Abstract
A DNA vaccine encoding sequence-conserved human immunodeficiency virus type 1 (HIV-1)-derived cytotoxic T-lymphocyte (CTL) epitopes from multiple HIV-1 gene products (designated EP HIV-1090) was evaluated in a placebo-controlled, dose escalation phase 1 clinical trial of HIV-1-infected subjects receiving potent combination antiretroviral therapy. Patients received four intramuscular immunizations with EP HIV-1090 over a 4-month period at one of four doses (0.5, 1.0, 2.0, or 4.0 mg) or received a placebo. The vaccine was determined to be safe and well tolerated at all doses tested. CTL responses were measured from cryopreserved peripheral blood mononuclear cells using gamma interferon enzyme-linked immunospot assays, with and without in vitro peptide stimulation (IVS). Responses to one or more vaccine epitopes were detected throughout the course of vaccination in 37.5% (12/32) and 47% (15/32) of vaccine recipients measured without and with IVS, respectively, indicating possible vaccine-induced priming of epitope-specific T cells. However, differences in rates of response to HIV-1 epitopes between vaccine and placebo recipients did not achieve statistical significance. The HIV-1 epitope-specific CTL responses measured in the peripheral blood after vaccination were often low level and short-lived, and therefore, alternative immunization schedules, routes of delivery, or vaccine formulations may be required to increase vaccine potency.
The use of potent combination antiretroviral therapy (ART) regimens to treat human immunodeficiency virus (HIV) infection has been effective at reducing viral loads, increasing CD4 cell counts, delaying HIV disease progression, reducing morbidity, and prolonging survival. However, the long-term success of chronic ART may be limited by costs, drug toxicity, development of viral resistance, and a persistent viral reservoir (6, 21). Thus, additional strategies for controlling HIV replication in chronically infected individuals are needed.
Most evidence indicates that HIV-specific cytotoxic CD8+ T lymphocytes play a role in controlling viral replication. The initial occurrence of virus-specific cytotoxic T-lymphocyte (CTL) responses correlates with the resolution of symptomatic acute primary HIV infection (20). An association between virologic control and the presence of CTL responses was documented more than a decade ago in studies of patients who progress to disease more slowly (4, 30). More recently, individuals capable of controlling viral replication in vivo without ART have been identified, and the breadth of epitope recognition, recognition of nondominant epitopes, HLA restriction, and types of cytokines produced may all affect virologic control (2, 11, 12, 33). Thus, the ability to induce new CTLs or to augment existing CTLs in HIV-infected individuals using therapeutic vaccination is likely to provide significant clinical benefit, particularly when the antiviral activity of drug therapy is threatened.
The induction of CTLs requires intracellular expression of the vaccine immunogen followed by proteolytic cleavage, mediated by proteosomes, to generate epitopes which are subsequently expressed bound to major histocompatibility class I antigens. This has focused most efforts within the field toward the use of viral vectors or DNA vaccines where the vaccine immunogen is transcribed and translated in vivo. Viral vectors, based on recombinant canarypox, modified vaccinia virus Ankara (MVA), and adenoviruses, and DNA plasmids encoding a variety of HIV type 1 (HIV-1) antigens have been and continue to be, tested in numerous clinical trials with HIV-1-infected volunteers (7, 8, 9, 13, 17, 19, 22, 23, 25). Both viral vectors and DNA vaccines have proved marginally effective at inducing measurable CTL responses, and some clinical benefit has been reported in the therapeutic setting through the use of ART cessation.
We developed an experimental therapeutic DNA vaccine that encodes 21 HLA class I supertype-restricted CTL epitopes; 7 epitopes restricted each to HLA-A2, -A3, and -B7 supertype allelic products; and the synthetic and universal helper T-lymphocyte (HTL) epitope, termed PADRE (32) (Table 1). The HIV epitopes are highly conserved and are derived from structural (Gag, Pol, and Env) and regulatory (Nef, Rev, and Vpr) proteins. The relevance of these epitopes was demonstrated based on their recognition by CD8+ T lymphocytes obtained from HIV-1-infected individuals; this immune recognition demonstrates the generation of these epitopes in vivo as a consequence of HIV-1 infection and the presence of the appropriate T-cell receptors for their recognition (1, 32). The vaccine is predicted to be immunogenic in approximately 85% of randomly selected individuals without ethnic bias, based on HLA-A2, -A3, and -B7 supertype allelic frequencies, but this is only a minimal estimate because many epitopes can be restricted through other, unrelated HLA products (32).
TABLE 1.
HIV-1-derived CTL epitopes encoded in the EP HIV-1090 vaccine
| Superfamily and HIV protein | Sequence |
|---|---|
| HLA-A2 | |
| Pol 498 | ILKEPVHGV |
| Gag 386 | VLAEAMSQV |
| Pol 448 | KLVGKLNWA |
| Env 134 | KLTPLCVTL |
| Vpr 62 | RILQQLLFI |
| Nef 221 | LTFGWCFKL |
| Gag 271 | MTNNPPIPV |
| HLA-A3 | |
| Env 47 | VTVYYGVPVWK |
| Pol 929 | QMAVFIHNFK |
| Pol 98 | VTIKIGGQLK |
| Pol 971 | KIQNFRVYYR |
| Pol 347 | AIFQSSMTK |
| Pol 722 | KVYLAWPAHK |
| Env 61 | TTLFCASDAK |
| HLA-B7 | |
| Nef 94 | FPVRPQVPL |
| Gag 545 | YPLASLRSLF |
| rev 75 | VPLQLPPL |
| Env 259 | IPIHYCAPA |
| Gag 237 | HPVHAGPIA |
| Pol 893 | IPYNPQSQGW |
| Env 250 | CPKVSFEPI |
The gene encoding the vaccine immunogen was codon optimized to promote translation in humans, and the protein sequence was engineered to include amino acid spacers to increase epitope processing efficiency (24). Here we describe the results of the initial phase 1 clinical trial with HIV-infected volunteers to investigate the safety and immunogenicity of the EP HIV-1090 therapeutic vaccine.
(This work was presented in part at the XV International AIDS Conference, Bangkok, Thailand, 11 to 16 July 2004 [abstr. no. ThPpA2088]; at the 12th Conference on Retroviruses and Opportunistic Infections, Boston, MA, 22 to 25 February 2005 [abstr. no. H-118]; and at AIDS Vaccine 2005, Montreal, Canada 6 to 9 September 2005.)
MATERIALS AND METHODS
EP HIV-1090 design and manufacturing.
The EP HIV-1090 vaccine plasmid carries only two open reading frames, those of the kanamycin resistance gene and the synthetic HIV epitope product. There are no known intact viral or oncogenic protein-coding sequences within the plasmid. EP HIV-1090 is 5,075 nucleotide pairs in length and has an approximate molecular mass of 3.3 MDa. A diagram of the EP HIV-1090 vector and the complete plasmid sequence are available in the supplemental material.
