Abstract
Flavobacterium psychrophilum is a serious pathogen in trout aquaculture, responsible for the diseases rainbow trout fry syndrome (RTFS) and cold water disease (CWD). Bacteriophage control of F. psychrophilum may constitute a realistic approach in the treatment of these diseases; however, a detailed understanding of the phage-host interactions is needed to evaluate the potential of F. psychrophilum bacteriophages for that purpose. Twenty-two F. psychrophilum phages from Danish rainbow trout farms were isolated and characterized. The phage genome sizes differed considerably and fell into three major size classes (8.5 to 12 kb, 48 kb, and 90 kb). The phage host ranges comprised from 5 to 23 of the 28 tested F. psychrophilum strains, and 18 of the phage isolates showed unique host ranges. Each bacterial strain had a unique pattern of susceptibility to the 22 phages, and individual strains also showed large variations (up to 107-fold differences) in susceptibility to specific phages. Phage burst size (7 to 162 phages infected cell−1) and latency period (4 to 6 h) also showed pronounced differences both between phages and, for a specific phage, between host strains. In general, the characterization documented the presence of diverse F. psychrophilum phage communities in Danish trout farms, with highly variable patterns of infectivity. The discovery and characterization of broad-host-range phages with strong lytic potential against numerous pathogenic F. psychrophilum host strains thus provided the foundation for future exploration of the potential of phages in the treatment of RTFS and CWD.
Flavobacterium psychrophilum is responsible for the diseases rainbow trout fry syndrome (RTFS) and cold water disease, causing considerable economic losses in salmonid aquaculture worldwide (27). Fish infected with F. psychrophilum have high mortality rates, and fry are especially affected, with mortalities of 50 to 60% (19). Treatment with antibiotics is required to limit economic losses; however, F. psychrophilum seems to be increasingly more resistant to the approved drugs (5). Today, florfenicol is the drug of choice, and no commercial vaccine is yet available. There is, therefore, a need for alternative treatments of F. psychrophilum infections in aquaculture, especially in infected fry.
Phage therapy may be a realistic alternative approach for controlling pathogenic bacteria in aquaculture. The use of bacteriophages to control bacterial pathogens has previously demonstrated promising potential for various types of bacteria in the food industry. Much of the recent research has focused on reducing the impacts of food-borne human bacterial pathogens, such as Escherichia coli in domestic animals (e.g., references 28 and 34) and Salmonella (11), Listeria (18), and Campylobacter (3) species in fruit, diary products, and poultry (reviewed in reference 12). However, in addition to the current attempts to apply phages in the control of human pathogens, fish and shellfish pathogens have also been investigated as a target for phage therapy.
A number of phages have been isolated for potential use in phage therapy against important fish and shellfish pathogens, such as Aeromonas salmonicida in brook trout (Oncorhynchus fontinalis) (15), Vibrio harveyi in shrimp (Penaeus monodon) (16, 31, 35), Pseudomonas plecoglossicida in ayu (Plecoglossus altivelis) (25, 29, 30), and Lactococcus garvieae in yellowtail (Seriola quinqueradiata) (26). All of these studies have demonstrated the potential of specific phages to significantly reduce the impacts of their bacterial hosts, with a resulting positive effect on fish survival. The studies emphasize the need for further investigations of the possibilities in using phages as an alternative to antibiotic treatment of other fish diseases in aquaculture.
The application of phages to control a certain bacterial pathogen is complicated by the high degrees of phenotypic and genotypic diversity within populations of both phages and bacteria (e.g., references 6 and 13). Consequently, individual strains of a pathogen may be more or less susceptible or even resistant to different cooccurring phages. For a successful phage control of pathogenic strains, it is therefore a prerequisite that a detailed characterization of isolated phages be obtained with respect to host range, adsorption rate, lytic potential, interaction with host, etc.
Flavobacterium psychrophilum bacteriophages have to our knowledge not previously been described in the literature. The purpose of the present study was to isolate and characterize a number of lytic phages that infect F. psychrophilum and to investigate their lytic properties toward their host bacterium under controlled conditions in the laboratory. The study thus provides the basis for an evaluation of the potential of phages to control the F. psychrophilum pathogen and consequently their potential application as a treatment of RTFS in aquaculture.
MATERIALS AND METHODS
Bacterial strains.
The Flavobacterium psychrophilum strains used in this study are listed in Table 1. The strains included the type strain NCIMB 1947T (serotype FpT, nonvirulent for rainbow trout) as well as two well-characterized Danish strains, 900406-1/3 (serotype Th, virulent) and 950106-1/1 (serotype Fd, virulent) (20, 21). An additional 23 strains were isolated from diseased rainbow trout in eight different Danish freshwater farms, and 2 strains were isolated from feral rainbow trout caught downstream from a rainbow trout farm. All strains were identified as F. psychrophilum biochemically (8) and by species-specific PCR with DNA primers against a sequence of the 16S rRNA gene (38).
TABLE 1.
Strains of Flavobacterium psychrophilum used in the study
| Bacterial strain | Growth ratea (h) | Enrichment mixture |
|---|---|---|
| NCIMB 1947T | 0.071 ± 0.018 | B |
| 900406-1/3 | 0.074 ± 0.017 | A, B |
| 950106-1/1 | 0.073 ± 0.016 | A, B |
| 040615-1/2D | 0.072 ± 0.014 | B |
| 040615-1/3A | 0.073 ± 0.015 | |
| 030522-1/1 | 0.075 ± 0.016 | |
| 030522-1/2 | 0.069 ± 0.015 | B |
| 030522-1/3 | 0.068 ± 0.014 | |
| 020612-2/1 | 0.062 ± 0.013 | B |
| 020612-2/2 | 0.062 ± 0.013 | |
| 020612-4/1 | 0.085 ± 0.021 | B |
| 020612-4/2 | 0.085 ± 0.020 | |
| 020529-2/1 | 0.090 ± 0.020 | |
| 020529-2/2 | 0.083 ± 0.015 | B |
| 010418-2/1 | 0.072 ± 0.016 | B |
| 010418-2/2 | 0.073 ± 0.017 | |
| 010418-2/3 | 0.081 ± 0.020 | |
| 990512-1/1B | 0.076 ± 0.017 | B |
| 990512-1/2A | 0.067 ± 0.015 | |
| 960625-3/1 | 0.078 ± 0.017 | B |
| 951004-1/1A | 0.098 ± 0.025 | |
| 951004-1/8A | 0.076 ± 0.018 | |
| 951004-1/11A | 0.073 ± 0.014 | B |
| 951004-1/14C | ND | |
| 001026-1/35C | 0.086 ± 0.022 | B |
| 001026-1/38B | 0.084 ± 0.022 | |
| 000720-1/59B | 0.097 ± 0.025 | |
| 000720-1/60C | 0.082 ± 0.022 |
Maximum growth rate at 15°C in TYB. ND, not done.
