Abstract
Highly specific borreliacidal antibodies are induced by infection with Borrelia burgdorferi, and the immunodominant response during early Lyme disease is specific for an epitope within the 7 amino acids nearest the C terminus of OspC. We evaluated the ability of an enzyme-linked immunosorbent assay (ELISA) based on a synthetic peptide (OspC7) that matched the region to detect the response and compared the sensitivity during early Lyme disease to that for an FDA-approved Western blot. When the optical density value was adjusted to 98% specificity based on the results from testing normal or uncharacterized sera (n = 236) or sera from patients with blood factors or illnesses that commonly produce antibodies that cross-react with B. burgdorferi antigens (n = 77), 115 (73%) of 157 sera from patients likely to have early Lyme disease were positive for immunoglobulin M (IgM) antibodies and 17 (11%) also had IgG antibodies. In addition, the IgM ELISA reactivities and the titers of antibodies detected by a flow cytometric borreliacidal antibody test correlated closely (r = 0.646). Moreover, the IgM ELISA was significantly more sensitive (P < 0.001) than the Western blot procedure. The findings therefore confirmed that the peptide IgM ELISA detected OspC borreliacidal antibodies and provided strong evidence that the test can eliminate the necessity for confirming early Lyme disease by a supplementary test such as Western blotting.
Lyme disease caused by transmission of Borrelia burgdorferi spirochetes from Ixodes sp. ticks remains prevalent throughout the United States. Diagnosis is based primarily on clinical manifestations and exposure to infected vector ticks. However, detecting specific antibodies in serum is often a necessary adjunct, because the clinical symptoms mimic a wide variety of other conditions and detecting the spirochetes by culture or PCR can be problematic. To increase the accuracy of serodiagnosis, the Centers for Disease Control and Prevention (CDC) recommend a two-test system where serum is screened with a nonspecific indirect immunofluorescence assay or an indirect enzyme-linked immunosorbent assay (ELISA), and equivocal or positive results are substantiated by a specific standardized Western blot procedure (8). The two-step system provides accurate confirmation of later stages of Lyme disease but is significantly less sensitive for detecting early infection (2). In addition, the technology and multiple testing requirements significantly increase the cost and turnaround time.
An alternative procedure for confirming early Lyme disease by serodiagnosis is a flow cytometric borreliacidal antibody test. Highly specific OspC borreliacidal antibodies are produced shortly after infection with B. burgdorferi (27), and detecting the response by use of flow cytometry and B. burgdorferi 50772, an ospA- and ospB-negative isolate sensitive to killing by OspC borreliacidal antibodies, is significantly more sensitive than Western blotting during early Lyme disease (3). Unfortunately, the complexity and requirement for live spirochetes make the test impractical for the routine laboratory. However, the immunodominant epitope recognized by human OspC borreliacidal antibodies is located within the carboxy (C)-terminal 7 amino acids of the protein (18), and the region is conserved among the pathogenic Borrelia spp. (18, 22). We therefore investigated the ability of an ELISA peptide that matched the epitope (OspC7) to serve as an antigen for detecting OspC borreliacidal antibodies and evaluated the diagnostic potential during early Lyme disease.
MATERIALS AND METHODS
Organism.
B. burgdorferi sensu stricto 50772 is a noninfectious isolate susceptible to killing by OspC borreliacidal antibodies because the spirochete lacks the plasmid containing ospA and ospB (1) and expresses high levels of OspC in laboratory culture medium (27). The spirochetes were cultured in Barbour-Stoenner-Kelly (BSK) medium at 35°C until reaching logarithmic phase, dispensed in 200-μl amounts, and stored at −70°C until used.
Sera.
Normal sera from individuals with no previous history (chart review) of Lyme disease or related symptoms (n = 36), uncharacterized sera from blood donors (n = 100) or individuals undergoing cholesterol screenings (n = 100), and sera from individuals with blood factors or illnesses that commonly cross-react with B. burgdorferi antigens, including antinuclear antibodies (n = 20), rheumatoid factor (n = 20), mononucleosis (n = 10), cytomegalovirus (CMV) (n = 10), syphilis (n = 13), or Rocky Mountain spotted fever (n = 4), were from archived samples stored at −20°C. Lyme disease sera were collected from patients evaluated at Gundersen Lutheran Medical Center during 2004 and 2005. The serum samples were from patients with a high likelihood of early Lyme disease. These included patients with significant tick exposures and erythema migrans (EM) (n = 86) that fulfilled the CDC surveillance criterion (7), atypical skin lesions (n = 22), or constitutional symptoms that included primarily headache, fever, myalgia, and arthralgia (n = 49). Sera were collected during the initial visit and stored at −20°C in a blind manner prior to testing. In addition, serum samples were obtained under the conditions provided by protocols established by the Gundersen Lutheran Medical Center institutional review board.
