Abstract
We developed an immunomagnetic separation (IMS) technique combined with real-time TaqMan reverse transcriptase PCR (RT-PCR), which allowed detection of norovirus at a level as low as 3 to 7 RT-PCR units from artificially contaminated strawberries. The inoculum recovery rate ranged from 14 to 30%. The data demonstrate that IMS combined with real-time RT-PCR will be useful as a rapid and sensitive method for detecting food-borne microbial contaminants.
Norovirus (NoV) is the leading cause of acute nonbacterial gastroenteritis worldwide that is transmitted via the fecal-oral route from contaminated food (2, 14, 19). One of the major limitations for monitoring NoV-contaminated foods and investigating outbreaks is the difficulty of detecting low levels of NoV in various food matrices. Unlike many bacterial and viral pathogens, NoV cannot be cultivated by conventional tissue culture methods; therefore, it is difficult to cultivate and enrich NoV from contaminated foods to monitor for NoV outbreaks (9). Molecular detection methods such as reverse transcriptase PCR (RT-PCR) are commonly used for detecting NoV in various foods (24). However, large volumes of elution buffers are typically necessary for eluting NoV from food, which requires further concentration and purification methods (8, 26). In addition, various PCR inhibitors that interfere with subsequent molecular assays also are present in elution buffers (25). Therefore, more-efficient elution, concentration, and purification methods are required for more-sensitive detection of NoV in food.
Immunomagnetic separation (IMS) is an established method for concentrating Escherichia coli, NoV, and Salmonella spp. from various foods (5, 16, 23). Antibodies specific for a particular microorganism are used, which gives the assay a higher specificity for concentrating and purifying microorganisms in both food and other environmental samples (1, 6). The aim of this study was to evaluate IMS combined with real-time TaqMan RT-PCR for rapid and quantitative detection of NoV from artificially contaminated strawberries (11, 20).
Production of polyclonal antibodies against NoV GI and GII.
The capsid genes of both GI-1 and GII-4 NoV were amplified by RT-PCR and cloned into either pGEX-4T-1 (Amersham) or pET-15b (Novagen) expression vectors. These expression vectors were transformed into E. coli strains BL21(DE3)pLysS and Rosetta2(DE3)pLysS. Cells were inoculated into individual flasks containing 500 ml of LB-ampicillin (50 μg/ml) medium with 0.5 mM isopropylthiogalactopyranoside (IPTG; Duchepa, Germany) and incubated at 37°C until the culture reached an optical density at 600 nm of 0.5 to 0.6. Cells were harvested by centrifugation at 8,500 × g at 4°C for 10 min. Harvested cells were washed with phosphate-buffered saline (PBS), suspended in 40 to 50 ml PBS, 1 mM phenylmethanesulfonyl fluoride (Sigma), and lysed by sonication (Sonicator XL2020; Misonic). Total, soluble, and insoluble proteins were collected and electrophoresed on two 12% sodium dodecyl sulfate (SDS)-polyacrylamide gels; one gel then was stained with Coomassie brilliant blue (Sigma) to visualize the protein bands, and the other gel was electrotransferred to a nitrocellulose membrane for Western blot analysis. After confirmation by Western blot analysis using antibodies against glutathione S-transferase (GST) or His tag, cells were expressed in a large volume and purified directly from gels. The appropriately sized band corresponding to the capsid protein was cut by a razor blade from SDS-polyacrylamide gels, and polyclonal antibodies against GI-1 and GII-4 NoV capsid proteins were generated from two rabbits by using these proteins. The specificity of polyclonal antibodies was confirmed by both enzyme-linked immunosorbent assay and Western blotting.
Test of elution buffers from artificially contaminated strawberries.
A 10-μl aliquot of NoV-containing stool suspension was inoculated onto 20 g of fresh strawberries by pipetting. A total of 4 × 103 to 104 RT-PCR units of NoV quantified by conventional RT-PCR assay was seeded onto the strawberries, which then were air dried on a clean bench for 30 min. The inoculated samples were mixed with 100 ml of three different elution buffers (100 mM Tris-HCl [pH 9.5], 50 mM glycine-50 mM MgCl2 [pH 9.5], or 3% Bacto beef extract [BD] [pH 7.4]) at 25°C with shaking (∼200 rpm) for 5 hours. An equal volume of chloroform (Sigma) was added to the elution buffer after soaking and the mixture was vortexed vigorously for 1 minute, followed by centrifugation at 530 × g at 4°C for 10 min. Supernatants were recovered and precipitated with 8% polyethylene glycol (PEG) 10,000 (Fluka, Germany) with 0.3 M NaCl at 4°C for 4 to 5 h. The resulting flocculate was precipitated by centrifugation at 13,400 × g at 4°C for 30 min, and the supernatant was discarded. The pellet was resuspended in 1 ml PBS (pH 7.4). Nucleic acid extraction was performed using the QIAamp viral RNA minikit (Qiagen). Nucleic acid extracts were serially diluted and subjected to RT-PCR as previously described for measuring the efficiencies of elution by each of the elution buffers tested (17).