The vaccine was produced in Escherichia coli DH5α using high-density fermentation and purified from bacterial lysate material by ethanol precipitation and anion-exchange column chromatography in accordance with current good manufacturing practice regulations by Althea Technologies Inc. (San Diego, CA). Plasmid DNA was filter sterilized and formulated with the biocompatible adhesive polymer excipient polyvinylpyrrolidone (PVP) (Plasdone; International Specialty Products, Wayne, NJ) at a ratio of 17 parts PVP to 1 part DNA in phosphate-buffered saline (PBS) (pH 7.0) at a DNA concentration of 2 mg/ml. The vaccine was tested in rabbit studies, following good laboratory practice guidelines, by SRI (Menlo Park, CA) to determine biodistribution, clearance, and general safety to support phase 1 clinical testing in HIV-1-infected patients (29). Tests on the clinical supply included appearance, pH, DNA concentration, DNA integrity, and immunogenicity measured using HLA-A2 transgenic mice (32). The vaccine was stored frozen (−20°C) in single-use borosilicate vials until used.
Clinical trial design.
This study was a randomized, double-blind phase 1 dose escalation trial conducted at the University of Colorado Health Sciences Center. The study was approved by the University of Colorado Health Sciences Center Institutional Review Board, and all subjects enrolled provided written informed consent. A total of 41 patients, 37 men and 4 women, were enrolled (Table 2). At entry, all subjects were receiving ART, typically consisting of three drugs, for a median duration of 152 weeks (range, 23 to 512 weeks); were required to have a preentry plasma HIV RNA level of <50 copies/ml for at least 3 months; and had a median CD4 count of 717 cells/μl (range, 403 to 1,518 cells/μl) and a median plasma HIV RNA level of <35 copies/ml (range, <20 to 2,210 copies/ml). Pre-ART CD4 nadirs were ≤100 cells/μl for 27% of the patients, 101 to 500 cells/μl for 63%, and >500 cells/μl for the remaining 10%. Patients were HLA typed (31).
TABLE 2.
Summary of patient characteristics by treatment group
| Characteristic | Value for group
|
||
|---|---|---|---|
| Total | Placebo | Treatment | |
| No. of patients randomized | 41a | 8 | 33a |
| Patients who completed protocolb | 38 (93) | 7 (87.5) | 31 (94) |
| Follow-up time (wk)c | 40 (8, 47) | 40 (27, 47) | 40 (8, 43) |
| Baseline CD4c | 717 (402.5, 1,517.5) | 851 (449.5, 1,095.5) | 709.5 (402.5, 1,517.5) |
| Baseline CD4 %c | 31.5 (19, 48.5) | 32.5 (27, 40) | 31.5 (19, 48.5) |
| Baseline CD8c | 1,066.5 (612.5, 2,716) | 1,048 (710, 2,716) | 1,066.5 (612.5, 2,474) |
| Baseline viral load (copies/ml)c | <35 (<20, 2,210) | <27.5 (<20, <224.5) | <35 (<20, 2,210) |
| HLA typingb | |||
| A2 | 27 (66) | 6 (75) | 21 (63.6) |
| A3 | 17 (41) | 3 (37.5) | 14 (42) |
| B7 | 25 (61) | 6 (75) | 19 (57.6) |
| All | 9 (22) | 2 (25) | 7 (21) |
| None | 4 (10) | 1 (12.5) | 3 (9) |
| Wks on ARV at baselinec | 152 (23, 512) | 151 (95, 364) | 160 (23, 512) |
| Protease inhibitor-based therapy at baselineb | 25 (61) | 5 (62.5) | 20 (61) |
| No. of drugsc | 3 (3, 6) | 3 (3, 5) | 4 (3, 6) |
| Previous AIDS-defining opportunistic infection(s)b | 13 (32) | 3 (37.5) | 10 (30) |
| Previous highly active ART change(s)b,d | 21 (51) | 2 (25) | 19 (58) |
| Nadir CD4b | |||
| ≤100 | 11 (27) | 3 (37.5) | 8 (24.3) |
| 101-200 | 12 (29) | 1 (12.5) | 11 (33.3) |
| 201-500 | 14 (34) | 3 (37.5) | 11 (33.3) |
| >500 | 4 (10) | 1 (12.5) | 3 (9.1) |
| Riskb,e | |||
| Male having sex with male, HIV positive | 32 (78) | 3 (37.5) | 29 (88) |
| Intravenous drug use | 2 (5) | 1 (12.5) | 1 (3) |
| Heterosexual sex | 5 (12) | 3 (37.5) | 2 (6) |
| Other | 2 (5) | 1 (12.5) | 1 (3) |
| Maleb | 37 (90) | 6 (75) | 31 (94) |
| Age (yr)c | 44 (23, 59) | 41 (32, 56) | 44 (23, 59) |
| Raceb | |||
| White | 33 (80) | 5 (62.5) | 28 (85) |
| Black | 1 (2.5) | 1 (12.5) | 0 (0) |
| Hispanic | 6 (15) | 2 (25) | 4 (12) |
| Other | 1 (2.5) | 0 (0) | 1 (3) |
Patient 343026 was replaced on 15 July 2003 (week 8) for noncompliance.
n (percent based on number of patients randomized).
Median (range).
Any addition or permanent discontinuation is considered a regimen change.
Treatment versus placebo, P = 0.009 by Fisher's exact test; no other table comparison is significant.
Patients were randomized and received either EP HIV-1090 at one of four doses (0.5, 1, 2, or 4 mg) or a placebo (PBS formulated with PVP) by intramuscular deltoid injections at 0, 4, 8, and 16 weeks. Ten subjects were tested at each dose level; eight received vaccine and two received placebo. Vaccine/placebo doses were administered as a single intramuscular injection of 1 ml under sterile conditions into the deltoid muscle using a 1-in., 22-gauge needle (Monoject Magellan safety needles; Kendall) for the 0-, 0.5-, 1-, and 2-mg dose groups, alternating opposite arms for injections at sequential time points. For the 4-mg dose group, two separate injections of 1 ml each were administered at two different intramuscular sites. The final vaccine/placebo administration was at week 16, with postvaccination follow-up for an additional 6 months. Safety laboratory assessments included hematology, serum creatine phosphokinase, serum creatinine, aspartate transaminase, alanine aminotransferase, alkaline phosphatase, total bilirubin, CD4 cell count, and plasma HIV RNA levels at screening, entry, and weeks 2, 6, 10, 18, 24, and 40 and an autoimmune profile at entry and week 24. Adverse events were assessed based on the Division of AIDS table for grading the severity of adverse events (http://www3.niaid.nih.gov/research/resources/DMIDClinRsrch/toxtables.htm). Immune response measurements were completed using peripheral blood mononuclear cells (PBMC) obtained at preentry, entry, and weeks 4, 8, 16, 18, 24, and 40. Primary gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assays were performed at all time points, with preentry and entry values used to define baseline responses. IFN-γ ELISPOT assays using PBMC following in vitro stimulation (IVS) with pooled epitope peptides were performed with baseline and week 24 samples.
