Abstract
Ethanol is a frequently abused drug that impairs cognitive processes such as learning. Varenicline, an α4β2 nicotinic receptor partial agonist and α7 nicotinic receptor full agonist prescribed for smoking cessation, has been shown to decrease ethanol consumption. The current study investigated whether varenicline could ameliorate ethanol-induced deficits in learning and whether varenicline alters blood alcohol concentration in C57BL/6 mice. Conditioning consisted of two auditory conditioned stimulus (CS; 30 seconds, 85 dB white noise)–foot shock unconditioned stimulus (US; 2 seconds, 0.57 mA) pairings. For all studies, saline or ethanol (1.0, 1.5, 2.0 g/kg i.p.) was administered 15 minutes before training, and saline or varenicline (0.05, 0.1, 0.2 mg/kg i.p.) was administered 60 minutes before either training or testing. For blood alcohol analysis, saline or varenicline (0.1 mg/kg) was administered 60 minutes before collection, and saline or ethanol (1.0, 1.5, 2.0 g/kg) was administered 15 minutes before collection. Varenicline dose-dependently ameliorated ethanol-induced conditioning deficits for all three doses of ethanol when administered before training but not when administered 24 hours later, before testing. In addition, varenicline did not alter blood alcohol concentration. The smoking cessation aid varenicline may have therapeutic uses for treating ethanol-associated disruptions in cognitive processes.
Keywords: Alcohol, Varenicline, Acetylcholine, Learning, Addiction, Nicotine
Introduction
Although the health risks associated with alcohol abuse and addiction are well-known, alcoholism remains a worldwide public health issue. The most common alcohol abstinence aids for patients are nonspecific opioid receptor antagonists such as naltrexone (Nava et al., 2006), and the glutamate receptor antagonist acamprosate, both of which decrease ethanol craving (Cowen et al., 2005). Although these drugs, alone and in combination, decrease the likelihood of relapse to drinking in abstinent alcoholics (Feeney et al., 2006), acamprosate has minimal effects on the cognitive deficits associated with acute and chronic ethanol consumption (Brasser et al., 2004), while naltrexone actually exacerbates ethanol-induced cognitive deficits (McCaul et al., 2000). As a result of the incomplete treatment options for alcohol cessation, research is ongoing to identify new therapies that treat both the cognitive-disruptive and addictive effects of ethanol.
One potential therapeutic target for treating alcoholism is the nicotinic acetylcholinergic system. There are strong correlations between susceptibilities to ethanol and nicotine dependence (Dawson, 2000; John et al., 2003) and between heavy consumption of both drugs (Dawson, 2000; John et al., 2003; Larsson & Engel, 2004) that may reflect a genetic link between alcoholism and nicotine addiction (Funk et al., 2006). In addition, both ethanol and nicotine act on nicotinic acetylcholine receptors (nAChRs) that underlie diverse neuronal processes (Davis & De Fiebre, 2006) such as those involved in reward/reinforcement (Jerlhag et al., 2006; Walters et al., 2006) and learning/memory (Chan et al., 2007; Davis et al., 2007; Gould, 2006). Drugs that target nAChRs have been shown to decrease both nicotine consumption (Rollema et al., 2007a; Tennant et al., 1984) and ethanol consumption (Blomqvist et al., 1996; Ericson et al., 1998; Le et al., 2000). Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist and α7 nicotinic receptor full agonist that is currently prescribed as a smoking cessation aid (under the names Chantix or Champix), has recently been shown to decrease ethanol consumption and craving (Steensland et al., 2007). It remains unclear, however, if varenicline also exerts an effect on ethanol-induced cognitive deficits.
The goal of the current study was to examine the dose-dependent interactive effects of acute varenicline and ethanol on contextual and cued fear conditioning. Fear conditioning is useful for investigating the effects of ethanol on learning because it tests two types of learning that are disrupted by ethanol (Gould, 2003; Gould & Lommock, 2003; Gulick & Gould, 2007); hippocampus-dependent contextual learning, in which contextual features are associated with an unconditioned stimulus (US) foot shock, and hippocampus-independent cued learning, in which an auditory conditioned stimulus (CS) is associated with the US (Logue et al., 1997; Phillips & LeDoux, 1992). We examined the effects of varenicline on acquisition and recall of conditioning in saline- and ethanol-treated mice. In addition, we examined whether varenicline altered blood alcohol levels following acute ethanol administration.
Materials and Methods
Subjects
Male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were tested at 8–12 weeks of age (20–30 g). Mice were housed in groups of 4 mice per cage and had ad libitum access to food and water. A 12-hr light–dark cycle (lights on at 0700) was maintained, with all testing done between 9:00 am and 5:00 pm. Procedures were approved by the Temple University Institutional Animal Care and Use Committee.
Apparatus
Training took place in identical conditioning chambers housed in sound-attenuating boxes (MED Associates, St. Albans, VT). Each 17.78 × 19.05 × 38.10 cm chamber consisted of Plexiglas panels in the front, back, and ceiling and two stainless-steel walls on the sides. The metal grid floor of each chamber, through which the foot-shock US (0.57 mA) was delivered, was connected to a shock generator and scrambler. Background noise and air exchange (69 dB) were provided by ventilation fans mounted on the right wall of each sound-attenuating box, and speakers that were used to deliver a white noise CS (85 dB) were mounted on the right wall of each chamber. A computer running MED Associates (St. Albans, VT) software controlled presentation of training and testing stimuli. Chambers were cleaned with 70% ethanol before each session.
