Abstract
Monocyte-derived dendritic cells (DCs) differentiate in the presence of Toll-like-receptor (TLR) ligands in the course of ongoing infections. A single-stranded RNA (ssRNA) sequence, corresponding to the sequence of the U5 region of human immunodeficiency virus type 1 RNA, was used to mimic viral activation of TLR7 in human DCs. We determined the effector potential of DCs differentiated in the presence of this ssRNA molecule (ssRNA-DCs). ssRNA-DCs phenotypically resembled mature DCs. In contrast, their capacity to allostimulate naive CD4+ T cells resembled that of conventional immature DCs and could be increased by TLR4 stimulation. Th1 polarization of CD4+ T cells and production of interleukin 12p70 (IL-12p70) by ssRNA-DCs were selectively abrogated in response to a late TLR4, but not in response to a CD40, maturation signal. Inhibition of p38 mitogen-activated protein kinase partially restored IL-12p70 secretion but did not restore Th1 polarization, whereas addition of exogenous IL-12 led to recovery of Th1 polarization. In contrast to lipopolysaccharide, ssRNA induced IL-12p70 production at the very earliest stages of DC differentiation, indicating a particular role for TLR7 in monocyte-derived DCs recently engaged in differentiation. These data demonstrate generation of phenotypically mature DCs with the ability to expand CD4+ T lymphocytes lacking Th1/2-polarizing capacity.
Dendritic cells (DCs) link innate recognition of pathogens with the subsequent development of a pathogen-specific adaptive immune response. One key event in the progression of the immune response is the initial recognition by DCs of conserved pathogen-derived patterns through receptors such as the Toll-like receptors (TLRs), which trigger the expression of different cytokines and costimulatory molecules (13). Different TLRs recognize specific bacterial, fungal, or viral molecules. In particular, TLR4 is required for Escherichia coli lipopolysaccharide (LPS)-mediated signal transduction (23), whereas TLR7/8 recognizes virus-derived single-stranded RNA (ssRNA) sequences (12). With the exception of TLR3, all the members of the human TLR family share signaling pathways dependent on the adaptor protein myeloid differentiation factor 88 (MyD88), which activates NF-κB and leads to the production of inflammatory cytokines and in most cases to the production of interleukin 12 (IL-12) (1). Instead, TLR3 together with TLR4 signals via the adaptor molecule TRIF, activating not only IL-12 production but also type 1 interferon (IFN) synthesis (1, 10). Production of IL-12 by DCs is critical for the polarization of CD4 T cells toward a Th1 phenotype (31).
The response driven by different TLR ligands in fully differentiated DCs has been widely described (24). However, the effect of TLR ligands on the effector function of DCs can be modified by factors such as the differentiation state of the cells. Several reports show that at early stages of in vitro differentiation, ligands for TLR4 or TLR9 allow DC differentiation while modifying their function (11, 22, 34). DCs differentiated in the presence of LPS have reduced antigen presentation capacity, partly due to early IL-10 production by the stimulated cells (34). In contrast, the presence of CpG oligonucleotides stimulates monocyte differentiation to DCs, which induce strong in vitro and in vivo antigen-specific T-cell responses (11).
In peripheral tissues, DCs are predominantly immature, and upon detecting danger signals and sampling antigens, they mature during migration to germinal centers. Under inflammatory conditions, recruitment and differentiation of blood monocytes allows replenishment of tissues with DCs (20, 25). The encounter of monocytes with pathogen-derived products is therefore likely to influence the conventional DC (cDC) differentiation process. We have recently shown that repeated addition of a synthetic TLR7/8 agonist, resiquimod, during DC differentiation modified the DC phenotype, partially inhibiting CD1a expression, and led to proinflammatory cytokine production (3). In the present study we examined the effector properties of DCs given ssRNA, a TLR7/8 agonist, from the beginning of differentiation (ssRNA-DCs). We show that ssRNA-DCs lost their ability to induce Th1 polarization despite maintaining an allostimulatory capacity. In addition, we show that very early in DC differentiation, and in contrast to TLR4, TLR7/8 induces production of IL-12p70.
MATERIALS AND METHODS
Reagents and antibodies.
