Abstract
The nodulation of Glycine max cv. Lambert and the nodulation-restricting plant introduction (PI) genotype PI 417566 by wild-type Bradyrhizobium japonicum USDA110 is regulated in a population-density-dependent manner. Nodulation on both plant genotypes was suppressed (inhibited) when plants received a high-density inoculum (109 cells/ml) of strain USDA110 grown in complex medium, and more nodules were produced on plants receiving a low-cell-density inoculum (105 cells/ml). Since cell-free supernatants from strain USDA110 grown to high cell density in complex medium decreased the expression of an nodY-lacZ fusion, this phenomenon was attributed to bradyoxetin-induced repression of nod gene expression. Inoculation of either the permissive soybean genotype (cv. Lambert) or PI 417566 with 109 cells/ml of the nodD2, nolA, nodW, and nwsB mutants of USDA110 enhanced nodulation (up to 24%) relative to that seen with inoculations done with 105 cells/ml of the mutants or the wild-type strain, indicating that these genes are involved in population-density-dependent nodulation of soybeans. In contrast, the number of nodules produced by an nodD1 mutant on either soybean genotype was less than those seen with the wild-type strain inoculated at a low inoculum density. The nodD2 mutant outcompeted B. japonicum strain USDA123 for nodulation of G. max cv. Lambert at a high or low inoculum density, and the results of root-tip-marking and time-to-nodulate studies indicated that the nolA and nodD2 mutants nodulated this soybean genotype faster than wild-type USDA110. Taken together, the results from these studies indicate that the nodD2 mutant of B. japonicum may be useful to enhance soybean nodulation at high inoculum densities and that NodD2 is a key repressor influencing host-controlled restriction of nodulation, density-dependent suppression of nodulation, perception of bradyoxetin, and competitiveness in the soybean-B. japonicum symbiosis.
Bradyrhizobium japonicum is the nitrogen-fixing, root nodule symbiont of soybeans. Results from several studies have shown that both the bacterial and host genotypes influence the symbiotic interaction of Bradyrhizobium with soybeans (32). Several soybean genotypes, including cultivars and plant introductions (PI), were shown to be differentially nodulated by specific strains or genotypes of Bradyrhizobium japonicum (4, 28). Cregan and colleagues (4, 5) and Lohrke et al. (22) identified two soybean PI genotypes, PI 377586 and PI 417566, which restricted nodulation by B. japonicum strains in serogroups 123 and 110, respectively. The results of several subsequent studies done using one or both PI genotypes indicated that host-controlled restriction of nodulation is strain and temperature dependent, determined by the root genotype, and conditioned by a single recessive host gene (22, 23). Moreover, results from preliminary microscopic studies done using both PI genotypes suggested that the nodulation restriction process occurred some time after the formation of nodule primordia in incompatible host-strain combinations (29). More recently, Lohrke and colleagues (21) showed that PI 417566-mediated restriction of nodulation by B. japonicum strain USDA110 was related to inoculum density; nodulation restriction was suppressed when plants were inoculated with 104 to 106 cells/ml and enhanced when plants were inoculated with 108 to 109 cells/ml.
In Bradyrhizobium japonicum and other rhizobia, nodulation requires the coordinated expression of many nodulation genes (nod, nol, and noe) leading to the production of lipochitooligosaccharidic Nod factors. The regulation of nod gene expression in the bradyrhizobia is complex and occurs via three regulatory pathways involving nodD, nodVW, and nolA (15). Bradyrhizobium japonicum produces two NodD proteins (NodD1 and NodD2) with distinctly different functions. NodD1, a LysR-type regulator, is a positive transcriptional activator and responds to plant-secreted isoflavones, such as genistein and daidzein (1, 8), while NodD2 acts as a repressor of nodD1 expression (16). Although the results of initial studies indicated that nodD2 did not have an obvious role in soybean nodulation (8), the results of subsequent studies done by the same authors indicated that an nodD2 deletion mutant had a delay in nodulation relative to the speed of nodulation by the wild-type strain (9). NodVW is essential for the nodulation of cowpeas, siratro, and mungbeans, but not for soybeans, and this two-component system provides an alternative pathway for nod gene activation in NodD1 mutants, which are still able to nodulate host plants (8, 9). The third pathway is regulated by NolA, a member of the MerR family of regulatory proteins, and was first identified as a genotype-specific nodulation gene that was required by B. japonicum serogroup 123 strains for the nodulation of soybean genotype PI 377578 (27). NolA was shown to activate the expression of NodD2, which in turn represses nod gene expression in Bradyrhizobium (7, 19).
Quorum sensing refers to the production and perception of extracellular signal molecules (previously called autoinducers) that signal elevated population density. This leads to the expression of genes that are active only at high population densities (25). Recently, an extracellular quorum-responsive signal molecule, bradyoxetin, was identified in the culture supernatant of B. japonicum USDA110 grown to high cell density (17, 20). Bradyoxetin was shown to be an inducer of NolA, which in turn leads to nod gene repression (16). The production of bradyoxetin was shown to be regulated in a population-density-dependent manner; the greatest production of bradyoxetin occurred in high-population-density and iron-depleted conditions (20), and this was correlated with elevated expression levels of nolA and nodD2. In addition, Loh and coworkers (14) also reported that NwsB is also involved in the cell-density-dependent regulation of nolA and nodD2 expression, and Pongsilp et al. (26) reported that many Bradyrhizobium strains also produce N-acyl homoserine lactone-like molecules, but their involvement in nodulation was not reported. Taken together, these data suggest that the expression of nod genes in the bradyrhizobia is modulated by bradyoxetin and, possibly, other quorum-responsive signal molecules.
