Abstract
The surface plasmon resonance (SPR) technique is a well-established method for the measurement of molecules binding to surfaces and the quantification of binding constants between surface-immobilized proteins and proteins in solution. In this paper we describe an extension of the methodology to study bacteriophage-bacterium interactions. A two-channel microfluidic SPR sensor device was used to detect the presence of somatic coliphages, a group of bacteriophages that have been proposed as fecal pollution indicators in water, using their host, Escherichia coli WG5, as a target for their selective detection. The bacterium, E. coli WG5, was immobilized on gold sensor chips using avidin-biotin and bacteriophages extracted from wastewater added. The initial binding of the bacteriophage was observed at high concentrations, and a separate, time-delayed cell lysis event also was observed, which was sensitive to bacteriophage at low concentrations. As few as 1 PFU/ml of bacteriophage injected into the chamber could be detected after a phage incubation period of 120 min, which equates to an approximate limit of detection of around 102 PFU/ml. The bacteriophage-bacterium interaction appeared to cause a structural change in the surface-bound bacteria, possibly due to collapse of the cell, which was observed as an increase in mass density on the sensor chip. These results suggest that this methodology could be employed for future biosensor technologies and for quantification of the bacteriophage concentration.
The monitoring of fecal pollution in wastewater and river water is becoming increasingly important as societies throughout the world become more urbanized. In order to diminish the risk of disease and a breakdown in public health, good fecal indicators are needed. Traditionally, Escherichia coli and/or Enterococcus spp. have been used as microbial indicators to monitor fecal pollution in water. However, it has been suggested that these bacterial strains are not good for predicting contamination of viruses transmitted via the fecal route. In the past two decades, it has been suggested that the presence of bacteriophages, which often exist synergistically with bacterial populations, may also be an indicator of the presence of viruses of fecal origin (7, 13, 21, 26, 27). A recent report from the U.S. Environmental Protection Agency stated that somatic coliphages have been included as fecal indicators of viral origin (28). Somatic coliphages are viruses that infect E. coli and are very good indicators of the presence of fecal contamination because they can infect only fecal bacteria. Hence, detection of somatic coliphages gives an indication of both bacterial and viral contamination levels in water.
Traditional methods to detect bacteriophages include the double-layer assay method, which is based on the deposition of a semisolid agar layer containing the host strain of the bacteriophage to be detected and the sample which is the object of study on an agar plate. Quantification of the lysis zones, visualized after an appropriate incubation time, is related to the number of bacteriophages present in the original sample (1). This method, used for the quantification of somatic coliphages, has recently been standardized (2, 3).
A wide variety of biosensors, including electrochemical (amperometric and impedimetric), piezoelectric (quartz crystal microbalance), and optical (surface plasmon resonance [SPR]) devices, have been developed for the detection of viral and bacterial pathogens (11, 20).
SPR is an evanescent-wave phenomenon whereby visible light can be coupled into the electron field (plasmon wave) in a thin metal film (usually gold or silver) to create an energy wave (SPR). This energy wave is not confined within the metal film but extends out into the dielectric above the metal film, where it decays exponentially with distance from the surface. In most commercial SPR instruments, such as the Ecochemie instrument used in this work or the well-known Biacore instrument, a glass prism is used to couple laser light into the metal film surface (19). At a certain angle of incidence of incident light with the metal prism, maximum coupling of the light with the surface plasmon occurs. This is the angle of resonance. The angle of resonance depends on the dielectric constant of (among other things) the material on the prism. Hence, binding of a film on the metal surface causes a change in the dielectric constant, which changes the angle of resonance. This change in the resonance angle can be followed in real time, thus providing kinetic information on film formation.