Lipopolysaccharide (LPS) from E. coli serotype 0111:B4, ionomycin, brefeldin A, phorbol myristate acetate (PMA), and phytohemagglutinin (PHA) lectin were purchased from Sigma Chemical Co. (St. Louis, MO). LPS was further purified by a phenol-chloroform-petroleum ether extraction (18). ssRNA (GCCCGUCUGUUGUGUGACUC) (12) was from IBA (Germany) and the Dotap liposomal reagent from Roche (Germany). The p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580 was from Calbiochem (San Diego, CA). Fluorescein isothiocyanate-, Alexa 488-, or phycoerythrin-conjugated antibodies and isotype controls were used. Monoclonal antibodies against CD1a were purchased from Dako (Denmark), those against CD40 were from Immunotech (Marseille, France), and those against CD11c, CD14, CD54, CD80, CD83, CD86, HLA-DR, gamma IFN (IFN-γ), and IL-4 were all from BD Biosciences (San Jose, CA).
Generation of monocyte-derived DCs.
Human mononuclear blood cells from healthy donors were isolated by density gradient centrifugation using lymphocyte separation medium (Eurobio, France), and monocytes were isolated by magnetically activated cell sorting of CD14+ cells (Miltenyi Biotech, Germany). Monocytes (2 × 106/ml) were cultured in RPMI 1640 supplemented with 10% fetal calf serum, 800 IU/ml human recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) (R&D Systems, France) and 1,000 IU/ml human recombinant IL-4 (R&D Systems). Half of the culture supernatant was renewed with fresh medium and cytokines on day 2. On day 5 the medium was completely renewed, and cells (2 × 106/ml) were further cultured for 24 h without any stimulus to maintain immature cDCs (iDCs) or with LPS (10 ng/ml) to generate mature cDCs (mDCs). For stimulation of DCs during differentiation, our previously described protocol (3) was modified and LPS (10 ng/ml) or ssRNA (500 ng/ml in 5 μg/ml Dotap) was added at day 0 of monocyte culture. Stimuli given at day 0 of DC differentiation are referred to as early, whereas those given at day 5 are referred to as late stimuli. Dotap alone was used as a control stimulus throughout and did not alter either the DC phenotype or function at the indicated concentration. In some assays, 1 μM SB203580 was added at the beginning of DC differentiation, 30 min before adding TLR agonists to the culture.
Immunophenotype analysis.
Staining of cells with antibodies was performed in phosphate-buffered saline containing 100 μg/ml human gamma-globulin (Calbiochem) and 5% fetal calf serum. Fluorescence-activated cell sorting (FACS) analysis was carried out with addition of propidium iodide to exclude dead cells from analysis. For intracellular staining, cells were fixed in 2% paraformaldehyde and permeabilized with 0.5% saponin before staining.
Allogeneic T-cell proliferation assay.
Purified naive CD4 T cells (naive CD4+ isolation kit; Miltenyi Biotec) from allogeneic donors were labeled with 5 μM carboxyfluorescein diacetate succinimidyl ester (Molecular Probes) and were cocultured with irradiated DCs (1.5 × 105 and 5 × 104 cells, respectively). On day 6, cells were stained with propidium iodide and were analyzed by FACS. Cell proliferation was calculated as the frequency of CD4 T-cell precursors in the original sample that were engaged in proliferation. In all proliferation assays, autologous naive CD4 T cells were included as controls (not shown).
T-cell polarization.
Irradiated DCs were cocultured with naive CD4 T cells under the conditions described above. In some experiments, 5 ng/ml of recombinant human IL-12p70 (R&D Systems) was added to the cell culture. On day 6, cells were restimulated with PMA (50 ng/ml) and ionomycin (1 μM) for 6 h, and brefeldin A (1 μM) was added during the last 2 h. Polarization of T cells toward a Th1 or Th2 phenotype was analyzed by intracellular detection of IFN-γ or IL-4, respectively.
Quantification of cytokine production.
Supernatants of DC cultures were collected at different times, and specific cytokines were measured by enzyme-linked immunosorbent assays (ELISAs). Commercial kits for IL-10 and IL-12p70 (detection limit, 4 pg/ml) were from BD Biosciences, and that for tumor necrosis factor alpha (TNF-α) (detection limit, 15.6 pg/ml) was from R&D Systems.