IMS and RT-PCR assay.
After the elution efficiency for each buffer was determined, the recovery efficiency and the sensitivity of IMS were determined. A total of 4 × 103 to 104 RT-PCR units of NoV (quantified by RT-PCR assay) was inoculated onto one strawberry and eluted with 100 ml of 3% beef extract buffer. The inoculated samples were mixed with 100 ml of 3% Bacto beef extract (pH 7.4; BD) at 25°C with shaking (∼200 rpm) for 5 hours. The eluate was subjected to chloroform extraction and PEG precipitation as described above. A total of 33 μl of magnetic beads (Dynabeads M-280, tosyl activated; Dynal, Norway) was washed three times with 0.1 M sodium phosphate buffer (pH 7.4) at room temperature. The washed beads were suspended gently in 5 μl rabbit immunoglobulin G serum against NoV in 1 ml of 0.1 M sodium phosphate buffer (pH 7.4) and incubated for 16 to 22 h at room temperature with slow tilt rotation on an MX4 sample mixer (Dynal, Norway). After being mixed to remove unbound immunoglobulin G, the beads were washed four times with PBS (pH 7.4) containing 0.1% (wt/vol) bovine serum albumin (BSA; Sigma) at 4°C. Antibody-bound magnetic beads were mixed gently with 1 ml of NoV elution buffer and incubated with rotation for 1 hour at room temperature. Magnetic beads were separated using a magnetic particle concentrator, washed three times in PBS (pH 7.4) containing 0.1% (wt/vol) BSA, and suspended in 50 μl diethyl pyrocarbonate water.
Conventional and real-time TaqMan RT-PCR assays for NoV.
In order to extract viral RNA, the suspension was incubated at 95°C for 5 min and chilled immediately on ice. The beads were pelleted by centrifugation at 13,500 × g at 4°C for 1.5 min. The supernatant was transferred to RNase-free tubes and used for either conventional RT-PCR or real-time PCR assays. In order to estimate the recovery efficiency and sensitivity of the assay, a standard curve was constructed using the control plasmid containing the NoV capsid gene insert. Plasmid standards containing partial capsid regions of the inoculated GI and GII NoVs were amplified with primer sets described in Table 1 and cloned into pMD18-T vector (Takara, Japan). Real-time PCR was carried out as described in the previous study (11, 12). Real-time RT-PCR assays were performed in a real-time PCR machine (Applied Biosystems 7300) using the TaqMan PCR reagent kit with AmpliTaq Gold DNA polymerase (Applied Biosystems) by following the manufacturer's protocol. In each experiment, NoV GI- or GII-specific standard curves were generated by a 10-fold serial dilution (approximately 107 copies of viral RNA) of purified NoV GI and GII cDNA plasmids. Conventional RT-PCR was performed using the Qiagen one-step RT-PCR kit (Qiagen) as described in a previous study (17). The experiments were repeated three times.
TABLE 1.