In recent years this technique has been improved, and a wide variety of SPR-based biosensors have been developed (see reference 15 for a review of advances in SPR technology). SPR, as well as being used to quantify protein-protein interactions (22, 29), has been shown to have wider applications beyond protein binding measurement, for example, the detection of hormones (14) and pesticides (23). It is also being used in applications previously studied exclusively using classical immunoassays, spectroscopic techniques, or chromatographic methods, since it can give real-time binding information and hence binding kinetics and enhanced quantification of the response (4). In recent years, some SPR detection protocols have been described for the detection of certain viruses (5, 6). However, they have focused mainly on the detection of animal virus particles rather than the intact form of viruses (30), while detection of bacteriophages, i.e., viruses that infect bacteria, remains an unexplored subject. Recent work by Jenkins has shown that SPR is sensitive to bacterial attachment, despite the fact that bacteria are larger than the evanescent-decay length of the resonant surface plasmons. Moreover, SPR could accurately discriminate between pil mutants of Pseudomonas aeruginosa and the wild type during ear-stage attachment (16).
In this study, the utility of SPR for the detection of somatic bacteriophages in water samples was investigated. This study showed that SPR could be used to monitor bacteriophage attachment on chips modified with surface-attached bacteria but also showed that SPR could measure the time-delayed bactericidal behavior of the bacteriophages, showing bacterial structural collapse on the surface.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Escherichia coli ATCC 700078, also known as WG5 (2, 12), was grown at 37°C with constant agitation at 100 rpm in modified Scholten's broth (MSB) supplemented with nalidixic acid (250 μg/ml) to prepare an overnight culture. This culture was diluted 1:100 into fresh MSB and incubated again until an optical density at 600 nm of 0.3 was reached. The culture was then used for either the measurement of somatic coliphage interaction or the preparation of stock cultures for bacteria immobilization.
Bacteriophage enumeration and preparation of stock cultures.
Bacteriophage φX174 (ATCC 13706-B1) was used as a reference bacteriophage for somatic coliphages. Enumeration of this bacteriophage was performed by the double-layer agar method (1). Enrichment cultures of this bacteriophage were prepared according to ISO 10705-2 (2). Briefly, 25 ml of an E. coli WG5 culture prepared as indicated above was diluted 1:100 in 25 ml of fresh MSB and incubated with a stock of 102 PFU/ml for 5 h at 37°C. Then, 2.5 ml of chloroform was added and the mixture was left overnight at 4°C. The aqueous phase was centrifuged at 3,000 × g for 20 min, and the supernatant was stored at 4°C. The supernatant was serially diluted (1:10) into phosphate-buffered saline (PBS) and enumerated with the double-layer method as indicated above before the SPR measurements were taken.
Preparation of stock cultures of biotinylated bacteria.
E. coli WG5 culture, prepared as previously described (optical density at 600 nm = 0.3), was used to prepare stock cultures for the immobilization of bacteria on gold-coated-glass sensor chips (Windsor Scientific Ltd., Slough, United Kingdom). Avidin was adsorbed directly onto the gold and bacteria subsequently immobilized on the chip via avidin-biotin interactions, with the bacteria being directly tagged with biotin labels. Biotinylation of bacteria was performed as follows: 10 ml of E. coli culture was centrifuged at 16,000 × g for 5 min and washed three times with chilled PBS (pH 8.0) to remove the culture medium and was incubated for 1 h in ice with 1 mg/ml of Sulfo-NHS-XX-Biotin (Invitrogen, Barcelona, Spain). The biotinylated cells were then washed three more times with chilled PBS to remove residual biotin. Finally, the cells were recovered and resuspended in PBS with 10% sterile glycerol and preserved in 200-μl aliquots at −20°C until used. Bacterial counts were performed in triplicate by 1:10 serially diluting the aliquot in PBS and incubating the lowest dilutions in MSB agar at 37°C for 24 h, giving a final concentration of 8.37 (±0.25) log (CFU/ml).
Assay protocol for SPR measurements.