OspC7 peptide.
The OspC7 peptide (AESPKKP) was synthesized at the University of Wisconsin Biotechnology Center (Madison, WI) by using an automated synthesizer (Protein Technologies, Tucson, AZ) using 9-fluorenylmethoxy carbonyl chemistry (10). Following synthesis, the amine-terminal end of the peptide was biotinylated manually by HBTU (2(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyl-uronium hexafluorophosphate) activation and purified by high-pressure liquid chromatography. Composition was confirmed using matrix-assisted laser desorption ionization-time of flight mass spectrometry (predicted mass, 1,095.4; observed mass, 1,095.8).
OspC7 ELISA.
The OspC7 ELISA was performed as described previously (21). Briefly, wells of microtiter plates (Immunolon 2 HB; Thermo Labsystems, Franklin, MA) were coated with 100 μl of a 4-μg/ml suspension of streptavidin (Pierce, Rockland, IL) contained in carbonate buffer (90 mM NaHCO3, 60 mM Na2CO3; pH 9.6) and incubated overnight at 4°C. Following incubation, plates were washed five times with Tris-buffered saline containing 0.05% Tween 20 (TBS-T; 13 mM Tris-HCl, 3 mM Tris base, 140 mM NaCl, 2.7 mM KCl; pH 7.4). After the washing, 200 μl of blocking buffer (15 mM NaCl, 10 mM Tris HCl, 3% fetal bovine serum, 0.05% Tween 20) containing 1 μg/ml of biotinylated OspC7 peptide was added to each well and incubated with rotation (150 rpm) for 1 h at room temperature. Plates were washed three times with TBS-T and reacted with 100-μl amounts of sera diluted 1:200 in blocking buffer for 1 h at room temperature. In some experiments, sera were serially diluted (1:200 to 1:409,600). Peroxidase-conjugated goat anti-human immunoglobulin M (IgM) (Kirkegaard & Perry Laboratories, Gaithersburg, MD) diluted 1:15,000 or IgG diluted 1:60,000 in blocking buffer was then added. After incubation for 1 h, plates were washed, 100 μl of o-phenylenediamine substrate (Sigma, St. Louis, MO) was added, and the optical density (OD) at 490 nm (SpectraMax 250; Molecular Devices, Sunnyvale, CA) was determined. Interassay variation was determined by including a positive control. The mean IgM OD value was 3.801 (standard deviation, 0.139; coefficient of variation, 3.7%) and the mean IgG OD value was 2.177 (standard deviation, 0.381; coefficient of variation, 17.5%).
Detection of borreliacidal antibodies.
Borreliacidal antibodies were detected by using the flow cytometric test (3, 6). Briefly, serum samples were diluted 1:25 in fresh BSK medium and filter sterilized by passage through a 0.2-μm microcentrifuge filter unit (Costar, Cambridge, MA). Following filtration, sera were serially twofold diluted (final dilution, 1:50 to 1:102,400) in fresh BSK medium and heat inactivated for 10 min at 56°C. Following inactivation, a fresh culture of B. burgdorferi 50772 in logarithmic growth phase was adjusted to a concentration of approximately 5 × 105 organisms/ml, and a 100-μl amount was combined with 100 μl of each serum dilution and 5 μl of complement (guinea pig serum [50% hemolytic complement, ≥200 units/ml]) and incubated. Following overnight incubation at 35°C, a 100-μl aliquot of each assay suspension was transferred to a polystyrene tube containing 400 μl of phosphate-buffered saline (0.01 M, pH 7.2) containing 1 μg of acridine orange/ml. Dead, blebbed spirochetes were then detected with a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA). Spirochetes were isolated by gating (CELLQuest software; Becton-Dickinson) and analyzed for 1 to 2 min with the flow rate set at low. Borreliacidal antibodies were detected by monitoring the increased side scatter and fluorescence that occurs when the acridine orange intercalates into the blebbed, nonviable spirochetes. Samples that yielded a >13% increase in fluorescence intensity compared to a normal serum control were considered positive (3, 4). In addition, positive samples were examined by dark-field microscopy to confirm the presence of characteristic blebbed organisms (5).