Testing for freezing to the CS occurred in a separate room in altered context chambers that were housed in sound-attenuating boxes. Speakers that delivered the white noise CS were mounted on the left wall of each chamber. The 20.32 × 22.86 × 17.78 cm chambers were constructed of Plexiglas walls and ceiling, and a metal grid floor covered with opaque white plastic. In addition, visual cues (e.g., white walls in the training context and black walls in the altered context boxes), chamber dimensions, floor construction, and a vanilla extract olfactory cue (no olfactory cue was present in the training chambers) further distinguished the altered context chambers from the original training chambers.
Procedure
Groups for all behavioral procedures consisted of 7–9 animals. Each animal was used in only one condition and one experiment. On training day, mice were placed in conditioning chambers. After a 120 second baseline period, they received two coterminating CS (30-s, 85 dB white noise)–US (2-s, 0.57 mA foot shock) pairings. At 120 and 270 seconds, the CS sounded for 30 seconds; the US occurred during the last 2 seconds of the CS. The mice remained in the chamber for 30 seconds after the second CS-US presentation, for a total of 5.5 minutes. Baseline freezing behavior was recorded during the first 120 seconds and immediate freezing was assessed between CS-US presentations (methods based on Gould and Higgins, 2003). Twenty-four hours after training, freezing to the context was assessed and then freezing to the CS was assessed one hour later. To evaluate freezing to the context, mice were placed in the training chamber for 5 minutes. To evaluate freezing to the CS, mice were placed in an altered context consisting of a modified conditioning chamber located in a different room. During the first 3 minutes of the altered context test, the CS was not presented (pre-CS test). During the last 3 minutes, the CS was continuously presented (CS test). Freezing was assessed during the entire 6-minute period.
We first tested whether a dose of varenicline (0.1 mg/kg) that preliminary data suggest may ameliorate nicotine withdrawal-associated deficits in fear conditioning would ameliorate acute ethanol-induced deficits in fear conditioning. Animals received saline or varenicline (0.1 mg/kg) via intraperitoneal (i.p.) injections 60 minutes before training (based on communications with Dr. Hans Rollema of Pfizer; Rollema et al., 2007) and saline or ethanol (1.0 g/kg) i.p. 15 minutes before training (based on Gould, 2003). The 1.0 g/kg dose of ethanol disrupts fear conditioning (Gould, 2003; Gulick & Gould, 2007) and administration before training may be altering processes involved in acquisition of learning. To determine whether the effects of varenicline are specific to acquisition, we also examined the effect of varenicline on testing day to assess changes in recall of learning. Animals received saline or ethanol (1.0 g/kg) i.p. 15 minutes before training, and saline or varenicline (0.1 mg/kg) i.p. 60 minutes before testing. For all drug conditions, contextual and cued fear conditioning was assessed at 24 hours post-training.
We then examined the interactive effects of multiple doses of ethanol and varenicline on fear conditioning. For the varenicline dose-response, saline or varenicline (0.05, 0.1, 0.2 mg/kg) was administered i.p. 60 minutes before training, and saline or ethanol (1.0 g/kg) was administered i.p. 15 minutes before training. For the ethanol dose-response, saline or varenicline (0.1 mg/kg) was administered i.p. 60 minutes before training and saline or ethanol (1.5, 2.0 g/kg) was administered i.p. 15 minutes before training.
Scoring
Freezing behavior was observed using a time-sampling procedure during training and testing sessions. At 10-second intervals, mice were assessed for freezing during a 1-second period. Freezing was defined as the absence of visible movement with the exception of respiration (methods based on Gould & Wehner, 1999).
Blood Alcohol Concentration
Finally, we examined changes in blood alcohol concentration (BAC) in response to ethanol, varenicline, and ethanol with varenicline treatment. Groups for BAC analysis consisted of 4–5 animals. Animals received saline or varenicline (0.1 mg/kg) i.p. 60 minutes before BAC analysis, and saline or ethanol (1.0, 1.5, 2.0 g/kg) i.p. 15 minutes before analysis to approximate BAC during training. Trunk blood was collected in a capillary tube (Analox Instruments, Lunenberg, MA) and spun down in a centrifuge for 2 minutes at 14,000 rev/min. The plasma was then analyzed by an AM1 Analyzer (Analox Instruments) to obtain BAC (mg/dl). To assure the accuracy of the reading, each sample was analyzed twice and a mean from the two readings was used for data analysis (Gould & Lommock, 2003).
Drugs
Varenicline was a gift from Pfizer (New York, NY). Ethanol was procured from Fisher Scientific (Pittsburgh, PA). Ethanol (1.0, 1.5, 2.0 g/kg diluted in saline) was given i.p. 15 minutes before each training session and varenicline was given i.p. 60 minutes before either training (0.05, 0.1, 0.2 mg/kg dissolved in saline) or testing (0.1 mg/kg). Injection volume for varenicline was 0.01 ml/g body weight, and ethanol was 20% vol/vol in saline. Controls received physiological saline.