Detection of reference bacteriophage φX174 and naturally occurring bacteriophages in wastewaters from urban treatment plants was conducted using the two-chamber SPR Autolab ESPRIT system (Eco Chemie, Utrecht, The Netherlands). The two chambers enabled two assays to be carried out simultaneously, although one chamber was always used as a control. To begin the measurement, the device was allowed to settle by injecting 50 μl of PBS into the chamber. Then, 50 μl of avidin (0.5 mg/ml) was injected and adsorbed onto the gold film for 10 min without using a gold-immobilized biotin monolayer. Avidin binding was monitored by SPR. After washing with PBS, 50 μl of biotinylated cells were incubated in the chamber for 1 h to allow the bacteria to be immobilized on the surface. Then, a wash step with PBS was performed to remove nonbound bacteria. The environmental samples containing bacteriophages were diluted 1:10 into MSB, and 50 μl of the mixture was injected into the chamber to allow the bacteriophages to replicate within their host, E. coli WG5. The SPR reflectance minima response was monitored over a minimum of 100 min, with a measurement being taken every 3 s. The instrument and all binding steps were kept at 37°C. All the experiments were performed in triplicate.
Wastewater sample collection and processing.
Three samples from different urban wastewater treatment plants were collected (Table 1) and transported to the laboratory according to standard methods (10). Samples were stored at −70°C to avoid inactivation of the bacteriophages (24) until used. Processing of the samples included a centrifugation step of 10 min at 1,000 × g and filtration through a sterile 0.22-μm-pore filter (Millipore Corporation, Barcelona, Spain). The filtered suspension was used for the enumeration and detection of somatic coliphages with the double-layer method and SPR, respectively.
TABLE 1.
Samples analyzed in this study
| Sample | Origin | Bacteriophage counts, log(PFU/ml) ± SD |
|---|---|---|
| GA2007 | Wastewater treatment plant 1 | 4.90 ± 0.01 |
| HM2001 | Wastewater treatment plant 2 | 3.81 ± 0.01 |
| HM1402 | Wastewater treatment plant 3 | 3.84 ± 0.02 |
| φX174 stock | Laboratory enrichment | 9.79 ± 0.02 |
RESULTS AND DISCUSSION
Bacteriophage φX174 and wastewater sample coliphage quantification by the double-layer agar method.
The bacteriophage φX174 stock culture prepared for the calibration of the SPR assay was enumerated by the double-layer agar method, giving rise to a final bacteriophage concentration of 9.79 log (PFU/ml) units (Table 1). Additionally, the concentrations of somatic coliphages present in three different raw wastewater samples were quantified and found to have values between 3.8 and 4.9 log (PFU/ml) units. These values are consistent with other previous reports of somatic coliphages in wastewater samples of similar characteristics (25).
Bacterial immobilization on avidin.
Functionalization of the gold disk with avidin was used to irreversibly attach biotinylated E. coli to avidin via avidin-biotin interactions (Fig. 1 and 2). Direct adsorption of E. coli to avidin has been previously documented (18). However, in this study direct adsorption of bacteria to avidin-coated-gold disks gave an insufficient density of attached bacteria. As a consequence, biotinylation of the surface proteins of the bacterial envelope was necessary, with the strong avidin-biotin binding being used to irreversibly immobilize the bacteria. The results obtained here suggest that biotinylation of the surface proteins did not alter the functionality of the bacteriophage receptor of the host strain. In relation to this procedure, it has been reported that avidin-biotin immobilization generally maintains biomolecule activity more effectively than other methods (9).
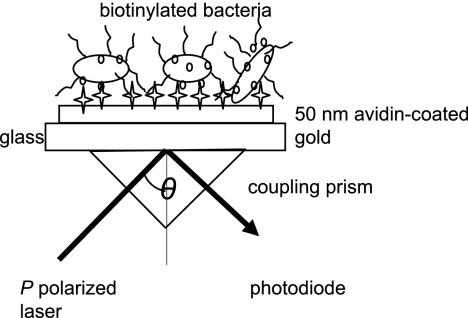
FIG. 1.