Primary IFN-γ ELISPOT assay.
PBMC were isolated from heparinized blood by density gradient centrifugation, immediately cryopreserved in 90% fetal bovine serum and 10% dimethyl sulfoxide, and transferred to liquid nitrogen for storage. IFN-γ ELISPOT assays were performed using cryopreserved PBMC as described previously (3). Briefly, unfractionated PBMC were thawed, resuspended at a concentration of 1 × 106 cells/ml in AIM V medium (Invitrogen Corp, Carlsbad, CA), and plated at 100 μl/well (1 × 105 cells/well) in 96-well, nitrocellulose-backed plates (Millipore Corp, Bedford, MA) previously coated with a PBS solution of anti-IFN-γ monoclonal antibody (1-D1K, 5 μg/ml; Mabtech Technologies, Nacka, Sweden). Cell stimuli used in these assays were either 1 μg/ml phytohemagglutinin (Sigma-Aldrich, St. Louis, MO) as the positive control, AIM-V medium alone as the negative control, or 21 individual epitope peptides at a concentration of 10 μg/ml as the epitope-specific test. Testing was completed using triplicate wells of cells, with the exception of the medium-only control, where six replicates were used. Plates were then incubated at 37°C for 40 h, harvested, dried, and read on Zeiss KS ELISPOT reader. A vaccine-induced response was defined as a ≥3-fold increase over the baseline response to the individual epitope peptide and with a minimum response magnitude of >50 spot-forming cells (SFC)/106 PBMC for the primary ELISPOT assay. The clinical protocol-defined immunological end point defining a “vaccine responder” was the measurement of significant responses using the primary ELISPOT assay for two or more epitopes at week 18.
IVS ELISPOT assay.
Cryopreserved PBMC collected prevaccine (baseline) and at week 24 postvaccination were thawed and resuspended in RPMI plus 10% human AB serum at a concentration of 5 × 106/ml in a six-well flat-bottom plate. A pool of the 21 epitope peptides was added to the cells at a final concentration of 1 μg/ml/peptide. Cells were incubated for 24 h at 37°C and then diluted with RPMI with 10% human AB serum supplemented with 50 U/ml interleukin-2 to a concentration of 1 × 106 cells/ml. Cultures were incubated for an additional 7 days before being harvested for the IVS ELISPOT assay; the assay was performed as for nonexpanded PBMC with individual epitope peptides by testing cells at a concentration of 1 × 104 cells/well, and a minimum of ≥500 SFC/106 PBMC was considered positive for response.
Statistical methods.
The primary outcome measure was safety, with immunologic end points defined as secondary. All analyses assumed a two-sided test of hypothesis with an overall significance level of 0.05. Immunologic end points and post hoc analyses, including IVS ELISPOT assays and outcomes, which included all postvaccine time points for the primary ELISPOT assay, were considered exploratory and were not adjusted for multiple comparisons. Nonparametric tests were used to compare characteristics across treatment groups. Fisher's exact test and McNemar's test were used to analyze two-by-two contingency tables.
RESULTS
Baseline patient characteristics.
A group of 41 patients, 37 men and 4 women, were enrolled (Table 2). At inclusion, patients had a median CD4 count of 717 cells/ml (range, 403 to 1,518 cells/ml) and a median plasma HIV RNA level of <35 copies/ml (range, <20 to 2,210 copies/ml). ART typically consisted of three drugs, with a median treatment duration of 152 weeks (range, 23 to 512 weeks). CD4 nadirs were ≤100 cells/ml for 27% of the patients, 101 to 500 cells/ml for 63% of the population, and >500 cells/ml for the remaining 10%.
Although HLA typing was not utilized as an inclusion criterion, subjects were molecularly HLA typed and 90% expressed at least one of the supertype alleles restricted by EP HIV-1090. As anticipated in a predominately (80%) Caucasian patient population, the majority of the patients (66%) expressed an HLA-A2 supertype allele. HLA-B7 patients were nearly equally represented at 61%, whereas HLA-A3-expressing patients were found at a frequency of 41%. Nine patients (22%) expressed allelic products representing all three HLA supertypes.
Safety and tolerability of EP HIV-1090.
EP HIV-1090 was generally safe and well tolerated in all 40 patients receiving the four scheduled intramuscular immunizations. One patient enrolled in the study was replaced at week 8 because of poor adherence to ART medication. CD4 counts and plasma HIV-1 RNA levels remained stable throughout the study, and no AIDS-defining clinical events were observed. There were no occurrences of grade 3 or 4 toxicity judged to be definitely or possibly related to the vaccine. There were two instances of grade 2 injection site pain reported in the vaccine recipients (Table 3). Both subjects were in the 1.0-mg dose group, and injection site discomfort was not observed in higher-dose groups, including the 4.0-mg cohort, who received twice the injection volume administered as two injections. Increases in creatine phosphokinase levels were also noted in three subjects, two vaccine recipients and one placebo recipient. These events were most likely the result of the intramuscular immunization route itself rather than an effect of EP HIV-1090.
TABLE 3.
Summary of possibly or definitely vaccine-related adverse events
| Adverse event(s) | No. of grade 2/3/4 for group
|
||
|---|---|---|---|
| Total | Placebo | Vaccine | |
| Ache, pain, discomfort, injection site pain | 2/0/0 | 0/0/0 | 2/0/0 |
| Creatine phosphokinase increase | 3/0/0 | 1/0/0 | 2/0/0 |
| Headache | 1/0/0 | 0/0/0 | 1/0/0 |
| Asthenia, fatigue, malaise | 1/0/0 | 0/0/0 | 1/0/0 |
| Fever, chills, rigors | 1/0/0 | 0/0/0 | 1/0/0 |
| Dreams, insomnia, sleep problems | 1/0/0 | 0/0/0 | 1/0/0 |
| Lethargy, mental status changes | 1/0/0 | 0/0/0 | 1/0/0 |
Immunogenicity of EP HIV-1090.
Baseline epitope-specific immune responses present prior to EP HIV-1090 immunizations were measured using primary IFN-γ ELISPOT assays (net SFC/106 PBMC). Preexisting epitope-specific responses to 9 of the 21 vaccine epitopes were detected in nine patients, for a total of 15 responses (Table 4). Three HLA-A2 epitopes (Gag 386, Nef 221, and Pol 498), five HLA-A3 epitopes (Env 61, Pol 347, Pol 722, Pol 929, and Pol 971), and one HLA-B7 epitope (Pol 893) were recognized at baseline. Four subjects had a measurable baseline response to one epitope, four additional subjects presented with responses to two epitopes, and one patient had responses to three vaccine epitopes before vaccination. Eighty percent of the baseline responses (12/15 epitopes recognized) were observed in patients expressing the correspondingly restricted HLA supertype allele.