The strains were kept at −80°C in tryptone-yeast extract-salts broth (TYES-B) (14) with 15 to 20% glycerol, and the strains were always grown in agitated cultures at 15°C. The strains were taken directly from −80°C and incubated in TYES-B for a minimum of 48 h before further inoculations were made either for liquid cultures in TYES-B or on solid agar consisting of TYES-B with 1.1% agar (TYES-A). The growth rates of the isolates were estimated from the exponential increases in optical density at 525 nm (OD525) during 50-h incubations in agitated liquid cultures at 15°C.
Isolation of bacteriophages.
Bacteriophages were isolated from water and water-sediment samples from Danish freshwater rainbow trout farms by using enrichment cultures. A total of 65 samples from 17 different farms were analyzed. Approximately 25 ml of a 0.2-μm-filtered sample was mixed with 3 ml 10× TYES-B and with 2 ml of a mixture of the strains to be used in the enrichment (Table 1). The enrichment cultures were incubated for 5 to 7 days at 15°C with agitation to allow amplification of lytic F. psychrophilum bacteriophages. Following incubation, the culture was chloroform (32 μl/ml) extracted and the presence of lytic bacteriophages in supernatant was detected by a modified version of the double-layer method (2). One hundred microliters of bacteriophages was mixed with 300 μl of F. psychrophilum cells in the exponential growth phase (OD525 = 0.2 to 0.5) and incubated at 15°C for approximately 30 min. Four milliliters of 48°C top agar (TYES-B with 0.4% agar) was added, and the mixture was poured onto a cold TYES-A plate, which was immediately placed at 15°C. After incubation of the plates for 3 to 5 days at 15°C, the presence of lytic bacteriophages in the form of plaques was detected. Unless otherwise mentioned, the F. psychrophilum strain 950106-1/1 was always used in the double-layer method for all detections, isolations, and purifications of phages.
Purification of bacteriophages.
The bacteriophages were eluted by adding 5 ml of SM buffer (50 mM Tris-Cl, pH 7.5, 99 mM NaCl, 8 mM MgSO4, 0.01% gelatin) on top of the plate and incubated for a minimum of 2 h with shaking, followed by chloroform (50 μl/ml) extraction. For purification of single bacteriophages, a single plaque was picked with a sterile glass Pasteur pipette and the phages were eluted with shaking for a minimum of 2 h in SM buffer. After chloroform (50 μl/ml) extraction and centrifugation (9,000 × g, 20 min, 4°C), the supernatant was transferred to a new tube. Bacteriophages were isolated as single bacteriophages by three repeated rounds of plaque purification and reinfection. For determination of phage concentrations, serial dilutions in SM buffer were used with the double-layer method.
Purification of bacteriophage DNA.
Bacteriophage DNA was isolated by the method described in Su et al. (32), modified to fit the conditions for F. psychrophilum bacteriophages with respect to incubation time and temperature. Infection of F. psychrophilum strain 950106-1/1 with bacteriophage was done as described earlier, but with plates containing agarose instead of agar and with top agarose. When possible, enough bacteriophages (100 to 200 μl) were added to ensure confluent lysis on the plate. Following incubation at 15°C for 3 to 5 days, bacteriophages were eluted with 5 ml SM buffer per plate. After eluting for at least 2 h, the buffer was transferred to a 50-ml tube and the bacterial debris was pelleted by centrifugation (9,000 × g, 20 min, 4°C). The supernatant was treated with DNase I and RNase A (both at a final concentration of 1 μg ml−1) for 30 min at room temperature, followed by precipitation of bacteriophages with 2 M ZnCl2 (0.2-μm-filtered sample) added at the ratio 1:50 (vol/vol) by incubation at 37°C for 5 min. The bacteriophages were pelleted by centrifugation (9,000 × g, 5 min, 20°C). The pellet was resuspended in approximately 1:25 of the original volume in TENS buffer (50 mM Tris-HCl, pH 8.00, 100 mM EDTA, 100 mM NaCl, 0.3% sodium dodecyl sulfate), and proteinase K was added to give a final concentration of 100 μg ml−1. Following incubation at 65°C for 10 min, the solution was deproteinated twice by extraction with an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1). After deproteination, the DNA in the aqueous phase was precipitated with an equal volume of cold isopropanol and incubated at room temperature for 5 min, followed by centrifugation at 16,000 × g (10 min, 4°C). The DNA was washed twice with 70% ethanol, and finally, the pellet was dissolved in 50 to 100 μl of water.
Determination of genome size.
The genome sizes of bacteriophages were determined by pulsed-field gel electrophoresis (PFGE) of purified bacteriophage DNA (13). The electrophoresis was performed with a Bio-Rad CHEF DRIII system and carried out with 1% agarose gels in 0.5× Tris-borate-EDTA at 14°C for 22 h, using switch times ranging from 0.5 to 5 s (the highest switch times were used with the largest genomes) and a voltage of 6 V/cm. After electrophoresis, the DNA was visualized by staining with Sybr green I in 0.5× Tris-borate-EDTA. The PFGE size standards used were an 8- to 48-kb standard (Bio-Rad) and MidRange PFGE Marker I (New England Biolabs).
Determination of the phage host range and efficiency of plating.
The host ranges of the isolated bacteriophages were determined by spotting 2 μl of bacteriophage concentrate on top of a TYES-A plate freshly prepared with 4 ml of top agar inoculated with 0.3 ml of the strain to be tested. The host range was determined with three separate plates for each phage-host combination, and all 28 bacterial strains were tested against all the phage isolates. To determine whether the efficiencies of phage infection differed between phages and hosts, a number of different hosts were exposed to a given titer of selected phages and phage infectivity was quantified by a plaque assay.
Restriction digest patterns of selected bacteriophages.
Purified bacteriophage DNA was digested with either ClaI or EcoRI according to the manufacturer's recommendations, except that the restrictions were carried out overnight in order to ensure that all restrictions were complete. Samples were analyzed along with undigested phage DNA, and band patterns were visualized by PFGE.
Electron microscopy of bacteriophages.
Eight microliters of a concentrated bacteriophage suspension (minimum 107 PFU/ml) in SM buffer was spotted on top of a Formvar-carbon-coated copper grid (Ted Pella, Inc.), and the bacteriophages were allowed to adsorb for 2 min. Excess sample was removed by carefully touching the side of the grid with filter paper. Bacteriophages were stained by addition of 8 μl of 2% sodium phosphotungstate (pH 7.6). After 2 min, excess stain was removed and the grid was allowed to air dry for 10 min. The grids were observed with a Zeiss EM 900 transmission electron microscope (TEM) at 80 kV.
In some cases, the plate eluates needed to be concentrated prior to loading on the grids to obtain sufficiently high phage titers for TEM analysis. Concentration of phages was performed by ultracentrifugation (100,000 × g, 98 min, 20°C; SW55 Beckman) of 4 ml phage stock, followed by resuspension of the pellet in 50 μl SM buffer.
Determination of latency time and burst size.