Schematic representation of the SPR setup used in this study (adapted from the work of Jenkins et al. [16]). P-polarized light is reflected, forming an energy wave at the gold surface. The angle (θ) of incident light which forms maximum excitation is sensitive to changes in the dielectric properties of the molecules attached to the gold film surface.
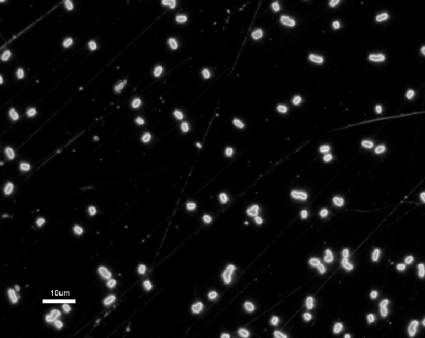
FIG. 2.
Adsorption of E. coli onto the gold through avidin-biotin interactions. Optical micrograph in dark field; magnification, ×1,000.
SPR measurements.
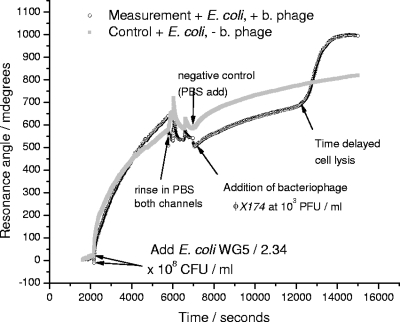
Figure 3 shows the measured SPR response as the biotin-tagged E. coli binds to the avidin-coated sensor chip. The reflectance angular minimum is proportional to the mass density of material on the chip surface. For clarity, the binding of avidin is not shown, but the initial binding of the bacteria and the subsequent, time-delayed increase in the reflectance angle minimum in the channel containing bacteriophage can be seen. The increase in the reflectance angle minimum for the control, following rinsing in PBS, is attributed to changes in the structure of the attached bacterial film.
FIG. 3.
A typical SPR reflectance angle minimum-time response used for the detection of bacteriophage (b. phage) φX174 and other somatic coliphages present in the wastewater samples. Arrows indicate the different steps from the cycle that were described in Materials and Methods: (i) incubation of the host strain E. coli WG5, (ii) PBS washing, and (iii) addition of the sample bacteriophage in one chamber (and negative control in the other chamber).
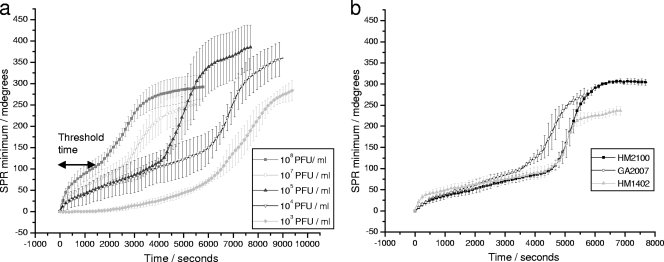
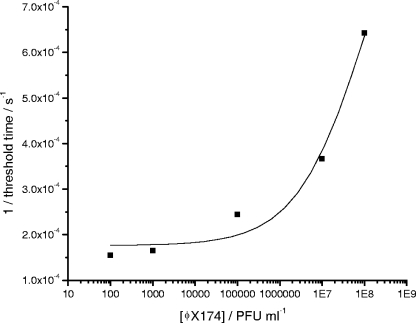
The time delay before the increase in the reflectance angle minimum for the system with bacteriophage was inversely proportional to the concentration of bacteriophages present in the sample (Fig. 4 and 5). As few as 102 PFU/ml in the initial sample could be detected after an incubation time of 120 min. Considering that a single bacteriophage can give rise to 20 to 50 new bacteriophages per infected host cell in a lapse time of around 20 min (17), it can be estimated that more than 106 bacteriophages could be produced in 120 min from a single bacteriophage. The shift in the reflectance angle minimum (m°) detected is hypothesized to be caused by the attachment of released phage particles and due to the collapse of bacterial cells with associated fragments of bacterial membranes and intracellular proteins adhering to the gold film. Choi et al. previously observed an increase in the SPR reflectance angle minimum following exposure of surface-attached E. coli to phenol. An increase of 60 m° was observed after exposure to different phenol concentrations due to bacterial membrane damage (8).