TABLE 4.
Baseline immune responses to EP HIV-1090 epitopes
| Patient | HLA type | Epitope (HLA type) | Net SFC/106 PBMC |
|---|---|---|---|
| 343007 | A2A3B7 | Pol 498 (A2) | 70 |
| 343009 | A2A3B7 | Gag 386 (A2) | 60 |
| 343033 | A3B7 | Pol 971 (A3) | 120 |
| 343044 | A2B7 | Pol 498 (A2) | 90 |
| 343031 | B7 | Nef 221 (A2) | 50 |
| Pol 929 (A3) | 80 | ||
| 343004 | A2 | Pol 498 (A2) | 130 |
| Pol 722 (A3) | 50 | ||
| 343038 | A2A3B7 | Env 61 (A3) | 50 |
| Pol 347 (A3) | 50 | ||
| 343023 | A2A3B7 | Gag 386 (A2) | 140 |
| Pol 893 (B7) | 360 | ||
| 343024 | A2A3B7 | Nef 221 (A2) | 310 |
| Pol 498 (A2) | 135 | ||
| Pol 971 (A3) | 425 |
The median baseline immune response to individual epitopes was 90 SFC/106 PBMC (range, 50 to 425). HLA-A2 epitopes were the most frequently recognized prevaccination and constituted 8 of 15 baseline responses directed against three epitopes. Moreover, each of the three HLA-A2 epitopes was recognized in a minimum of two patients, whereas other epitopes were generally recognized in a single individual, with the exception of the HLA-A3 epitope Pol 971, against which two patients responded. Interestingly, subjects expressing alleles from all three vaccine HLA supertypes seemed somewhat overrepresented in the baseline responder group, as five of the nine patients were HLA-A2, -A3, and -B7 positive (P = 0.014 by Fisher's exact test).
The clinical protocol-defined immunological end point used to define an individual subject as a “vaccine responder” was the measurement of significant responses against at least two epitopes at week 18, 2 weeks after the final immunization. By this criterion, two subjects, one subject each from the 0.5- and 1-mg dose groups, were considered to have responded to EP HIV-1090 immunization, and no subjects who received placebo immunization were classified as vaccine responders (P = 1.0) (Table 5). Three additional subjects (dose groups 0.5, 2, and 4 mg) responded to a single epitope at week 18. Differences in response frequencies between dose groups for one or more epitope responses at week 18 did not achieve statistical significance (Fisher's exact test, P = 0.950). Of the eight epitope-specific responses identified using PBMC and the primary ELISPOT assay at week 18, 75% were found in patients expressing the predicted HLA restricting allele.
TABLE 5.
Fresh ELISPOT assay responses to one or more peptides at week 18
| Patient | Dose group (mg) | HLA type | No. of responses | Corresponding peptide(s) |
|---|---|---|---|---|
| 343002a | 0.5 | A2A3 | 3 | Gag 545, Pol 347, Pol 498 |
| 343013 | 0.5 | A2 | 1 | Env 250 |
| 343033a | 1.0 | A3B7 | 2 | Pol 929, Pol 971 |
| 343038 | 2.0 | A2A3B7 | 1 | Gag 386 |
| 343025 | 4.0 | A2A3B7 | 1 | Gag 386 |
Met vaccine responder criteria of ≥3 times baseline level and at least 50 net SFC per 106 PBMC for two or more peptides.
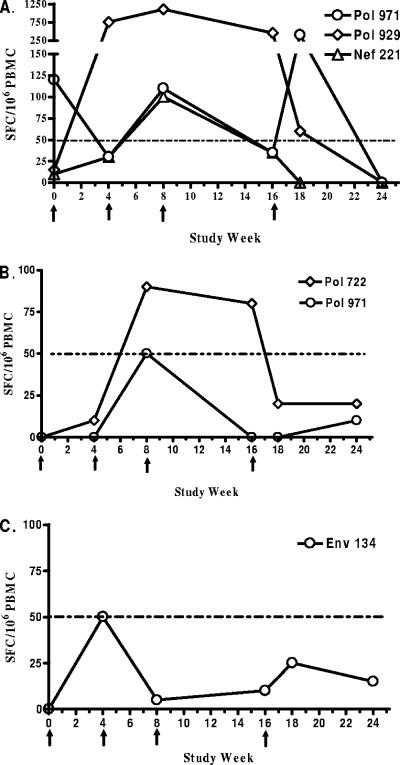
When primary ELISPOT responses were evaluated using sequentially collected PBMC samples after each vaccination, the transient nature of vaccine-induced T-cell responses in the peripheral blood compartment became apparent. In the representative example shown in Fig. 1, responses to epitopes that were ≥3 times baseline and at least ≥50 net SFC/106 PBMC at any time point from week 4 through 24 are shown for three subjects: subject 343033 (1-mg dose group) (Fig. 1A), subject 343007 (0.5-mg dose group) (Fig. 1B), and subject 343020 (placebo group) (Fig. 1C). For subject 343033, a significant baseline response to Pol 971 was measured prior to vaccination, and the magnitude of the response varied during the vaccination period. The response to Pol 929 increased 50-fold from 15 SFC/106 PBMC at baseline to 750 SFC/106 PBMC 4 weeks after the first immunization. The Pol 929 response persisted throughout the active immunization phase of the clinical trial, averaging 768 SFC/106 PBMC. When the response to this epitope was measured at week 18, the magnitude had fallen over 12-fold to 60 SFC/106 PBMC, indicating the highly variable nature of responses measured in this study. Similarly, responses to Nef 221 were detected with magnitudes of up 100 SFC/106 PBMC but only during the immunization period, indicating a transient nature of the response. Responses to Pol 971 for subject 343007 and to Env 134 for subject 343020 barely reached the minimum significance level of 50 net SFC/106 PBMC at a single time point tested before dropping back to baseline levels.
FIG. 1.
Examples of epitope-specific responses in subjects 343033 (1-mg dose group) (A), 343007 (0.5-mg dose group) (B), and 343020 (placebo group) (C) over the course of vaccination. PBMC were stimulated with individual peptides corresponding to EP HIV-1090 epitopes in an overnight IFN-γ ELISPOT assay. Epitopes for which specific responses were ≥3 times baseline and at least ≥50 net SFC/106 PBMC at any time point tested from week 4 through week 24 are shown. The times of vaccine immunizations are indicated by arrows. The horizontal dashed line represents the lower limit of significant responses.
