of Fe-S clusters. FUM C has been shown to be critical in maintaining TCA cycle flux during Fe depletion and oxidative stress (23). Indeed, this enzyme may compensate for the decrease in the activity of both FUM A and SDH by ensuring that the TCA cycle remains active. Furthermore, the activation of an isozyme of FUM devoid of Fe confers a unique strategy to combat Al and Ga toxicity. It is interesting to note that an Fe-free FUM can perform as an enzyme while ACN devoid of Fe behaves as a sensor for oxygen tension and Fe levels. Hence, it is not surprising that, in this microbial system, FUM C was upregulated while no similar isozyme of ACN was detected (34). This adaptation involving FUM C would allow the cells to compensate for the ineffectiveness of SDH and enable ACN to perform its oxygen-sensing role. In the presence of Fe, only FUM A was expressed.
The pro-oxidant attributes of Al and Ga are derived from their ability to increase the labile-Fe pool. Labile Fe can participate in the formation of ROS since this redox-active metal can donate and accept electrons. Previous work in our laboratory has shown that P. fluorescens cells exposed to either Al or Ga upregulate several antioxidant enzymes such as catalase and superoxide dismutase in order to cope with the pro-oxidant properties of these metals (3, 31). In addition, enzymes involved in the production of NADPH display a generous increase in activity following exposure to either Al or Ga (17). In fact, NADPH availability is the underlying force which promotes antioxidant defense and ROS detoxification. We have reported recently that NADK and NADPase are increased and decreased, respectively, in activity following oxidative stress in order to provide enough NADP for antioxidant defense (32). In the present study, complexes I and IV appeared to be severely affected by Al and Ga, thus sharply diminishing oxidative phosphorylation. Interestingly, this scenario would expose the cells to less ROS. However, it is essential that the NADH, albeit in smaller amounts in the metal-stressed cells, be oxidized. In this study, it appeared that the organism invoked the participation of an H2O-forming NOX to ensure its survival. This would allow for the generation of NAD to propel degradative metabolic reactions and alleviate the generation of ROS. Indeed, the Al- and Ga-challenged cells consumed NADH without a significant increase in the generation of ROS. In addition, the oxidation of NADH by this H2O-forming alternative oxidase would regenerate NAD, which can be used to prime metabolic reactions or be phosphorylated to produce NADP for antioxidant defense. The increase and decrease in the activities of NADK and NADPase, respectively, observed in the Al-treated cells would shift the metabolic balance in favor of a reductive environment, a key ingredient for the survival of the organism in conditions of limited Fe availability, metal toxicity, and oxidative stress.
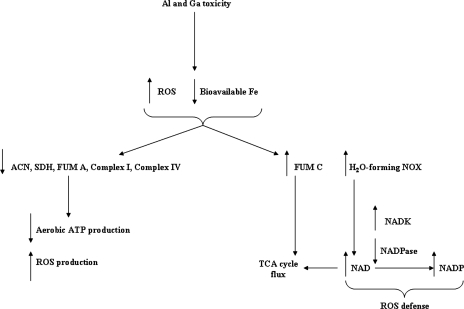
In conclusion, this study provides a novel insight into part of a global metabolic response invoked by Pseudomonas fluorescens to adapt to the toxic effects of the environmental pollutants Al and Ga. These two toxic metals perturbed the activity of several Fe-dependent enzymes involved in aerobic metabolism such as ACN, SDH, FUM A, complex I, and complex IV. In an effort to adapt to this situation, the microbe promotes the syntheses of a FUM (FUM C) devoid of Fe and an NOX that can oxidize NADH without producing ROS. These enzymes were absent in media supplemented with Fe. Furthermore, the modulation of NADK and NADPase helps maintain a reductive environment. Figure 6 depicts a scheme of the metabolic adaptation evoked by these two metal pollutants. Hence, FUM C, H2O-forming NOX, and NADK confer a unique metabolic strategy to combat the deleterious effects of Al and Ga.
FIG. 6.
FUM C, H2O-forming NOX, NADK, and NADPase play a key role in the adaptive response of P. fluorescens to Al- and Ga-induced bioavailable-Fe deprivation.
Acknowledgments
This work was funded in part by grants from NATO and Northern Heritage Fund.
Footnotes
Published ahead of print on 9 May 2008.