One-step growth experiments were performed to determine the latency periods and burst sizes of selected bacteriophages (2). Phages were transferred to 1 ml of F. psychrophilum in the exponential growth phase at a multiplicity of infection (MOI) of approximately 0.001. Following incubation at 15°C for 10 min, the cells were harvested by centrifugation at 10,000 × g for 10 min at 15°C. Cells were then resuspended in 1 ml of cold TYES-B and inoculated to 60 ml of cold TYES-B. From this point on, samples were collected every 30 to 60 min for a minimum of 8 h. The concentration of bacteriophages was determined immediately by the double-layer method.
Phage adsorption rate.
The rate of adsorption of FpV-9 to its host cells was determined by adding the phage to a 30-ml culture of exponentially growing F. psychrophilum strain 950106-1/1 at an MOI of 0.0005 and incubating the infected culture at 15°C with agitation (36). Samples (150 μl) were taken at 0, 5, 10, 15, 20, 30, 40, 50, and 60 min, immediately diluted 1:10 in SM buffer with chloroform (50 μl ml−1), mixed thoroughly, and centrifuged. The supernatant was transferred to a new tube, and the number of unadsorbed bacteriophages on F. psychrophilum strain 950106-1/1 was determined by the double-layer method. Phage adsorption rate was determined as the exponential rate of decrease in PFU over time during incubation.
RESULTS
Bacteriophage isolation.
A total of 22 bacteriophages (Table 2) were isolated from rainbow trout farms both with and without outbreaks of RTFS at the time of sampling. F. psychrophilum phages were found in 31 of the 59 samples examined and in 9 out of 17 examined fish farms. The bacteriophages were all isolated following enrichment of water, water-sediment, or water-fish samples with either 2 (mixture A) or 13 (mixture B) F. psychrophilum strains in liquid cultures (Table 1).
TABLE 2.
Overview of the isolated Flavobacterium psychrophilum phagesa
| Group | Bacteriophage | Sample type | Fish farm | Sample mo and yr | Enrichment mixture | Genome size (kb) |
|---|---|---|---|---|---|---|
| I | FpV-1 | Water | C | June 2005 | A | 90 |
| FpV-2 | Water | C | June 2005 | A | 90 | |
| FpV-3 | Water with feces | K | June 2005 | A | 90 | |
| FpV-4 | Water with feces | L | June 2005 | A | 90 | |
| II | FpV-5 | Water | E | June 2005 | A | 48 |
| FpV-6 | Water | E | June 2005 | A | 48 | |
| FpV-7 | Water | E | June 2005 | A | 48 | |
| FpV-8 | Water | E | June 2005 | A | 48 | |
| FpV-9 | Water | F | July 2005 | A | 48 | |
| FpV-10 | Waterb | H | July 2005 | A | 48 | |
| FpV-11 | Waterb | H | July 2005 | A | 48 | |
| III | FpV-12 | Water | F | November 2005 | B | 12 |
| FpV-13 | Water | F | November 2005 | B | 12 | |
| FpV-14 | Water with feces | I | June 2005 | A | 12 | |
| FpV-15 | Water with feces | I | June 2005 | A | 12 | |
| FpV-16 | Water | O | November 2005 | B | 12 | |
| FpV-17 | Water | O | November 2005 | B | 12 | |
| FpV-18 | Water | P | November 2005 | B | 10 | |
| FpV-19 | Water with feces | L | June 2005 | A | 8 | |
| FpV-20 | Water with feces | L | June 2005 | A | ND | |
| FpV-21 | Water with feces | L | June 2005 | A | ND | |
| FpV-22 | Water | O | November 2005 | B | ND |
Letters C, E, F, H, I, K, L, O, and P indicate the different fish farms where the phages were isolated. ND, not done.
A dead fish was present in the water from the sampling time until the processing of the samples (∼24 h).
Phage genome sizes.
As a first approach to distinguish the isolated phages, they were separated according to genome size as determined by PFGE of phage DNA. The phages all fell into one of three genome size groups: group I (∼90 kb), group II (∼48 kb), or group III (∼8 to 12 kb). Several phages had similar genome sizes, and only two phages had unique genome sizes (Table 2). The resolution of genome size as obtained by PFGE is limited, especially in the high-genome-size ranges (probably determined at a 4- to 5-bp accuracy level), and it is therefore not possible to determine whether the genome sizes within the respective groups are exactly identical.
Host range and efficiency of plating.
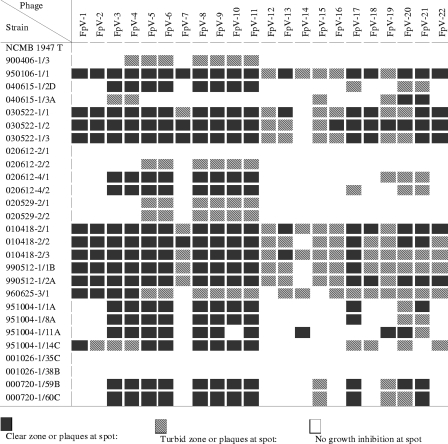
The host ranges of the isolated phages were tested for 28 F. psychrophilum strains originating from different fish (Table 1). Together, the phages were able to lyse 24 of the 28 strains tested, but with highly variable host ranges (Fig. 1). For example, phage FpV-14 infected only 5 out of 28 strains and showed only clear plaques on 1 of these strains (951004-1/11A), whereas turbid plaques were observed on the remaining 4 strains, indicating lysogeny or low probability of killing per infected cell. At the other end of the range, phage FpV-9 was able to lyse 23 of 28 strains, and of these, 18 were lysed efficiently (Fig. 1, clear plaques).
FIG. 1.
Host ranges of the isolated bacteriophages against selected F. psychrophilum strains.
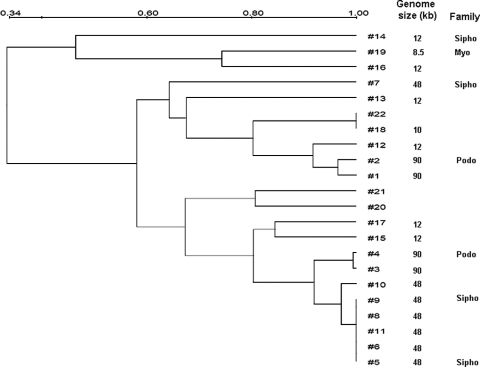
Based on the matrix of host ranges (lysis/no lysis) (Fig. 1), a similarity analysis (Dice's correlation coefficients) was performed using the clustering algorithm based on the unweighted-pair group method using average linkages (Quantity One, Bio-Rad) (Fig. 2). According to the analysis, the low-genome-size phages FpV-14, FpV-16, and FpV-19 seemed to branch out as a separate group quite different from the others. The remaining phages clustered into two groups with a similarity coefficient of about 0.6, which contained the full range of observed genome sizes as well as different viral morphotypes. Highly variable host ranges were observed within groups of phages with identical genome sizes. For example, all four phages in group 1 (90 kb) had unique host ranges, thus demonstrating that a given genome size may include many different phages. For the group 2 phages (48 kb), on the other hand, FpV-5, -6, -8, -9, and -11 had identical host ranges, and FpV-10 had a very similar host range, whereas FpV-7 showed little similarity in host range to the other six phages (Fig. 2). In general, the high-genome-size phages (FpV-1 to FpV-11) had the highest host ranges as well as the largest fractions of lytic infections of the selected F. psychrophilum strains and, therefore, the strongest lytic potential of the isolated phages.