FIG. 4.
Variation of the reflectance angle minimum in response time detection with different concentrations of bacteriophages. (a) Detection of various concentrations of bacteriophage φX174 as measured by the SPR minimum angle change with time. Threshold time for 108 PFU/ml φX174 is marked on the plot for illustration. (b) Mean values of SPR minimum angle with time for detection of somatic coliphages in wastewater samples. Error bars show standard errors for triplicate repeats.
FIG. 5.
Plot of reciprocal of threshold time (i.e., when SPR reflectance angle minimum starts to rapidly increase due to bacteriophage replication) versus bacteriophage concentration. Fitting is a best-fit sigmoidal function.
To further test this hypothesis, the membrane-lysing enzyme phospholipase A2 (1 μg/ml) was added to bound bacteria in a separate experiment, with a very similar increase in mass density following enzyme binding and cell membrane lysis being observed.
Detection of somatic coliphages in wastewater samples.
Three samples from different wastewater treatment plants were evaluated for the presence of bacteriophages using both the SPR method and the traditional double-layer agar method. The curve shapes obtained after the addition of the wastewater samples were similar to those of the bacteriophage φX174. Moreover, the time lapse before the increase of the reflectance angle minimum was similar to that expected according to the calibration of bacteriophage φX174 and concentration (PFU/ml) of bacteriophages in the samples as estimated by the double-layer agar method (Table 1).
As far as we know, this is the first attempt at real-time monitoring of the presence of somatic coliphages in wastewater samples using SPR measurements. Moreover, the method described here possesses the two characteristics which are desired for the development of a biosensor: a high analyte specificity, which relies on the specificity of the host-phage interaction, and a high sensitivity, in this case realized by using the SPR technique. This new approach could in principle be applied in the development of miniaturized and portable SPR biosensors to detect other bacteriophages of interest in industry, which need to be detected in a short period of time to prevent the lysis of starter cultures, by just adapting the host strain to the bacteriophage to be detected.
Conclusions.
In this study, we have shown that the presence of bacteriophages in water samples can be monitored in real time by the use of the SPR technique using their host bacteria adsorbed to gold films as a biorecognition element. The detection limit of the method was estimated to be around 102 PFU/ml, requiring a minimum incubation time of 120 min for detection. All the experiments were performed in a closed, nonflowing system in a semiautomated process. However, automation of the whole process could in principle be achieved in most SPR instruments. These results are very encouraging for the future development of portable biosensors for detection of bacteriophage and other fecal indicators because it offers the possibility of making automated and real-time measurements in the field.
Acknowledgments
We are grateful to the team of Nick Waterfield from the Biology and Biochemistry Department for their scientific and technical assessments.
This work was supported by the international mobility program of the Consejo Superior de Investigaciones Cientificas. Cristina García-Aljaro is a beneficiary of the Beatriu de Pinós program from the Departament d'Universitats, Recerca i Societat de la Informació de la Generalitat de Catalunya.
Footnotes
Published ahead of print on 9 May 2008.
REFERENCES
- 1.Adams, M. H. 1959. Bacteriophages. Interscience Publishers, Inc., New York, NY.
- 2.Anonymous. 2000. ISO 10705-2: water quality. Detection and enumeration of bacteriophages—part 2: enumeration of somatic coliphages. International Organisation for Standardisation, Geneva, Switzerland.