REFERENCES
- 1.Anderson, S., V. D. Appanna, J. Huang, and T. Viswanatha. 1992. A novel role for calcite in calcium homeostasis. FEBS Lett. 308:94-96. [DOI] [PubMed] [Google Scholar]
- 2.Beriault, R., D. Chenier, R. Singh, J. Middaugh, R. Mailloux, and V. Appanna. 2005. Detection and purification of glucose 6-phosphate dehydrogenase, malic enzyme, and NADP-dependent isocitrate dehydrogenase by blue native polyacrylamide gel electrophoresis. Electrophoresis 26:2892-2897. [DOI] [PubMed] [Google Scholar]
- 3.Beriault, R., R. Hamel, D. Chenier, R. J. Mailloux, H. Joly, and V. D. Appanna. 2007. The overexpression of NADPH-producing enzymes counters the oxidative stress evoked by gallium, an iron mimetic. Biometals 20:165-176. [DOI] [PubMed] [Google Scholar]
- 4.Berndt, C., C. H. Lillig, M. Wollenberg, E. Bill, M. C. Mansilla, D. de Mendoza, A. Seidler, and J. D. Schwenn. 2004. Characterization and reconstitution of a 4Fe-4S adenylyl sulfate/phosphoadenylyl sulfate reductase from Bacillus subtilis. J. Biol. Chem. 279:7850-7855. [DOI] [PubMed] [Google Scholar]
- 5.Chakraborty, R., E. Storey, and D. van der Helm. 2007. Molecular mechanism of ferricsiderophore passage through the outer membrane receptor proteins of Escherichia coli. Biometals 20:263-274. [DOI] [PubMed] [Google Scholar]
- 6.Chen, H., T. Davidson, S. Singleton, M. D. Garrick, and M. Costa. 2005. Nickel decreases cellular iron level and converts cytosolic aconitase to iron-regulatory protein 1 in A549 cells. Toxicol. Appl. Pharmacol. 206:275-287. [DOI] [PubMed] [Google Scholar]
- 7.Exley, C., and M. M. Esiri. 2006. Severe cerebral congophilic angiopathy coincident with increased brain aluminium in a resident of Camelford, Cornwall, UK. J. Neurol. Neurosurg. Psychiatry 77:877-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fillebeen, C., and K. Pantopoulos. 2002. Redox control of iron regulatory proteins. Redox Rep. 7:15-22. [DOI] [PubMed] [Google Scholar]
- 9.Gray, N. K., K. Pantopoulos, T. Dandekar, B. A. Ackrell, and M. W. Hentze. 1996. Translational regulation of mammalian and Drosophila citric acid cycle enzymes via iron-responsive elements. Proc. Natl. Acad. Sci. USA 93:4925-4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassett, D. J., M. L. Howell, P. A. Sokol, M. L. Vasil, and G. E. Dean. 1997. Fumarase C activity is elevated in response to iron deprivation and in mucoid, alginate-producing Pseudomonas aeruginosa: cloning and characterization of fumC and purification of native FumC. J. Bacteriol. 179:1442-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawasaki, S., J. Ishikura, D. Chiba, T. Nishino, and Y. Niimura. 2004. Purification and characterization of an H2O-forming NADH oxidase from Clostridium aminovalericum: existence of an oxygen-detoxifying enzyme in an obligate anaerobic bacteria. Arch. Microbiol. 181:324-330. [DOI] [PubMed] [Google Scholar]
- 12.Kruszewski, M. 2003. Labile iron pool: the main determinant of cellular response to oxidative stress. Mutat. Res. 531:81-92. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 14.Lemire, J., R. Mailloux, and V. D. Appanna. 2008. Zinc toxicity alters mitochondrial metabolism and leads to decreased ATP production in hepatocytes. J. Appl. Toxicol. 28:175-182. [DOI] [PubMed] [Google Scholar]
- 15.Lemire, J., R. J. Mailloux, and V. D. Appanna. 2008. Mitochondrial lactate dehydrogenase is involved in oxidative-energy metabolism in human astrocytoma cells (CCF-STTG1). PLoS ONE 3:e1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mailloux, R., J. Lemire, and V. Appanna. 2007. Aluminum-induced mitochondrial dysfunction leads to lipid accumulation in human hepatocytes: a link to obesity. Cell. Physiol. Biochem. 20:627-638. [DOI] [PubMed] [Google Scholar]
- 17.Mailloux, R. J., R. Beriault, J. Lemire, R. Singh, D. R. Chenier, R. D. Hamel, and V. D. Appanna. 2007. The tricarboxylic acid cycle, an ancient metabolic network with a novel twist. PLoS ONE 2:e690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mailloux, R. J., R. Hamel, and V. D. Appanna. 2006. Aluminum toxicity elicits a dysfunctional TCA cycle and succinate accumulation in hepatocytes. J. Biochem. Mol. Toxicol. 20:198-208. [DOI] [PubMed] [Google Scholar]