FIG. 2.
Tree based on the unweighted-pair group method using average linkages for the F. psychrophilum phage isolates (“#” is followed by FpV number) based on their host ranges of infectivity against the 28 bacterial isolates. The data in Fig. 1 were scored and converted to pairwise distances by using the Dice similarity coefficient. Groupings of phages according to genome size and morphology are inserted to facilitate comparison.
Plate lysates produced from narrow-host-range phages (FpV-7 and FpV-14) were generally found to contain 103- to 107-fold fewer infective units than those from broad-host-range phages (FpV-4, FpV-9, and FpV-10) when tested against selected host strains (Table 3). Moreover, the efficiency of plating (i.e., the number of PFU in a given plate lysate on the lawn of a given host strain) also differed several orders of magnitude between phage-host pairs (Table 3). For example, a given plate lysate of FpV-4 yielded 7.8 × 108 ± 1.2 × 108 PFU on a lawn of F. psychrophilum type strain 950106-1/1 and only 3.5 × 102 ± 1.2 × 102 PFU on strain 900406-1/3.
TABLE 3.
Efficiencies of phage infection
| Phage | Result for indicated straina
|
|||||
|---|---|---|---|---|---|---|
| 950106-1/1 | 010418-2/3 | 900406-1/3 | 960625-3/1 | 951004-1/11a | 95100-1/14c | |
| FpV-4 | 7.8 × 108 ± 1.2 × 108 | 2.7 × 109 ± 4.2 × 108 | 3.5 × 102 ± 1.2 × 102 | 1.5 × 109 ± 3.3 × 108 | 1.5 × 108 ± 5.3 × 107 | ND |
| FpV-7 | 3.2 × 104 ± 7.8 × 103 | 4.1 × 104 ± 0.5 × 103 | ND | ND | ND | ND |
| FpV-9 | >1012 | 6.9 × 109 ± 3.5 × 108 | 1.5 × 105 ± 3.3 × 104 | ND | 2.8 × 1010 ± 4.3 × 109 | 2.8 × 1010 ± 2.3 × 109 |
| FpV-10 | 3.7 × 109 ± 2.5 × 108 | 5.4 × 109 ± 5.6 × 108 | ND | ND | ND | ND |
| FpV-14 | 6.4 × 103 ± 1.5 × 103 | 6.4 × 103 ± 0.4 × 103 | ND | 6.5 × 103 ± 0.7 × 103 | 5.7 × 103 ± 2.9 × 103 | ND |
Number of infecting units in phage plate lysates produced after infection of strain 950106-1/1 and subsequently exposed to selected host strains (i.e., the different strains were exposed to the same titer of the given phage). ND, not done.
Restriction digest patterns.
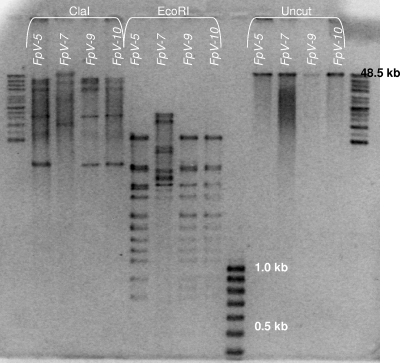
In order to examine whether phage isolates with similar genome sizes were genetically different, restriction analyses were performed for phages with genome sizes of 48 kb (Fig. 3). Of the analyzed group 2 phages (48 kb), FpV-5 and FpV-9 had identical host ranges, while FpV-10 showed 97.2% similarity to FpV-5 and FpV-9 (Fig. 2). In contrast, FpV-7 had a narrower host range. Both ClaI and EcoRI digests produced similar DNA fragment patterns for of FpV-5, FpV-9, and FpV-10, while phage FpV-7 differed from the other phages (Fig. 3).
FIG. 3.
Restriction digest analysis of selected bacteriophages with 48-kb genomes after treatment with enzymes ClaI and EcoRI. Lanes to the right are uncut phage DNA. The top band in the FpV-7 digest probably represents a fraction of undigested phage DNA.
Phage morphology.
Phage morphology was examined by TEM for seven different phages, representing the different genome size classes (Fig. 4 and Table 4). The separation of phages according to genome size also reflected systematic differences in morphology. The analyzed large-genome phages (90 kb, group 1) were characterized by having no or only short tails and were classified as Podoviridae according to the International Committee on Taxonomy of Viruses (1), whereas group 2 phages with smaller genomes (48 kb) had very distinct morphologies, with long flexible tails (172 to 240 nm), and belonged to Siphoviridae. Of the three analyzed group 2 phages, FpV-9 and FpV-10 were not statistically different, according to head and tail sizes (Table 3), although there seemed to be small morphological differences between them (Fig. 4), while FpV-7 showed significantly longer tails than the other two groups (Table 4). The last group, containing phages with small genomes (8 to 12 kb), were more variable but generally had larger heads (>80 nm) and tail diameters (>20 nm) than the other two groups and probably belonged to Myoviridae.
FIG. 4.