Statistical Testing
Data were analyzed using a two-way analysis of variance. Tukey’s post hoc analysis was used to detect significant differences at the p<0.05 level. Statistics were calculated with SPSS (Version 13; SPSS, Chicago, IL).
Results
Varenicline on training day ameliorates ethanol-induced deficits in conditioning
To examine whether varenicline ameliorates ethanol-induced deficits in acquisition, animals received saline or varenicline (0.1 mg/kg) 60 minutes before training, and saline or ethanol (1.0 g/kg) 15 minutes before training. The levels of baseline freezing, measured before the first CS presentation, and the levels of immediate freezing, measured between stimulus presentations, were similar across groups. There was a significant main effect of ethanol on freezing to the context, F(1, 31)=29.52, p<0.001, but not on freezing to the cue, and there were significant main effects of varenicline on freezing to both the context, F(1, 31)=10.61, p<0.01 and to the cue, F(1, 31)=5.55, p<0.05. There was also a significant interactive effect of ethanol and varenicline on freezing to the cue, F(1, 31)= 13.84, p<0.01 but not on freezing to the context. There was no significant difference in freezing during the pre-CS period (Figure 1).
Figure 1.
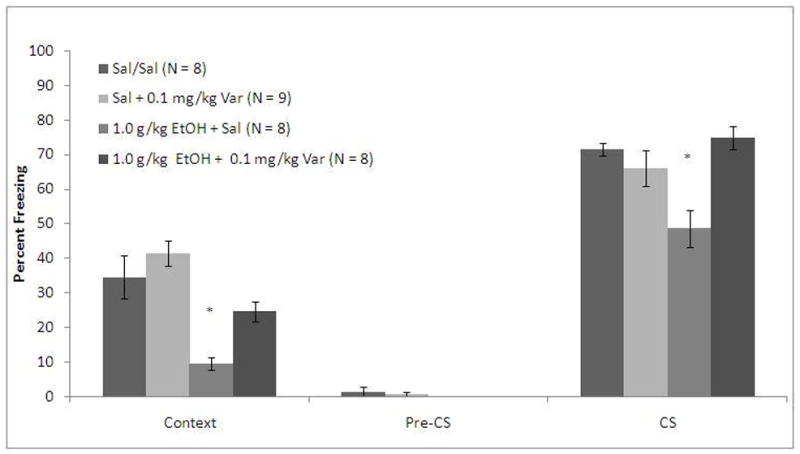
Varenicline ameliorates ethanol-induced deficits in acquisition of both contextual and cued fear conditioning (Mean ± SEM; * indicates significant difference from controls, p<0.05).
Post-hoc analysis revealed that the group administered ethanol alone froze significantly less than saline controls to both the context (p<0.001) and the cue (p<0.01). The group administered varenicline alone was not significantly different from saline controls in freezing to the context or the cue, but the group administered varenicline and ethanol froze significantly more than the group administered ethanol alone to both the context (p<0.05) and to the cue (p<0.001). Furthermore, the varenicline and ethanol group was not significantly different from saline controls in freezing to the context or to the cue.
To determine whether the effects of varenicline on ethanol-induced conditioning deficits are specific to acquisition as opposed to recall, animals received saline or ethanol (1.0 g/kg) 15 minutes before training and saline or varenicline (0.1 mg/kg) 60 minutes before testing. The levels of baseline freezing and of immediate freezing were similar across all groups. There were significant main effects of ethanol on freezing during context testing, F(1, 30)=42.41, p<0.001, and on freezing during cued testing, F(1, 30)=31.67, p<0.001, and a significant main effect of varenicline on freezing during context testing, F(1, 30)=5.58, p<0.05, but not on freezing during cued testing. There were also significant interactive effects of ethanol and varenicline on freezing to the context, F(1, 31)=5.58, p<0.05 and on freezing to the cue, F(1,31)=5.50, p<0.05. There was no significant difference in freezing during the pre-CS period (Figure 2).
Figure 2.
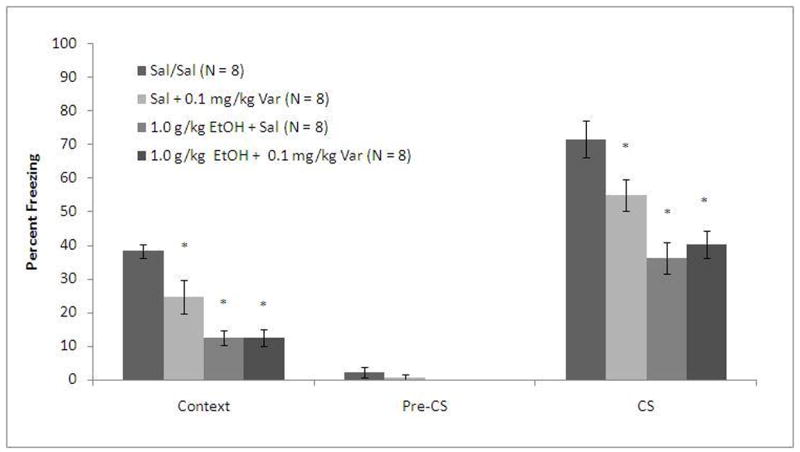
Varenicline does not reverse ethanol (1.0 g/kg)-induced deficits in fear conditioning by altering recall (Mean ± SEM; * indicates significant difference from controls, p<0.05).