The aim of this study was to determine if nodulation gene regulation and quorum sensing are involved in the restriction of nodulation by B. japonicum USDA110 on soybean PI genotype 417566. Mutations in several nodulation-regulating genes were also evaluated to determine if repressors of nod gene induction enhanced the nodulation and competitiveness of Bradyrhizobium japonicum strains on plants grown in artificial media and in natural soil.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
The strains and plasmids used in this study are listed in Table 1. For normal growth and inoculation studies, B. japonicum strains were cultured in arabinose-gluconate (AG) medium (28) at 30°C. Minimal medium (2) was also used for β-galactosidase activity assays as previously described (17). When appropriate, antibiotics were added to the culture medium to maintain selection of plasmids. The nodD2 insertion mutant JD21 was constructed (J. Loh and G. Stacey, unpublished data) by inserting the Ω interposon from pHP45Ω into the ClaI site of the cloned nodD2 gene, and this was subsequently recombined into the genome of B. japonicum USDA110 as previously described (7). The fidelity of the nodD2 mutation in USDA110 was verified by using PCR with primers NodD2F 5′-CGATTCAGGATCGTCCTTTC-3′, NodD2R 5′-GTTGTGAAGTGAGGGCCATT-3′, AadaF 5′-TGATTTGCTGGTTACGGTGA-3′, and AadaR 5′-TACTGCGCTGTACCAAATGC-3′, in both orientations, that are specific for the nodD2 gene and the aadA gene of the Ω interposon, respectively.
TABLE 1.
Bacterial strains and plasmid used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| B. japonicum strains | ||
| USDA110 | Wild type | USDA, Beltsville, MD |
| BjB3 | nolA Ω insertion; Smr Spr | 7 |
| JD21 | nodD2 Ω insertion; Smr Spr | G. Stacey, University of Missouri |
| JNW21 | nwsB Smr Spr | 14 |
| Bj586 | nodD1 small deletion; Kmr | 8 |
| Bj613 | nodW Ω insertion; Smr | 10 |
| ZB977 | USDA110(pZB32) | 1 |
| Plasmid | ||
| pZB32 | nodY-lacZ translational fusion | 1 |
Nodulation suppression studies.
The B. japonicum wild-type strain USDA110 and the nod gene mutants JNW21 (nwsB), Bj586 (nodD1), Bj613 (nodW), BjB3 (nolA), and JD21 (nodD2) were grown in AG broth medium to late log phase. Cultures were serially diluted to 108, 107, 106, 105, and 104 cells/ml in sterile AG medium, and 1-ml aliquots of these dilutions were added to sterile Glycine max (L.) Merr. seeds. Plant assays were done in sterile Leonard jar assemblies containing a 3:1 mixture of vermiculite and perlite as previously described (28) or in nonsterile soil (see below). Seeds of G. max cv. Lambert or PI 417566 (22) were surface sterilized in 0.2% acidic HgCl2 (31). After inoculation, seeds were covered with a 1-cm layer of sterilized, paraffin-coated sand and thinned to two seedlings per hill after germination. Plants were watered with N-free plant nutrient solution (13) or tap water, as needed, and incubated in a plant growth chamber at 20°C with a photoperiod of 16 h. The number of nodules and their dry mass were determined at 31 days postinoculation. Uninoculated plants and those inoculated with B. japonicum strain USDA110 served as negative and positive controls, respectively. The experiment was carried out using a completely randomized experimental design, with three replications.
Nodulation restriction studies were also done using natural, nonsterile, Verndale sandy loam soil (Typic Argiudoll) collected in Staples, Minnesota. This soil had no detectable B. japonicum, as determined by using the most-probable-number method (31) and G. max cv. Lambert as a trap host. The soil was allowed to partially air dry (to about 5% moisture), sieved (<2 mm), and adjusted to near-neutral pH (6.5 to 7.0) with CaCO3. A completely randomized experimental design with three replications was used in this study, consisting of two soybean genotypes (G. max cv. Lambert and PI 417566), three strains of B. japonicum (wild type and nolA and nodD2 mutants), six levels of wild-type population density (from 104 to 109 cells/ml), and two levels of population density for the mutants (105 and 109 cells/ml). Seeds were surface sterilized in acidified HgCl2 as described above, and three seeds were planted in 11.5-cm (diameter) plastic pots with a total volume of 1 kg dry soil. Plants were thinned to two seedlings per pot 3 days after emergence and inoculated with 1.0 ml of late log phase, AG-grown cultures of each strain at each population density described above. Uninoculated plants and those inoculated with B. japonicum wild-type strain USDA110 served as negative and positive controls, respectively. Plants were grown and watered in a plant growth chamber as described above. The number of nodules and their dry mass were determined 31 days after inoculation.
Nodule distribution and timing of nodulation.
Studies done to examine the location of nodules on the primary root (distance from root tip mark) and the timing of nodulation were done in growth pouches (Mega International, Minneapolis, MN) as previously described (3, 21). Seeds of G. max cv. Lambert and PI 417566 were surface sterilized as described above and germinated in the dark on 0.75% water agar at 25°C for 2 to 3 days, until the radicle was between 1.0 and 1.5 cm in length. Two seedlings of each genotype were placed in each sterile growth pouch, containing 10 ml of nitrogen-free plant nutrient solution, and plant roots were inoculated with either 105 or 109 cells/ml of USDA110 or the nolA or nodD2 mutant. Twenty growth pouches were inoculated with each culture at each cell population density. At the time of inoculation, the location of the root tip was marked on the outside of the plastic growth pouch. Plants were incubated at 20°C in a plant growth chamber as described above and were watered with nitrogen-free nutrient solution every day, and roots were examined daily for the appearance of nodules. The number of nodules on each root, both above and below the root tip mark, was recorded at 30 days postinoculation.
Competition studies.
Studies were done to examine the influence of bradyoxetin signal perception on competition for nodulation on G. max cv. Lambert. Experiments were done in triplicate Leonard jar assemblies using two cell densities, 105 and 109 cells/ml, of wild-type USDA110 alone, the nolA or nodD2 mutant alone, or USDA123 alone and each strain plus USDA123 at a 1:1 ratio. Plants were grown for 31 days as described above. Twenty nodules from each replication of each treatment, or about 50% of the total nodules, were randomly picked to examine nodule occupancy. The nodules were washed, surface sterilized (31), and crushed in 100 μl of sterile water in 96-well microtiter plates. The strains in the nodule homogenates were determined by using fluorescent antibodies specific for strains USDA110 and USDA123 (30).
Effect of conditioned AG culture medium on expression of nodY-lacZ.