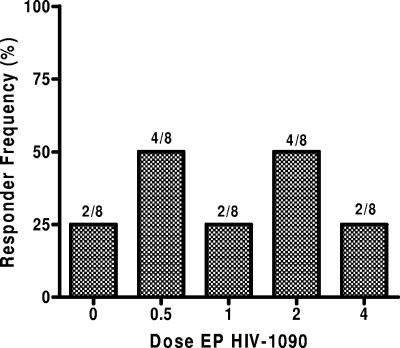
Epitope-specific responses measured in the vaccine follow-up period, weeks 2 through 40, for each dose group are depicted in Fig. 2. Overall, 12 of 32 subjects (37.5%) receiving active vaccine responded to at least one new epitope over the course of vaccination, whereas two of eight placebo recipients (25%) responded similarly (P = 1.0 by Fisher's exact test). The highest frequencies of responses over time were observed in the 0.5- and 2-mg dose groups, where four subjects responded in each group. However, the differences in response frequencies throughout the vaccination period between dose groups did not achieve statistical significance (Fisher's exact test, P = 0.815). Thus, there was no evidence of a dose effect on the response rate.
FIG. 2.
Frequency of subjects exhibiting one or more epitope-specific responses of ≥50 net SFC/106 PBMC and a ≥3-fold increase over baseline in the PBMC ELISPOT assay during vaccine treatment at weeks 2 to 40. The number of responders out of total subjects for each dose group is also indicated. The following samples were missing: week 4 for subject 343038 (2 mg), week 24 for subject 343034 (2 mg), and week 40 for subjects 343014 (placebo), 34301 (0.5 mg), and 343005 (1 mg).
Measurement of epitope-specific responses using the IVS ELISPOT assay.
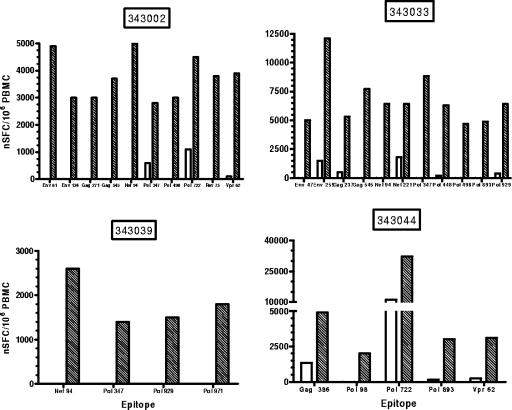
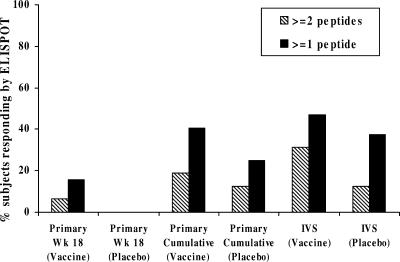
To test for the presence of HIV-1-specific T-cell responses primed by the vaccine but present in the blood at a frequency below the detection limit of the primary ELISPOT assay, an in vitro restimulation step was included in the assay. Figure 3 shows examples of pre- and postvaccine expanded ELISPOT peptide responses in four vaccine recipients. Using the criteria for a vaccine-induced epitope-specific response of a ≥3-fold increase at week 24 over the baseline response to the corresponding peptide and a minimum magnitude of ≥500 SFC/106 expanded cells, 10 vaccinees and 1 placebo recipient (P = 0.41) recognized two or more peptides, and 15 vaccinees and 3 placebo recipients (P = 0.71) recognized one or more vaccine peptides when measured using the IVS ELISPOT assay. This is in comparison to two vaccinees and no placebo recipients for two or more peptides (P = 1.0) and five vaccinees and no placebo recipients for one or more responses (P = 0.56) in the primary ELISPOT assay at week 18. These results are summarized in Fig. 4. Among vaccine recipients, the expanded ELISPOT assay at week 24 detected a significantly greater number of vaccine responders than the primary ELISPOT assay at week 18 (P = 0.013 for responses to one or more peptides and P = 0.008 for responses to two or more peptides) (Fig. 4). Concordance between these two assays was very good in terms of detecting vaccine responders. As an example, the 2 responders to two or more peptides in the primary ELISPOT assay at week 18 were among the 11 responders to two or more peptides detected by the IVS ELISPOT assay at week 24.
FIG. 3.
Expanded ELISPOT peptide responses for patients 343002 (0.5 mg), 343033 (1 mg), 343039 (2.0 mg), and 343044 (4 mg). Cryopreserved PBMC from before vaccine administration (clear bars) and week 24 after vaccine administration (shaded bars) were stimulated with a pool of the 21 vaccine-encoded epitope peptides for 8 days in culture and then assayed for responses using an IFN-γ ELISPOT assay as described for nonexpanded PBMC. Responses to epitopes that were ≥3 times baseline with a magnitude of ≥500 net SFC/106 PBMC at week 24 are illustrated.
FIG. 4.
Summary of vaccine (n = 32) and placebo (n = 8) recipients recognizing vaccine peptides using primary and expanded ELISPOT assays. The percentage of recipients responding to one or more (solid bars) or two or more (hatched bars) epitope peptides using the following criteria are shown: primary ELISPOT assay (prevaccine versus week 18, unexpanded PBMCs), primary cumulative (prevaccine versus any postvaccine time point, unexpanded PBMCs), or IVS ELISPOT assay (prevaccine versus week 24, expanded PBMCs).
Using both the primary week 18 and expanded ELISPOT assays to measure epitope-specific responses, 31% (10/32) of vaccine recipients responded to two or more epitope peptides and 17/32 (53%) responded to one or more vaccine peptides, although these response rates were not statistically significant compared to those measured using PBMC from placebo patients (P = 0.41 and P = 0.69, respectively). Using both the primary and expanded ELISPOT assays and including all postvaccine time points for the primary ELISPOT assay, 41% (13/32) of vaccine recipients responded to two or more peptides and 66% (21/32) responded to one or more vaccine peptides, although this again failed to achieve statistical significance compared to placebo (P = 0.22 and P = 0.44, respectively).
DISCUSSION
The EP HIV-1090 DNA vaccine is an experimental, first-generation product designed to induce responses to highly conserved, HLA class I supertype-restricted, HIV-1 derived CTL epitopes (32). Our initial preclinical studies showed these conserved epitopes to be antigenic, recognized in vitro using PBMC from a cohort of HIV-1-infected patients, suggesting that they are processed, presented, and immunogenic during natural infection. However, the magnitude of naturally occurring CD8+ T-cell responses targeting these epitopes was low in treated subjects, and the breadth of recognition for a given individual was relatively narrow. Thus, we hypothesized that immunization of HIV-1-infected subjects receiving effective ART with EP HIV-1090 DNA vaccine would induce a stronger and more broadly directed CTL response capable of recognizing autologous HIV-1 variants in vivo.