Western blotting.
The IgM Western blotting procedure was performed using an FDA-approved commercial kit (Marblot; Mardx Diagnostics, Carlsbad, CA), and the results were interpreted according to the manufacturer's instructions.
Statistical analyses.
The strength of the relationship between borreliacidal antibody titers and OspC7 ELISA reactivity was determined by use of the Pearson correlation coefficient. McNemar's test was used to compare the diagnostic sensitivities between tests. P values of <0.05 were considered significant. The kappa statistic was also used to evaluate the level of agreement between tests.
RESULTS
OspC7 ELISA reactivities of sera from patients with early Lyme disease.
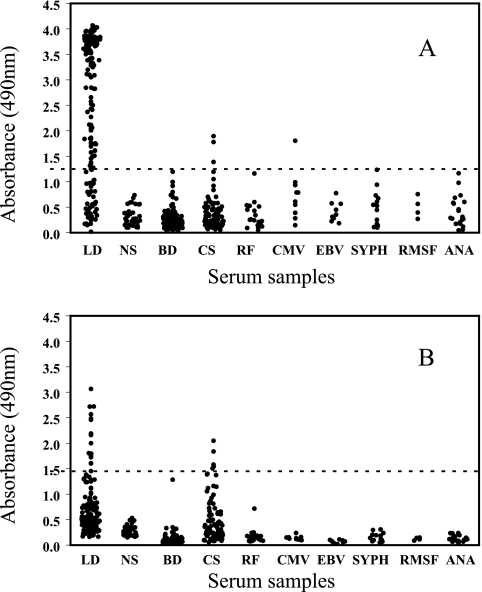
The reactivities of normal sera and of sera from patients with common factors or illnesses that commonly contain antibodies that cross-react with B. burgdorferi were used to establish the diagnostic cutoff levels, and a specificity of 98% was obtained when the OD of the IgM ELISA was 1.25 (Fig. 1A) and the OD of the IgG ELISA was 1.45 (Fig. 1B). By use of the cutoff values, IgM and IgG antibodies were detected for 3 and 5 uncharacterized sera, respectively, from patients undergoing cholesterol screening at Gundersen Lutheran. In addition, a single serum sample from a patient with CMV was positive for IgM OspC7 antibodies. In contrast, 17 (11%) early Lyme disease sera yielded positive IgG results, and 115 (73%) sera, including the IgG-positive sera, were positive by the IgM ELISA.
FIG. 1.
Anti-OspC7 IgM (A) and IgG (B) in 157 early Lyme disease patient (LD) sera and in sera from 36 individuals with no previous exposure to or history of Lyme disease (NS), 100 blood donors (BD), 100 individuals getting cholesterol screens (CS), 20 patients with rheumatoid factor (RF), 10 patients with CMV, 10 patients with mononucleosis (EBV), 13 patients with syphilis (SYPH), 4 patients with Rocky Mountain spotted fever (RMSF), and 20 patients with antinuclear antibodies (ANA). The horizontal lines mark the diagnostic cutoff levels for 98% specificity.
Correlation of the OspC7 ELISA and the borreliacidal-antibody test.
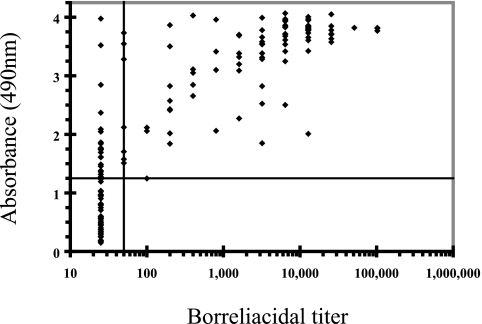
Because of the increased levels of IgM antibodies, we then determined the borreliacidal-antibody levels in the sera of early Lyme disease patients and compared the responses to the IgM ELISA reactivities. Borreliacidal antibodies were not detected for ELISA-negative sera (Fig. 2). In contrast, 22 sera from patients with tick exposures and only constitutional complaints were positive by the IgM ELISA and negative by the borreliacidal-antibody test. In addition, 93 (59%) sera contained borreliacidal and OspC7-specific antibodies, and the levels of borreliacidal antibodies and the reactivities of the sera in the ELISA correlated closely (r = 0.646).
