Abstract
We used a combination of stable isotope probing (SIP), gas chromatography-mass spectrometry-based respiration, isolation/cultivation, and quantitative PCR procedures to discover the identity and in situ growth of soil microorganisms that metabolize benzoic acid. We added [13C]benzoic acid or [12C]benzoic acid (100 μg) once, four times, or five times at 2-day intervals to agricultural field plots. After monitoring 13CO2 evolution from the benzoic acid-dosed soil, field soils were harvested and used for nucleic acid extraction and for cultivation of benzoate-degrading bacteria. Exposure of soil to benzoate increased the number of culturable benzoate degraders compared to unamended soil, and exposure to benzoate shifted the dominant culturable benzoate degraders from Pseudomonas species to Burkholderia species. Isopycnic separation of heavy [13C]DNA from the unlabeled fraction allowed terminal restriction fragment length polymorphism (T-RFLP) analyses to confirm that distinct 16S rRNA genes were localized in the heavy fraction. Phylogenetic analysis of sequenced 16S rRNA genes revealed a predominance (15 of 58 clones) of Burkholderia species in the heavy fraction. Burkholderia sp. strain EBA09 shared 99.5% 16S rRNA sequence similarity with a group of clones representing the dominant RFLP pattern, and the T-RFLP fragment for strain EBA09 and a clone from that cluster matched the fragment enriched in the [13C]DNA fraction. Growth of the population represented by EBA09 during the field-dosing experiment was demonstrated by using most-probable-number-PCR and primers targeting EBA09 and the closely related species Burkholderia hospita. Thus, the target population identified by SIP not only actively metabolized benzoic acid but reproduced in the field upon the addition of the substrate.
Soil environments are commonly carbon limited (1), and carbon input through decomposition, industrial spills, or other disturbances can lead to an increase in microbial activity (36). In order to understand the population dynamics of bacteria in soils, it is necessary to understand which organisms respond to increases in carbon availability and how the population changes. Investigations using stable isotope probing (SIP) are particularly suited to identifying bacteria that metabolize a specific carbon compound because cellular biomarkers used to identify organisms become 13C labeled when organisms metabolize and incorporate 13C from the labeled substrate (6, 7, 28, 32, 33, 35, 40). The growth of bacteria identified by DNA-SIP can be inferred because, at minimum, two generations are required for DNA to be fully labeled with 13C due to semiconservative DNA replication. Researchers can use phylogenetic analysis of labeled sequences, along with chemical knowledge of both the labeled compound and the experimental environment, to gain further insight into the physiology of the metabolically active population. Furthermore, the successful isolation of a strain representative of an active population identified by SIP allows for more detailed genetic and physiological investigation (23, 24, 25, 30, 39).
Benzoic acid is a naturally occurring aromatic acid that enters soil environments through plant decomposition and root exudates. Soil with growing or decomposing quackgrass contained approximately 30 or 80 nmol benzoic acid g−1 dry soil, respectively (4). Although a diverse number of bacteria are known to metabolize benzoic acid (Biodegradative Strain Database, http://bsd.cme.msu.edu/jsp/InfoController.jsp?object=Chemical&id=C_benzo8), to our knowledge there are no in situ studies identifying populations that metabolize benzoic acid in soil. Aerobic metabolism of benzoic acid occurs either through dihydroxylation of benzoate by benzoate 1,2-dioxygenase (BenABC) with subsequent ortho cleavage of catechol by catechol 1,2-dioxygenase (21) or through conversion to benzoyl-coenzyme A with subsequent ring cleavage (17). Benzoic acid is also a common intermediate metabolite in metabolic pathways for aromatic pollutants such as benzonitrile, biphenyl, and toluene. The extensively studied biphenyl degrader Burkholderia xenovorans LB400 encodes both the catechol ortho cleavage pathway and the benzoyl-coenzyme A pathway (11). In B. xenovorans LB400, the benzoate dioxygenase pathway converted benzoate more rapidly than metabolism via coenzyme A activation and resulted in faster growth (11).
In this study, we investigated the dynamics of a benzoic acid-metabolizing population in an agricultural field during multiple amendments of benzoic acid. To discover the identity of microorganisms that metabolize benzoic acid and measure their in situ growth in soil, we used a combination of SIP, gas chromatography-mass spectrometry (GC/MS)-based respiration, and isolation/cultivation. Furthermore, to confirm the growth of the active population identified by SIP, we used most-probable-number (MPN)-PCR to quantify the population in situ.
MATERIALS AND METHODS
Bacterial strains.
B. xenovorans LB400 was originally isolated from a site contaminated with polychlorinated biphenyls (5) and was a gift from G. Zylstra, Rutgers University. Additional strains, isolated during this study and used to verify the specificity of PCR primers used to detect Burkholderia strain EBA09, included Burkholderia sp. strains EBA01, EBA02, EBA03, EBA04, EBA05, EBA07, EBA11, EBA14, EBA15, and EBA16; Pseudomonas sp. strain EBA13; Arthrobacter sp. strain EBA06; and Cupriavidus sp. strain EBA17.
Soil field treatments for respiration, SIP, cultivation, and population monitoring.
The application of SIP in the field is based on methods developed by Padmanabhan et al. (38) and DeRito et al. (12). A small field plot (∼1 m2) of Collamer silt loam at the Cornell University Agricultural Experiment Station, Ithaca, NY, was leveled and cleared of vegetation several days prior to the experiment. A grid of dosing points (on 15-cm centers marked by screw-cap canning jar bands) was laid out to accommodate all treatments in triplicate. For the SIP experiment, soil in the field was dosed four times with 100 μg of benzoic acid in 50 μl H2O every 48 h and a fifth time 24 h after the fourth dose. Three treatments varied in the amount of [13C]benzoic acid added to the soil. For the first treatment, soils received 5 doses of [12C]benzoic acid, the second treatment received 4 doses of [12C]benzoic acid with a final dose of [13C]benzoic acid, and the third treatment received 5 doses of [13C]benzoic acid. Immediately following the final dose, septum-covered chambers were placed over the treated soils to allow headspace analysis. In parallel with the respiration/SIP procedures, additional soil plot treatments were dosed with unlabeled benzoic acid or left unamended. These soils were used in experiments using cultivation and MPN-PCR techniques. A table was placed over the plot (0.8 m high) to protect the experiment from rain and direct exposure to sunlight.