Bradyrhizobium japonicum strain USDA110 was cultured in AG or minimal medium at 30°C to various population densities as determined by measuring the optical density at 600 nm (OD600). Cells were harvested by centrifugation at 8,000 × g, and the supernatant was filtered through a 0.45-μm filter (Millipore) as described previously (15, 17). The filtrate was subsequently concentrated 100-fold by vacuum evaporation and stored at −20°C until needed. Studies done to examine the induction of nodY-lacZ fusion expression were performed essentially as described by Loh and Stacey (15), with slight modifications. Bradyrhizobium japonicum strain ZB977 (USDA110 harboring the nodY-lacZ fusion) was grown for 2 days in minimal medium (2) with 100 μg of tetracycline per ml at 30°C until the OD600 was 0.5 and subcultured in sterile fresh minimal medium until the OD600 was 0.2. The induction of the nodY-lacZ fusion was initiated by the addition of 2 μM genistein and 10 to 100 μl of supernatants from AG- or minimal medium-grown cells collected at each population density. Cultures were incubated at 30°C for 14 h, and β-galactosidase assays were used to measure the induction of the nodY-lacZ fusion as described previously (15, 24). Uninduced cultures served as negative controls.
Statistical analyses.
Data were log transformed prior to analysis and analyzed by analysis of variance (ANOVA) using the Statistical Analysis computer package, version 9.1, of SAS (SAS Institute, Inc., Cary, NC). Mean values were compared by using Duncan-Waller multiple-range analysis with an α value of 0.05.
RESULTS
Population-density-dependent suppression of soybean nodulation.
It was previously reported that a high population density of B. japonicum strain USDA110 suppressed nodulation on G. max cv. Kasota (21). To determine if a high cell population density also suppressed nodulation on other soybean varieties, G. max cv. Lambert seeds were inoculated with 108, 107, 106, 105, or 104 cells/ml of B. japonicum USDA110 in sterile vermiculite-perlite growth medium. The results given in Table 2 show that the number of nodules on cv. Lambert was indirectly related to the inoculum density; the greatest numbers of nodules were produced on plants inoculated with 105 cells/ml, and nodulation was significantly suppressed when plants were inoculated with 109 cells/ml. Thus, density-dependent suppression of soybean nodulation is not limited to cv. Kasota. Since the nodule number was reduced approximately 42% at 109 cells/ml relative to that seen when seeds were inoculated with 105 cells/ml, these two inoculum densities were used in subsequent studies.
TABLE 2.
Population density suppression of nodulation on G. max cv. Lambert by B. japonicum strain USDA110a
| Inoculum population density (cells/ml) | No. of nodules | Nodule dry mass (mg) |
|---|---|---|
| Uninoculated | 0 D | 0 D |
| 104 | 25.2 B | 20.0 BC |
| 105 | 31.2 A | 40.2 A |
| 106 | 24.5 B | 26.0 B |
| 107 | 26.5 AB | 17.9 C |
| 108 | 25.3 B | 15.1 C |
| 109 | 18.0 C | 18.7 C |
Plants were grown in vermiculite in sterile Leonard jar assemblies. Values are means of results for three replicate experiments. Nodule number and dry mass values are per plant. Numbers in a column not followed by the same letter differ significantly at a P value of 0.05 as tested by ANOVA and the Duncan-Waller multiple-range test.
Culture supernatants suppress nod gene expression in a density-dependent manner.
The results of previous studies demonstrated that the expression of nodC-lacZ and nodY-lacZ fusions was a function of the initial population density of strain USDA110 (17) and that bradyoxetin induces the expression of nolA, leading to repression of the expression of nodY and other nodulation genes (14). These studies, however, were done using USDA110 grown in minimal medium. Since previous results showed that AG-grown USDA110 induced fewer nodules on plants inoculated with a high inoculum density, it was hypothesized that supernatants from AG culture grown to a high population density might also produce bradyoxetin that subsequently represses the expression of nod genes. To test this, the expression of an nodY-lacZ fusion was examined, in the presence of genistein, as a function of the addition of supernatant from various population densities of B. japonicum USDA110 grown in AG or minimal medium. The results presented in Table 3 show that the genistein-induced expression of the nodY-lacZ strain of USDA110 was similar whether cells were grown in AG or minimal medium. In AG medium, nodY-lacZ expression was maximal at an OD600 of 0.08, corresponding to approximately 8 × 107 cells/ml. In contrast, when supernatants (20 μl) from AG cultures grown to an OD600 of 2.0 (about 2 × 109 cells/ml) were added to tester strain ZB977, the level of nodY-lacZ expression in the presence of genistein was reduced approximately 53%, relative to that seen with the lower-density cultures. This result strongly suggested that bradyoxetin, an inducer of nolA expression and indirect repressor of Bradyrhizobium nod genes, was likely involved in the inoculum-density-dependent inhibition of nodulation of the soybean genotypes examined here.
TABLE 3.
Expression of nodY-lacZ fusion in response to the addition of supernatants from B. japonicum USDA110 cultures grown to various population densities in complex or minimal medium
| OD600 of culture | β-Galactosidase activity ± SD (units) with supernatant from:
|
|
|---|---|---|
| Complex mediuma | Minimal mediumb | |
| 0.08 | 126 ± 0.5 | 140 ± 22.2 |
| 0.20 | 112 ± 5.5 | 132 ± 2.2 |
| 0.50 | 105 ± 1.3 | 128 ± 1.0 |
| 1.00 | 72 ± 4.7 | 82 ± 2.3 |
| 1.50 | 71 ± 4.2 | 82 ± 1.8 |
| 2.00 | 59 ± 5.0 | 78 ± 3.6 |
| 2.50 | 45 ± 6.5 | 52 ± 4.7 |
| Uninducedc | 22 ± 4.4 | 21 ± 0.6 |
Involvement of nodulation genes in restriction of nodulation and nodulation suppression.
To investigate whether Bradyrhizobium nod genes were directly involved in the restriction of nodulation by strain USDA110 on PI 417566 and the density-dependent nodulation suppression on G. max cv. Lambert, plants were cultivated in sterile vermiculite-perlite plant growth medium and inoculated with 105 or 109 cells/ml of nodD1, nodD2, nolA, nodW, or nwsB mutant cultures grown to late log phase in AG medium. The results presented in Table 4 show that the population densities of B. japonicum USDA110 and the nod gene mutants significantly (P = 0.05) influenced nodule numbers on the soybean genotypes. On both soybean genotypes inoculated with wild-type USDA110, the greatest number of nodules was found on plants receiving a population density of 105 cells/ml. Moreover, the inoculation of PI 417566 with 109 cells/ml of wild-type USDA110 produced 42.8% fewer nodules than did inoculation at 105 cells/ml, indicating that the nodulation of this genotype was susceptible to density-dependent regulation of nodulation.