A major challenge of this type of therapeutic immunization is viral variation, as multiple viral variants, or quasispecies, exist that can evade immune detection through changes in amino acid sequences of CTL epitopes. The evolution of such viral escape mutants, in concert with well-documented HIV-associated T-cell dysfunction, contributes to the loss of immune system control associated with disease progression. With this in mind, the EP HIV-1090 DNA vaccine was designed to induce CTLs that will recognize not only amino acid sequence conserved epitopes but also epitopes with minor changes in sequence (27). The working hypothesis is that these epitopes cannot be altered significantly without affecting viral fitness, and as such, they are logical targets for therapeutic vaccination.
In this, the first phase 1 trial with HIV-1 infected subjects, the vaccine proved to be safe and very well tolerated but not as immunogenic as was predicted based on animal testing and clinical testing data reported by others. It is difficult to compare the results obtained from the clinical testing of other DNA vaccines because different study designs and types of immune response assays were used. For example, the use of peptide pools rather than individual CTL or HTL epitope peptides complicates comparison, as peptide length can effect rate of detection (10). The use of ELISPOT assays or intracellular cytokine staining, with and without peptide or other forms of cellular activation or restimulation, also varies between laboratories. Additionally, the variation reported between studies involving the same vaccine products is significant, indicating variability between laboratory capabilities. Finally, preexisting HIV-specific immune responses induced during natural infection may vary between target patient populations and can complicate measurement of vaccine-induced immunity in the therapeutic vaccination setting. However, given all of these variables, the vaccine immunogenicity observed in our studies appears to be comparable to, but certainly not greater than, that reported for other DNA vaccines encoding intact HIV viral gene products or epitopes or for peptide-based products tested clinically.
For example, a vaccine composed of HIV-1 subtype A-derived CTL epitopes fused to the intact Gag p24 gene product and delivered as a DNA vaccine was reported to induce weak and transient CTL responses, measured using the ELISPOT assay, in the PBMC of 14 of 18 healthy uninfected volunteers (78%) (28). However, a significantly reduced response rate of <15% was reported following subsequent clinical testing, and the immunogenicity of this DNA vaccine could not be demonstrated in HIV-infected volunteers, where preexisting immune responses complicated the analysis (8, 16). Cellular immune responses measured using an ELISPOT following vaccination of normal volunteers with a similarly designed DNA product encoding Plasmodium falciparum CTL epitopes fused to the thrombospondin-related adhesion protein appear to be comparable in rate and magnitude and mediated predominantly by CD4+ T lymphocytes (26). Immune responses were increased significantly following a booster immunization with the P. falciparum products delivered using an MVA, indicating immune system priming by administration of the DNA vaccine. This is similar to the results we report in this paper, where additional responses could be detected using a culture step to expand and activate responding T lymphocytes to detectable levels.
Higher rates of T-lymphocyte responses were observed using DNA vaccines encoding largely intact but inactivated HIV gene products; response rates of 36 to 40% for CD8+ T lymphocytes and 93 to 97% for CD4+ T lymphocytes were reported (5, 15). These finding could be interpreted to indicate that DNA vaccines encoding large or intact viral gene products are more immunogenic than epitope-based vaccines such as the product tested in our study. The increased rates of response to the intact Gag p24 protein and TRAP over the defined CTL epitopes may support this interpretation (26, 28). However, an alternative possibility is that HTL epitopes and the responses induced to them may increase vaccine potency with respect to inducing CTL responses. It should be noted that other experimental vaccines encoding both HTL and CTL epitopes have been developed and are being evaluated clinically; the results of these studies may provide some insight into the results from this first trial.
The use of the primary ELISPOT assay for analysis of PBMC samples from multiple study time points indicated the induction of transient HLA phenotype-restricted CTL responses rather than long-lived circulating and activated CTLs. This has been reported for DNA- and MVA-vectored vaccines by others (16, 28) and interpreted largely to indicate low-level responses and limitations with assay sensitivity or specificity (18). Transient CTL responses may also indicate loss in the periphery resulting from homing of lymphocytes to lymphoid tissues, a reduction in cellular function and activation in the absence of continued epitope stimulation or proinflammatory signals, or potentially the progression of vaccine-induced CTLs to memory cells. To investigate these possibilities, an IVS assay was used. The sensitivity of the IVS ELISPOT assay significantly increased our ability to detect responses, resulting in the detection of multiple responses to vaccine-encoded epitopes.
The EP HIV-1090 DNA vaccine was also tested in non-HIV-infected volunteers by the HIV Vaccine Trials Network (HVTN-048) (14). The product was observed to be safe and well tolerated. Similar to the case for this study, immune responses measured using a primary ELISPOT assay or chromium release assay were only rarely observed; responses were detected in 4 of 35 of vaccine recipients (11%) using PBMC sets from three or four study time points. Further testing using other assays was not completed for the HIV Vaccine Trials Network study, and therefore, the two studies cannot be compared further.
The EP HIV-1090 DNA vaccine proved to be safe and tolerable, and transient new CTL responses were observed in a small subset of vaccinated subjects using prespecified response criteria. However, overall differences in response rates between vaccine and placebo recipients did not achieve statistical significance. The transient nature of the measured CTLs may be a concern; however, additional assays will be needed to better characterize the vaccine-induced T-cell responses to determine if memory CTL responses were effectively primed. As this study was focused on safety and not powered to detect significant differences in vaccine-induced immune responses, additional clinical testing will also be needed to assess vaccine schedules and methods that may augment vaccine potency in HIV-infected subjects. Similarly, larger trials will be necessary to determine whether epitope-based therapeutic vaccine approaches result in better control of HIV-1 replication. Analysis of the combined data from the studies completed with HIV-infected and uninfected volunteers using this vaccine indicates the likely need to increase vaccine immunogenicity through the use of vaccine delivery devices, adjuvants, or potentially the incorporation of HIV-1-derived HTL epitopes. Further studies with second-generation products and vaccine delivery devices are ongoing.
Supplementary Material
Acknowledgments
We thank the study subjects for their generous participation.
This work was supported by NIH grant P01AI48238 and was facilitated by the infrastructure and resources provided by the Colorado Center for AIDS Research (grant AI054907).
M.J.N. and B.D.L. were employed by the company that produced the vaccine (Pharmexa-Epimmune, Inc.) during implementation of the trial. The other authors report no conflicts of interest.
Footnotes
Published ahead of print on 9 April 2008.