GC/MS analysis of CO2.
Analysis of CO2 respired from soil was conducted as previously described (12). A Hewlett-Packard HP5890 gas chromatograph (Hewlett-Packard, Wilmington, DE) equipped with an HP5971A mass-selective detector was used for the respiration analyses. With high-purity helium as the carrier gas, a Hewlett-Packard Pora Plot Q column (25 m by 0.32 mm, 10-μm film thickness) was used to separate carbon dioxide from other gaseous components. The detector was operated at an electron energy of 70 eV and a detector voltage of 2,000 V. The ion source pressure was maintained at 1 × 10−5 torr. A splitless injection was used, and the GC oven was isothermal at 60°C. CO2 eluted at 1.18 min. Single-ion monitoring allowed simultaneous quantification of both 12CO2 (m/z = 44) and 13CO2 (m/z = 45). The concentration of 13CO2 was quantified by using calibration curves prepared with external standards (Scott Specialty Gases, Plumsteadville, PA). The net 13CO2 produced from metabolism of the [13C]benzoic acid was calculated by subtracting the background 13CO2 produced by the native microbial community from soil organic matter. Background 13CO2 was inferred from direct measurement of 12CO2 adjusted to the known fixed ratio of 12C to 13C in naturally occurring carbon (1.11%). This ratio was confirmed analytically. Net 13CO2 values from replicate chambers were averaged at each time point.
DNA extraction.
DNA was extracted from soil according to the method of Griffiths et al. (18). Extractions were performed by combining 0.5 g (wet weight) of soil with 0.5 ml of cetyltrimethylammonium bromide (CTAB) extraction buffer and 0.5 ml of phenol-chloroform-isoamyl alcohol (25:24:1, pH = 8.0) in Lysing Matrix E tubes (Mo Bio Laboratories, Carlsbad, CA). CTAB extraction buffer is prepared by mixing equal volumes of 10% (wt/vol) CTAB in 0.7 M NaCl with 240 mM potassium phosphate buffer, pH 8.0. Samples were homogenized with a BeadBeater (BioSpec Products, Bartlesville, OK) for 30 s, and the aqueous phase was separated by centrifugation (16,000 × g) for 5 min at 4°C. The aqueous phase was moved to a 1.5-ml tube, and phenol was removed by mixing an equal volume of chloroform-isoamyl alcohol (24:1), followed by centrifugation (16,000 × g) for 5 min at 4°C. DNA was precipitated from the aqueous layer with 2 volumes of 30% (wt/vol) polyethylene glycol 6000 for 2 h at room temperature. The precipitated DNA was pelleted by centrifugation (16,000 × g), washed with ice-cold 70% ethanol, and air dried prior to resuspension in Tris-EDTA (TE) (pH = 8.0).
Isopycnic centrifugation and gradient fractionation.
Density gradient ultracentrifugation and subsequent fractionation were performed according to the method of Lueders et al. (31). One hundred nanograms of extracted DNA was added to a CsCl solution in TE buffer (pH = 8.0) to a final volume of 5.5 ml and an average density of 1.729 g ml−1. The ultracentrifugation tubes were sealed and centrifuged at 146,000 × gav and 20°C for at least 60 h. The centrifuged gradients were fractionated from the bottom to the top into ∼300-μl fractions by displacing the gradient with sterile water from the top of the tube with a high-performance liquid chromatography pump. The density of each fraction was determined by measuring the refractive index of a subsample with an AR200 digital refractometer (Leica Microsystems). DNA was precipitated from the CsCl fractions with polyethylene glycol as described above, and precipitated DNA was washed with 70% ethanol and suspended in 35 μl TE. Primarily two fractions (1.70 to 1.71 and 1.747 g ml−1) were analyzed.
T-RFLP.
Terminal restriction fragment length polymorphism (T-RFLP) protocols and analyses of the fragments were performed as described by Cadillo-Quiroz et al. (8). 6-Carboxyfluorescein-labeled amplicons were generated from community DNA from 1 μl of selected gradient fractions with primers FAM-27f (5′-AGAGTTTGATCCTGGCTCAG), which was fluorescently labeled on the 5′ end with 6-carboxyfluorescein, and 1492r (5′-TACGGYTACCTTGTTACGACTT) (Integrated DNA Technologies). The labeled 16S rRNA genes were generated with Thermo-Start PCR Master Mix (ABgene, United Kingdom), with each primer at 0.5 μM and 1.5 mM MgCl2. The PCR conditions used were 94°C for 15 min; 34 cycles consisting of 94°C for 30s, 55°C for 1 min, and 72°C for 1 min; and 72°C for 5 min. Amplicons were purified (Qiagen PCR purification kit), and 100-ng samples of pooled triplicate PCR mixtures were digested with the enzyme MspI (5 U; New England BioLabs) for 3 h at 37°C. Purified, digested products were concentrated in a vacuum centrifuge and then resuspended with a mixture of Hi Di-Formamide (Applied Biosystems) and Gene Scan 500-Liz marker (12 μl ml−1; Applied Biosystems). Fragments were resolved with an Applied Biosystems 3730xl DNA analyzer (Bio Resources Center, Cornell University). Terminal restriction fragment length and peak height were determined with Peak Scanner (Applied Biosystems).
PCR cloning, restriction digestion, and sequencing.