Primers used to generate constructs for NoV capsid expression system and real-time PCR standard curve plasmids in this study
| Group and genotype | Primer | Sequence (5′ to 3′) | Polaritya | Restriction enzyme site | Locationd |
|---|---|---|---|---|---|
| NoV capsid expression vectors | |||||
| GI-1 | GIF | GAA TTC ATG ATG ATG GCG TCT AAG G | + | EcoRI | 5358-5376 |
| GIR | GCG GCC GCC AGT GTG ATG GAT A | − | NotI | TOPO vectore | |
| GII-4 | GIIF | CAT ATG AAG ATG GCG TCG AGT G | + | NdeI | 5058-5103 |
| GIIR | TAA ACT GGA AGG AAG CTT GAT G | − | HindIII | 6827-6842 | |
| Real-time PCR standard curve plasmids | |||||
| GI-1 | GI SCCF | TGG ARA TGT ATG TCC CAG G | + | 5256-5274 | |
| GI SCCR | CCA ACC CAR CCA TTR TAC Ab | − | 5653-5671 | ||
| GII-4 | GII SCCF | RGC TNT NGA AAT NAT GGTc | + | 4338-4355 | |
| GII SCCR | CCR CCN GCA TRH CCR TTR TAC ATb | − | 5367-5389 |
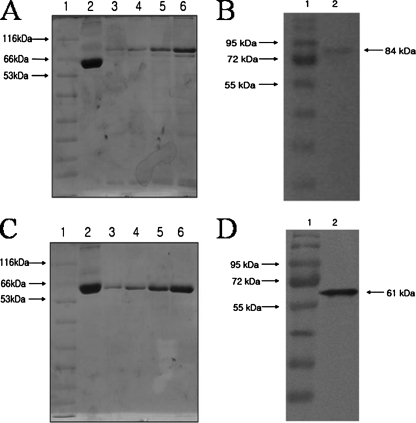
Expression of either GST or His-tagged recombinant capsid proteins was confirmed by SDS-polyacrylamide gel electrophoresis (PAGE) and Western blot analysis (Fig. 1). Both the GST-GI NoV capsid protein (84 kDa) and the His-tagged GII NoV capsid protein (61 kDa) were detected with polyclonal antibodies against either GST or His tag, respectively (unpublished data). The specificity of the polyclonal antibodies raised in rabbits against NoV capsid proteins was confirmed with Western blotting (Fig. 1) and enzyme-linked immunosorbent assay (unpublished data).
FIG. 1.
Quantitative analysis of NoV GI-1 (A and B) and GII-4 (C and D) recombinant capsid proteins expressed in E. coli by SDS-PAGE and Western blotting. (A and C) SDS-PAGE of recombinant capsid proteins expressed in E. coli with NoV GI-1 (A) and GII-4 (B). Lanes: 1, protein size marker; 2, BSA, 1 μl (4 mg/ml); 3, 0.5 μl each capsid protein; 4, 1 μl each capsid protein; 5, 2 μl each capsid protein; 6, 4 μl each capsid protein. (B and D) Western blot probed with polyclonal antisera produced from this study to each capsid protein. Lanes: 1, protein size marker; 2, recombinant capsid protein.
Among tested elution buffers, 3% beef extract resulted in a higher viral recovery rate (85%) of NoV eluted from inoculated strawberries than did either 100 mM Tris-HCl (8.5%) or 50 mM glycine-50 mM MgCl2 (8.5%). Therefore, 3% beef extract was chosen for the elution of NoV from contaminated strawberries.
Conventional RT-PCR on the eluate demonstrated a 5% inoculum recovery rate for both GI and GII NoV (Table 2). The recovery rate for both GI and GII NoV was 5%, and the limit of detection of IMS combined with conventional RT-PCR was 20 RT-PCR units. For the real-time RT-PCR assay, the dilution curves were distributed over 5 and 6 orders of magnitude for the GI-1 and GII-4 control plasmids in our assays, respectively. In all cases, excellent correlation between the amount of target templates and the threshold cycle values was obtained for both GI-1 (R2 = 0.99) and GII-4 NoV (R2 = 0.99). The initial GI-1 and GII-4 NoV copy numbers used in the inoculum were 2.58 × 104 and 5.87 × 106, respectively.
TABLE 2.
Recovery rate and sensitivity of IMS combined with conventional or real-time TaqMan RT-PCR for detecting NoV from artificially contaminated strawberries
| RT-PCR type and genogroup | Recovery rate (%)a | Limit of detectionb | Sensitivity per RT-PCRc |
|---|---|---|---|
| Conventional | |||
| GI | 5 | 20 | 400 |
| GII | 5 | 20 | 400 |
| Real-time TaqMan | |||
| GI | 29.50 | 3.36 | 67.2 |
| GII | 14.14 | 7.07 | 141.4 |
Recovery rate (percent) was estimated from a percent ratio of recovered RT-PCR units in a final volume of 50 μl nucleic acid extraction solution and initially inoculated RT-PCR units.
The limit of detection was estimated for the final 50-μl volume of nucleic acid extraction solution. It was calculated from 100 (percent recovery rate).
Sensitivity for RT-PCR. Only 2.5 μl out of a 50-μl nucleic acid extraction solution was used for nucleic acid amplification.