Statistical analysis.
The statistical significance of the data was analyzed by using paired t tests. P values smaller than 0.05 were considered significant. All experiments were carried out with cells from a minimum of three different donors.
RESULTS
ssRNA-DCs develop a mature phenotype.
During an infection or an inflammatory process, local DCs emigrate to draining lymph nodes and monocytes are recruited (6, 28) which differentiate into DCs (20) in an environment rich in inflammatory and microbial molecules. Here we have assessed the functional effects of a virus-derived ssRNA sequence, a TLR7/8 agonist, on DC differentiation. We compared the functional potential of DCs differentiated in the presence of E. coli LPS (LPS-DCs), a TLR4 ligand, for which changes in phenotypic and cytokine responses have been characterized (34). Low concentrations of ssRNA or LPS were present from the beginning of the DC differentiation process. Additionally, on day 5, LPS was added in order to test the potential of these DCs to undergo further maturation.
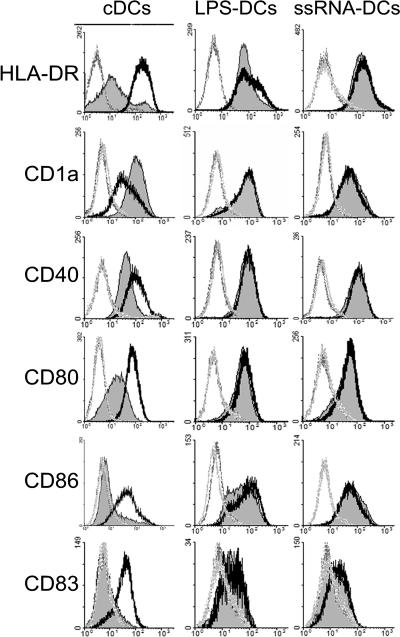
Differences in the cultured cells were observed at early culture times. On day 2, control cultures of DC were mainly composed of single-cell suspensions whereas addition of ssRNA led to formation of small cell aggregates (not shown). Early ssRNA priming constantly led to a lower cell yield, which, depending on the donor, was reduced by 18% to 64% compared to that of cDCs. The phenotype of DCs was analyzed on day 6, 24 h after addition or not of a late maturation stimulus. cDCs had the typical phenotype of in vitro-differentiated monocyte-derived DCs (29), with high expression of CD1a and low surface expression of HLA-DR and costimulatory molecules including CD40, CD80, and CD86 (Fig. 1). CD14 was not detected (data not shown). Day 5 LPS-DCs and ssRNA-DCs expressed lower levels of CD1a but higher levels of HLA-DR, CD40, CD80, CD86, and CD83, indicating maturation of these DCs compared to control iDCs (Fig. 1). However, in response to the late LPS stimulus, cDCs markedly up-regulated surface expression of different maturation markers (HLA-DR, CD40, CD80, CD86, and CD83) while the expression level of these markers was unchanged in ssRNA-DCs (Fig. 1, thick lines). Control DC cultures differentiated in the presence of Dotap were included here and in all experiments presented below; they were not significantly different from nonstimulated DCs (data not shown). These results confirm data from our group and from others showing that an early LPS or ssRNA stimulus (3, 34) leads to DCs with a mature phenotype that remains stable even after addition of a late maturation signal. Moreover, in contrast to results in our previous study, these data reveal that a single ssRNA stimulus was sufficient to induce phenotypic modification.
FIG. 1.
ssRNA-DCs and LPS-DCs display a mature phenotype. Monocyte-derived DCs were differentiated in the presence or not (cDCs) of LPS (LPS-DCs) or ssRNA/Dotap (ssRNA-DCs). On day 5, DCs were kept for 24 h in medium (filled histograms) or stimulated with LPS (thick lines). Expression of surface HLA-DR, CD1a, CD14, CD40, CD80, CD83, and CD86 was determined by FACS analysis. The background signal of an Ig isotypic control is shown (dotted lines). The phenotype of DCs differentiated in the presence of Dotap did not differ from that of cDCs (not shown).