PCR amplification of 16S rRNA genes from gradient fractions with universal eubacterial primers 27f and 1492r was performed as described previously (3, 12). The product was ligated into the vector pCR2.1 (TA cloning; Invitrogen) by following the manufacturer's recommended protocol. Following the transformation of plasmids into host cells and blue-white screening, colonies with inserts were verified by PCR with vector-specific primers (5′-GTAACGGCCGCCAGTGTGCT and 5′-CAGTGTGATGGATATCTGCA) that flanked the cloning region. Amplicons were digested with HaeIII and HhaI, and RFLP patterns were analyzed on 3% MetaPhore agarose gels (BioWhittaker Molecular Applications, Rockland, ME) with a 100-bp ladder (Promega) as a marker. Clones that were selected for sequencing were grown overnight in 5 ml of Luria-Bertani broth with kanamycin (50 μg/ml) and pelleted, and plasmids were purified (QiaPrep spin miniprep kit; Qiagen, Santa Clarita, CA).
Sequencing (Cornell University DNA Sequencing Facility) was conducted with four primers, M13 forward (5′-TGTAAAACGACGGCCAGT-3′), M13 reverse (5′-AACAGCTATGACCATG-3′), 531 reverse (5′-TACCGCGGCTGCTGGCAC-3′), and 533 forward (5′-GTGCCAGCMGCCGCGG-3′). Raw sequence data were assembled into full-length sequences with the program SEQMAN II (DNASTAR, Inc.). After assembly, the consensus sequence was verified manually by referring to the corresponding ABI chromatograms of the sequencing reactions, and Bellerophon (22) was used to check for chimeras. Phylogenetic relationships were discerned by the neighbor-joining method with the computational tools of Lasergene (DNASTAR, Inc., Madison, WI) and ClustalX (44).
Isolation of benzoate-metabolizing bacteria.
Benzoate-metabolizing bacteria were isolated from soils that were left unamended or treated with benzoic acid as in the field experiment. For both treatments, 1 g soil was serially diluted in a phosphate-buffered solution, spread plated onto MSB agar plates (41) with 0.01% (0.7 mM) sodium benzoate in duplicate, and incubated at room temperature. For each treatment, 30 individual colonies from the two highest dilutions were randomly selected and checked for purity by streaking on R2A agar plates. Confirmed pure cultures were grown again on MSB agar with 0.01% benzoate, and isolates that maintained the benzoate-metabolizing phenotype were stored at −80°C for later characterization of the cultures. Eleven genetically distinct isolates were distinguished by 16S rRNA gene fingerprinting as described above.
Amplification of the benzoate 1,2-dioxygenase large-subunit gene in isolated bacteria.
A 540-bp fragment of the benzoate 1,2-dioxygenase alpha subunit gene was amplified from selected isolates with primers BAf1 (5′-GCRCARGAYAGCCAGATTCCC) and BAr2 (5′-GGTGGCMGCYTAGTTCCAGTG), which were designed from the homologous regions of benA of Acinetobacter baylyi ADP1 and xylX of Pseudomonas putida TOL plasmid pWW0 (13, 20, 34). The benA fragment was amplified with the following program: 95°C for 15 min with 5 initial cycles of 94°C for 30 s, 60°C for 30 s (−1°C per cycle), and 70°C for 45 s, followed by 35 cycles of 95°C for 30 s, 54°C for 45 s, and 70°C for 45 s and a 1-min extension at 68°C. The resulting amplicons were sequenced directly with the PCR primers.
MPN-PCR.
Quantification of bacteria identified by SIP by MPN-PCR was performed as previously described (14). In parallel with the field SIP experiment, three sets of triplicate soils were dosed with 100 μg benzoic acid in 50 μl water every 48 h. Triplicate 0.5-g soil samples were collected and stored at −80°C at times 0 (untreated), 48 (1 dose), 144 (3 doses), and 192 h (5 doses). DNA was extracted as described above from each soil sample, and the primer Burkhospita1F (5′-AAAGGCCTCGCGCTCAAG) was designed with the 16S rRNA gene sequence from isolate EBA09 and cloned 16S rRNA sequences D603, D617, and D638 (generated in this study [GenBank entries EU677389, EU677396, and EU677406, respectively]) with Primrose software (2). A 180-bp amplicon was generated when Burkhospita1F was used in combination with the universal eubacterial primer 342r (5′-CTGCTGCSYCCCGTAG). Tenfold dilution series of the extracted DNA were made, and 1 μl was used as the template in a 20-μl PCR mixture with the following program: 15 min at 95°C; 32 cycles of 30 s at 95°C, 30 s at 60°C, and 45 s at 70°C; 4 min at 70°C; and a final hold at 4°C. The second highest dilution to produce the target 180-bp amplicon was used as a starting point for triplicate threefold dilution series. The freeware MPN Calculator (VB6 version; Michael Curiale [http://www.i2workout.com/mcuriale/mpn/index.html]) was used to calculate the MPN abundance of populations related to strain EBA09 in soil samples dosed with benzoate.
Nucleotide sequence accession numbers.
The nucleotide sequence data reported here have been submitted to GenBank under accession no. EU677388 to EU677420.
RESULTS
Benzoic acid metabolism at a field site.
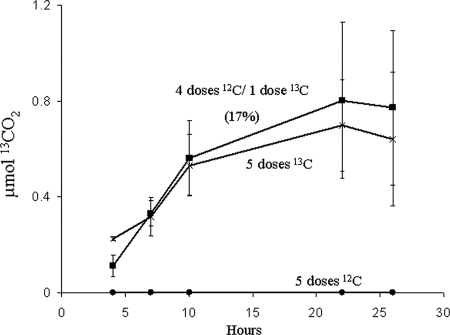
In order to investigate the dynamics of the benzoic acid-metabolizing community in the field agricultural study site, we carried out a dosing regimen based on that established by DeRito et al. (12). For the first treatment, soils received 5 doses of [12C]benzoic acid, the second treatment received 4 doses of [12C]benzoic acid with a final dose of [13C]benzoic acid, and the third treatment received 5 doses of [13C]benzoic acid. Monitoring of benzoic acid metabolism in the field was determined by GC/MS analysis of 13CO2 respired from the soil after the final dose (Fig. 1). In soils exposed to [13C]benzoic acid, a net increase in respired 13CO2 above the background level was observed, whereas no increase was seen in soils receiving [12C]benzoic acid. The 13CO2 respired from soils receiving a single dose of [13C]benzoic acid amounted to 17% of the labeled carbon added to the soil. Respired 13CO2 was nearly identical between soils that received 5 doses of 13[C]benzoic acid and soils that received 4 doses of 12[C]benzoic acid with a final dose of 13[C]benzoic acid. This suggested that (i) benzoic acid was metabolized quickly—little or no residual 13[C]benzoic acid remained in soils receiving prior doses—and (ii) the 13C-labeled biomass (produced via assimilation of early doses of 13[C]benzoic acid) was relatively stable and not subject to further metabolism during the experiment.