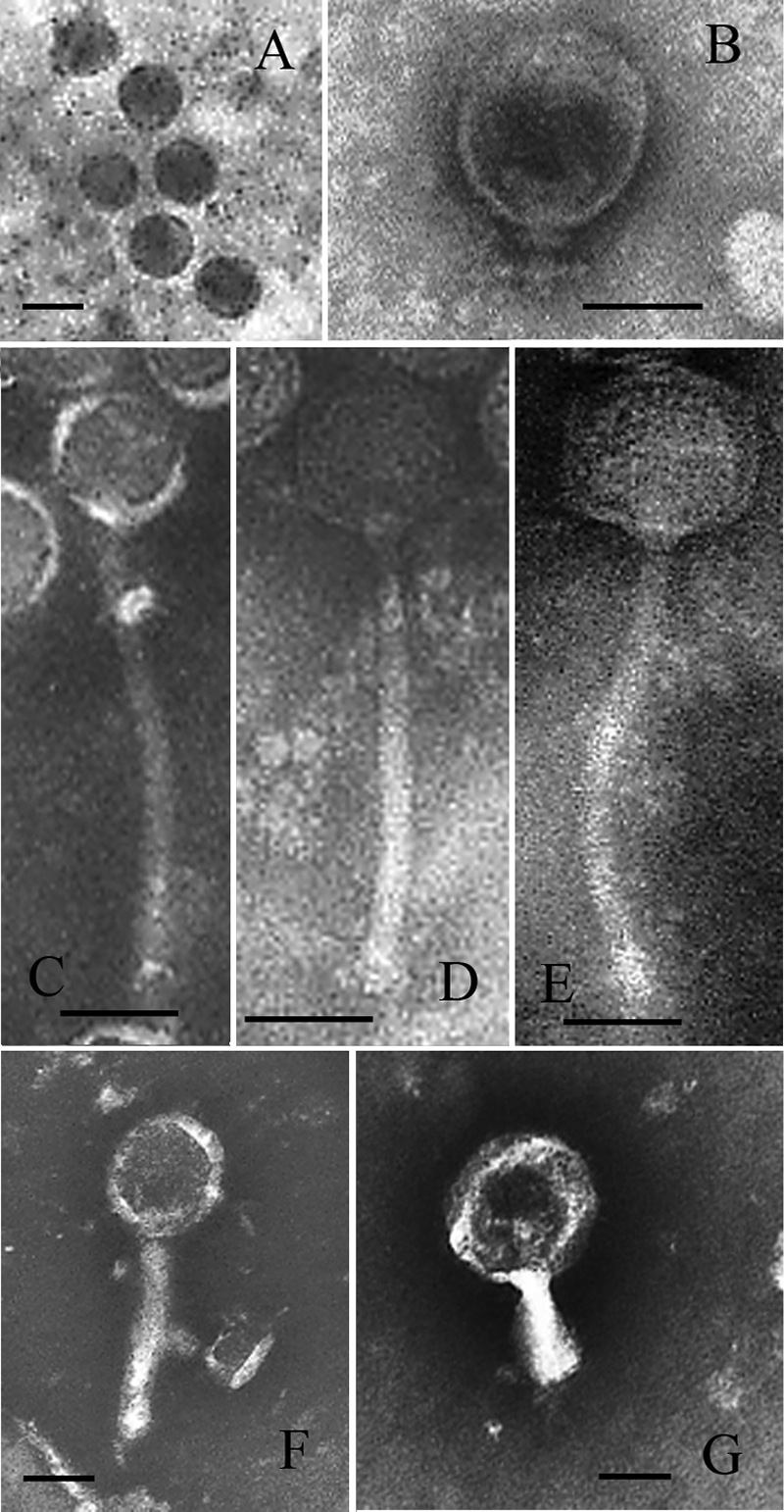
TEM pictures of selected bacteriophages. (A) FpV-2; (B) FpV-4; (C) FpV-7; (D) FpV-9; (E) FpV-10; (F) FpV-14; (G) FpV-19. Scale bar, 50 nm.
TABLE 4.
Morphological characteristics of selected phages
| Bacteriophage | Head diam (nm) | Head length (nm) | Tail diam (nm) | Tail length (nm) | Family |
|---|---|---|---|---|---|
| FpV-2 | 50.1 ± 2.2 | 57.5 ± 3.3 | None | None | Podoviridae |
| FpV-4 | 76.0 ± 4.0 | 75.0 ± 6.2 | 14.6 ± 3.6 | 26.1 ± 3.8 | Podoviridae |
| FpV-7 | 67.2 ± 9.0 | 68.0 ± 5.7 | 13.1 ± 1.4 | 239.6 ± 11.0 | Siphoviridae |
| FpV-9 | 60.1 ± 2.6 | 63.1 ± 3.1 | 10.8 ± 1.4 | 172.6 ± 10.7 | Siphoviridae |
| FpV-10 | 67.7 ± 4.8 | 65.8 ± 5.3 | 11.3 ± 0.8 | 181.8 ± 5.6 | Siphoviridae |
| FpV-14 | 86.0 ± 8.3 | 94.9 ± 11.5 | 24.3 ± 7.6 | 147.8 ± 18.3 | Myoviridae |
| FpV-19 | 127.2 ± 4.6 | 128.7 ± 7.1 | 28.7 ± 4.4 | 102.9 ± 2.5 | Myoviridae |
Susceptibility of F. psychrophilum to phage infection.
Of the 28 F. psychrophilum strains tested, only 4 were not infected by any of the isolated phages. These four strains included the type strain originally isolated from coho salmon, two strains isolated from feral rainbow trout without disease signs, and one strain that was isolated from a diseased rainbow trout. The remaining 24 bacterial strains showed large variations in phage susceptibility, ranging from susceptibility to all 22 isolates, found in 3 strains (950106-1/1, 010418-2/1, and 010418-2/3), to infection by only 6 phages and development of turbid plaques upon exposure to the phages, found in 3 strains (020612-2/2, 020529-2/1, and 020529-2/2).
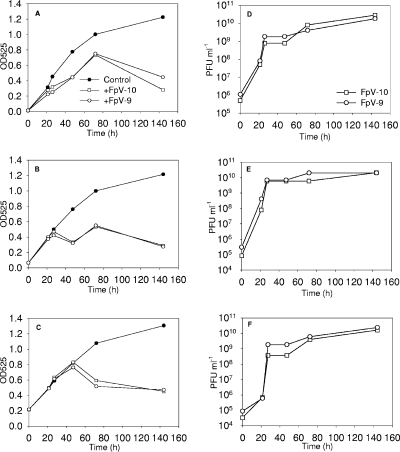
Infection experiments with FpV-9 and FpV-10 and F. psychrophilum strain 950106-1/1 performed in batch cultures at different MOI showed rapid propagation during the first 20 h of incubation for both phages, resulting in phage titers of 1.6 × 1010 to 2.8 × 1010 PFU ml−1, irrespective of the initial bacterial density (Fig. 5). However, reducing the initial OD525 from 0.216 to 0.013 delayed the maximum OD in the culture by approximately 24 h. In all the experiments, the ODs reached similar levels, corresponding to 64 to 77% reductions in OD relative to levels for control cultures without phages, suggesting that phage infection had a significant impact on host cell density.
FIG. 5.
Development in F. psychrophilum densities measured as changes in OD in the cultures (OD525) (A to C) and abundances of phages FpV-9 and FpV-10 measured as numbers of PFU (D to F) in enrichment cultures with various initial bacterial densities.
Bacterial growth and phage-host interactions.
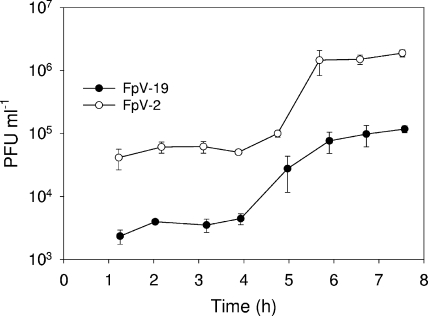
F. psychrophilum growth was relatively slow in the enriched liquid cultures, with maximum growth rates at 15°C, ranging from 0.062 ± 0.013 h−1 (020612-2/1) to 0.098 ± 0.025 h−1 (951004-1/1A). The one-step growth experiments showed no systematic changes in latency period and burst size with phage genome size (Fig. 6 and Table 5). In fact, FpV-2, FpV-9, and FpV-19, representing the different genome size groups, had very similar latency periods (4.0 to 4.5 h) and burst sizes (37 to 51 viruses infection−1). FpV-7, however, were less efficient than the other investigated phages and produced only 7 ± 1 phages after a latency period of 6 h. Interestingly, the lytic properties of individual phages were dependent on the host strain, as the burst sizes of phage FpV-9 were four times higher with strain 010418-2/3 than with strain 950106-1/1 used as a host strain (Table 5). The rate of adsorption of FpV-9 to F. psychrophilum strain 950106-1/1 was estimated at 0.016 ± 0.002 h−1, corresponding to the exponential decline of free phages during the incubation (Table 5).