- 3.Anonymous. 2001. Method 1602: male-specific (F+) and somatic coliphage in water by single agar layer (SAL) procedure. EPA 821-R-01-029. Office of Water, U.S. Environmental Protection Agency, Washington, DC.
- 4.Atkins, K. L., J. Burman, E. Deavin, J. E. Cooper, B. Poutrel, A. T. A. Jenkins, E. J. Feil, and J. M. H. van den Elsen. 2008. Staphylococcus aureus immunoglobulin-binding proteins SpA and Sbi utilize differential molecular mechanisms in the formation of insoluble immune complexes and show no host-specificity in IgG binding. Mol. Immunol. 45:1600-1611. [DOI] [PubMed] [Google Scholar]
- 5.Baac, H., J. P. Hajos, J. Lee, D. Kim, S. J. Kim, and M. L. Shuler. 2006. Antibody-based surface plasmon resonance detection of intact viral pathogen. Biotechnol. Bioeng. 94:815-819. [DOI] [PubMed] [Google Scholar]
- 6.Boltovets, P. M., B. A. Snopok, V. R. Boyko, T. P. Shevchenko, N. S. Dyachenko, and Y. M. Shirshov. 2004. Detection of plant viruses using a surface plasmon resonance via complexing with specific antibodies. J. Virol. Methods 121:101-106. [DOI] [PubMed] [Google Scholar]
- 7.Borrego, J., M. Moriñigo, A. De Vicente, R. Córnax, and P. Romero. 1987. Coliphages as an indicator of faecal pollution in water. Its relationship with indicator and pathogenic microorganisms. Water Res. 21:1473-1480. [Google Scholar]
- 8.Choi, J. W., K. W. Park, D. B. Lee, W. Lee, and W. H. Lee. 2005. Cell immobilization using self-assembled synthetic oligopeptide and its application to biological toxicity detection using surface plasmon resonance. Biosens. Bioelectron. 20:2300-2305. [DOI] [PubMed] [Google Scholar]
- 9.Da Silva, S., L. Grosjean, N. Ternan, P. Mailley, T. Livache, and S. Cosnier. 2004. Biotinylated polypyrrole films: an easy electrochemical approach for the reagentless immobilization of bacteria on electrode surfaces. Bioelectrochemistry 63:297-301. [DOI] [PubMed] [Google Scholar]
- 10.Eaton, A. D., L. S. Clesceri, E. W. Rice, and E. Greenberg. 2005. Standard methods for the examination of water and wastewater. American Public Health Association, American Water Works Association & Water Environment Federation, Washington, DC.
- 11.Gau, J. J., E. H. Lan, B. Dunn, C. M. Ho, and J. C. Woo. 2001. A MEMS based amperometric detector for E. coli bacteria using self-assembled monolayers. Biosens. Bioelectron. 16:745-755. [DOI] [PubMed] [Google Scholar]
- 12.Grabow, W. O. K., and P. Coubrough. 1986. Practical direct plaque assay for coliphages in 100-milliliter samples of drinking water. Appl. Environ. Microbiol. 52:430-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grabow, W. O. K. 2001. Bacteriophages: update on application as model for viruses in water. Water SA 27:251-268. [Google Scholar]
- 14.Habauzit, D., J. Chopineau, and B. Roig. 2007. SPR-based biosensors: a tool for biodetection of hormonal compounds. Anal. Bioanal. Chem. 387:1215-1223. [DOI] [PubMed] [Google Scholar]
- 15.Hoa, X. D., A. G. Kirk, and M. Tabrizian. 2007. Towards integrated and sensitive surface plasmon resonance biosensors: a review of recent progress. Biosens. Bioelectron. 23:151-160. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins, A. T. A., A. Buckling, M. McGhee, and R. H. French-Constant. 2005. Surface plasmon resonance shows that type IV pili are important in surface attachment by Pseudomonas aeruginosa. J. R. Soc. Interface 22:255-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kokjohn, T. A., G. S. Sayler, and R. V. Miller. 1991. Attachment and replication of Pseudomonas aeruginosa bacteriophages under conditions simulating aquatic environments. J. Gen. Microbiol. 137:661-666. [Google Scholar]