FIG. 2.
Correlation of OspC7 IgM ELISA reactivities of and borreliacidal-antibody titers in early Lyme disease patient sera (n = 157). The horizontal line represents the diagnostic cutoff of the borreliacidal-antibody test, and the vertical line represents the cutoff of the IgM OspC7 ELISA.
Sensitivities of the IgM OspC7 ELISA and Western blotting.
We then tested the early Lyme disease sera with an FDA-approved IgM Western blotting procedure and compared the findings to the ELISA results. There was little agreement between the tests (κ = 0.410), and the discrepancy could be explained primarily by the increased sensitivity of the IgM ELISA (Table 1). The ELISA was significantly more sensitive regardless of the severity of the symptoms (73% versus 41%; P < 0.001), and the increased sensitivity was highly significant (P < 0.001), even when the sera were from patients with EM lesions sufficient to fulfill the CDC Lyme disease surveillance criterion (7).
TABLE 1.
Reactivities of IgM OspC7 ELISA and IgM Western blotting using sera from patients with early Lyme disease characterized by tick exposure and constitutional symptoms, atypical lesions, or EM lesions
| Patient group (no. of sera tested) | Testa | No. of positive sera (%) | No. of negative sera (%) | r valueb | P valuec |
|---|---|---|---|---|---|
| Constitutional symptom (49) | OspC7 | 27 (55) | 22 (45) | 0.204 | <0.001 |
| WB | 6 (12) | 43 (88) | |||
| Atypical lesion (22) | OspC7 | 19 (86) | 3 (14) | 0.233 | 0.003 |
| WB | 10 (45) | 12 (55) | |||
| EM lesion (86) | OspC7 | 69 (80) | 17 (20) | 0.492 | <0.001 |
| WB | 49 (57) | 37 (43) | |||
| Total (157) | OspC7 | 115 (73) | 42 (27) | 0.410 | <0.001 |
| WB | 65 (41) | 92 (59) |
OspC7, OspC7 ELISA; WB, Western blotting.
Kappa statistic.
McNemar's test.
DISCUSSION
B. burgdorferi increases the production of OspC shortly after an infected tick begins taking a blood meal (28), and the expression of OspC is critical for establishing a mammalian infection (11, 32). OspC antibodies are therefore a hallmark of human Lyme disease (8), and researchers have evaluated the ability of ELISAs based on OspC to provide serodiagnostic confirmation of the illness (19, 25, 33). However, the ELISAs have lacked specificity because cross-reactive antibodies bind to heterogeneous regions of the protein (13, 31). The flow cytometric OspC borreliacidal antibody test circumvents this shortcoming (3, 4), but the necessity for viable spirochetes, the potential for interference from antimicrobial agents, and the technical complexity limit the usefulness. We therefore evaluated the ability of a synthetic-peptide ELISA based on the immunodominant epitope (18) to provide serodiagnostic confirmation of early Lyme disease by detecting OspC borreliacidal antibodies.
The ELISA reactivities of sera from patients with other infections or autoimmune diseases were negligible, so the IgM and IgG tests could be adjusted to high specificity (98%) by using relatively low diagnostic OD cutoff values. At this level, false-positive IgM reactivity was detected for a single serum sample from a patient with CMV, and false-positive reactions were detected for three and five uncharacterized sera from patients undergoing cholesterol screening tests by the IgM and IgG ELISAs, respectively. The false-positive result from the CMV patient can be accounted for by the ability of the infection to cause polyclonal B-cell stimulation, but explanations for the reactivities in the other sera are less clear. It should be noted, however, that at least some reactivity may have been due to unrecognized Lyme disease. In support, the reactivities of sera from healthy blood donors who resided in a focus of nonendemicity (Milwaukee) were negligible. In addition, the Gundersen Medical Center is located in a focus of high endemicity (12), and the identities and clinical histories of the patients being tested for cholesterol were unknown. Moreover, one of the IgM and IgG OspC7 ELISA-positive serum samples was also positive by the C6 ELISA (data not shown).
In contrast to the minimal reactivity detected in the potentially cross-reactive sera, the early Lyme disease sera reliably contained IgM OspC7-specific antibodies and some also contained IgG antibodies. Furthermore, the results from the IgM ELISA closely paralleled the findings from the flow cytometric borreliacidal-antibody test, and the ELISA was even more sensitive during the earliest stages of the illness. The latter finding is likely because of the increased efficiency of using purified “target” antigen rather than viable intact organisms. This result therefore corroborated previous reports (3, 14, 18) that human borreliacidal antibodies detected by using B. burgdorferi 50772 are IgM or IgG OspC borreliacidal antibodies specific for the OspC C terminus and also confirmed that the ELISA could be used to detect the response.