TABLE 4.
Nodulation phenotypes of two soybean genotypes in vermiculite plant growth assays in response to population density of the wild type and nod gene insertion or deletion mutants of B. japonicum USDA110
| Strain and inoculum density (cells/ml) | Nodulation response on G. max genotypea
|
|||
|---|---|---|---|---|
| cv. Lambert
|
PI 417566
|
|||
| No. of nodules | Nodule dry mass (mg) | No. of nodules | Nodule dry mass (mg) | |
| Wild type | ||||
| 105 | 27.0 E | 72.2 CDE | 9.0 F | 20.3 E |
| 109 | 20.3 F | 68.8 DE | 6.3 G | 15.5 F |
| nodD1 mutant | ||||
| 105 | 19.0 F | 56.7 F | 6.3 G | 20.6 E |
| 109 | 19.3 F | 68.6 DE | 6.7 G | 19.2 E |
| nodD2 mutant | ||||
| 105 | 29.0 DE | 91.0 B | 15.0 BC | 34.4 BC |
| 109 | 36.0 A | 78.1 C | 18.0 A | 31.8 C |
| nolA mutant | ||||
| 105 | 28.7 E | 92.5 B | 13.0 DE | 31.5 C |
| 109 | 31.0 CD | 94.0 B | 15.3 BC | 32.7 BC |
| nodW mutant | ||||
| 105 | 28.3 E | 65.9 E | 12.0 E | 40.7 A |
| 109 | 33.3 BC | 117.0 A | 14.7 BC | 35.3 B |
| nwsB mutant | ||||
| 105 | 28.3 E | 76.6 CD | 14.0 CD | 24.4 D |
| 109 | 34.0 AB | 88.8 B | 16.0 B | 24.9 D |
| Uninoculated | 0 G | 0 G | 0 H | 0 G |
Values are means of results of three replicate experiments. Nodule number and dry mass values are per plant. Numbers in a column not followed by the same letter differ significantly at a P value of 0.05 as tested by ANOVA and the Duncan-Waller multiple-range test.
In contrast, the results presented in Table 4 show that the inoculation of either the permissive soybean genotype (cv. Lambert) or PI 417566 with 109 cells/ml of the nodD2, nolA, nodW, and nwsB mutants enhanced nodule numbers (up to 24%) relative to those seen with inoculations done with 105 cells/ml. In addition, the nodule dry mass and the number of nodules produced by the B. japonicum strains on both plant host genotypes were found to be significantly related, with correlation coefficients of 0.85 and 0.71 for cv. Lambert and PI 417566, respectively (data not shown). At either inoculation density, the nodD2, nolA, nodW, and nwsB mutants overcame the nodulation restriction conditioned by PI 417566, producing more nodules than were seen when the PI genotype was inoculated with 105 cells/ml of the wild-type strain. In general, the sizes of nodules formed by the mutants on cv. Lambert inoculated with 109 cells/ml were larger than those seen when the nodulation-restricting host PI 417566 was inoculated at the same level.
Interestingly, while the number of nodules produced by the nodD1 mutant was less than that seen with the wild-type strain on cv. Lambert, generally the nodule number was the same at both inoculum densities regardless of the genotype. Thus, in contrast to what was seen with the other mutants tested, an increased inoculation dosage of the nodD1 mutant did not result in enhanced nodulation on either soybean genotype. Overall, the ability of the mutants to enhance the nodulation of cv. Lambert soybeans at a high inoculation density can be ranked as follows: nodD2 > nwsB > nodW > nolA > nodD1. Taken together, these results indicated that the suppression of nodulation at high cell densities on soybeans can be alleviated by using the tested nodulation gene mutants of B. japonicum USDA110, with the exception of the nodD1 mutant, and that density-dependent nodulation restriction is likely conditioned by bradyoxetin and, perhaps, other quorum-induced molecules which act to repress nodulation gene function.
Enhancement of nodulation in natural soil.
Since it was previously shown that nodulation studies done using artificial plant growth media frequently do not mimic what happens in soils, further experiments were done to determine if the nodulation gene mutants had enhanced nodulation in a natural, nonsterile soil system. The results presented in Table 5 show that nodule numbers were suppressed when the wild-type USDA110 strain was applied to soybean PI genotype 417566 at 109 cells/ml. However, the dilution of wild-type USDA110 cells to 105 cells/ml before application to plants yielded an approximately twofold increase (59%) in nodule number relative to that seen when plants were inoculated with 109 cells/ml (Table 5). However, the reverse was not true on the nonrestrictive cv. Lambert soybean plants. In this case, when USDA110 was inoculated at 109 cells/ml, nodulation was slightly enhanced relative to that seen at 105 cells/ml. In contrast, and similar to what was seen in studies done in artificial plant growth media, nodulation by the nodD2 and nolA mutants in soil was enhanced approximately 9 to 54% at the higher inoculum density on both the nodulation-permissive and -restrictive soybean genotypes. Nodule dry mass, on a per-plant basis, was significantly correlated with nodule number in both host plant genotypes tested. While fewer nodules were generally produced on the unimproved PI genotype than on cv. Lambert, as expected, the nodules were larger on the PI genotype. Taken together, the results of these studies indicate that nodulation enhancement by the nod gene mutants can be realized in natural nonsterile soil and that host-controlled restriction of nodulation can be overcome by using the nolA and nodD2 mutants, even at high inoculum densities.
TABLE 5.
Nodulation response of two soybean genotypes in response to population density of the wild type and nolA and nodD2 gene mutants of B. japonicum USDA110 in natural, nonsterile soil
| Strain and inoculum density (cells/ml) | Nodulation response on G. max genotypea
|
|||
|---|---|---|---|---|
| cv. Lambert
|
PI 417566
|
|||
| No. of nodules | Nodule dry mass (mg) | No. of nodules | Nodule dry mass (mg) | |
| Wild type | ||||
| 105 | 20.3 E | 32.6 BC | 6.7 DE | 36.3 A |
| 109 | 24.3 B | 20.3 F | 2.7 H | 12.0 E |
| nolA mutant | ||||
| 105 | 22.3 CD | 37.9 AB | 8.0 CD | 34.0 A |
| 109 | 24.3 BC | 28.7 CD | 12.3 B | 36.0 A |
| nodD2 mutant | ||||
| 105 | 23.0 BC | 35.2 B | 9.7 C | 35.7 A |
| 109 | 27.3 A | 45.6 A | 15.0 A | 37.3 A |
| Uninoculated | 0 H | 0 G | 0 I | 0 F |
Values are means of results for three replicate experiments. Nodule number and dry mass values are per plant. Numbers in a column not followed by the same letter differ significantly at a P value of 0.05 as tested by ANOVA and the Duncan-Waller multiple-range test.