After elution and concentration of viral particles from strawberries by using IMS, the mean GI-1 and GII-4 copy numbers detectable by real-time RT-PCR in 50-μl-final-volume nucleic acid extraction solutions were 7.68 × 103 and 8.30 × 105, respectively. The mean recovery rate (29.50%) of GI was slightly higher than that of GII (14.14%) (Table 2). Based on these recovery rates, the limit of detection of the NoV RT-PCR assay was calculated to be three to seven copies of viral genome. As shown in Table 2, as few as 3.36 copies of GI viral RNA could be detected by IMS combined with the real-time RT-PCR assay, and as few as 7.07 copies of GII viral RNA were detected from NoV-spiked strawberries.
This is the first study that utilizes IMS combined with real-time RT-PCR to quantitatively detect NoV from artificially contaminated food. This study demonstrated that IMS can be used to isolate and concentrate NoV from complex media and will efficiently detect low copy numbers of a viral genome present in contaminated food when combined with TaqMan real-time RT-PCR. The methods presented in this study potentially could be applied to the detection of NoV or other viruses from various food matrices.
The sensitivity of methods used for detecting NoV in food is highly dependent upon the efficiency of the elution buffer and the sensitivity of subsequent concentration techniques. Reported recovery efficiencies of elution methods range from 9 to 85%; the efficiency of concentration by PEG (10,000-molecular-weight) precipitation was estimated to be 85% in our study (unpublished data) using conventional RT-PCR. Our data demonstrated that the sensitivity of real-time TaqMan RT-PCR was three- to fivefold superior to that of conventional RT-PCR assays (Table 2). Thus, further concentration by IMS combined with real-time TaqMan RT-PCR proved to be a useful tool for detecting NoV in food. One significant advantage of applying IMS methods is that the sample volume for the subsequent RT-PCR assay is reduced.
In the present study, an E. coli expression system was used to produce NoV capsid proteins. Bacterial expression systems are easier and more convenient than other expression systems (baculovirus and Saccharomyces cerevisiae) and can express high levels of recombinant NoV proteins (27, 29). We found that the low efficiency of viral capsid protein expression in the typical E. coli BL21 host strain likely was due to differences in codon usage frequencies between NoV and E. coli (4, 29). However, the GII NoV capsid protein was successfully expressed at a high yield when E. coli strain Rosetta2(DE3)pLysS with eukaryotic protein codon usage was used. Therefore, these E. coli expression systems modified for eukaryotic codon usage were successful for rapidly producing high concentrations of protein.
IMS methods have been applied for detecting various microorganisms in different environmental samples (10, 22, 23, 28). In addition, IMS methods combined with conventional RT-PCR have been applied to detect NoV (7, 16, 22). However, no previous study characterized the sensitivity of this combined method for detecting NoV on contaminated food. The detection limit of IMS combined with conventional RT-PCR for NoV in a 10% stool suspension was estimated at 250 to 740 genomic copies/ml (7). The specificity and sensitivity of IMS methods depend on (i) antibody specificity, (ii) incubation time and temperature, (iii) washing conditions, and (iv) subsequent RT-PCR assays. Our experiment demonstrated that real-time RT-PCR could be combined effectively with IMS methods to allow high specificity. Therefore, prior to applying IMS to food and other environmental samples, the choice of experimental conditions such as the volume ratio of magnetic bead to antibody, incubation temperature, and incubation time should be optimized. Our developed methods could be applied to the detection of other microbial pathogens from various foods.
The polyclonal antibody used for IMS methods could react with other common enteric viruses and proteins. However, nonspecific binding to other proteins was not an issue in our study because an additional level of specificity is provided by subsequent real-time RT-PCR using primers and probes specific for NoV capsid proteins. However, the sensitivity of the assay depends heavily on the sensitivity of the polyclonal antibodies produced for IMS. The polyclonal antibodies used in the present study were raised against recombinant proteins from the GI-1 and GII-4 genotypes of NoV. More than 31 genotypes of NoV have been identified (13, 30), and different genotypes of NoV have highly diverse antigenic properties (3, 15, 21), which could affect the sensitivity of this assay. Highly sensitive and specific antibody binding to common epitopes of different NoV genotypes is critical for the success of IMS methods. In future experiments, a panel of monoclonal and polyclonal antibodies against various genotypes of NoV will be evaluated thoroughly. Our results demonstrate that multiplex IMS combined with real-time RT-PCR for simultaneous detection of both GI and GII NoV in food is very promising and should be developed further.