In addition, the IgM OspC7 ELISA was significantly (P < 0.001) more sensitive than an FDA-approved Western blotting procedure for confirming early Lyme disease. This is extremely significant, because the two-test system mandated currently by the CDC is cumbersome, time-consuming, and expensive. In response, alternative tests have been developed, and two in particular, the VlsE (2, 15) and C6 (17, 23) ELISAs, offer promise as stand-alone tests that confirm Lyme disease by detecting specific antibodies induced by multiple pathogenic Borrelia genospecies (15, 16). However, the tests primarily detect IgG antibodies (2, 17) and lack sensitivity during early Lyme disease (2, 20, 29). In contrast, the OspC7 ELISA detects an immunodominant IgM borreliacidal antibody response produced early during infection. Therefore, the combination of high specificity and sensitivity provided by testing serum with both the IgM OspC7 ELISA and either the VlsE or C6 ELISA could conceivably eliminate the necessity for two-tiered testing.
Moreover, a BLAST search confirmed (18) that the amino acid sequence recognized by the OspC7 antibodies is conserved among the pathogenic Borrelia spp., despite the well-documented (13, 31) diversity of OspC alleles (13, 31) both within and among pathogenic Borrelia genospecies. In addition, the OspC borreliacidal antibodies were detected by using a B. burgdorferi sensu stricto isolate (50772) recovered from a host-seeking Ixodes scapularis tick captured from a focus of endemicity in Connecticut (1). Therefore, the OspC7 ELISA should be effective for confirming early Lyme disease in patients from throughout the United States and may also be useful for detecting the illness among European Lyme disease patients.
Interestingly, researchers (2, 21) previously evaluated an IgM ELISA based on a 10-amino-acid peptide (pepC10) that contained a sequence identical to that used in the OspC7 ELISA. The investigators confirmed that the ELISA detected specific antibodies in sera from patients infected with B. garinii (21) or B. burgdorferi (2), but the test appeared less sensitive than Western blotting when testing sera from patients with an EM lesion. We believe that there is a simple explanation for the discrepancy. The sera in our study were collected immediately prior to antibiotic therapy and stored frozen for short amounts of time without thawing. In contrast, many sera tested in the previous studies (2, 21) were stored and used for years, and IgM activity can be reduced significantly after one freeze-thaw cycle (26). Furthermore, some sera were collected after the patient completed antibiotic therapy, and there is compelling evidence that the levels of borreliacidal antibodies, such as those detected by the pepC10 and OspC7 ELISAs, decrease rapidly after the antigen is eliminated. For example, Lyme disease vaccines that provide protection by inducing OspA borreliacidal antibodies to eliminate spirochetes from feeding ticks (9) are effective for only a few months because the response disappears rapidly (24, 30).
In summary, additional studies to more completely define the utility of the OspC7 ELISA remain necessary. However, the findings confirm that the peptide based on the 7 C-terminal amino acids of OspC detects borreliacidal antibodies efficiently and provide strong evidence that the test can eliminate the necessity for two-tiered testing during early Lyme disease.
Acknowledgments
Financial support was provided by the Gundersen Lutheran Medical Foundation.
Footnotes
Published ahead of print on 16 April 2008.