FIG. 6.
Temporal development in PFU during one-step growth experiments with F. psychrophilum phages as exemplified by FpV-2 and FpV-19.
TABLE 5.
Estimated latency times, burst sizes, and adsorption rates of selected F. psychrophilum phages
| Phage | F. psychrophilum strain | Genome size (kb) | Latency time (h) | Burst size | Adsorption ratea (h) |
|---|---|---|---|---|---|
| FpV-2 | 950106-1/1 | 90 | 4.5 | 38 ± 6 | ND |
| FpV-7 | 950106-1/1 | 48 | 6.0 | 7 ± 1 | ND |
| FpV-9 | 950106-1/1 | 48 | 4.0 | 37 ± 12 | 0.016 ± 0.002 |
| FpV-9 | 010418-2/3 | 48 | 4.0 | 162 ± 12 | ND |
| FpV-19 | 950106-1/1 | 8 | 4.0 | 51 ± 7 | ND |
ND, not done.
DISCUSSION
Diversity of F. psychrophilum phages.
This study presents the first characterization of bacteriophages infecting the fish pathogen Flavobacterium psychrophilum. The high occurrence of F. psychrophilum phages in the investigated samples (48% of all samples) and fish farms (53% of all farms) suggested that F. psychrophilum phages are widespread in Danish rainbow trout aquaculture, even in periods without RTFS outbreaks. Eighteen of the 22 isolated phages showed unique host ranges of infectivity against 24 strains of F. psychrophilum, which all had unique phage susceptibility patterns. This high phage and host diversity within the Flavobacterium psychrophilum group supports recent studies of Cellulophaga baltica (13), Vibrio parahaemolyticus (6, 7), and E. coli (e.g., references 10 and 24) and indicates that interactions between F. psychrophilum and their phages are characterized by a high degree of complexity at the strain level. The facts that phages were found in more than 50% of the analyzed samples and that 18 of 22 F. psychrophilum phage isolates were unique with respect to host range suggested that the obtained phage isolates most likely represent only a small fraction of the actual F. psychrophilum phage diversity in aquaculture. However, the phages FpV-5, FpV-6, FpV-8, FpV-9, FpV-10, and FpV-11 all had similar genome sizes, very similar host ranges, and (for the analyzed FpV-5, FpV-9, and FpV-10 phages) similar restriction patterns but were isolated from three different fish farms (Table 2 and Fig. 1 and 3), indicating a higher occurrence of this phage or group of phages with relatively broad host ranges. None of the isolated phages were infective toward the type strain NCIMB 1947T. This strain was isolated in Washington State from Coho salmon, whereas all other strains originated from Danish fish farms, suggesting that the host ranges of F. psychrophilum phages are restricted to potentially cooccurring, and thus likely genetically related, host strains.
According to genome sizes, F. psychrophilum phages fell into at least three distinct groups of phages, which also showed some characteristic differences with respect to morphology, host range, and lytic potential. The intermediate-genome-size group (group II) had the largest host range among the three groups and together infected all susceptible hosts in the present study, with the exception of F. psychrophilum strain 040615-1/3A, which was susceptible only to group 1 and group 3 phages. Also, the large-genome phages in group 1 (90 kb) had relatively broad host ranges, whereas the small-genome phages in group 3 were much more restricted in host range among the tested hosts. In contrast to the generally positive correlation between phage head size and phage genome size (9), we found the smallest phage genomes in the relatively large phages belonging to Myoviridae.
The Dice correlation analysis of phage host ranges showed clustering of group 2 phages, suggesting that the phages in this group, with the exception of FpV-7, were indeed closely genetically related. Host range data were supported by the restriction analysis, as the restriction digest banding patterns of FpV-5, FpV-9, and FpV-10 were very similar but different from that of FpV-7. Despite a similar restriction pattern, FpV-10 showed a different pattern of infectivity against strain 951004-1/11A (Fig. 1), which indicates that FpV-10 is not identical to FpV-5 and FpV-9. The observation perhaps also indicates that determining the host ranges of closely related phages may be a more sensitive way of distinguishing between these phages than performing a restriction analysis. Generally, however, we cannot exclude on the basis of the used methods that FpV-5, FpV-6, FpV-8, FpV-9, and FpV-10 are not identical phages.
Small-genome phages did not show any systematic similarities in host range, and generally, the study demonstrated that similarity in genome size does not imply similarity in other phenotypic or genotypic characteristics of the phages, although this sometimes is the case. Likewise, similar host range does not imply that phages are genetically related, although that also tended to be the case for certain groups of phages. One consequence of these results is that analyses of natural viral communities based on genome size using PFGE fingerprinting severely underestimate actual viral diversity and should therefore be used only for comparative studies.
TEM analysis of selected phages from groups 1 and 2 to some extent supported their separation according to genome size in that these phages belonged to the families Podoviridae and Siphoviridae, respectively. Within the groups, however, different phages had distinct sizes and characteristics. Podoviridae and Siphoviridae phages are generally considered to have restricted host ranges compared with phages belonging to Myoviridae (33, 37). Thus, in contrast to results from these previous, mainly marine studies, these morphotypes are apparently characterized by infecting a wide range of F. psychrophilum hosts in trout aquaculture.
Phage-host interactions.
In addition to differences in host range, as discussed above, the individual F. psychrophilum phages also showed large differences in their lytic potentials against susceptible hosts. This was illustrated by the variability in efficiencies of infection against different F. psychrophilum strains, which covered 7 orders of magnitude. The generally low efficiencies of infection of FpV-4 and FpV-9 on strain 900406-1/3 were probably due to a lysogenic relationship between the phages and the host, as indicated by the turbid plaques produced after spotting (Fig. 1). Interestingly, the phages which produced the lowest plate lysate titers with F. psychrophilum strains 950106-1/1, FpV-7, and FpV-14 both had narrow host ranges, and FpV-7 also had the lowest burst size and the longest latency period of the examined phages. Generally, results from one-step growth experiments emphasized that burst size and latency period are important and variable parameters that show both phage specificity and host strain specificity. The mechanisms underlying these differences in lytic properties are not revealed in the present study. There is no indication of a relation between lytic potential and either the genome sizes or the morphological characteristics of the investigated phages, and the observed differences are probably to a large extent determined by the differences in phage receptor properties between host strains.
Each bacterial isolate had a distinct pattern of susceptibility to the 22 isolated phages and therefore probably represented unique F. psychrophilum strains, and phage susceptibilities are probably a highly variable and dynamic parameter among F. psychrophilum strains. Combined with the large host range heterogeneity in F. psychrophilum phages, this suggests that the clonal composition and dynamics of F. psychrophilum and their phages in Danish fish farms are influenced by a complex network of phage-host interactions, as was also found for another bacterial host, Cellulophaga baltica, also belonging to the Flavobacteriaceae group (13). Our results thus confirm the emerging view of highly variable strength in phage-host interactions and extremely diverse patterns of infectivity and susceptibility even within relatively narrow phylogenetic groups of bacteria (13).