The capacity of ssRNA-DCs to stimulate CD4 T-cell proliferation is unrelated to their phenotype.
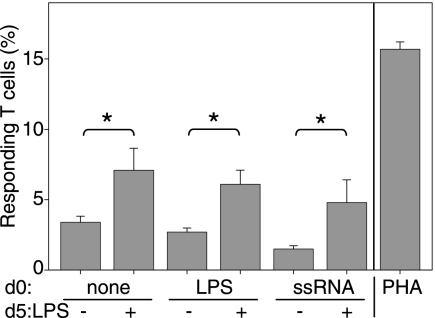
We next determined the ability of ssRNA-DCs to stimulate allogeneic naive CD4 T-cell proliferation. Figure 2 shows that in all cases, DCs induced the proliferation of allogeneic naive CD4 T cells. Conventional iDCs stimulated significantly less T-cell proliferation than mDCs did (Fig. 2), in strict correlation with their phenotypic maturation state (Fig. 1). In contrast, the allostimulatory capacity of LPS-DCs and ssRNA-DCs did not correlate with the expression level of activation markers. Although the proportions of CD4 T cells activated by LPS-DCs and ssRNA-DCs varied from donor to donor, they were constantly equivalent to the proportions activated by conventional iDCs (Fig. 2). Thus, ssRNA-DCs which were provided with a late TLR4 stimulus consistently stimulated significantly higher levels of T-cell proliferation (Fig. 2), despite their phenotypic similarity with ssRNA-DCs, which had not received a late TLR4 stimulus (Fig. 1). These results indicate that the expression level of maturation and/or activation markers is therefore not predictive of the capacity of ssRNA-DCs to induce a high level of CD4 T-cell proliferation. In addition, the phenotypic similarity between ssRNA-DCs which had or had not received a late TLR4 stimulus suggests that other functional changes are triggered which allow these DCs to display a stronger capacity to stimulate CD4 T cells.
FIG. 2.
ssRNA-DCs and LPS-DCs induce proliferation of allogeneic T cells. cDCs, LPS-DCs, and ssRNA-DCs (day 0 treatment) not receiving further stimuli or receiving a late LPS maturation stimulus (day 5) were cocultured with carboxyfluorescein diacetate succinimidyl ester-loaded naive CD4 T cells (ratio, 1:3) from one allogeneic donor. At day 6 of coculture, the different division cycles undergone by T cells were detected by FACS analysis and the number of stimulated cells on the initial sample was calculated. Proliferation induced by cells receiving a late LPS stimulus was significantly greater in all DC preparations (P < 0.05). Results are representative of experiments with at least six different donors. The frequency of responding autologous naive CD4 T cells was in all cases under 2% (not shown). Control peripheral blood mononuclear cells stimulated with PHA are also shown. DCs differentiated in the presence of Dotap induced T-cell proliferation which was not significantly different from that induced by cDCs (not shown).
ssRNA-DCs have low potential to polarize the CD4 T-cell response.
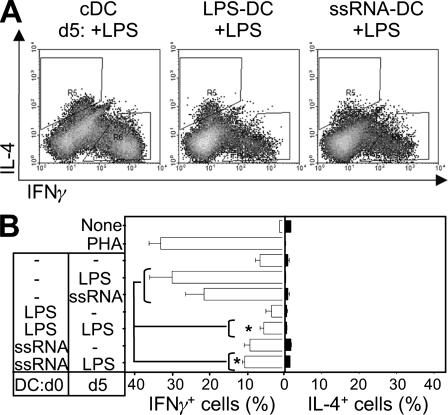
To further characterize the effector potential of ssRNA-DCs, we tested their ability to polarize T cells toward a Th1 or Th2 phenotype. Naive CD4 T cells were stimulated with DCs, and on day 6, the cytokine profile of the T cells was assessed. Figure 3A shows the gating of conventionally produced mDCs for IFN-γ and IL-4 measurements. Figure 3B shows that cDCs given a late maturation stimulus (LPS or ssRNA) strongly induced a Th1 profile increasing the frequency of IFN-γ-producing cells from 6.8% ± 1.2% for iDCs to either 30.3% ± 6% for LPS or 20.8% ± 5.9 for ssRNA. The frequency of IL-4-producing cells in all cases was less than 1%. This result concords with the reported Th1-polarizing potential of E. coli LPS and TLR7/8 ligands (24).