The nodD2 mutant is highly competitive for nodulation of soybeans.
To determine if the USDA110 nod gene mutants showing enhanced nodulation at high culture densities were competitive for soybean nodulation, paired, equal-concentration competition assays were done using B. japonicum USDA123 as the challenge strain. The results of these studies, done in sterile plant growth medium with the nodulation-permissive soybean cv. Lambert, are shown in Table 6. When equal concentrations of wild-type USDA110 and USDA123 were used as the inoculum, 20 and 30% of the nodules were occupied by USDA110 and USDA123, respectively. In contrast, 46.7% of the nodules were occupied by the nodD2 mutant when competition studies were done using USDA123 as the challenge strain. This represents a 133% increase in competitive ability relative to that of the wild-type USDA110 strain. The nolA mutant strain appeared to be about equally as competitive for nodulation as USDA110 against USDA123. Due to the relatively high inoculum density used, a large proportion, up to 50%, of the tested nodules contained both strains. However, in competition with USDA123, cooccupancy by the nodD2 mutant and USDA123 or the nolA mutant strain and USDA123 was reduced approximately 25% relative to that seen when wild-type USDA110 was competed against USDA123. While these results suggest that the competitive ability of the nodD2 mutant was increased relative to that seen with the wild-type strain, additional studies with more replications are needed to assess whether statistically significant increases in competitive ability occur as a result of the mutations.
TABLE 6.
Competition for nodulation between wild-type and mutant strains of B. japonicum on G. max cv. Lamberta
| Inoculant strain | % Nodule occupancy by strain:
|
Total no. of nodules | ||
|---|---|---|---|---|
| USDA110 | USDA123 | Both | ||
| USDA110 alone | 100 A | 0 E | 0 B | 20.3 F |
| 110-BjB3 (nolA mutant) alone | 100 A | 0 E | 0 B | 28.0 BC |
| 110-JD21 (nodD2 mutant) alone | 100 A | 0 E | 0 B | 28.3 BCD |
| USDA123 alone | 0 D | 100 A | 0 B | 25.3 CDE |
| USDA110 and USDA123 | 20.0 C | 30.0 BC | 50.0 A | 24.7 E |
| 110-BjB3 and USDA123 | 21.7 C | 40.0 B | 38.3 A | 30.7 B |
| 110-JD21 and USDA123 | 46.7 B | 16.7 D | 36.6 A | 34.7 A |
Plants were inoculated with 109 cells/ml of each bacterium (1:1 ratio) and grown in a vermiculite-perlite mixture (3:1) in sterile Leonard jar assemblies. Values are means of results for ≥50% of total number of nodules. Means within a column not followed by the same letter differ significantly at a P value of 0.05 as tested by ANOVA and the Duncan-Waller multiple-range test.
Speed of nodulation and distribution of nodules.
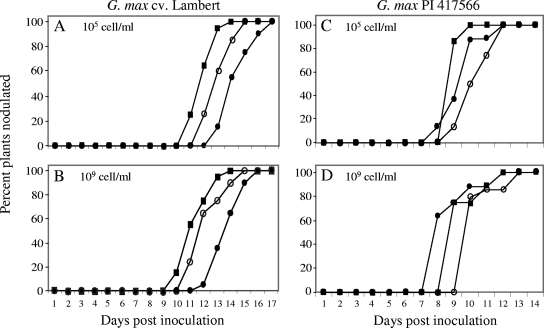
Since the previous results showed that mutations in the nolA and nodD2 genes enhanced nodule formation and competitiveness at high cell densities on both nodulation-restrictive and -permissive soybean genotypes, it was of interest to determine whether mutations in these two regulatory genes also influenced the timing of nodule formation in a population-density-dependent manner. The results presented in Fig. 1A and B show that less time was required by the nolA and nodD2 mutant strains to begin nodule formation, at both population densities, on the permissive soybean genotype G. max cv. Lambert, relative to that seen with the wild-type strain. When the nodD2 mutant strain was inoculated at 105 cells/ml on cv. Lambert, the first nodules appeared 10 to 11 days after inoculation; 50% of the plants were nodulated by day 11, and 100% of plants were nodulated after 13 days. In contrast, nodulation by the nolA mutant and the wild-type strain did not begin until after 12 or 13 days, and 100% of the plants were not nodulated until 15 or 17 days postinoculation, respectively. A similar nodulation profile was seen when wild-type and mutant cells were inoculated on cv. Lambert at 109 cells/ml. The ranking of the tested strains for fastest nodulation of cv. Lambert was as follows: nodD2 mutant > nolA mutant > wild type.
FIG. 1.
Percentage of G. max cv. Lambert and PI 417566 genotype plants nodulated by wild-type B. japonicum USDA110 (•) and the nolA (○) and nodD2 (▪) mutant strains at two population densities as indicated. Twenty plants with each treatment were analyzed at each time point.
The nodD2 mutant strain formed nodules faster on PI 417566 than on cv. Lambert when plants were inoculated at 105 cells/ml (Fig. 1A and C). Moreover, 100% of the plants were nodulated 10 days after inoculation. At the 105-cells/ml inoculation rate, nodulation by the nolA mutant and wild-type strains was delayed 1 to 2 days relative to that seen with the nodD2 mutant strain. In contrast, the timing of complete infection (100% plants nodulated) was different for the tested strains when examined at 109 cells/ml (Fig. 1D). In this case, the wild-type USDA110 strain was the first to form nodules on PI 417566, and about 60% of the plants were nodulated at 8 days postinoculation. Nodulation by the nodD2 mutant on PI 417566 was generally faster and more complete at 105 cells/ml than at 109 cells/ml (Fig. 1C and D), and all the strains examined formed nodules faster and more completely on PI 417566 than on cv. Lambert.