The results suggest that a given bacterial species at all times may be represented by multiple strains, each with a unique pattern of susceptibility to a diverse community of cooccurring phages, which also have highly variable potentials for controlling the available host.
In culture systems, bacteria rapidly acquire resistance to cooccurring phages (e.g., references 4, 17, and 22), and model simulations have proposed that under well-defined growth conditions, populations of sensitive and resistant strains of a given bacterial phylotype may coexist but fluctuate as a function of phage abundance and differences in competitiveness for substrate (23). However, the accumulation of data that document an immense diversity in phage efficiency and host susceptibility, even within a narrow phylogenetic group of bacteria, suggests that phage-host interactions are even more complex than previously proposed from model studies and that any given phage-and-host community is characterized by a diversity of different properties. As phage infections represent a strong selection pressure on bacterial communities, we suggest that phage activity constitutes an important driving force for the large clonal diversity of F. psychrophilum strains found in Danish fish farms.
Potential for phage therapy treatment of RTFS.
Successful application of bacteriophages in the treatment of bacterial diseases requires a group of broad-host-range phages in order to cover a possible broad spectrum of potential pathogenic host strains associated with a disease outbreak. A combination of phages with different properties may thus provide the best starting point for further exploration of the potential of phage therapy for controlling pathogenic bacteria. In the present study, we have isolated a suite of lytic F. psychrophilum phages that were able to infect and lyse a wide range of F. psychrophilum strains. The most potent phages belonged to genome size group 1 and group 2, which together infected 24 of the 28 F. psychrophilum strains examined, including strain 950106-1/1, which is highly pathogenic to rainbow trout. This range of host strains was mainly covered by phages FpV-5, FpV-6, FpV-8, FpV-9, and FpV-11, which all had the same (very broad) host range. Moreover, FpV-9 also had the highest infection efficiency of the analyzed phages, and apparently, a combination of FpV-4, FpV-9, and FpV-21 would seem to constitute the most potent cocktail of the isolated phages, together infecting 24 of the 27 Danish F. psychrophilum strains, with 20 of the 24 phage-host interactions being lytic.
Infection experiments with FpV-9 and FpV-10 demonstrated that addition of phages to cultures of the pathogenic strain 950106-1/1 over a range of bacterial densities in all cases caused the population to crash and kept it at reduced densities for 6 days, while phages maintained high (∼1010 PFU ml−1) titers throughout the incubations. Consequently, phage infection indeed has the lytic capacity to build up large titers and control an exponentially growing F. psychrophilum population at 15°C, thus emphasizing the potential of using phages to reduce the impact of pathogenic bacteria. Obviously, as these experiments are carried out under optimal conditions for phage infection and propagation, it is not evident from these results to what extent significant phage control of F. psychrophilum can be obtained by addition of phages to infected fish in their natural environment.
All the isolated phages were infective to the pathogenic strain 950106-1/1, probably reflecting that the strain was used in all enrichment cultures for phage isolation. This observation suggested that it is probably possible to isolate phages for most bacterial strains that are or have been present in a Danish trout farm just by introducing them in enrichment cultures. In a phage therapy context, this is interesting, as it suggests that it is possible to assemble a phage library representing phages for the majority of F. psychrophilum strains present.
Detailed characterization of phage properties and phage-host interactions is a prerequisite for evaluating the potential of phages as controllers of a pathogenic host. In conclusion, the current characterization of F. psychrophilum phages demonstrated that phage isolates with strong lytic potential against pathogenic F. psychrophilum could be obtained from fish farms, thus providing a foundation for future exploration of their potential in the treatment of RTFS in rainbow trout aquaculture.
Acknowledgments
The study was supported by the Directorate for Food, Fisheries and Agribusiness and The Danish Natural Sciences Research Council.
We thank Jette Mundus Nikolajsen and Kirsten Kaas for excellent technical support.
Footnotes
Published ahead of print on 9 May 2008.
REFERENCES
- 1.Ackermann, H.-W. 2005. Bacteriophage classification, p. 67-90. In E. Kutter and A. Sulakvelidze (ed.), Bacteriophages: biology and applications. CRC Press, Boca Raton, FL.
- 2.Adams, M. H. 1959. Bacteriophages. Interscience Publishers, New York, NY.
- 3.Atterbury, R. J., P. L. Connerton, C. E. R. Dodd, C. E. D. Rees, and I. F. Connerton. 2003. Application of host-specific bacteriophages to the surface of chicken leads to reduction in the recovery of Campylobacter jejuni. Appl. Environ. Microbiol. 69:6302-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohannan, B. J. M., and R. E. Lenski. 2000. Linking genetic change to community evolution: insights from studies of bacteria and bacteriophage. Ecol. Lett. 3:362-377. [Google Scholar]
- 5.Bruun, M. S., A. S. Schmidt, L. Madsen, and I. Dalsgaard. 2000. Antimicrobial resistance patterns in Danish isolates of Flavobacterium psychrophilum. Aquaculture 187:201-212. [Google Scholar]
- 6.Comeau, A. M., A. M. Chan, and C. A. Suttle. 2006. Genetic richness of vibriophages isolated in a coastal environment. Environ. Microbiol. 8:1164-1176. [DOI] [PubMed] [Google Scholar]
- 7.Comeau, A. M., E. Buenaventura, and C. A. Suttle. 2005. A persistent, productive, and seasonally dynamic vibriophage population within Pacific oysters (Crassostrea gigas). Appl. Environ. Microbiol. 71:5324-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalsgaard, I., and L. Madsen. 2000. Bacterial pathogens in rainbow trout Oncorhyncus mykiss reared at Danish freshwater farms. J. Fish Dis. 23:199-209. [Google Scholar]
- 9.De Paepe, M., and F. Taddei. 2006. Viruses' life history: towards a mechanistic basis for a trade-off between survival and reproduction among phages. PLoS Biol. 4:1248-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer, C. R., M. Yoichi, H. Unno, and Y. Tanji. 2004. The coexistence of Escherichia coli serotype O157:H7 and its specific bacteriophage in continuous culture. FEMS Microbiol. Lett. 241:171-177. [DOI] [PubMed] [Google Scholar]
- 11.Goode, D., V. M. Allen, and P. A. Barrow. 2003. Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of bacteriophages. Appl. Environ. Microbiol. 69:5032-5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greer, G. G. 2005. Bacteriphage control of foodborne bacteria. J. Food Prot. 68:1102-1111. [DOI] [PubMed] [Google Scholar]
- 13.Holmfeldt, K., M. Middelboe, O. Nybroe, and L. Riemann. 2007. Large variabilities in host strain susceptibility and phage host range govern interactions between lytic marine phages and their Flavobacterium hosts. Appl. Environ. Microbiol. 73:6730-6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holt, R. A., J. S. Rohovec, and J. L. Fryer. 1993. Bacterial coldwater disease, p. 3-23 In V. Englis, R. J. Roberts, and N. R. Bromage (ed.), Bacterial diseases of fish. Blackwell Scientific Publications, Oxford, United Kingdom.