Post-hoc analysis revealed that the groups administered ethanol froze significantly less than saline controls to the context (p<0.001) and to the cue (p<0.001) regardless of varenicline treatment. The group administered varenicline alone froze significantly more to both the context and the cue than the groups administered ethanol (p<0.05) but significantly less than the group administered saline alone (p<0.05). These results suggest that varenicline ameliorates ethanol-induced learning deficits by altering processes involved in acquisition rather than recall.
Dose-dependent effects of varenicline and ethanol on conditioning
To further examine the interactive effects of varenicline and ethanol on fear conditioning, multiple doses of each drug were tested. For the varenicline dose-response, animals received saline or varenicline (0.05, 0.1, 0.2 mg/kg) 60 minutes before training and saline or ethanol (1.0 g/kg) 15 minutes before training. The levels of baseline freezing and immediate freezing were similar across all groups. There were significant main effects of ethanol on freezing during context testing, F(1, 61)=9.89, p<0.05, and on freezing during cued testing, F(1, 61)=40.00, p<0.001, as well as significant main effects of varenicline on freezing during context testing, F(3, 59)=15.23, p<0.001, and on freezing during cued testing, F(3, 59)=19.73, p<0.001. There were significant interactive effects of ethanol and varenicline on freezing to the context, F(3, 59)=6.75, p<0.01, and on freezing to the cue, F(3, 59)=12.05, p<0.001. There was no significant difference in freezing during the pre-CS period (Figure 3).
Figure 3.
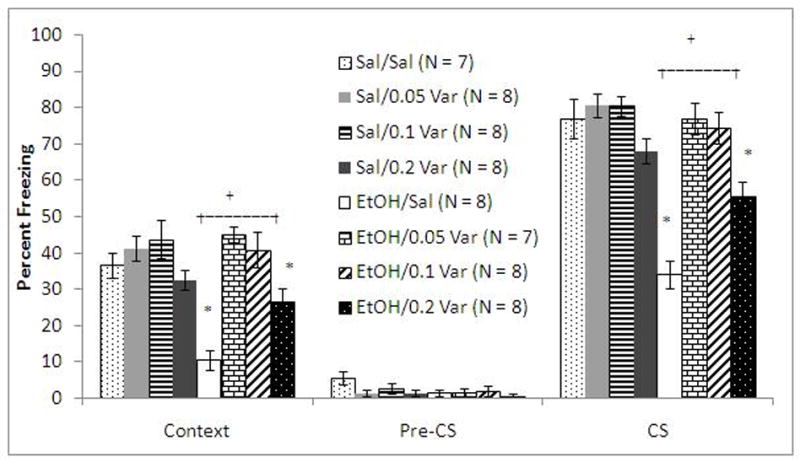
Varenicline dose-dependently ameliorates ethanol (1.0 g/kg)-induced deficits in fear conditioning but does not alter conditioning when administered alone (Mean ± SEM; * indicates significant difference from controls, + indicates differences between starred groups p<0.05).
Post-hoc analysis revealed that the groups administered varenicline alone were not significantly different from saline controls in freezing to the context or to the cue. The group administered ethanol alone froze significantly less than controls to the context and to the cue (p<0.001). The groups administered ethanol and 0.05 or 0.1 mg/kg varenicline were not significantly different from controls in freezing to the context or to the cue. The group administered ethanol and 0.2 mg/kg varenicline froze significantly more than the group administered ethanol alone to the context (p<0.05) and to the cue (p<0.01) but froze significantly less than saline controls to the cue (p<0.005).
To test if varenicline would ameliorate deficits due to higher doses of ethanol, animals received saline or varenicline (0.1 mg/kg) 60 minutes before training and saline or ethanol (1.5 or 2.0 g/kg) 15 minutes before training. The levels of baseline freezing and of immediate freezing were similar across all groups. There were significant main effects of ethanol on freezing during context testing, F(2, 69)=115.33, p<0.001, and on freezing during cued testing, F(2, 69)=6175.55, p<0.001. There were significant main effects of varenicline on freezing to the context, F(1, 70)=11.78, p<0.05 and on freezing to the cue, F(1, 70)=26.17, p<0.001. There were also significant interactive effects of ethanol and varenicline on freezing to the context, F(2, 69)=4.88, p<0.05, and on freezing to the cue, F(2, 69)=10.82, p<0.001. There was no significant difference in freezing during the pre-CS period (Figure 4).
Figure 4.
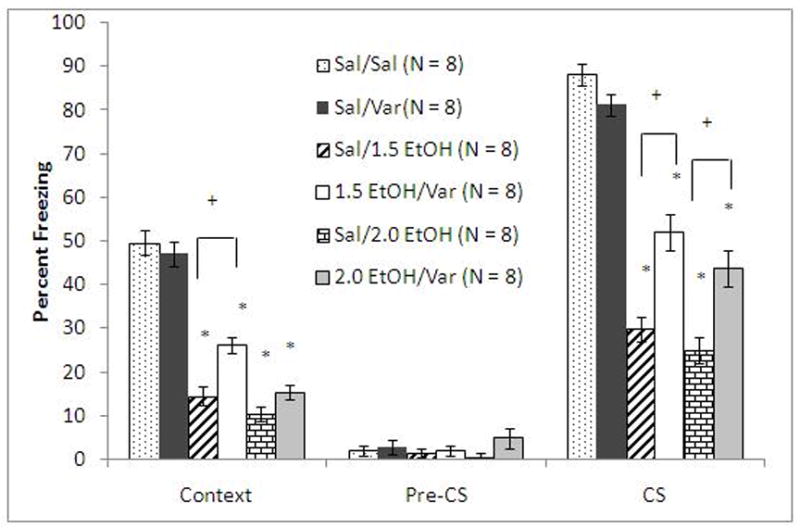
Varenicline (0.1 mg/kg) ameliorates the deficits in fear conditioning associated with higher doses of ethanol (Mean ± SEM; * indicates significant difference from controls and + indicates significant difference between groups, p<0.05).