REFERENCES
- 1.Anderson, J. F., R. A. Flavell, L. A. Magnarelli, S. W. Barthold, S. S. Kantor, R. Wallich, D. H. Persing, D. Mathiesen, and E. Fikrig. 1996. Novel Borrelia burgdorferi isolates from Ixodes scapularis and Ixodes dentatus ticks feeding on humans. J. Clin. Microbiol. 34:524-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacon, R. M., B. J. Bickerstaff, M. E. Schriefer, R. D. Gilmore, Jr., M. T. Philipp, A. C. Steere, G. P. Wormser, A. R. Marques, and B. J. B. Johnson. 2003. Serodiagnosis of Lyme disease by kinetic enzyme-linked immunosorbent assay using recombinant VlsE1 or peptide antigens of Borrelia burgdorferi compared with 2-tiered testing using whole-cell lysates. J. Infect. Dis. 187:1187-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callister, S. M., D. A. Jobe, W. A. Agger, R. F. Schell, T. J. Kowalski, S. D. Lovrich, and J. A. Marks. 2002. Ability of the borreliacidal antibody test to confirm Lyme disease in clinical practice. Clin. Diagn. Lab. Immunol. 9:908-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callister, S. M., D. A. Jobe, R. F. Schell, C. S. Pavia, and S. D. Lovrich. 1996. Sensitivity and specificity of the borreliacidal-antibody test during early Lyme disease: a gold standard? Clin. Diagn. Lab. Immunol. 3:399-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callister, S. M., R. F. Schell, K. L. Case, S. D. Lovrich, and S. P. Day. 1993. Characterization of the borreliacidal antibody response to Borrelia burgdorferi in humans: a serodiagnostic test. J. Infect. Dis. 167:158-164. [DOI] [PubMed] [Google Scholar]
- 6.Callister, S. M., R. F. Schell, L. C. L. Lim, D. A. Jobe, K. L. Case, G. L. Bryant, and P. E. Molling. 1994. Detection of borreliacidal antibodies by flow cytometry: an accurate, highly specific serodiagnostic test for Lyme disease. Arch. Intern. Med. 154:1625-1632. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 1990. Case definitions for public health surveillance. MMWR Morb. Mortal. Wkly. Rep. 39:19-21. [Google Scholar]
- 8.Centers for Disease Control and Prevention. 1995. Recommendations for test performance and interpretations from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb. Mortal. Wkly. Rep. 44:590-591. [PubMed] [Google Scholar]
- 9.de Silva, A. M., S. R. Telford III, L. R. Brunet, S. W. Barthold, and E. Fikrig. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 83:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fields, C. G., D. H. Lloyd, R. L. MacDonald, K. M. Otteson, and R. L. Noble. 1991. HBTU activation for automated Fmoc solid-phase peptide synthesis. Peptide Res. 4:95-101. [PubMed] [Google Scholar]
- 11.Grimm, D., K. Tilly, R. Byram, P. E. Stewart, J. G. Krum, D. M. Bueschel, T. G. Schwan, P. F. Policastro, A. F. Elias, and P. A. Rosa. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. 101:3142-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson, C. A., S. D. Lovrich, W. A. Agger, and S. M. Callister. 2002. Reassessment of a midwestern Lyme disease focus for Borrelia burgdorferi and the human granulocytic ehrlichiosis agent. J. Clin. Microbiol. 40:2070-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jauris-Heipke, S., R. Fuchs, M. Motz, V. Preac-Mursic, E. Schwab, E. Soutschek, G. Will, and B. Wilske. 1993. Genetic heterogeneity of the genes coding for the outer surface protein C (OspC) and the flagellin of Borrelia burgdorferi. Med. Microbiol. Immunol. 182:37-50. [DOI] [PubMed] [Google Scholar]
- 14.Jobe, D. A., S. D. Lovrich, R. F. Schell, and S. M. Callister. 2003. C-terminal region of outer surface protein C binds borreliacidal antibodies in sera from patients with Lyme disease. Clin. Diagn. Lab. Immunol. 10:573-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawrenz, M. B., J. M. Hardham, R. T. Owens, J. Nowakowski, A. C. Steere, G. P. Wormser, and S. J. Norris. 1999. Human antibody responses to VlsE antigenic variation protein of Borrelia burgdorferi. J. Clin. Microbiol. 37:3997-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang, F. T., E. Aberger, M. Cinco, L. Gern, C. M. Hu, Y. N. Lobet, M. Ruscio, P. E. Voet, Jr., V. E. Weynants, and M. T. Philipp. 2000. Antigenic conservation of an immunodominant invariable region of the VlsE lipoprotein among European pathogenic genospecies of Borrelia burgdorferi SL. J. Infect. Dis. 182:1455-1462. [DOI] [PubMed] [Google Scholar]