- 15.Imbeault, S., S. Parent, M. Lagacé, C. F. Uhland, and J.-F. Blais. 2006. Using bacteriophages to prevent furunculosis caused by Aeromonas salmonicida in farmed brook trout. J. Aquat. Anim. Health 18:203-214. [Google Scholar]
- 16.Karunasagar, I., M. M. Shivu, S. K. Girisha, G. Krohne, and I. Karunasagar. 2007. Biocontrol of pathogens in shrimp hatcheries using bacteriophages. Aquaculture 268:288-292. [Google Scholar]
- 17.Lenski, R. E. 1984. Coevolution of bacteria and phage: are there endless cycles of bacterial defenses and phage counterdefenses? J. Theor. Biol. 108:319-325. [DOI] [PubMed] [Google Scholar]
- 18.Leverentz, B., W. S. Conway, W. Janisiewich, and M. J. Camp. 2004. Optimizing concentration and timing of a phage spray application to reduce Listeria monocytogenes on honeydew melon tissue. J. Food Prot. 67:1682-1686. [DOI] [PubMed] [Google Scholar]
- 19.Lorenzen, E., I. Dalsgaard, J. From, E. M. Hansen, V. Hørlyck, H. Korsholm, S. Mellergaard, and N. J. Olesen. 1991. Preliminary investigations of fry mortality syndrom in rainbow trout. Bull. Eur. Assoc. Fish Pathol. 11:77-79. [Google Scholar]
- 20.Madsen, L., and I. Dalsgaard. 2000. Comparative studies of Danish Flavobacterium psychrophilum strains concerning ribotypes, plasmid profiles, serotypes and virulence. J. Fish Dis. 23:211-218. [Google Scholar]
- 21.Madsen, L., and I. Dalsgaard. 1999. Reproducible methods for experimental infection with Flavobacterium psychrophilum in rainbow trout Oncorhyncus mykiss. Dis. Aquat. Organ. 36:169-176. [DOI] [PubMed] [Google Scholar]
- 22.Middelboe, M. 2000. Bacterial growth rate and marine virus-host dynamics. Microb. Ecol. 40:114-124. [DOI] [PubMed] [Google Scholar]
- 23.Middelboe, M., Å. Hagstrom, N. Blackburn, B. Sinn, U. Fischer, N. H. Borch, J. Pinhassi, K. Simu, and M. G. Lorenz. 2001. Effects of bacteriophages on the population dynamics of four strains of pelagic marine bacteria. Microb. Ecol. 42:395-406. [DOI] [PubMed] [Google Scholar]
- 24.Mizoguchi, K., M. Morita, C. R. Fischer, M. Yoichi, Y. Tanji, and H. Unno. 2003. Coevolution of bacteriophage PP01 and Escherichia coli O157:H7 in continuous culture. Appl. Environ. Microbiol. 69:170-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakai, T., and K.-H. Park. 2002. Bacteriophage therapy of infectious diseases in aquaculture. Res. Microbiol. 153:13-18. [DOI] [PubMed] [Google Scholar]
- 26.Nakai, T., R. Sugimoto, K.-H. Park, K. Mori, T. Nishioka, and K. Maruyama. 1999. Protective effects of bacteriophage on experimental Lactococcus garvieae infection in yellowtail. Dis. Aquat. Organ. 37:33-41. [DOI] [PubMed] [Google Scholar]
- 27.Nematollahi, A., A. Decostere, F. Pasmans, and F. Haesebrouck. 2003. Flavobacterium psychrophilum infections in salmonid fish. J. Fish Dis. 26:563-574. [DOI] [PubMed] [Google Scholar]
- 28.O'Flynn, G., R. P. Ross, G. F. Fitzgerald, and A. Coffey. 2004. Evaluation of a cocktail of three bacteriophages for biocontrol of Escherichia coli O157:H7. Appl. Environ. Microbiol. 70:3417-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park, S. C., and T. Nakai. 2003. Bacteriophage control of Pseudomonas plecoglossicidae infection in ayu Plecoglossus altivelis. Dis. Aquat. Organ. 53:33-39. [DOI] [PubMed] [Google Scholar]
- 30.Park, S. C., I. Shimamura, M. Fukunaga, K.-I. Mori, and T. Nakai. 2000. Isolation of bacteriophages specific to a fish pathogen, Pseudomonas plecoglossicidae, as a candidate for fish disease control. Appl. Environ. Microbiol. 66:1416-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shivu, M. M., B. C. Rajeeva, S. K. Girisha, I. Karunasagar, G. Krohne, and I. Karunasagar. 2007. Molecular characterisation of Vibrio harveyi bacteriophages isolated from aquaculture environments along the coast of India. Environ. Microbiol. 9:322-331. [DOI] [PubMed] [Google Scholar]
- 32.Su, M.-T., T. V. Venkatesh, and R. Bodmer. 1998. Large- and small-scale preparation of bacteriophage λ lysate and DNA. BioTechniques 25: 44-46. [DOI] [PubMed] [Google Scholar]
- 33.Suttle, C. A., and A. M. Chan. 1993. Marine cyanophages infecting oceanic and costal strains of Synechococcus: abundance, morphology, cross-infectivity and growth characteristics. Mar. Ecol. Prog. Ser. 92:99-109. [Google Scholar]
- 34.Tanji, Y., T. Shimada, M. Yoichi, K. Miyanaga, K. Hori, and H. Unno. 2004. Toward rational control of Escherichia coli O157:H7 by a phage cocktail. Appl. Microbiol. Biotechnol. 64:270-274. [DOI] [PubMed] [Google Scholar]
- 35.Vinod, M. G., M. M. Shivu, K. R. Umesha, B. C. Rajeeva, G. Krohne, I. Karunasagar, and I. Karunasagar. 2006. Isolation of Vibrio harveyi bacteriophage with a potential for biocontrol of luminous vibriosis in hatchery environments. Aquaculture 255:117-124. [Google Scholar]
- 36.Weiss, B. D., M. A. Capage, M. Kessel, and S. A. Benson. 1994. Isolation and characterization of a generalized transducing phage for Xanthomonas campestris pv. campestris. J. Bacteriol. 176:3354-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wichels, A., S. S. Biel, H. R. Gelderblom, T. Brinkhoff, G. Muyzer, and C. Schütt. 1998. Bacteriophage diversity in the North Sea. Appl. Environ. Microbiol. 64:4128-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiklund, T., L. Madsen, M. S. Bruun, and I. Dalsgaard. 2000. Detection of Flavobacterium psychrophilum from fish tissue and water samples by PCR amplification. J. Appl. Microbiol. 87:299-307. [DOI] [PubMed] [Google Scholar]