Post-hoc analysis revealed that all ethanol-treated groups froze significantly less than saline controls to the context and to the cue (p<0.001). The group administered varenicline alone was not significantly different from controls in freezing to the context and to the cue. The group administered varenicline and 1.5 g/kg ethanol froze significantly more than the group administered 1.5 g/kg ethanol alone to the context (p<0.005) and to the cue (p<0.001). The group administered varenicline and 2.0 g/kg ethanol froze significantly more than the group administered 2.0 g/kg ethanol alone to the cue (p<0.005) but not to the context; this may be due to the greater impairment of contextual conditioning compared to cued conditioning by ethanol, which may be too great to be fully overcome by varenicline. These results demonstrate that varenicline ameliorates ethanol-induced conditioning deficits associated with higher doses of ethanol but that this effect decreases as ethanol doses increase.
Varenicline does not alter blood alcohol concentration
In order to determine whether the modulation of ethanol-induced learning deficits may be due to changes in BAC after varenicline treatment, animals were administered saline or varenicline (0.1 mg/kg) 60 minutes before analysis and saline or ethanol (1.0, 1.5, 2.0 g/kg) 15 minutes before analysis. There was a significant main effect of ethanol, F(3, 35)=258.25, p<0.001 but no significant main effect of varenicline and no significant interaction between ethanol and varenicline. Post-hoc analysis revealed that ethanol treatment significantly increased BAC above baseline levels (p<0.001) but varenicline treatment did not alter BAC in any group. Thus, changes in BAC by varenicline cannot explain the amelioration of ethanol-induced learning deficits by varenicline across the range of ethanol doses used in the current study (Figure 5).
Figure 5.
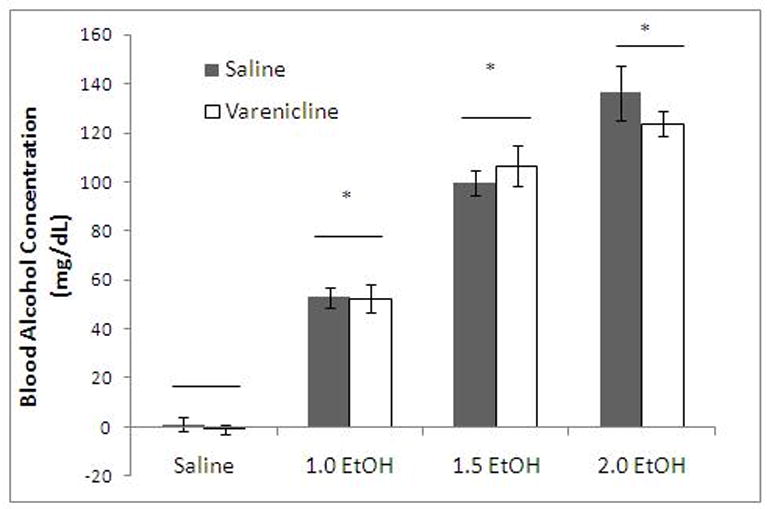
Varenicline (1.0 g/kg) does not decrease blood alcohol (Mean ± SEM; * indicates significant difference from controls and line indicates no significant difference across varenicline conditions, p<0.05).
Discussion
Ethanol interacts with many neurotransmitter systems (for review, see Chastain, 2006) and these diverse effects may contribute to an equally diverse range of drug treatments for alcoholism. This is the first study to demonstrate that varenicline, which has previously been shown to decrease ethanol consumption and seeking (Steensland et al., 2007), ameliorates ethanol-induced cognitive deficits without altering blood alcohol concentration. Specifically, varenicline ameliorated deficits in acquisition of contextual and cued associative learning. Interestingly, varenicline impaired recall in ethanol-naïve mice when administered before testing; this impairment may be due to an over-activation of the acetylcholinergic system by varenicline. Indeed, it is well-established that nicotinic drugs produce inverted, u-shaped dose-response curves (for review, Picciotto, 2003). In addition, the highest dose of varenicline used in the current study only partially reversed ethanol-induced learning impairments, further suggesting that the dose-response curve for varenicline on ethanol-induced deficits may be curvilinear. It should be noted that the effective dose range in the present study (0.05–0.2 mg/kg) differed from the effective dose range in the Steensland and colleagues study (1–2 mg/kg) (2007). However, the current study used mice whereas the Steensland study used rats, and the differences between studies could be due to differences in drug effects across species. Nonetheless, the ability of varenicline to modulate the effects of ethanol on systems underlying both reward and learning may make it an effective treatment for patients who struggle with multiple behavioral consequences of alcohol abuse.