- 17.Liang, F. T., A. C. Steere, A. R. Marques, B. J. B. Johnson, J. N. Miller, and M. T. Philipp. 1999. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of VlsE. J. Clin. Microbiol. 37:3990-3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovrich, S. D., D. A. Jobe, R. F. Schell, and S. M. Callister. 2005. Borreliacidal OspC antibodies specific for a highly conserved epitope are immunodominant in human Lyme disease and do not occur in mice or hamsters. Clin. Diagn. Lab. Immunol. 12:746-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magnarelli, L. A., J. W. Ijdo, S. J. Padula, R. A. Flavell, and E. Fikrig. 2000. Serologic diagnosis of Lyme borreliosis by using enzyme-linked immunosorbent assays with recombinant antigens. J. Clin. Microbiol. 38:1735-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marangoni, A., M. Sparacino, V. Mondarini, F. Cavrini, E. Storni, M. Donati, R. Cevenini, and V. Sambri. 2005. Comparative evaluation of two enzyme linked immunosorbent assay methods and three Western blot methods for the diagnosis of culture-confirmed early Lyme borreliosis in Italy. New Microbiol. 28:37-43. [PubMed] [Google Scholar]
- 21.Mathiesen, M. J., M. Christiansen, K. Hansen, A. Holm, W. Asbrink, and M. Theisen. 1998. Peptide-based OspC enzyme-linked immunosorbent assay for serodiagnosis of Lyme borreliosis. J. Clin. Microbiol. 36:3474-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathiesen, M. J., A. Holm, M. Christiansen, J. Blom, E. Asbrink, and M. Thiesen. 1998. The dominant epitope of Borrelia garinii outer surface protein C recognized by sera from patients with neuroborreliosis has a surface-exposed conserved structural motif. Infect. Immun. 66:4073-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mogilyansky, E., C. C. Loa, M. E. Adelson, E. Mordechai, and R. C. Tilton. 2004. Comparison of Western immunoblotting and the C6 Lyme antibody test for laboratory detection of Lyme disease. Clin. Diagn. Lab. Immunol. 11:924-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Padilla, M. L., S. M. Callister, R. F. Schell, G. L. Bryant, D. A. Jobe, S. D. Lovrich, B. K. DuChateau, and J. R. Jensen. 1996. Characterization of the protective borreliacidal antibody response in humans and hamsters after vaccination with a Borrelia burgdorferi outer surface protein A vaccine. J. Infect. Dis. 174:739-746. [DOI] [PubMed] [Google Scholar]
- 25.Padula, S. J., F. Dias, A. Sampieri, R. B. Craven, and R. W. Ryan. 1994. Use of recombinant OspC from Borrelia burgdorferi for serodiagnosis of early Lyme disease. J. Clin. Microbiol. 32:1733-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrakis, N. L. 1985. Biologic banking in cohort studies, with special reference to blood. NCI Monogr. 67:193-198. [PubMed] [Google Scholar]
- 27.Rousselle, J. C., S. M. Callister, R. F. Schell, S. D. Lovrich, D. A. Jobe, J. A. Marks, and C. A. Wieneke. 1998. Borreliacidal antibody production against outer surface protein C of Borrelia burgdorferi. J. Infect. Dis. 178:733-741. [DOI] [PubMed] [Google Scholar]
- 28.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smismans, A., V. J. Goossens, E. Nulens, and C. A. Bruggeman. 2006. Comparison of five different immunoassays for the detection of Borrelia burgdorferi IgM and IgG antibodies. Clin. Microbiol. Infect. 12:648-655. [DOI] [PubMed] [Google Scholar]
- 30.Steere, A. C., V. K. Sikand, F. Meurice, D. L. Parenti, E. Fikrig, R. T. Schoen, J. Nowakowski, C. H. Schmid, S. Laukamp, C. Buscarino, and D. S. Krause. 1998. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. N. Engl. J. Med. 339:209-215. [DOI] [PubMed] [Google Scholar]
- 31.Theisen, M., B. Frederiksen, A. M. Lebech, J. Vuust, and K. Hansen. 1993. Polymorphism in ospC gene of Borrelia burgdorferi and immunoreactivity of OspC protein: implications for taxonomy and for use of OspC protein as a diagnostic antigen. J. Clin. Microbiol. 31:2570-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tilly, K., J. G. Krum, A. Bestor, M. W. Jewett, D. Grimm, D. Bueschel, R. Byram, D. Dorward, P. Stewart, and P. A. Rosa. 2006. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect. Immun. 74:3554-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wieneke, C. A., S. D. Lovrich, S. M. Callister, D. A. Jobe, J. A. Marks, and R. F. Schell. 2000. Evaluation of whole-cell and OspC enzyme-linked immunosorbent assays for discrimination of early Lyme borreliosis from OspA vaccination. J. Clin. Microbiol. 38:313-317. [DOI] [PMC free article] [PubMed] [Google Scholar]