FIG. 3.
ssRNA-DCs and LPS-DCs have a reduced Th-polarizing capacity. Allogeneic naive CD4 T cells were stimulated for 6 days with the different DC preparations: cDCs, LPS-DCs, and ssRNA-DCs not receiving or receiving a late LPS maturation stimulus. Control cells were also stimulated with ssRNA. The phenotype of activated T cells was determined by FACS analysis after stimulation with PMA-ionomycin and intracellular staining of IFN-γ and IL-4. Results are shown as dot plots (A) or bar histograms (B) representative of experiments with different donors. Compared to cDCs, LPS-DCs and ssRNA-DCs receiving a late TLR4-mediated signal induced significantly fewer IFN-γ-producing cells (indicated with asterisks [P < 0.05]).
In contrast, the Th1-polarizing potential of ssRNA-DCs was significantly diminished. Thus, ssRNA-DCs induced 9.5% ± 1.5% IFN-γ-producing cells (LPS-DCs induced 3.7% ± 1.5%, for comparison), and the polarizing potential of these DCs failed to increase after a late LPS maturation stimulus (10.9% ± 0.6% IFN-γ producing cells) (Fig. 3B). Overall, the Th1-polarizing potential of ssRNA-DCs was equivalent to that of conventional iDCs but was significantly lower than that of conventional mDCs (P < 0.05).
Interestingly, the absence of Th1 polarization by ssRNA-DCs did not result in increased Th2-polarizing potential. Whereas cDCs, immature and mature, led to frequencies of IL-4-producing cells of 2.55 ± 0.1% and 3.15 ± 0.2%, respectively, ssRNA-DCs generated IL-4-producing cell frequencies of 3.8% ± 1% (Fig. 3). Overall, these results demonstrate that ssRNA-mediated activation of DCs can lead to distinct functional outcomes depending on the differentiation stage of the DC. Whereas both ssRNA and LPS activate Th1-polarizing potential in cDCs, addition of these stimuli at the beginning of differentiation results in a DC population which fails to induce Th polarization even in response to a late TLR4-mediated signal.
Early ssRNA addition to differentiating DCs is accompanied by strong IL-10 production and late IL-12p70 suppression.
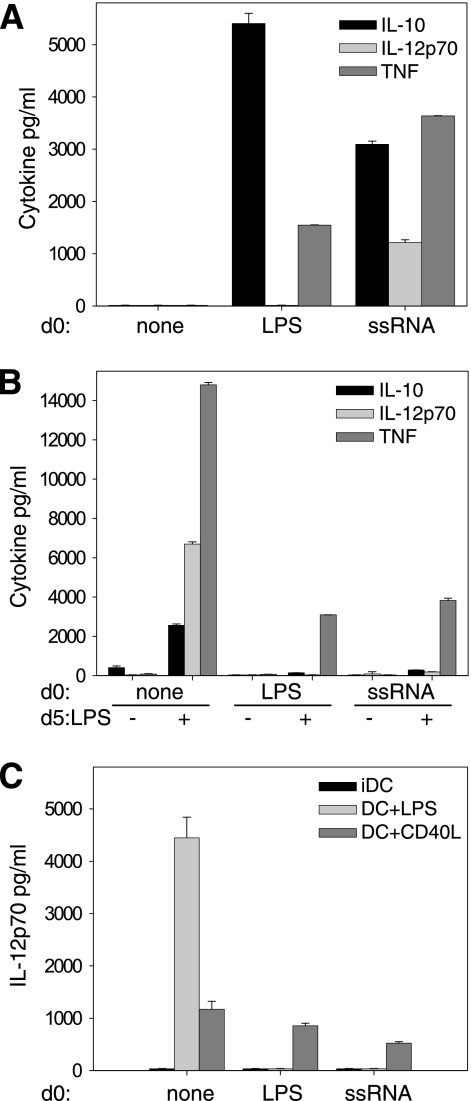
The response of DCs to TLR ligands involves the production of cytokines which participate in the polarization of primed CD4 T cells (21, 24, 26). IL-12p70 and IL-10 are particularly associated with Th1- and Th2-polarizing potential, respectively (24, 26). Since ssRNA-DCs failed to induce Th polarization, we examined whether this failure was due to modified production of IL-12 and/or IL-10. Differentiation of DCs in the absence of TLR stimuli was not accompanied by production of IL-10 or IL-12 either at early (day 1) or at later (day 6) culture times (Fig. 4A and B: no TLR agonist at day 0 or 5). After 5 days of differentiation, cDCs responded to LPS or to ssRNA by production of IL-10, IL-12p70, and proinflammatory cytokines, such as TNF-α (Fig. 4B, no TLR agonist at day 0 and LPS at day 5). Conversely, addition of ssRNA or LPS at the beginning of DC differentiation induced a distinct cytokine response. Both stimuli induced production of TNF-α and IL-10 within 24 h (Fig. 4A, LPS or ssRNA at day 0) at levels comparable to those produced by cDCs in response to the same stimuli (Fig. 4B).