FIG. 1.
Confirmation of [13C]benzoic acid metabolism measured as net 13CO2 respiration from the agricultural field study site. 13CO2 was produced from soils receiving 5 doses of 100 μg [12C]benzoic acid (•), 4 doses of 100 μg [12C]benzoic acid and a final 100 μg dose of [13C]benzoic acid (▪), or 5 doses of 100 μg [13C]benzoic acid (×). The percentage in parentheses shows the proportion of the total added 13C recovered as 13CO2 from the treatment receiving a single dose of [13C]benzoic acid. Experimental values represent the mean and standard deviation of triplicate samples.
Community profiles of density-resolved DNA.
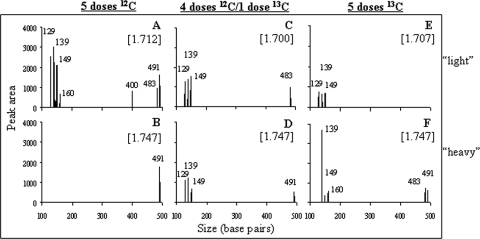
Triplicate soil samples from each of the three field treatments were collected and pooled. DNA was extracted, and the [13C]DNA was separated from [12C]DNA by isopycnic centrifugation. We used T-RFLP to compare community profiles from heavy (1.747 g ml−1) and light (1.70 to 1.71 g ml−1) fractions taken from the CsCl gradient of each field treatment (Fig. 2). Figure 2A and B show results of our positive- and negative-control treatments, respectively, that received unlabeled benzoate. As expected, the light fraction (Fig. 2A) featured a complex T-RFLP pattern typical of heterogeneous populations in a soil community and the heavy fraction (Fig. 2B) was virtually free of signals (except for a spurious peak at 491 bp that was also present in our reagent blank). All other T-RFLP patterns should be interpreted relative to Fig. 2B and according to contrasts between heavy and light fractions in a given treatment.
FIG. 2.
Microbial community composition represented by T-RFLP profiles from three fractions collected from CsCl gradients containing DNA extracted from soils receiving 5 doses of 100 μg [12C]benzoic acid (A), 4 doses of 100 μg [12C]benzoic acid and a final 100 μg dose of [13C]benzoic acid (B), or 5 doses of 100 μg [13C]benzoic acid (C). The buoyant densities (grams per milliliter) of the fractions are shown in brackets.
For the three treatments, the distribution of T-RFLP signals within a gradient was consistent with the type of benzoic acid label (13C or 12C) the treatment received. In contrast to the treatment receiving only [12C]benzoic acid (discussed above), T-RFLP signals in the DNA from soil receiving 5 doses of [13C]benzoic acid were enhanced in the heavy fraction (Fig. 2F) compared to the light fraction of the gradient (Fig. 2E). The peak corresponding to a fragment length of 139 bp, in particular, showed enrichment as the density of the gradient increased (Fig. 2F). This suggested that the population represented by the 139-bp fragment was metabolizing the benzoic acid and fully incorporated the 13C label into its biomass. Three peaks corresponding to fragment sizes of 128, 139, and 149 bp were shared by all treatments, which suggests that one or all of the organisms represented by these fragments were members of the benzoic acid-metabolizing community. The T-RFLP signals in the DNA from the soil that received 4 doses of [12C]benzoic acid followed by a single dose of [13C]benzoic acid were evenly distributed throughout the gradient (Fig. 2C and D), suggesting that the single dose of [13C]benzoic acid supplied to the enriched population resulted in a spectrum of unlabeled, partially labeled, and fully labeled populations.
Identifying active benzoic acid-metabolizing bacteria.
We pursued two methods to investigate the identities of active benzoic acid-metabolizing bacteria, (i) cultivation and (ii) cloning plus sequencing of 16S rRNA genes. Prior to the cultivation of benzoic acid-metabolizing isolates, soil was either dosed with benzoic acid as in the SIP experiment or left unamended. Plate counts showed that culturable benzoic acid-metabolizing isolates from the unamended soil were present at 3.9 × 105 CFU g−1 soil, while successive benzoate additions increased the number of benzoic acid degraders nearly 10-fold to 3.3 × 106 CFU g−1 soil. Comparison of RFLP patterns generated from 16S rRNA gene amplicons digested with HaeIII and HhaI and subsequent 16S rRNA gene sequencing revealed that 16 of 24 selected isolates from the highest dilutions of the unamended soil were Pseudomonas species. However, when the soil was exposed to benzoic acid, 14 of 17 isolates from the highest dilutions were Burkholderia species.
In addition to the above culture-based investigation, a 16S rRNA gene clone library was prepared from the heavy (1.747 g ml−1) fraction (Fig. 2F) of the treatment that received 5 doses of [13C]benzoic acid. Fifty-eight clones were screened by comparing RFLP patterns following double digestion with HhaI and HaeIII. There were 25 unique RFLP patterns, and 22 clones representing 12 unique RFLP patterns were sequenced. No chimeras were detected by Bellerophon. BLAST analysis of cloned sequences revealed that clones representing the predominant RFLP pattern (15 of 58 clones) were Burkholderia species.