The efficacy with which the wild-type and mutant strains nodulated the restrictive and permissive soybean genotypes was also examined, by using the root tip marking (RTM) technique (3). Consistent with the speed of nodulation data presented in Fig. 1, RTM analyses indicated that there was a marked change in the nodule distribution pattern observed with PI 417566 with regard to the inoculum strain, but not with respect to population density (data not shown). The nodules formed on PI 417566 by wild-type USDA110 at the 105-cells/ml inoculum density were mostly located about 1 to 7 cm below the root tip mark, whereas those produced by the nolA and nodD2 mutants were more scattered along the plant roots, 3 to 11 cm below the root tip mark. In contrast, when PI 417566 was inoculated with the nodD2 mutant at 109 cells/ml, some nodules were produced above the root tip mark, indicating faster nodulation. When inoculated on cv. Lambert, however, USDA110 formed nodules from 2 cm above to 11 cm below the root tip mark (data not shown). Moreover, on cv. Lambert, the nolA and nodD2 mutant strains produced a much-greater number of nodules that were above the RTM, at either inoculum density, than was seen when the wild-type strain was used as the inoculum (data not shown). Taken together, the results of these studies indicated that the nolA and nodD2 mutant strains formed nodules more rapidly than the wild-type strain on the permissive soybean genotype.
DISCUSSION
The results of several studies have shown that soybean genotypes are differentially nodulated by Bradyrhizobium strains (4, 5, 21) and that B. japonicum strain USDA110 is restricted for nodulation by soybean genotype PI 417566 (22). Although the results of previous studies showed that the restriction of nodulation by USDA110 on soybean PI genotypes is temperature dependent, determined by the root genotype, conditioned by a single recessive host gene, and related to inoculum density (21-23, 29), the molecular basis for restriction of strain USDA110 has largely been unexplored. Moreover, the influence of nod gene mutations on inoculum-density-dependent nodulation of soybeans is not well understood. While it was previously demonstrated that USDA110 displayed density-dependent nodulation on soybean cv. Kasota, this phenotype can also be seen on cv. Lambert. Taken together with the studies done by Loh and coworkers (14) and Ferrey et al. (6) using soybean cvs. Essex and Peking, respectively, these results indicate that density-dependent nodulation suppression is not limited to particular soybean genotypes but is a phenomenon that can be generalized to different soybean cultivars.
The results of the present studies also indicated that the population-density-dependent suppression of nodulation by USDA110 on cv. Lambert was more evident in sterile plant growth medium than in natural, nonsterile soil. In contrast, suppression of nodulation by USDA110 on PI 417566 at the high inoculum density was quite evident in soil, suggesting that both the host genotype and soil abiotic and biotic factors play major roles in density-dependent nodulation restriction. It is well known that soil is a heterogeneous environment containing many bacterial species that are in close proximity and compete with each other for nutrients and space. Thus, it is reasonable to assume that the soil environment may have limited the number of bradyrhizobia, typically 104 to 106 cells/g (33), coming into contact with the plant root system and thus obscured the nodulation suppression phenotype in soil. Alternately, it may also be possible that bradyoxetin, which mediates the population-density-dependent suppression of nodulation of soybeans, was reduced to a subcritical level by either adsorption to soil particles or organic matter or degradation by soil microorganisms to render quorum sensing ineffective with this particular host and bacterial combination. Consequently, caution must be used to define which plant genotypes actually restrict nodulation by B. japonicum strains, as this phenotype is conditioned by the genomes of both symbionts, by several abiotic factors, by plant growth conditions, and by the medium used for nodulation studies.
The nodulation of soybeans by B. japonicum occurs via the coordinated interaction of five regulatory pathways, involving nodD1, nodD2, nodVW, nwsB, and nolA (10-12, 16, 18), and is influenced by the density of the inoculant strain (21, 17). The most-dramatic effect on density-dependent nodulation suppression occurred when the nodD2 and nolA mutants were inoculated on nodulation-restrictive and -permissive soybean genotypes. In almost all cases, plants receiving a greater number of mutant B. japonicum cells had enhanced nodulation relative to that seen with wild-type USDA110. Thus, under these conditions, nodulation suppression at a high inoculum rate was not seen.
While the data presented here support results from previous studies showing that an nodD1 mutant retains the capacity to nodulate soybean plants (9), this mutant produced significantly fewer nodules on cv. Lambert at a high inoculum density than the other mutants tested. Moreover, the nodD1 mutant did not display density-dependent suppression of nodulation at a higher inoculum rate. This is likely a direct result of the lack of a functional nodD1 gene, whose repression is normally mediated via the action of NolA and NodD2 (16).
In contrast to nodD1, the nodW and nwsB mutants examined here showed slightly and statistically significantly enhanced nodulation at the higher inoculum level on both soybean genotypes. NodVW was previously shown to be required for nodulation of mungbeans, cowpeas, and siratro (10), but not for soybeans, and NwsB was previously shown to be required for the population-density-dependent expression of nolA and nodD2 (14). The mechanism by which the NodW mutant enhances nodulation at a high cell density is not currently known, but it has been shown that NwsB is also required for the population-density-dependent expression of nolA and nodD2 and that mutations in nwsB can be complemented by the overexpression of NodW (14). This suggests that the multiple nodulation pathways in B. japonicum may be interactive and coordinately regulate the density-dependent suppression of soybean nodulation. In addition, these results indicate that a functional copy of the nodD1 gene is apparently required for the density-dependent enhanced nodulation of soybeans and that B. japonicum strains with mutations in nolA and nodD2 can be used to enhance the nodulation of soybeans at high inoculum densities.
The regulation of nodulation gene expression in B. japonicum is relatively complex and is controlled by both activators and repressors (16). The population-density-dependent nodulation of soybeans was previously shown to be regulated by the extracellular concentration of bradyoxetin, a novel Fe-regulated and secreted quorum-responsive signal molecule that induces the expression of nolA (20). Bradyrhizobium nolA, which was initially identified as a genotype-specific nodulation gene allowing the nodulation of soybean PI 377578 (27), activates the expression of nodD2, which in turn represses the expression of common and host-specific nodulation genes in Bradyrhizobium (7, 15, 19). Similar to what was previously reported for cells grown in minimal medium (17), B. japonicum cells grown to high density in complex (AG) medium also display population-density-dependent repression of nod gene expression. Thus, it is likely that the composition of the medium and the growth rate play minor roles in the synthesis and perception of bradyoxetin. Moreover, the suppression of soybean nodulation at high inoculum densities can be eliminated or severely reduced by the use of several nod gene mutants that interrupt the perception and transmission of the nodulation-gene-repressing, quorum-responsive signal molecule bradyoxetin. While the concentrations of bradyoxetin in supernatants of wild-type USDA110-grown cells were not directly measured, results from both sets of studies suggest that nolA, acting through nodD2, plays a prominent role in density-dependent nodulation suppression and that deletions in either of these genes can be used to enhance soybean nodulation. Moreover, consistent with results from previous studies done using nolA-lacZ, nodC-lacZ, and nodD2-lacZ expression (14) and mutational analyses (10, 11, 18), mutations in the response regulators NwsB and NodW result in enhanced nodulation at high cell densities on soybean cv. Lambert.