Acknowledgments
This study was supported partially by a grant from the Korean Centers for Disease Control and Prevention (grant 900-20060001) and by the Seoul R&BD Program (grant 10580).
Footnotes
Published ahead of print on 25 April 2008.
REFERENCES
- 1.Abd El Galil, K. H., M. A. El Sokkary, S. M. Kheira, A. M. Salazar, M. V. Yates, W. Chen, and A. Mulchandani. 2004. Combined immunomagnetic separation-molecular beacon-reverse transcription-PCR assay for detection of hepatitis A virus from environmental samples. Appl. Environ. Microbiol. 70:4371-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adler, J. L., and R. Zickl. 1969. Winter vomiting disease. J. Infect. Dis. 119:668-673. [DOI] [PubMed] [Google Scholar]
- 3.Ando, T., J. S. Noel, and R. L. Fankhauser. 2000. Genetic classification of “Norwalk-like” viruses. J. Infect. Dis. 181(Suppl. 2):S336-S348. [DOI] [PubMed] [Google Scholar]
- 4.Batten, C. A., I. N. Clarke, S. L. Kempster, S. L. Oliver, J. C. Bridger, and P. R. Lambden. 2006. Characterization of a cross-reactive linear epitope in human genogroup I and bovine genogroup III norovirus capsid proteins. Virology 356:179-187. [DOI] [PubMed] [Google Scholar]
- 5.Drysdale, M., M. MacRae, N. J. Strachan, T. M. Reid, and I. D. Ogden. 2004. The detection of non-O157 E. coli in food by immunomagnetic separation. J. Appl. Microbiol. 97:220-224. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Aljaro, C., X. Bonjoch, and A. R. Blanch. 2005. Combined use of an immunomagnetic separation method and immunoblotting for the enumeration and isolation of Escherichia coli O157 in wastewaters. J. Appl. Microbiol. 98:589-597. [DOI] [PubMed] [Google Scholar]
- 7.Gilpatrick, S. G., K. J. Schwab, M. K. Estes, and R. L. Atmar. 2000. Development of an immunomagnetic capture reverse transcription-PCR assay for the detection of Norwalk virus. J. Virol. Methods 90:69-78. [DOI] [PubMed] [Google Scholar]
- 8.Guevremont, E., J. Brassard, A. Houde, C. Simard, and Y. L. Trottier. 2006. Development of an extraction and concentration procedure and comparison of RT-PCR primer systems for the detection of hepatitis A virus and norovirus GII in green onions. J. Virol. Methods 134:130-135. [DOI] [PubMed] [Google Scholar]
- 9.Hedberg, C. W., K. L. Palazzi-Churas, V. J. Radke, C. A. Selman, and R. V. Tauxe. 2008. The use of clinical profiles in the investigation of foodborne outbreaks in restaurants: United States, 1982-1997. Epidemiol. Infect. 136:65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jothikumar, N., D. O. Cliver, and T. W. Mariam. 1998. Immunomagnetic capture PCR for rapid concentration and detection of hepatitis A virus from environmental samples. Appl. Environ. Microbiol. 64:504-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jothikumar, N., J. A. Lowther, K. Henshilwood, D. N. Lees, V. R. Hill, and J. Vinje. 2005. Rapid and sensitive detection of noroviruses by using TaqMan-based one-step reverse transcription-PCR assays and application to naturally contaminated shellfish samples. Appl. Environ. Microbiol. 71:1870-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kageyama, T., S. Kojima, M. Shinohara, K. Uchida, S. Fukushi, F. B. Hoshino, N. Takeda, and K. Katayama. 2003. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 41:1548-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kageyama, T., M. Shinohara, K. Uchida, S. Fukushi, F. B. Hoshino, S. Kojima, R. Takai, T. Oka, N. Takeda, and K. Katayama. 2004. Coexistence of multiple genotypes, including newly identified genotypes, in outbreaks of gastroenteritis due to norovirus in Japan. J. Clin. Microbiol. 42:2988-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapikian, A. Z., R. G. Wyatt, R. Dolin, T. S. Thornhill, A. R. Kalica, and R. M. Chanock. 1972. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J. Virol. 10:1075-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katayama, K., H. Shirato-Horikoshi, S. Kojima, T. Kageyama, T. Oka, F. Hoshino, S. Fukushi, M. Shinohara, K. Uchida, Y. Suzuki, T. Gojobori, and N. Takeda. 2002. Phylogenetic analysis of the complete genome of 18 Norwalk-like viruses. Virology 299:225-239. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi, S., K. Natori, N. Takeda, and K. Sakae. 2004. Immunomagnetic capture rt-PCR for detection of norovirus from foods implicated in a foodborne outbreak. Microbiol. Immunol. 48:201-204. [DOI] [PubMed] [Google Scholar]