Compared to naltrexone and acamprosate, varenicline may have different therapeutic indices because of the diverse substrates on which these drugs act. Naltrexone is an opioid receptor antagonist; by blocking the opioid receptors involved in producing the positive effects of ethanol, naltrexone may decrease the motivation to consume alcohol (Stromberg et al., 2001). Naltrexone, however, may not ameliorate the negative effects of ethanol on learning. Opioid antagonists impair memory formation (Freeman & Young, 2000), possibly by altering the protein synthesis processes that underlie learning and memory (Cole &McNally, 2007; Freeman & Young, 2000). Thus, because ethanol also disrupts learning, naltrexone in combination with ethanol could produce greater memory impairments in alcoholics than either drug alone. In support, naltrexone exacerbates ethanol-induced learning deficits in heavy drinkers (McCaul et al., 2000) and potentiates the memory-impairing effects of another depressant, chlordiazepoxide (Silva & Frussa-Filho, 2002). The other commonly-prescribed treatment for alcohol dependence, acamprosate, is an n-methyl-d-aspartate (NMDA) receptor antagonist (al Qatari et al., 1998). Although the mechanisms underlying the behavioral effects of acamprosate remain unclear, NMDA receptors are an important substrate underlying learning (for review, Sweatt, 1999), and the effects of acamprosate on glutamatergic processes may produce learning deficits. Indeed, chronic acamprosate treatment impairs delayed recall in non-alcoholic subjects (Schneider et al., 1999), although acamprosate has no effect on working memory (Okulicz-Kozaryn et al., 2001) or short-term memory (Mikolajczak et al., 2002) in rats. Although studies are needed to directly compare the effects of alcohol cessation aids on learning, if naltrexone and acamprosate fail to ameliorate ethanol-induced cognitive deficits and varenicline can ameliorate these deficits, varenicline may be a more complete therapeutic agent for treating alcohol abuse and addiction.
The mechanism by which varenicline ameliorates ethanol-induced learning deficits has not yet been elucidated. Varenicline is both an α4β2 nAChR partial agonist and α7 nAChR full agonist; thus, either receptor subtype could mediate the effects of varenicline on ethanol-induced impairments in learning. Both receptors also exist in large numbers in the hippocampus (Séguéla et al., 1993; Marks et al., 2006; Wada et al., 1989) and amygdala (Addy et al., 2003; Han et al., 2003), brain areas involved in fear conditioning (Logue et al., 1997; Phillips and LeDoux, 1992). As a partial agonist, varenicline has high affinity for α4β2 receptors but has lower efficacy at these receptors compared to other nAChR agonists such as nicotine and acetylcholine (Coe et al., 2005; Mihalak et al., 2006; Rollema et al., 2007a; Rollema et al., 2007b). The α4β2 nAChR underlies the enhancement of learning by nicotine (Davis et al., 2007) and animals lacking the β2 subunit of this receptor show neither enhancement of learning by nicotine nor amelioration of ethanol-induced learning deficits by nicotine (Wehner et al., 2004). Thus, evidence supports the possibility that varenicline may be acting at α4β2 nAChRs to ameliorate ethanol-induced learning deficits. Varenicline also binds to α7 receptors and, as a full agonist, has a high level of efficacy at these receptors (Mihalak et al., 2006); however, varenicline has a much lower affinity for α7 receptors compared to α4β2 receptors (Coe et al., 2005, Mihalak et al., 2006; Rollema et al., 2007b). Furthermore, although α7 nAChRs have been implicated in the effects of ethanol on learning, α7 knockout mice show a decreased sensitivity to the memory-impairing effects of ethanol (Wehner et al., 2004). Therefore, a drug acting primarily as anα7 agonist may increase sensitivity to the memory-impairing effects of ethanol and, thus, may fail to reverse learning deficits in animals administered ethanol. This, combined with the lower affinity of varenicline for a7 receptors, suggests that the a4b2 rather than a7 receptors are mediating the effects of varenicline on ethanol-induced deficits in learning. Further research identifying the mechanism by which varenicline exerts its effects on ethanol-induced learning deficits may increase understanding of the effects of ethanol on learning and may also lead to new treatments for alcoholism.