FIG. 4.
Early ssRNA or LPS priming suppresses late IL-12p70 response of DCs to LPS but not to CD40LT. Freshly purified blood monocytes were cultured with GM-CSF and IL-4 to induce DC differentiation. (A) Medium (none), 10 ng/ml LPS, or 500 ng/ml ssRNA was added at the beginning of the culture (day 0). (B) On day 5 (d5), cells either were left in medium or were stimulated with 10 ng/ml LPS or 200 ng/ml CD40LT. (C) On day 5, cells either were left in medium or were stimulated with 200 ng/ml CD40LT. The amounts of IL-10, IL-12p70, and TNF-α released during the 24 h following stimulation were determined by ELISA: culture supernatants were collected on day 1 (A) or day 6 (B and C).
However, LPS-DCs failed to produce either IL-12p40 (not shown) or IL-12p70, whereas ssRNA DCs produced IL-12p70 detected within 24 h of culture (Fig. 4A). Although neither ssRNA DCs nor LPS-DCs produced IL-10 or IL-12p70 in response to a late maturation stimulus (Fig. 4B), the same cells did produce TNF-α and were therefore selectively rather than totally refractory to a late TLR4-mediated signal.
In order to enhance a potentially low level of expression of IL-12p70, we stimulated LPS-DCs and ssRNA-DCs together with LPS and IFN-γ; IL-12p70 was still not produced (data not shown).
To further test the extent of nonresponsiveness, we evaluated the ability of soluble CD40L, a non-TLR-mediated maturation stimulus, to stimulate LPS-DCs and ssRNA-DCs. The CD40-mediated signal led to production of IL-12p70 (Fig. 4C, DC+CD40L). This result is important, since it reveals that the inhibition of the IL-12p70 response to a late TLR4-mediated signal is not due to an IL-12 exhaustion phenomenon, as occurs in models of repetitive DC stimulation (15). Taken together, these results indicate that ssRNA-DCs maintain a selective ability to signal via TLR4 and that IL-12 production remains possible in response to certain stimuli.
Finally, whereas LPS-DCs failed to produce significant IL-12p40 (not shown) or IL-12p70, ssRNA-DCs produced IL-12p70 detected within 24 h of culture (Fig. 4A).
Addition of exogenous IL-12p70, but not recovery of endogenous IL-12 production, supports Th1 polarization by ssRNA-DCs.