- 18.Korpela, J., E. M. Salonen, P. Kuusela, M. Sarvas, and A. Vaheri. 1984. Binding of avidin to bacteria and to the outer membrane porin of Escherichia coli. FEMS Microb. Lett. 22:3-10. [Google Scholar]
- 19.Kretschmann, E. Z. 1971. Determination of the optical constants of metals by excitation of surface plasmons. Physics 241:313-324. [Google Scholar]
- 20.Kurosawa, S., J. W. Park, H. Aizawa, S. Wakida, H. Tao, and K. Ishihara. 2006. Quartz crystal microbalance immunosensors for environmental monitoring. Biosens. Bioelectron. 22:473-481. [DOI] [PubMed] [Google Scholar]
- 21.Lasobras, J., J. Dellunde, J. Jofre, and F. Lucena. 1999. Occurrence and levels of phages proposed as surrogate indicators of enteric viruses in different types of sludges. J. Appl. Microbiol. 86:723-729. [DOI] [PubMed] [Google Scholar]
- 22.Liu, S., M. L. M. Vareiro, S. Fraser, and A. T. A. Jenkins. 2005. Control of attachment of bovine serum albumin to pulse plasma-polymerized maleic anhydride by variation of pulse conditions. Langmuir 21:8572-8575. [DOI] [PubMed] [Google Scholar]
- 23.Mauriz, E., A. Calle, J. J. Manclús, A. Montoya, and L. M. Lechuga. 2007. Multi-analyte SPR immunoassays for environmental biosensing of pesticides. Anal. Bioanal. Chem. 387:1449-1458. [DOI] [PubMed] [Google Scholar]
- 24.Mendez, J., J. Jofre, F. Lucena, N. Contreras, K. Mooijman, and R. Araujo. 2002. Conservation of phage reference materials and water samples containing bacteriophages of enteric bacteria. J. Virol. Methods 106:215-224. [DOI] [PubMed] [Google Scholar]
- 25.Mocé-Llivina, L., M. Muniesa, H. Pimenta-Vale, F. Lucena, and J. Jofre. 2003. Survival of bacterial indicator species and bacteriophages after thermal treatment of sludge and sewage. Appl. Environ. Microbiol. 69:1452-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mocé-Llivina, L., M. Muniesa, H. Pimenta-Vale, F. Lucena, and J. Jofre. 2005. Enteroviruses and bacteriophages in bathing waters. Appl. Environ. Microbiol. 71:6838-6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Traub, F., S. K. Spillmann, and R. Wyler. 1986. Method for determining virus inactivation during sludge treatment processes. Appl. Environ. Microbiol. 52:498-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. Environmental Protection Agency. 2006. National primary drinking water regulations; ground water rule; final rule (40 CFR parts 9, 141, 142). Fed. Regist. 71:65573-65660. [Google Scholar]
- 29.Vareiro, M. L., M. J. Liu, W. Knoll, K. Zak, D. Williams, and A. T. A. Jenkins. 2005. Surface plasmon fluorescence measurements of human chorionic gonadotrophin: role of antibody orientation in obtaining enhanced sensitivity and limit of detection. Anal. Chem. 77:2426-2431. [DOI] [PubMed] [Google Scholar]
- 30.Wittekindt, C., B. Fleckenstein, K. Wiesmuller, B. R. Eing, and J. E. Kuhn. 2000. Detection of human serum antibodies against type-specifically reactive peptides from the N-terminus of glycoprotein B of herpes simplex virus type. J. Virol. Methods 87:133-144. [DOI] [PubMed] [Google Scholar]