Acknowledgments
The authors would like to acknowledge grant support from the National Institute on Drug Abuse (DA017949 TG), the National Institute on Alcohol Abuse and Alcoholism (AA015515 TG), and the National Cancer Institute (P5084718 PI: Caryn Lerman Ph.D.), Jon Raybuck for laboratory assistance, and Pfizer for donating varenicline.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addy N, Nakajama A, Levin E. Nicotinic mechanisms of memory: effects of acute local DHbE and MLA infusions in the basolateral amygdala. Cognitive Brain Research. 2003;16:51–57. doi: 10.1016/s0926-6410(02)00209-4. [DOI] [PubMed] [Google Scholar]
- Al Qatari M, Bouchenafa O, Littleton J. Mechanism of action of acamprosate. Part II. Ethanol dependence modifies effects of acamprosate on NMDA receptor binding in membranes from rat cerebral cortex. Alcohol Clinical and Experimental Research. 1998;22(4):810–814. [PubMed] [Google Scholar]
- Blomqvist O, Ericson M, Johnson DH, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. European Journal of Pharmacology. 1996;314:257–267. doi: 10.1016/s0014-2999(96)00583-3. [DOI] [PubMed] [Google Scholar]
- Brasser SM, McCaul ME, Houtsmuller EJ. Alcohol effects during acamprosate treatment: a dose-response study in humans. Alcohol Clinical and Experimental Research. 2004;28:1074–1083. doi: 10.1097/01.alc.0000130802.07692.29. [DOI] [PubMed] [Google Scholar]
- Chan WK, Wong PT, Sheu FS. Frontal cortical alpha7 and alpha4beta2 nicotinic acetylcholine receptors in working and reference memory. Neuropharmacology. 2007;52:1641–1649. doi: 10.1016/j.neuropharm.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Chastain G. Alcohol, neurotransmitter systems, and behavior. Journal of General Psychology. 2006;133:329–335. doi: 10.3200/GENP.133.4.329-335. [DOI] [PubMed] [Google Scholar]
- Clements JD, Feltz A, Sahara Y, Westbrook GL. Activation kinetics of AMPA receptor channels reveal the number of functional agonist binding sites. Journal of Neuroscience. 1998;18:119–127. doi: 10.1523/JNEUROSCI.18-01-00119.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O’Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. Journal of Medicinal Chemistry. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Cole S, McNally GP. Opioid receptors mediate direct predictive fear learning: evidence from one-trial blocking. Learning and Memory. 2007;14(4):229–235. doi: 10.1101/lm.489507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen MS, Adams C, Kraehenbuehl T, Vengeliene V, Lawrence AJ. The acute anti-craving effect of acamprosate in alcohol-preferring rats is associated with modulation of the mesolimbic dopamine system. Addiction Biology. 2005;10:233–242. doi: 10.1080/13556210500223132. [DOI] [PubMed] [Google Scholar]
- Davis TJ, de Fiebre CM. Alcohol’s actions on neuronal nicotinic acetylcholine receptors. Alcohol Research and Health. 2006;29:179–185. [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. beta2 subunit-containing nicotinic receptors mediate the enhancing effect of nicotine on trace cued fear conditioning in C57BL/6 mice. Psychopharmacology (Berl) 2007;190:343–352. doi: 10.1007/s00213-006-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. Journal of Neuroscience. 2007;27:10870–10877. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA. Drinking as a risk factor for sustained smoking. Drug and Alcohol Dependence. 2000;59:235–249. doi: 10.1016/s0376-8716(99)00130-1. [DOI] [PubMed] [Google Scholar]
- Ericson M, Blomqvist O, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. European Journal of Pharmacology. 1998;358:189–196. doi: 10.1016/s0014-2999(98)00602-5. [DOI] [PubMed] [Google Scholar]
- Feeney GF, Connor JP, Young RM, Tucker J, McPherson A. Combined acamprosate and naltrexone, with cognitive behavioural therapy is superior to either medication alone for alcohol abstinence: a single centres’ experience with pharmacotherapy. Alcohol and Alcoholism. 2006;41:321–327. doi: 10.1093/alcalc/agl007. [DOI] [PubMed] [Google Scholar]
- Freeman FM, Young IG. Identification of the opioid receptors involved in passive-avoidance learning in the day-old chick during the second wave of neuronal activity. Brain Research. 2000;864:230–239. doi: 10.1016/s0006-8993(00)02181-8. [DOI] [PubMed] [Google Scholar]
- Funk D, Marinelli PW, Le AD. Biological processes underlying co-use of alcohol and nicotine: neuronal mechanisms, cross-tolerance, and genetic factors. Alcohol Research and Health. 2006;29:186–192. [PMC free article] [PubMed] [Google Scholar]
- Gould TJ. Ethanol disrupts fear conditioning in C57BL/6 mice. Journal of Psychopharmacology. 2003;17:77–81. doi: 10.1177/0269881103017001702. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Nicotine and hippocampus-dependent learning: implications for addiction. Molecular Neurobiology. 2006;34:93–107. doi: 10.1385/MN:34:2:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Stephen Higgins J. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiology of Learning and Memory. 2003;80:147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Lommock JA. Nicotine enhances contextual fear conditioning and ameliorates ethanol-induced deficits in contextual fear conditioning. Behavioral Neuroscience. 2003;117:1276–1282. doi: 10.1037/0735-7044.117.6.1276. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behavioral Brain Research. 1999;102:31–39. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. Acute ethanol has biphasic effects on short- and long-term memory in both foreground and background contextual fear conditioning in C57BL/6 mice. Alcohol Clinical and Experimental Research. 2007;31:1528–1537. doi: 10.1111/j.1530-0277.2007.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han ZY, Zoli M, Cardona A, Bourgeois JP, Changeux JP, Le Novere N. Localization of [3H]Nicotine, [3H]Cytisine, [3H]Epibatidine, and [125I]_-Bungarotoxin Binding Sites in the Brain of Macaca mulatta. Journal of Comparative Neurology. 2003;461:49–60. doi: 10.1002/cne.10659. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Grotli M, Luthman K, Svensson L, Engel JA. Role of the subunit composition of central nicotinic acetylcholine receptors for the stimulatory and dopamine-enhancing effects of ethanol. Alcohol and Alcoholism. 2006;41:486–493. doi: 10.1093/alcalc/agl049. [DOI] [PubMed] [Google Scholar]