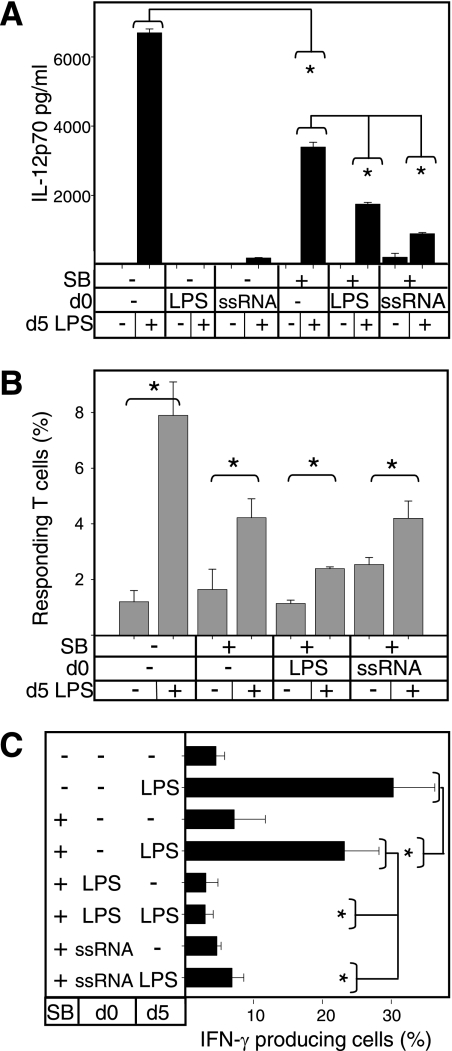
It has been previously reported that the capacity of LPS-DCs to produce IL-12p70 could be increased by pretreatment with the p38 MAPK inhibitor SB203580 (34). We therefore tested whether SB203580 increased the ability of ssRNA-DCs to induce CD4 T-cell polarization. We examined LPS-DCs for comparison. Adding SB203580 at the beginning of the DC differentiation process resulted in an overall decrease in effector function: IL-12p70 production, T-cell proliferation, and Th1 polarization (Fig. 5, SB pretreatment, no LPS at day 0). However, SB203580 pretreatment only partially restored the capacity of ssRNA-DCs to produce IL-12p70 in response to a late LPS stimulus (Fig. 5A). This was significantly low (P < 0.05), limited to around 22% of the capacity of pretreated DCs (Fig. 5A, SB pretreatment, LPS at day 5). SB203580 pretreatment was more effective in restoring the IL-12p70 production of LPS-DCs, which reached 50% of that of cDCs, though remaining significantly lower (P < 0.05).
FIG. 5.
Pretreatment of LPS-DCs and ssRNA-DCs with the p38 MAPK inhibitor SB203580 recovers the late IL-12p70 response but not a Th1-polarizing potential. For pretreatment, on day 0 (d0), monocytes cultured in GM-CSF and IL-4 to induce DC differentiation were treated for 30 min with 1 μM SB203580 (SB) or left untreated before medium, LPS, or ssRNA was added. For maturation stimulus, on day 5 (d5), DCs were stimulated with LPS for 24 h or left untreated. (A) Day 6 supernatants were tested for IL-12p70 by ELISAs. (B and C) DCs were cocultured with allogeneic carboxyfluorescein diacetate succinimidyl ester-labeled naive CD4 T cells. On day 6, T-cell proliferation (B) and intracellular IFN-γ accumulation (C) were determined by FACS analysis. In the proliferation assay (B), the frequency of responding autologous naive CD4 T cells was under 1.0% in all cases (not shown). Statistically significant differences (P < 0.05) between the control and a given test are indicated with an asterisk.
Regarding the ability to activate T cells, cDCs pretreated with the p38 MAPK inhibitor induced a slightly reduced proliferation of T cells compared with nontreated DCs (Fig. 5B). Moreover, addition of the p38 MAPK inhibitor restored CD4 T-cell proliferation levels (to those of cDCs) when ssRNA-DCs were given a late LPS stimulus. However, despite restored IL-12p70 production and proliferation, ssRNA-DC pretreatment with the p38 MAPK inhibitor failed to restore Th1 polarization in response to a late LPS stimulus (Fig. 5C).