Competition for nodulation is, and remains, a critical problem for enhancing nitrogen fixation in legumes (32). It is widely recognized that B. japonicum strain USDA123 and serocluster 123 members are more competitive for the nodulation of soybeans than strain USDA110 in Midwestern United States soils (34). This result was also borne out in the studies reported here using soybean cv. Lambert inoculated at both low and high inoculum densities. While the results of the equal-concentration competition studies done here suggest that the nodD2 mutant of strain USDA110 is more competitive for soybean nodulation than the wild-type USDA110 strain, additional studies done with more replications and at several inoculation levels are needed to more thoroughly assess whether statistically significant increases in competitive ability occur as a result of the mutation. This competitive advantage may in part be due to the fact that the nodD2 mutant of USDA110 nodulated the permissive soybean genotype faster than the wild-type strain and displayed enhanced nodulation at high population densities. These results are also consistent with those reported by Loh and colleagues (14) in which a B. japonicum nwsB mutant was able to better compete with the wild-type strain for nodule occupancy at a high population density, further indicating that alterations in the nodulation gene repression system can be used to enhance competitiveness.
Interestingly, the competitive advantage for soybean nodulation was not seen with the nolA mutant strain at low or high inoculum densities, despite results showing that this mutant forms nodules faster than wild-type USDA110 on cv. Lambert. This suggests that other genes may be acting through nodD2 to influence nodulation and the competitiveness of this strain and that the regulation of the nodulation genes in B. japonicum involves complex, interactive circuitry. It should also be noted that the results obtained from the nodulation speed assays are in contrast with those reported by Garcia et al. (7), who reported that an nolA mutant had a slight delay in nodulation on soybeans compared to the speed of nodulation of wild-type USDA110. This inconsistency may simply reflect differences in the soybean genotypes used, the way plants were grown, and the types of mutants used. For example, while Göttfert et al. (9) reported that an nodD2 deletion mutant (Δ370) was delayed in nodulation on soybean cv. Williams relative to its speed of nodulation on USDA110, the mutant also contained a 600-bp deletion in sequences downstream of the nodD2 coding region, perhaps altering the regulation of downstream genes, including nolA.
The results of nodulation assays done using PI 417566, however, also indicated that host genotype and cell density significantly influenced the speed of nodulation of the mutants and the wild-type strain. For example, while the nodD2 mutant strain formed nodules faster on PI 417566 at 105 cells/ml, the wild-type USDA110 strain nodulated the PI genotype faster than the nodD2 or nolA mutants when the strains were inoculated at 109 cells/ml. Despite this, the results of the present studies showed that nodulation by the nodD2, nolA, nodW, and nwsB mutants at high inoculum levels was enhanced relative to that seen with the wild-type strain. This may in part be due to the controlling influence of the single recessive host gene in PI 417566 (23) which conditions nodulation by USDA110 on this genotype. Further studies done using isogenic soybean genotypes lacking this allele would shed more light on the host and microbial factors controlling nodulation with this host. Despite this limitation, however, these results indicate that NodD2 is a key repressor influencing host-controlled restriction of nodulation, density-dependent suppression of nodulation, perception of the bradyoxetin quorum-sensing molecule, and competitiveness in the soybean-B. japonicum symbiosis.
Acknowledgments
We thank Gary Stacey for helpful suggestions and critical reading of the manuscript. We also thank Gary and Minviluz Stacey for providing the nodulation gene mutants used in these studies, Carl Rosen for providing soils, Matthew Hamilton and Masayuki Sugawara for help analyzing the nodD2 mutant, and Andrew Scobbie and Daniel Norat for help with statistical analyses.
This work was supported, in part, by a fellowship from the Royal Thai Government, Ministry of Agriculture and Cooperatives (to S.J.), and by funding from the University of Minnesota Agricultural Experiment Station (to M.J.S.).
Footnotes
Published ahead of print on 25 April 2008.