- 19.Mailloux, R. J., R. Singh, and V. D. Appanna. 2006. In-gel activity staining of oxidized nicotinamide adenine dinucleotide kinase by blue native polyacrylamide gel electrophoresis. Anal. Biochem. 359:210-215. [DOI] [PubMed] [Google Scholar]
- 20.Middaugh, J., R. Hamel, G. Jean-Baptiste, R. Beriault, D. Chenier, and V. D. Appanna. 2005. Aluminum triggers decreased aconitase activity via Fe-S cluster disruption and the overexpression of isocitrate dehydrogenase and isocitrate lyase: a metabolic network mediating cellular survival. J. Biol. Chem. 280:3159-3165. [DOI] [PubMed] [Google Scholar]
- 21.Oexle, H., E. Gnaiger, and G. Weiss. 1999. Iron-dependent changes in cellular energy metabolism: influence on citric acid cycle and oxidative phosphorylation. Biochim. Biophys. Acta 1413:99-107. [DOI] [PubMed] [Google Scholar]
- 22.Olakanmi, O., B. E. Britigan, and L. S. Schlesinger. 2000. Gallium disrupts iron metabolism of mycobacteria residing within human macrophages. Infect. Immun. 68:5619-5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park, S. J., and R. P. Gunsalus. 1995. Oxygen, iron, carbon, and superoxide control of the fumarase fumA and fumC genes of Escherichia coli: role of the arcA, fnr, and soxR gene products. J. Bacteriol. 177:6255-6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinlan, G. J., T. W. Evans, and J. M. Gutteridge. 2002. Iron and the redox status of the lungs. Free Radic. Biol. Med. 33:1306-1313. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez, G. M., and I. Smith. 2003. Mechanisms of iron regulation in mycobacteria: role in physiology and virulence. Mol. Microbiol. 47:1485-1494. [DOI] [PubMed] [Google Scholar]
- 26.Saks, V., R. Favier, R. Guzun, U. Schlattner, and T. Wallimann. 2006. Molecular system bioenergetics: regulation of substrate supply in response to heart energy demands. J. Physiol. 577:769-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sapan, C. V., R. L. Lundblad, and N. C. Price. 1999. Colorimetric protein assay techniques. Biotechnol. Appl. Biochem. 29(Pt. 2):99-108. [PubMed] [Google Scholar]
- 28.Schagger, H., and K. Pfeiffer. 2001. The ratio of oxidative phosphorylation complexes I-V in bovine heart mitochondria and the composition of respiratory chain supercomplexes. J. Biol. Chem. 276:37861-37867. [DOI] [PubMed] [Google Scholar]
- 29.Schagger, H., and G. von Jagow. 1991. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal. Biochem. 199:223-231. [DOI] [PubMed] [Google Scholar]
- 30.Seznec, H., D. Simon, C. Bouton, L. Reutenauer, A. Hertzog, P. Golik, V. Procaccio, M. Patel, J. C. Drapier, M. Koenig, and H. Puccio. 2005. Friedreich ataxia: the oxidative stress paradox. Hum. Mol. Genet. 14:463-474. [DOI] [PubMed] [Google Scholar]
- 31.Singh, R., R. Beriault, J. Middaugh, R. Hamel, D. Chenier, V. D. Appanna, and S. Kalyuzhnyi. 2005. Aluminum-tolerant Pseudomonas fluorescens: ROS toxicity and enhanced NADPH production. Extremophiles 9:367-373. [DOI] [PubMed] [Google Scholar]
- 32.Singh, R., R. J. Mailloux, S. Puiseux-Dao, and V. D. Appanna. 2007. Oxidative stress evokes a metabolic adaptation that favors increased NADPH synthesis and decreased NADH production in Pseudomonas fluorescens. J. Bacteriol. 189:6665-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stookey, L. 1970. Ferrozine—a new spectrophotometric reagent for iron. Anal. Chem. 42:779-781. [Google Scholar]
- 34.Varghese, S., Y. Tang, and J. A. Imlay. 2003. Contrasting sensitivities of Escherichia coli aconitases A and B to oxidation and iron depletion. J. Bacteriol. 185:221-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yumoto, I., D. Ichihashi, H. Iwata, A. Istokovics, N. Ichise, H. Matsuyama, H. Okuyama, and K. Kawasaki. 2000. Purification and characterization of a catalase from the facultatively psychrophilic bacterium Vibrio rumoiensis S-1T exhibiting high catalase activity. J. Bacteriol. 182:1903-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zatta, P., E. Lain, and C. Cagnolini. 2000. Effects of aluminum on activity of Krebs cycle enzymes and glutamate dehydrogenase in rat brain homogenate. Eur. J. Biochem. 267:3049-3055. [DOI] [PubMed] [Google Scholar]