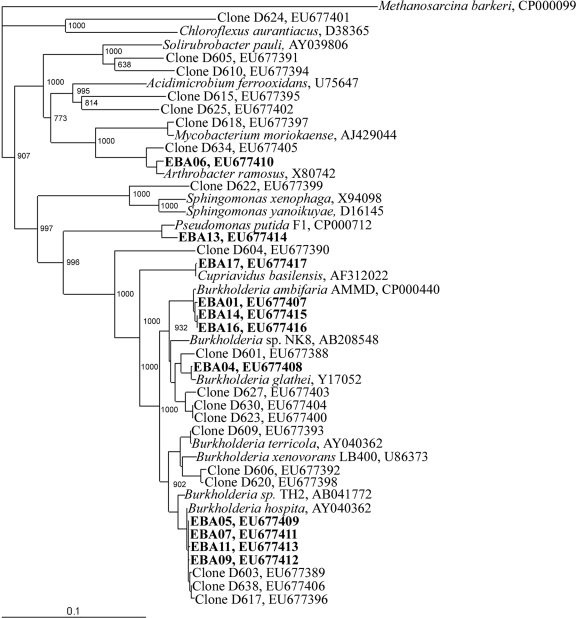
A phylogenetic tree was generated with complete 16S rRNA gene sequences from 11 isolates cultured from soil amended with benzoic acid and 19 cloned 16S rRNA sequences (Fig. 3). One group of isolated Burkholderia strains, EBA05, EBA07, EBA09, and EBA11, clustered near Burkholderia hospita and Burkholderia sp. strain TH2. The latter is known to metabolize benzoate and chlorobenzoates (42). Remarkably, this group of isolates clustered together with three clones representing the dominant RFLP pattern from the [13C]DNA fraction, suggesting that we had successfully isolated representatives of the benzoic acid-metabolizing population that was active in the field. Isolate EBA09 was found to have 99.5% sequence similarity to this group of clones. Furthermore, the T-RFLP pattern for isolate EBA09 and clones from that cluster revealed a 139-bp fragment (data not shown) which matches the fragment enriched in the [13C]DNA fraction from the field (Fig. 2).
FIG. 3.
Phylogenetic analysis of 11 benzoate-metabolizing isolates and 19 cloned 16S rRNA genes (∼1,400 bp) derived from 13C-labeled DNA from soils receiving 5 doses of [13C]benzoic acid. Isolates are in bold, clones are in plain text, and 17 reference strains are in italics. Accession numbers are given for the sequences.
Phylogenetic analysis of benzoate 1,2-dioxygenase genes.
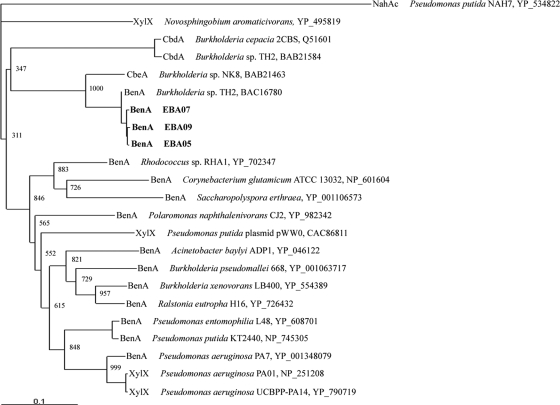
A 540-bp fragment of the gene encoding the benzoate 1,2-dioxygenase alpha subunit was amplified from isolates EBA05, EBA07, and EBA09. The amplified benA fragments were sequenced and subjected to phylogenetic analysis along with other benzoate 1,2-dioxygenase, toluate 1,2-dioxygenase, and chlorobenzoate dioxygenase genes (Fig. 4). The resulting phylogenetic tree shows that the benA genes from the isolates are closely related to the nonfunctional benA gene in Burkholderia sp. strain TH2 (43), as well as chlorobenzoate dioxygenase genes from Burkholderia sp. strain NK8 (13), Burkholderia sp. strain TH2, and Burkholderia cepacia 2CBS (19), which are able to metabolize chlorinated benzoates.
FIG. 4.
Phylogenetic analysis inferred from the alignment of 175 amino acid positions predicted from benA sequences amplified from isolates (EBA05, EBA07, and EBA09; accession numbers EU677418, EU677419, and EU677420, respectively; in bold), as well as 19 reference sequences for benzoate 1,2-dioxygenase (BenA), toluate 1,2-dioxygenase (XylX), and chlorobenzoate dioxygenase (CbdA and CbeA) sequences from GenBank. The naphthalene dioxygenase, NahAc, from P. putida G7 is the outgroup. Accession numbers are given for the reference sequences.
Growth of Burkholderia populations in the field during the benzoate-dosing regimen.
To demonstrate growth of the population represented by EBA09 upon the addition of benzoic acid in the field, we performed MPN-PCR on archived DNA extracts with a primer targeting the 16S rRNA gene of EBA09 and the closely related species B. hospita (Fig. 5). The specificity of the primer was tested by performing PCR on unrelated Burkholderia species, as well as all of the isolates from the benzoic acid-amended soil, which included Burkholderia, Pseudomonas, Arthrobacter, and Cupriavidus species. We only obtained the expected 180-bp amplicon from isolates EBA05, EBA07, and EBA09, which show at least 99.4% sequence similarity. The DNA used in the MPN-PCR assay was extracted from three sets of triplicate soils that were dosed with 100 μg benzoic acid in 50 μl water every 48 h and triplicate soils that were unamended. The soil treatments for the MPN-PCR assay occurred simultaneously with the field SIP experiment, so the field conditions for each were the same. At time zero, the target population was present at slightly under 100,000 copies/g of soil, with little change after 48 h. However, after 144 h, the population underwent approximately two doublings, as the target population had increased to 4.5 × 105 to 4.8 × 105 copies/g of soil. Clearly, the target population identified by SIP not only actively metabolized benzoic acid but also reproduced in the field after the addition of the substrate.
FIG. 5.