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1.Altfeld, M. A., B. Livingston, N. Reshamwala, P. T. Nguyen, M. M. Addo, A. Shea, M. Newman, J. Fikes, J. Sidney, P. Wentworth, R. Chesnut, R. L. Eldridge, E. S. Rosenberg, G. K. Robbins, C. Brander, P. E. Sax, S. Boswell, T. Flynn, S. Buchbinder, P. J. Goulder, B. D. Walker, A. Sette, and S. A. Kalams. 2001. Identification of novel HLA-A2-restricted human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte epitopes predicted by the HLA-A2 supertype peptide-binding motif. J. Virol. 75:1301-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boritz, E., B. E. Palmer, B. Livingston, A. Sette, and C. C. Wilson. 2003. Diverse repertoire of HIV-1 p24-specific, IFN-gamma-producing CD4+ T cell clones following immune reconstitution on highly active antiretroviral therapy. J. Immunol. 170:1106-1116. [DOI] [PubMed] [Google Scholar]
- 4.Cao, Y., L. Qin, L. Zhang, J. Safrit, and D. D. Ho. 1995. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N. Engl. J. Med. 332:201-208. [DOI] [PubMed] [Google Scholar]
- 5.Catanzaro, A. T., M. Roederer, R. A. Koup, R. T. Bailer, M. E. Enama, M. C. Nason, J. E. Martin, S. Rucker, C. A. Andrews, P. L. Gomez, J. R. Mascola, G. J. Nabel, and B. S. Graham. 2007. Phase I clinical evaluation of a six-plasmid multiclade HIV-1 DNA candidate vaccine. Vaccine 25:4085-4092. [DOI] [PubMed] [Google Scholar]
- 6.Clough, L. A., E. D'Agata, S. Raffanti, and D. W. Haas. 1999. Factors that predict incomplete virological response to protease inhibitor-based antiretroviral therapy. Clin. Infect. Dis. 29:75-81. [DOI] [PubMed] [Google Scholar]
- 7.Cosma, A., R. Nagaraj, S. Buhler, J. Hinkula, D. H. Busch, G. Sutter, F. D. Goebel, and V. Erfle. 2003. Therapeutic vaccination with MVA-HIV-1 nef elicits Nef-specific T-helper cell responses in chronically HIV-1 infected individuals. Vaccine 22:21-29. [DOI] [PubMed] [Google Scholar]
- 8.Dorrell, L., H. Yang, A. K. Iversen, C. Conlon, A. Suttill, M. Lancaster, T. Dong, I. Cebere, A. Edwards, S. Rowland-Jones, T. Hanke, and A. J. McMichael. 2005. Therapeutic immunization of highly active antiretroviral therapy-treated HIV-1-infected patients: safety and immunogenicity of an HIV-1 gag/poly-epitope DNA vaccine. AIDS 19:1321-1323. [DOI] [PubMed] [Google Scholar]
- 9.Dorrell, L., H. Yang, B. Ondondo, T. Dong, K. di Gleria, A. Suttill, C. Conlon, D. Brown, P. Williams, P. Bowness, N. Goonetilleke, T. Rostron, S. Rowland-Jones, T. Hanke, and A. McMichael. 2006. Expansion and diversification of virus-specific T cells following immunization of human immunodeficiency virus type 1 (HIV-1)-infected individuals with a recombinant modified vaccinia virus Ankara/HIV-1 Gag vaccine. J. Virol. 80:4705-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubey, S., J. Clair, T. M. Fu, L. Guan, R. Long, R. Mogg, K. Anderson, K. B. Collins, C. Gaunt, V. R. Fernandez, L. Zhu, L. Kierstead, S. Thaler, S. B. Gupta, W. Straus, D. Mehrotra, T. W. Tobery, D. R. Casimiro, and J. W. Shiver. 2007. Detection of HIV vaccine-induced cell-mediated immunity in HIV-seronegative clinical trial participants using an optimized and validated enzyme-linked immunospot assay. J. Acquir. Immune. Defic. Syndr. 45:20-27. [DOI] [PubMed] [Google Scholar]
- 11.Frahm, N., S. Adams, P. Kiepiela, C. H. Linde, H. S. Hewitt, M. Lichterfeld, K. Sango, N. V. Brown, E. Pae, A. G. Wurcel, M. Altfeld, M. E. Feeney, T. M. Allen, T. Roach, M. A. St John, E. S. Daar, E. Rosenberg, B. Korber, F. Marincola, B. D. Walker, P. J. Goulder, and C. Brander. 2005. HLA-B63 presents HLA-B57/B58-restricted cytotoxic T-lymphocyte epitopes and is associated with low human immunodeficiency virus load. J. Virol. 79:10218-10225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frahm, N., P. Kiepiela, S. Adams, C. H. Linde, H. S. Hewitt, K. Sango, M. E. Feeney, M. M. Addo, M. Lichterfeld, M. P. Lahaie, E. Pae, A. G. Wurcel, T. Roach, M. A. St John, M. Altfeld, F. M. Marincola, C. Moore, S. Mallal, M. Carrington, D. Heckerman, T. M. Allen, J. I. Mullins, B. T. Korber, P. J. Goulder, B. D. Walker, and C. Brander. 2006. Control of human immunodeficiency virus replication by cytotoxic T lymphocytes targeting subdominant epitopes. Nat. Immunol. 7:173-178. [DOI] [PubMed] [Google Scholar]
- 13.Giri, M., K. E. Ugen, and D. B. Weiner. 2004. DNA vaccines against human immunodeficiency virus type 1 in the past decade. Clin. Microbiol. Rev. 17:370-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorse, G. J., L. R. Baden, M. Wecker, M. J. Newman, G. Ferrari, K. J. Weinhold, B. D. Livingston, T. L. Villafana, H. L. Li, E. Noonan, and N. D. Russell. 2008. Safety and immunogenicity of cytotoxic T-lymphocyte poly-epitope, DNA plasmid (EP HIV-1090) vaccine in healthy, human immunodeficiency virus type 1 (HIV-1)-uninfected adults. Vaccine. 26:215-223. [DOI] [PubMed] [Google Scholar]
- 15.Graham, B. S., R. A. Koup, M. Roederer, R. T. Bailer, M. E. Enama, Z. Moodie, J. E. Martin, M. M. McCluskey, B. K. Chakrabarti, L. Lamoreaux, C. A. Andrews, P. L. Gomez, J. R. Mascola, and G. J. Nabel. 2006. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 DNA candidate vaccine. J. Infect. Dis. 194:1650-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanke, T., A. J. McMichael, and L. Dorrell. 2007. Clinical experience with plasmid DNA- and modified vaccinia virus Ankara-vectored human immunodeficiency virus type 1 clade A vaccine focusing on T-cell induction. J. Gen. Virol. 88:1-12. [DOI] [PubMed] [Google Scholar]