- John U, Meyer C, Rumpf HJ, Hake U. Probabilities of alcohol high-risk drinking, abuse, or dependence estimated on grounds of tobacco smoking and nicotine dependence. Addiction. 2003;98:805–814. doi: 10.1046/j.1360-0443.2003.00381.x. [DOI] [PubMed] [Google Scholar]
- Larsson A, Engel JA. Neurochemical and behavioral studies on ethanol and nicotine interactions. Neuroscience and Biobehavioral Reviews. 2004;27:713–720. doi: 10.1016/j.neubiorev.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Le AD, Corrigall WA, Harding JW, Juzytsch W, Li TK. Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clinical and Experimental Research. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behavioral Neuroscience. 1997;111:104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Whiteaker P, Collins AC. Deletion of the alpha7, beta2, or beta4 nicotinic receptor subunit genes identifies highly expressed subtypes with relatively low affinity for [3H]epibatidine. Molecular Pharmacology. 2006;70(3):947–959. doi: 10.1124/mol.106.025338. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Eissenberg T, Rohde CA, Cheskin LJ. Naltrexone alters subjective and psychomotor responses to alcohol in heavy drinking subjects. Neuropsychopharmacology. 2000;22:480–492. doi: 10.1016/S0893-133X(99)00147-5. [DOI] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Corodimas KP, Wilson MC, Ledoux JE. Hippocampal-dependent learning and experience-dependent activation of the hippocampus are preferentially disrupted by ethanol. Neuroscience. 1996;74:313–322. doi: 10.1016/0306-4522(96)00138-8. [DOI] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Molecular Pharmacology. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Mikolajczak P, Okulicz-Kozaryn I, Kaminska E, Niedopad L, Polanska A, Gebka J. Effects of acamprosate and some polyamine site ligands of NMDA receptor on short-term memory in rats. European Journal of Pharmacology. 2002;444:83–96. doi: 10.1016/s0014-2999(02)01276-1. [DOI] [PubMed] [Google Scholar]
- Nava F, Premi S, Manzato E, Lucchini A. Comparing treatments of alcoholism on craving and biochemical measures of alcohol consumptionst. Journal on Psychoactive Drugs. 2006;38:211–217. doi: 10.1080/02791072.2006.10399846. [DOI] [PubMed] [Google Scholar]
- Okulicz-Kozaryn I, Midolajczak P, Szczawinska K, Kaminska E, Kus K. Effects of acamprosate and scopolamine on the working memory of rats in a three-panel runway task. Journal of Basic Clinical Physiology and Pharmacology. 2001;12:197–216. doi: 10.1515/jbcpp.2001.12.3.197. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Picciotto M. Nicotine as a modulator of behavior: Beyond the inverted U. TRENDS in Pharmacological Sciences. 2003;24(9):493–499. doi: 10.1016/S0165-6147(03)00230-X. [DOI] [PubMed] [Google Scholar]
- Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of alpha4beta2 nACh receptors for smoking cessation. Trends in Pharmacological Sciences. 2007;28:316–325. doi: 10.1016/j.tips.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, 3rd, Williams KE. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Schneider U, Wohlfarth K, Schulze-Bonhage A, Haacker T, Muller-Vahl KR, Zedler M, Becker H, Dengler R, Emrich HM. Effects of acamprosate on memory in healthy young subjects. Journal of Studies on Alcohol. 1999;60:172–175. doi: 10.15288/jsa.1999.60.172. [DOI] [PubMed] [Google Scholar]
- Séguéla P, Wadiche J, Dineley-MIller K, Dani JA, Patrick JW. Molecular cloning, functional properties and distribution of rat brain a7: a nicotinic cation channel highly permeable to calcium. Journal of Neuroscience. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva RH, Frussa-Filho R. Naltrexone potentiates both amnestic and anxiolytic effects of chlordiazepoxide in mice. Life Sciences. 2002;72(6):721–30. doi: 10.1016/s0024-3205(02)02298-1. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proceedings of the National Academy of Science, U S A. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg MF, Mackler SA, Volpicelli JR, O’Brien CP. Effect of acamprosate and naltrexone, alone or in combination, on ethanol consumption. Alcohol. 2001;23:109–116. doi: 10.1016/s0741-8329(00)00137-3. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Toward a molecular explanation for long-term potentiation. Learning and Memory. 1999;6:399–416. doi: 10.1101/lm.6.5.399. [DOI] [PubMed] [Google Scholar]
- Tennant FS, Jr, Tarver AL, Rawson RA. Clinical evaluation of mecamylamine for withdrawal from nicotine dependence. NIDA Research Monograph. 1984;49:239–246. [PubMed] [Google Scholar]
- Wada E, Wada K, Boutler J, Deneris E, Heinemann S, Patrick J, Swanson L. Distribution of alpha 2, alpha3, alpha4, and beta2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridisation histochemical study in the rat. Journal of Computational Neurology. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology (Berl) 2006;184:339–344. doi: 10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]
- Wehner JM, Keller JJ, Keller AB, Picciotto MR, Paylor R, Booker TK, Beaudet A, Heinemann SF, Balogh SA. Role of neuronal nicotinic receptors in the effects of nicotine and ethanol on contextual fear conditioning. Neuroscience. 2004;129:11–24. doi: 10.1016/j.neuroscience.2004.07.016. [DOI] [PubMed] [Google Scholar]