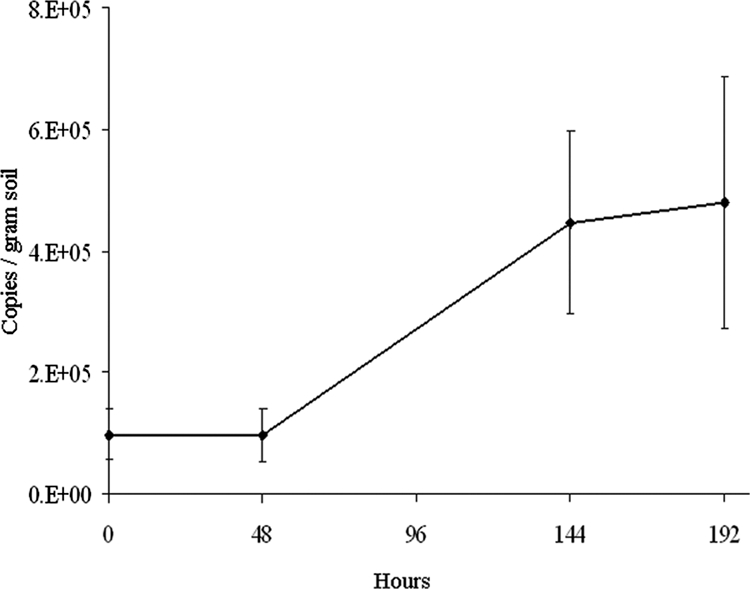
Quantification of a Burkholderia hospita EBA09-like population in the field by MPN-PCR during growth on benzoic acid. Experimental values represent the average copy number of the target 16S rRNA gene from triplicate soil samples. Error bars represent standard deviations.
DISCUSSION
In this study, we found that successive addition of benzoic acid to a field soil resulted in the growth of Burkholderia species that were also identified as the primary degraders of benzoic acid in situ by SIP. Although identifying an organism by DNA-SIP implies growth, MPN-PCR quantification of the population identified in our SIP experiment confirmed that the population underwent approximately two doublings. Furthermore, enumeration of culturable benzoic acid degraders suggested that Pseudomonas species were initially the more abundant benzoic acid degraders, but the addition of benzoic acid to the soil caused a shift in the community yielding greater numbers of Burkholderia species. According to the rRNA operon copy number database (27; http://ribosome.mmg.msu.edu/rrndb/index.php), species from the genus Burkholderia have an average of 4.94 16S rRNA gene copies. Assuming that the EBA09 population detected in the MPN-PCR assay has 5 16S rRNA gene copies per cell, the population increased from about 2 × 104 cells/g of soil to just over 9 × 104 cells/g of soil during the experiment. Following the final dose of benzoic acid, the number of culturable benzoate-degrading Burkholderia bacteria was approximately 35 to 40 times higher than that detected by MPN-PCR, which used a primer designed to detect only populations closely related to Burkholderia sp. strain EBA09. This discrepancy between the final number of culturable Burkholderia species and the EBA09 population measured by MPN-PCR is likely due to the specificity of the PCR primers used in the MPN-PCR assay and the likelihood that the EBA09 population is a subset of the total benzoic acid-metabolizing community. Despite this difference, a measured increase in Burkholderia species after the addition of benzoic acid is consistent between culture-based and non-culture-based methods.
The population numbers obtained by MPN-PCR indicated a slowdown in growth between 144 and 196 h (Fig. 5), which could indicate that nutrients other than carbon were becoming scarce and limiting the growth of the EBA09-related population. If this were the case, it could have implications for microbial communities in soils receiving repeated carbon inputs. The high rRNA gene copy number found in many Burkholderia species is associated with bacteria that use a copiotrophic ecological strategy (26). Copiotrophic populations are expected to respond quickly to the added carbon and exhaust other nutrients (e.g., N and P), which may subsequently limit their activity. This could explain why there was not greater enrichment of the 139-bp fragment in the heavy DNA fraction from the sample that received only 1 dose of [13C]benzoic acid after 4 doses of [12C]benzoic acid (Fig. 2). By the time the 13C-labeled substrate was added, nutrients other than carbon may have been limiting, causing the Burkholderia sp. strain EBA09 population to grow at a slower rate. If the experiment had been extended another 48 to 96 h, it is possible that oligotrophic populations, which tend to have higher affinities for nutrients, would have replaced the Burkholderia population as the active benzoic acid degraders within the community.
In order for bacteria to take advantage of an introduced carbon source, they must have a combination of genes and physiology that enables them to compete for and utilize a substrate. Species within the genus Burkholderia are widespread in soil environments, ecologically versatile, and capable of metabolizing aromatic compounds (10, 37). Other studies have suggested that Burkholderia species are the primary degraders of aromatic compounds in soil environments. For example, in soils amended with 2- and 3-chlorobenzoate, Burkholderia species were the dominant indigenous culturable degraders of the added compounds (15, 16). Another study using SIP found Burkholderia species to be the primary degraders of polychlorinated biphenyls (45). The ecological versatility of Burkholderia bacteria is attributed to their multireplicon genomes, which can be larger than 9 Mb (9). Such large genomes allow metabolic versatility, and the presence of mobile genetic elements can promote genomic plasticity and general adaptability (29). The isolation of Burkholderia sp. strain EBA09 will allow subsequent experimentation to examine the genetic and physiological characteristics that enabled ecological success of the bacterium upon the introduction of benzoic acid in the field.
Acknowledgments
This research was supported by NIEHS grants 1-R21-ES012834 and 5-T32-ES00752-28.
Footnotes
Published ahead of print on 9 May 2008.