- 17.Harrer, E., M. Bauerle, B. Ferstl, P. Chaplin, B. Petzold, L. Mateo, A. Handley, M. Tzatzaris, J. Vollmar, S. Bergmann, M. Rittmaier, K. Eismann, S. Muller, J. R. Kalden, B. Spriewald, D. Willbold, and T. Harrer. 2005. Therapeutic vaccination of HIV-1-infected patients on HAART with a recombinant HIV-1 nef-expressing MVA: safety, immunogenicity and influence on viral load during treatment interruption. Antivir. Ther. 10:285-300. [PubMed] [Google Scholar]
- 18.Jamieson, B. D., F. J. Ibarrondo, J. T. Wong, M. A. Hausner, H. L. Ng, M. Fuerst, C. Price, R. Shih, J. Elliott, P. M. Hultin, L. E. Hultin, P. A. Anton, and O. O. Yang. 2006. Transience of vaccine-induced HIV-1-specific CTL and definition of vaccine “response”. Vaccine 24:3426-3431. [DOI] [PubMed] [Google Scholar]
- 19.Jin, X., M. Ramanathan, Jr., S. Barsoum, G. R. Deschenes, L. Ba, J. Binley, D. Schiller, D. E. Bauer, D. C. Chen, A. Hurley, L. Gebuhrer, R. El Habib, P. Caudrelier, M. Klein, L. Zhang, D. D. Ho, and M. Markowitz. 2002. Safety and immunogenicity of ALVAC vCP1452 and recombinant gp160 in newly human immunodeficiency virus type 1-infected patients treated with prolonged highly active antiretroviral therapy. J. Virol. 76:2206-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lederberg, J. 2000. Infectious history. Science 288:287-293. [DOI] [PubMed] [Google Scholar]
- 22.Levy, Y., C. Durier, A. S. Lascaux, V. Meiffredy, H. Gahery-Segard, C. Goujard, C. Rouzioux, M. Resch, J. G. Guillet, M. Kazatchkine, J. F. Delfraissy, and J. P. Aboulker. 2006. Sustained control of viremia following therapeutic immunization in chronically HIV-1-infected individuals. AIDS 20:405-413. [DOI] [PubMed] [Google Scholar]
- 23.Levy, Y., H. Gahery-Segard, C. Durier, A. S. Lascaux, C. Goujard, V. Meiffredy, C. Rouzioux, R. E. Habib, M. Beumont-Mauviel, J. G. Guillet, J. F. Delfraissy, and J. P. Aboulker. 2005. Immunological and virological efficacy of a therapeutic immunization combined with interleukin-2 in chronically HIV-1 infected patients. AIDS 19:279-286. [PubMed] [Google Scholar]
- 24.Livingston, B. D., M. Newman, C. Crimi, D. McKinney, R. Chesnut, and A. Sette. 2001. Optimization of epitope processing enhances immunogenicity of multiepitope DNA vaccines. Vaccine 19:4652-4660. [DOI] [PubMed] [Google Scholar]
- 25.MacGregor, R. R., J. D. Boyer, K. E. Ugen, P. Tebas, T. J. Higgins, Y. Baine, R. B. Ciccarelli, R. S. Ginsberg, and D. B. Weiner. 2005. Plasmid vaccination of stable HIV-positive subjects on antiviral treatment results in enhanced CD8 T-cell immunity and increased control of viral “blips”. Vaccine 23:2066-2073. [DOI] [PubMed] [Google Scholar]
- 26.McConkey, S. J., W. H. Reece, V. S. Moorthy, D. Webster, S. Dunachie, G. Butcher, J. M. Vuola, T. J. Blanchard, P. Gothard, K. Watkins, C. M. Hannan, S. Everaere, K. Brown, K. E. Kester, J. Cummings, J. Williams, D. G. Heppner, A. Pathan, K. Flanagan, N. Arulanantham, M. T. Roberts, M. Roy, G. L. Smith, J. Schneider, T. Peto, R. E. Sinden, S. C. Gilbert, and A. V. Hill. 2003. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat. Med. 9:729-735. [DOI] [PubMed] [Google Scholar]
- 27.McKinney, D. M., R. Skvoretz, B. D. Livingston, C. C. Wilson, M. Anders, R. W. Chesnut, A. Sette, M. Essex, V. Novitsky, and M. J. Newman. 2004. Recognition of variant HIV-1 epitopes from diverse viral subtypes by vaccine-induced CTL. J. Immunol. 173:1941-1950. [DOI] [PubMed] [Google Scholar]
- 28.Mwau, M., I. Cebere, J. Sutton, P. Chikoti, N. Winstone, E. G. Wee, T. Beattie, Y. H. Chen, L. Dorrell, H. McShane, C. Schmidt, M. Brooks, S. Patel, J. Roberts, C. Conlon, S. L. Rowland-Jones, J. J. Bwayo, A. J. McMichael, and T. Hanke. 2004. A human immunodeficiency virus 1 (HIV-1) clade A vaccine in clinical trials: stimulation of HIV-specific T-cell responses by DNA and recombinant modified vaccinia virus Ankara (MVA) vaccines in humans. J. Gen. Virol. 85:911-919. [DOI] [PubMed] [Google Scholar]
- 29.Nicolaou, M., P. Chang, and M. J. Newman. 2005. Use of poly(N-vinyl pyrrolidone) with noncondensed plasmid DNA formulations for gene therapy and vaccines, p. 329-344. In M. M. Amiji (ed.), Polymeric gene delivery: principles and applications. CRC Press, Boca Raton, FL.
- 30.Pantaleo, G., S. Menzo, M. Vaccarezza, C. Graziosi, O. J. Cohen, J. F. Demarest, D. Montefiori, J. M. Orenstein, C. Fox, and L. K. Schrager. 1995. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N. Engl. J. Med. 332:209-216. [DOI] [PubMed] [Google Scholar]
- 31.Sette, A., and J. Sidney. 1999. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics 50:201-212. [DOI] [PubMed] [Google Scholar]
- 32.Wilson, C. C., D. McKinney, M. Anders, S. MaWhinney, J. Forster, C. Crimi, S. Southwood, A. Sette, R. Chesnut, M. J. Newman, and B. D. Livingston. 2003. Development of a DNA vaccine designed to induce cytotoxic T lymphocyte responses to multiple conserved epitopes in HIV-1. J. Immunol. 171:5611-5623. [DOI] [PubMed] [Google Scholar]
- 33.Zuniga, R., A. Lucchetti, P. Galvan, S. Sanchez, C. Sanchez, A. Hernandez, H. Sanchez, N. Frahm, C. H. Linde, H. S. Hewitt, W. Hildebrand, M. Altfeld, T. M. Allen, B. D. Walker, B. T. Korber, T. Leitner, J. Sanchez, and C. Brander. 2006. Relative dominance of Gag p24-specific cytotoxic T lymphocytes is associated with human immunodeficiency virus control. J. Virol. 80:3122-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.