- 17.Kojima, S., T. Kageyama, S. Fukushi, F. B. Hoshino, M. Shinohara, K. Uchida, K. Natori, N. Takeda, and K. Katayama. 2002. Genogroup-specific PCR primers for detection of Norwalk-like viruses. J. Virol. Methods 100:107-114. [DOI] [PubMed] [Google Scholar]
- 18.Laverick, M. A., A. P. Wyn-Jones, and M. J. Carter. 2004. Quantitative RT-PCR for the enumeration of noroviruses (Norwalk-like viruses) in water and sewage. Lett. Appl. Microbiol. 39:127-136. [DOI] [PubMed] [Google Scholar]
- 19.Lees, D. N., K. Henshilwood, J. Green, C. I. Gallimore, and D. W. Brown. 1995. Detection of small round structured viruses in shellfish by reverse transcription-PCR. Appl. Environ. Microbiol. 61:4418-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohamed, N., S. Belak, K. O. Hedlund, and J. Blomberg. 2006. Experience from the development of a diagnostic single tube real-time PCR for human caliciviruses, norovirus genogroups I and II. J. Virol. Methods 132:69-76. [DOI] [PubMed] [Google Scholar]
- 21.Myrmel, M., and E. Rimstad. 2000. Antigenic diversity of Norwalk-like viruses: expression of the capsid protein of a genogroup I virus, distantly related to Norwalk virus. Arch. Virol. 145:711-723. [DOI] [PubMed] [Google Scholar]
- 22.Myrmel, M., E. Rimstad, and Y. Wasteson. 2000. Immunomagnetic separation of a Norwalk-like virus (genogroup I) in artificially contaminated environmental water samples. Int. J. Food Microbiol. 62:17-26. [DOI] [PubMed] [Google Scholar]
- 23.Rijpens, N., L. Herman, F. Vereecken, G. Jannes, J. De Smedt, and L. De Zutter. 1999. Rapid detection of stressed Salmonella spp. in dairy and egg products using immunomagnetic separation and PCR. Int. J. Food Microbiol. 46:37-44. [DOI] [PubMed] [Google Scholar]
- 24.Sair, A. I., D. H. D'Souza, C. L. Moe, and L. A. Jaykus. 2002. Improved detection of human enteric viruses in foods by RT-PCR. J. Virol. Methods 100:57-69. [DOI] [PubMed] [Google Scholar]
- 25.Schultz, A. C., P. Saadbye, J. Hoorfar, and B. Norrung. 2007. Comparison of methods for detection of norovirus in oysters. Int. J. Food Microbiol. 114:352-356. [DOI] [PubMed] [Google Scholar]
- 26.Schwab, K. J., R. De Leon, and M. D. Sobsey. 1996. Immunoaffinity concentration and purification of waterborne enteric viruses for detection by reverse transcriptase PCR. Appl. Environ. Microbiol. 62:2086-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan, M., W. Zhong, D. Song, S. Thornton, and X. Jiang. 2004. E. coli-expressed recombinant norovirus capsid proteins maintain authentic antigenicity and receptor binding capability. J. Med. Virol. 74:641-649. [DOI] [PubMed] [Google Scholar]
- 28.Tomoyasu, T. 1998. Improvement of the immunomagnetic separation method selective for Escherichia coli O157 strains. Appl. Environ. Microbiol. 64:376-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoda, T., Y. Terano, A. Shimada, Y. Suzuki, K. Yamazaki, N. Sakon, I. Oishi, E. T. Utagawa, Y. Okuno, and T. Shibata. 2000. Expression of recombinant Norwalk-like virus capsid proteins using a bacterial system and the development of its immunologic detection. J. Med. Virol. 60:475-481. [DOI] [PubMed] [Google Scholar]
- 30.Zheng, D. P., T. Ando, R. L. Fankhauser, R. S. Beard, R. I. Glass, and S. S. Monroe. 2006. Norovirus classification and proposed strain nomenclature. Virology 346:312-323. [DOI] [PubMed] [Google Scholar]