REFERENCES
- 1.Aldén, L., F. Demoling, and E. Bååth. 2001. Rapid method of determining factors limiting growth in soil. Appl. Environ. Microbiol. 67:1830-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashelford, K. E., A. J. Weightman, and J. C. Fry. 2002. PRIMROSE: a computer program for generating and estimating the phylogenetic range of 16S rRNA oligonucleotide probes and primers in conjunction with the RDP-II database. Nucleic Acids Res. 30:3481-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakermans, C., and E. L. Madsen. 2002. Diversity of 16S rDNA and naphthalene dioxygenase genes from coal-tar-waste-contaminated aquifer waters. Microb. Ecol. 44:95-106. [DOI] [PubMed] [Google Scholar]
- 4.Baziramakenga, R., R. R. Simard, and G. D. Leroux. 1995. Determination of organic acids in soil extracts by ion chromatography. Soil Biol. Biochem. 27:349-356. [Google Scholar]
- 5.Bedard, D. L., R. Unterman, L. H. Bopp, M. J. Brennan, M. L. Haberl, and C. Johnson. 1986. Rapid assay for screening and characterizing microorganisms for the ability to degrade polychlorinated biphenyls. Appl. Environ. Microbiol. 51:761-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boschker, H. T. S., S. C. Nold, P. Wellsbury, D. Bos, W. de Graaf, R. Pel, R. J. Parkes, and T. E. Cappenburg. 1998. Direct linking of microbial populations to specific biogeochemical processes by 13C-labelling of biomarkers. Nature 392:801-804. [Google Scholar]
- 7.Buckley, D. H., V. Huangyutitham, S.-F. Hsu, and T. A. Nelson. 2007. Stable isotope probing achieved by disentangling the effects of genome G+C content and isotope enrichment on DNA density. Appl. Environ. Microbiol. 73:3189-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cadillo-Quiroz, H., S. Bräuer, E. Yashiro, C. Sun, J. Yavitt, and S. Zinder. 2006. Vertical profiles of methanogenesis and methanogens in two contrasting acidic peatlands in central New York State, USA. Environ. Microbiol. 8:1428-1440. [DOI] [PubMed] [Google Scholar]
- 9.Chain, P. S., V. J. Denef, K. T. Konstantinidis, L. M. Vergez, L. Agulló, V. L. Reyes, L. Hauser, M. Córdova, L. Gómez, M. González, M. Land, V. Lao, F. Larimer, J. J. LiPuma, E. Mahenthiralingam, S. A. Malfatti, C. J. Marx, J. J. Parnell, A. Ramette, P. Richardson, M. Seeger, D. Smith, T. Spilker, W. J. Sul, T. V. Tsoi, L. E. Ulrich, I. B. Zhulin, and J. M. Tiedje. 2006. Burkholderia xenovorans LB400 harbors a multi-replicon, 9.73-Mbp genome shaped for versatility. Proc. Natl. Acad. Sci. USA 103:15280-15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coenye, T., and P. Vandamme. 2003. Diversity and significance of Burkholderia species occupying diverse ecological niches. Environ. Microbiol. 5:719-729. [DOI] [PubMed] [Google Scholar]
- 11.Denef, V. J., J. A. Klappenbach, M. A. Patrauchan, C. Florizone, J. L. M. Rodrigues, T. V. Tsoi, W. Verstraete, L. D. Eltis, and J. M. Tiedje. 2006. Genetic and genomic insights into the role of benzoate-catabolic pathway redundancy in Burkholderia xenovorans LB400. Appl. Environ. Microbiol. 72:585-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeRito, C. M., G. M. Pumphrey, and E. L. Madsen. 2005. Use of field-based stable isotope probing to identify adapted populations and track carbon flow through a phenol-degrading soil microbial community. Appl. Environ. Microbiol. 71:7858-7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francisco, P. B., N. Ogawa, K. Suzuki, and K. Miyashita. 2001. The chlorobenzoate dioxygenase genes of Burkholderia sp. NK8 involved in the catabolism of chlorobenzoates. Microbiology 147:121-133. [DOI] [PubMed] [Google Scholar]
- 14.Fredslund, L., E. Flemming, C. S. Jacobsen, and K. Johnsen. 2001. Development and application of a most-probable-number-PCR assay to quantify flagellate populations in soil samples. Appl. Environ. Microbiol. 67:1613-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gentry, T. J., D. T. Newby, K. L. Josephson, and I. L. Pepper. 2001. Soil microbial population dynamics following bioaugmentation with a 3-chlorobenzoate-degrading bacterial culture. Biodegradation 12:349-357. [DOI] [PubMed] [Google Scholar]
- 16.Gentry, T. J., G. Wang, C. Rensing, and I. L. Pepper. 2004. Chlorobenzoate-degrading bacteria in similar pristine soils exhibit different community structures and population dynamics in response to anthropogenic 2-, 3-, and 4-chlorobenzoate levels. Microb. Ecol. 48:90-102. [DOI] [PubMed] [Google Scholar]
- 17.Gescher, J., A. Zaar, M. Mohamed, H. Schägger, and G. Fuchs. 2002. Genes coding for a new pathway of aerobic benzoate metabolism in Azoarcus evansii. J. Bacteriol. 184:6301-6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffiths, R. I., A. S. Whiteley, A. G. O'Donnell, and M. J. Bailey. 2000. Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Appl. Environ. Microbiol. 66:5488-5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haak, B., S. Fetzner, and F. Lingens. 1995. Cloning, nucleotide sequence, and expression of the plasmid-encoded genes for the two-component 2-halobenzoate1,2-dioxygenase from Pseudomonas cepacia 2CBS. J. Bacteriol. 177:667-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harayama, S., M. Rekik, A. Bairoch, E. L. Neidle, and L. N. Ornston. 1991. Potential DNA slippage structures acquired during evolutionary divergence of Acinetobacter calcoaceticus chromosomal benABC and Pseudomonas putida TOL pWW0 plasmid xylXYZ, genes encoding benzoate dioxygenases. J. Bacteriol. 173:7540-7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harwood, C. S., and R. E. Parales. 1996. The β-ketoadipate pathway and the biology of self-identity. Annu. Rev. Microbiol. 50:553-590. [DOI] [PubMed] [Google Scholar]