REFERENCES
- 1.Banfalvi, Z., A. Nieuwkoop, M. Schell, L. Besl, and G. Stacey. 1988. Regulation of nod gene expression in Bradyrhizobium japonicum. Mol. Gen. Genet. 214:420-424. [DOI] [PubMed] [Google Scholar]
- 2.Bergersen, F. J. 1961. The growth of Rhizobium in synthetic media. Aust. J. Biol. Sci. 14:349-360. [Google Scholar]
- 3.Bhuvaneswari, T. V., B. G. Turgeon, and W. D. Bauer. 1980. Early events in the infection of soybean (Glycine max (L.) Merr.) by Rhizobium japonicum. Plant Physiol. 66:1027-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cregan, P. B., and H. H. Keyser. 1986. Host restriction of nodulation by Bradyrhizobium japonicum strain USDA 123. Crop Sci. 26:911-916. [Google Scholar]
- 5.Cregan, P. B., H. H. Keyser, and M. J. Sadowsky. 1989. Host plant effects on nodulation and competitiveness of the Bradyrhizobium japonicum serotype strains constituting serocluster 123. Appl. Environ. Microbiol. 55:2532-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrey, M. L., P. H. Graham, and M. P. Russelle. 1994. Nodulation efficiency of Bradyrhizobium japonicum strains with genotypes of soybean varying in the ability to restrict nodulation. Can. J. Microbiol. 40:456-460. [Google Scholar]
- 7.Garcia, M. L., J. Dunlap, J. Loh, and G. Stacey. 1996. Phenotypic characterization and regulation of the nolA gene of Bradyrhizobium japonicum. Mol. Plant-Microbe Interact. 9:625-635. [DOI] [PubMed] [Google Scholar]
- 8.Göttfert, M., D. Holzhauser, and H. Hennecke. 1992. Structural and functional analysis of two different nodD genes in Bradyrhizobium japonicum USDA110. Mol. Plant-Microbe Interact. 5:257-265. [DOI] [PubMed] [Google Scholar]
- 9.Göttfert, M., J. W. Lamb, R. Gasser, J. Semenza, and H. Hennecke. 1989. Mutational analysis of the Bradyrhizobium japonicum common nod genes and further nod box-linked genomic DNA regions. Mol. Gen. Genet. 215:407-415. [DOI] [PubMed] [Google Scholar]
- 10.Göttfert, M., P. Grob, and H. Hennecke. 1990. Proposed regulatory pathway encoded by the nodV and nodW genes, determinants of host specificity in Bradyrhizobium japonicum. Proc. Natl. Acad. Sci. USA 87:2680-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grob, P., H. Hennecke, and M. Göttfert. 1994. Cross-talk between the two-component regulatory systems NodVW and NwsAB of Bradyrhizobium japonicum. FEMS Microbiol. Lett. 120:349-354. [Google Scholar]
- 12.Grob, P., P. Michel, H. Hennecke, and M. Göttfert. 1993. A novel response regulator is able to suppress the nodulation defect of a Bradyrhizobium japonicum nodW mutant. Mol. Gen. Genet. 241:531-541. [DOI] [PubMed] [Google Scholar]
- 13.Keyser, H. H., and P. B. Cregan. 1987. Nodulation and competition for nodulation of selected soybean genotypes among Bradyrhizobium japonicum serogroup 123 isolates. Appl. Environ. Microbiol. 53:2631-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loh, J., D. P. Lohar, B. Andersen, and G. Stacey. 2002. A two-component regulator mediates population-density-dependent expression of the Bradyrhizobium japonicum nodulation genes. J. Bacteriol. 184:1759-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loh, J., and G. Stacey. 2001. Feedback regulation of the Bradyrhizobium japonicum nodulation genes. Mol. Microbiol. 41:1357-1364. [DOI] [PubMed] [Google Scholar]
- 16.Loh, J., and G. Stacey. 2003. Nodulation gene regulation in Bradyrhizobium japonicum: a unique integration of global regulatory circuits. Appl. Environ. Microbiol. 169:10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loh, J., J. P. Y. Yuen-Tsai, A. Welborn, and G. Stacey. 2001. Population density-dependent regulation of the Bradyrhizobium japonicum nodulation genes. Mol. Microbiol. 42:37-46. [DOI] [PubMed] [Google Scholar]
- 18.Loh, J., M. Garcia, and G. Stacey. 1997. NodV and NodW, a second flavonoid recognition system regulating nod gene expression in Bradyrhizobium japonicum. J. Bacteriol. 179:3013-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loh, J., M. G. Stacey, M. J. Sadowsky, and G. Stacey. 1999. The Bradyrhizobium japonicum nolA gene encodes three functionally distinct proteins. J. Bacteriol. 181:1544-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loh, J., R. W. Carlson, W. S. York, and G. Stacey. 2002. Bradyoxetin, a unique chemical signal involved in symbiotic gene regulation. Proc. Natl. Acad. Sci. USA 99:14446-14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lohrke, S. M., C. J. Madrzak, H. G. Hur, A. K. Judd, J. H. Orf, and M. J. Sadowsky. 2000. Inoculum density-dependent restriction of nodulation in the soybean-Bradyrhizobium japonicum symbiosis. Symbiosis 29:59-70. [Google Scholar]
- 22.Lohrke, S. M., J. H. Orf, E. Martinez-Romero, and M. J. Sadowsky. 1995. Host-controlled restriction of nodulation by Bradyrhizobium japonicum strains in serogroup 110. Appl. Environ. Microbiol. 61:2378-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lohrke, S. M., J. H. Orf, and M. J. Sadowsky. 1996. Inheritance of host-controlled restriction of nodulation by Bradyrhizobium japonicum strain USDA 110. Crop Sci. 36:1271-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 25.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 26.Pongsilp, N., E. W. Triplett, and M. J. Sadowsky. 2005. Detection of homoserine lactone-like quorum sensing molecules in Bradyrhizobium strains. Curr. Microbiol. 51:250-254. [DOI] [PubMed] [Google Scholar]
- 27.Sadowsky, M. J., P. B. Cregan, M. Göttfert, A. Sharma, D. Gerhold, F. Rodriguez-Quiñones, H. H. Keyser, H. Hennecke, and G. Stacey. 1991. The Bradyrhizobium japonicum nolA gene and its involvement in the genotype specific nodulation of soybeans. Proc. Natl. Acad. Sci. USA 88:637-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadowsky, M. J., R. E. Tully, P. B. Cregan, and H. H. Keyser. 1987. Genetic diversity in Bradyrhizobium japonicum serogroup 123 and its relation to genotype-specific nodulation of soybeans. Appl. Environ. Microbiol. 53:2624-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadowsky, M. J., R. M. Kosslak, B. Golinska, C. J. Madrzak, and P. B. Cregan. 1995. Restriction of nodulation by B. japonicum is mediated by factors present in the roots of Glycine max. Appl. Environ. Microbiol. 61:832-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt, E. L., R. O. Bankole, and B. B. Bohlool. 1968. Fluorescent antibody approach to study of rhizobia in soil. J. Bacteriol. 95:1987-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Somasegaran, P., and H. J. Hoben. 1994. Handbook for rhizobia: methods in legume-Rhizobium technology. Springer-Verlag, New York, NY.
- 32.Triplett, E. W., and M. J. Sadowsky. 1992. Genetics of competition for nodulation. Annu. Rev. Microbiol. 46:399-428. [DOI] [PubMed] [Google Scholar]
- 33.Viteri, S. E., and E. L. Schmidt. 1987. Ecology of indigenous soil rhizobia: response of Bradyrhizobium japonicum to readily available substrates. Appl. Environ. Microbiol. 53:1872-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viteri, S. E., and E. L. Schmidt. 1996. Ecology of indigenous soil rhizobia: selective response of Bradyrhizobium japonicum to a soybean meal. Appl. Soil Ecol. 3:187-195. [DOI] [PMC free article] [PubMed] [Google Scholar]