- 22.Huber, T., G. Faulkner, and P. Hugenholtz. 2004. Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics 20:2317-2319. [DOI] [PubMed] [Google Scholar]
- 23.Jeon, C. O., W. Park, P. Padmanabhan, C. DeRito, J. R. Snape, and E. L. Madsen. 2003. Discovery of a bacterium, with distinctive dioxygenase, that is responsible for in situ biodegradation in contaminated sediment. Proc. Natl. Acad. Sci. USA 100:13591-13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeon, C. O., M. Park, H. S. Ro, W. Park, and E. L. Madsen. 2006. The naphthalene catabolic (nag) genes of Polaromonas naphthalenivorans CJ2: evolutionary implications for two gene clusters and novel regulatory control. Appl. Environ. Microbiol. 72:1086-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasai, Y., Y. Takahata, M. Manefield, and K. Watanabe. 2006. RNA-based stable isotope probing and isolation of anaerobic benzene-degrading bacteria from gasoline-contaminated groundwater. Appl. Environ. Microbiol. 72:3586-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klappenbach, J. A., J. M. Dunbar, and T. M. Schmidt. 2000. rRNA operon copy number reflects ecological strategies of bacteria. Appl. Environ. Microbiol. 66:1328-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klappenbach, J. A., P. R. Saxman, J. R. Cole, and T. M. Schmidt. 2001. rrnDB: the ribosomal RNA operon copy number database. Nucleic Acids Res. 29:181-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leigh, M. B., V. Pellizari, O. Uhlí-k, R. Sutka, J. Rodrigues, N. E. Ostrom, J. Zhou, and J. M. Tiedje. 2007. Biphenyl-utilizing bacteria and their functional genes in a pine root zone contaminated with polychlorinated biphenyls (PCBs). ISME J. 1:134-148. [DOI] [PubMed] [Google Scholar]
- 29.Lessie, T. G., W. Hendrickson, B. D. Manning, and R. Devereux. 1996. Genomic complexity and plasticity of Burkholderia cepacia. FEMS Microbiol. Lett. 144:117-128. [DOI] [PubMed] [Google Scholar]
- 30.Liou, J. S.-C., C. M. DeRito, and E. L. Madsen. 21 April 2008, posting date. Field-based and laboratory stable isotope probing surveys of the identities of both aerobic and anaerobic benzene-metabolizing microorganisms in freshwater sediment. Environ. Microbiol. doi: 10.1111/j.1462-2920.2008.01612.x. [DOI] [PubMed]
- 31.Lueders, T., M. Manfield, and M. W. Friedrich. 2004. Enhanced sensitivity of DNA-and rRNA-based stable isotope probing by fractionation and quantitative analysis of isopycnic centrifugation gradients. Environ. Microbiol. 6:73-78. [DOI] [PubMed] [Google Scholar]
- 32.Madsen, E. L. 2006. The use of stable isotope probing techniques in bioreactor and field studies on bioremediation. Curr. Opin. Biotechnol. 17:92-97. [DOI] [PubMed] [Google Scholar]
- 33.Manefield, M., A. S. Whitely, R. I. Griffiths, and M. J. Bailey. 2002. RNA stable isotope probing, a novel means of linking community function to phylogeny. Appl. Environ. Microbiol. 68:5367-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neidle, E. L., C. Hartnett, L. N. Ornston, A. Bairoch, M. Rekik, and S. Harayama. 1991. Nucleotide sequences of the Acinetobacter calcoaceticus benABC genes for benzoate 1,2-dioxygenase reveal evolutionary relationships among multicomponent oxygenases. J. Bacteriol. 173:5385-5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neufeld, J. D., J. Vohra, M. G. Dumont, T. Lueders, M. Manefield, M. W. Friedrich, and J. C. Murrell. 2007. DNA stable-isotope probing. Nat. Protoc. 2:860-866. [DOI] [PubMed] [Google Scholar]
- 36.Nyman, J. A. 1999. Effect of crude oil and chemical additives on metabolic activity of mixed microbial populations in fresh marsh soils. Microb. Ecol. 37:152-162. [DOI] [PubMed] [Google Scholar]
- 37.O'Sullivan, L. A., and E. Mahenthiralingam. 2005. Biotechnology potential within the genus Burkholderia. Lett. Appl. Microbiol. 41:8-11. [DOI] [PubMed] [Google Scholar]
- 38.Padmanabhan, P., S. Padmanabhan, C. DeRito, A. Gray, D. Gannon, J. R. Snape, C. S. Tsai, W. Park, C. Jeon, and E. L. Madsen. 2003. Respiration of 13C-labeled substrates added to soil in the field and subsequent 16S rRNA gene analysis of 13C-labeled soil DNA. Appl. Environ. Microbiol. 69:1614-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pumphrey, G. M., and E. L. Madsen. 2007. Naphthalene metabolism and growth inhibition by naphthalene in Polaromonas naphthalenivorans strain CJ2. Microbiology 153:3730-3738. [DOI] [PubMed] [Google Scholar]
- 40.Radajewski, S., P. Ineson, N. H. Parekh, and J. C. Murrell. 2000. Stable-isotope probing as a tool in microbial ecology. Nature 403:646-649. [DOI] [PubMed] [Google Scholar]
- 41.Stanier, R. Y., N. J. Palleroni, and M. Doudoroff. 1966. The aerobic pseudomonads: a taxonomic study. J. Gen. Microbiol. 43:159-271. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki, K., N. Ogawa, and K. Miyashita. 2001. Expression of 2-halobenzoate dioxygenase genes (cbdSABC) involved in the degradation of benzoate and 2-halobenzoate in Burkholderia sp. TH2. Gene 262:137-145. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki, K., A. Ichimura, N. Ogawa, A Hasebe, and K. Miyashita. 2002. Differential expression of two catechol 1,2-dioxygenases in Burkholderia sp. strain TH2. J. Bacteriol. 184:5714-5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The Clustal_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tillmann, S., C. Strömpl, K. N. Timmis, and W.-R. Abraham. 2005. Stable isotope probing reveals the dominant role of Burkholderia species in aerobic degradation of PCBs. FEMS Microbiol. Ecol. 52:207-217. [DOI] [PubMed] [Google Scholar]