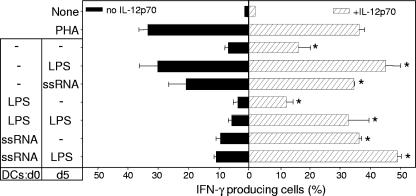
Although IL-12 expression was partially restored by SB203580, we cannot exclude that the lack of restoration of Th1 polarization was due to other effects of the inhibitor. Therefore, we tested the effect of exogenous IL-12p70 on the Th1 polarization capacity of LPS-DCs and ssRNA-DCs. Addition of IL-12p70 led to an overall and statistically significant increase in the percentage of IFN-γ-producing T cells (Fig. 6), although neither the baseline level nor the high proportion of Th1 induced by PHA was significantly increased. However, the Th1 population induced by conventional iDCs increased 2.4 times (from 6.8% ± 1.2% to 16.3% ± 3.9%), while that induced by cDCs matured with LPS or with ssRNA increased 1.5 or 1.7 times, respectively (from 20.8% ± 5.9% to 34.6% ± 0.1% and from 20.8% ± 5.9% to 34.6% ± 0.1%).
FIG. 6.
Exogenous IL-12p70 recovers the Th1 polarization mediated by LPS-DCs and ssRNA-DCs. Allogeneic naive CD4 T cells were stimulated with the different DC preparations either without (black bars) or in the presence of (hatched bars) 5 ng/ml recombinant IL-12p70. After 6 days, cell were stimulated with PMA-ionomycin, stained for intracellular IFN-γ, and analyzed by FACS. Percentages of IFN-γ-producing cells significantly different (P < 0.05) between IL-12p70-treated and nontreated cultures are indicated by asterisks.
Addition of IL-12p70 led to a marked increase in the Th1-polarizing potential of ssRNA-DCs or LPS-DCs. The percentage of IFN-γ-producing cells induced in the presence of exogenous IL-12p70 by LPS-DCs receiving or not receiving a late LPS stimulus (32.8% ± 6.8% or 12.35% ± 2.2%, respectively) corresponded to an expansion of 5.7 times and 3.3 times, respectively. The population in which addition of IL-12p70 made the most difference was ssRNA-DCs. The Th1-polarizing potential was increased 4.1 times for ssRNA-DCs (9.5% ± 1.5% versus 39.3% ± 0.8%) and 4.5 times for ssRNA-DCs which had received a late LPS stimulus (10.9% ± 0.6% versus 49% ± 1.2%). Therefore, although Th1 polarization by ssRNA was not recovered by the p38 MAPK inhibitor, addition of exogenous IL-12 can be used to compensate.
The IL-12 response of DCs recently engaged in differentiation is differentially regulated by LPS and ssRNA.
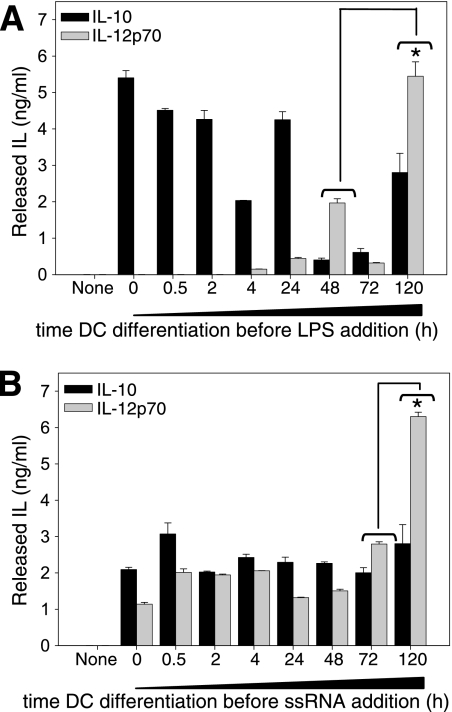
We showed above that in early DC differentiation, ssRNA induces production of IL-12p70 whereas LPS does not (Fig. 4A). To identify the point in differentiation at which DCs begin to respond to either TLR4 or TLR7 by IL-12 production, we allowed DC differentiation for different periods of time before adding LPS or ssRNA. IL-10 and IL-12p70 production was tested 24 h after addition of either. Monocytes recently engaged in DC differentiation produced IL-10 immediately after LPS addition, whereas IL-12p70 production was detected only when the LPS stimulus was given after at least 4 h of differentiation (Fig. 7A). After this time point, the response to LPS of DCs at early differentiation times was accompanied by relatively low IL-12p70 production, with a peak level consistently observed after 48 h of DC differentiation. At day 5 of differentiation, the DC response to LPS resulted in significantly higher IL-12p70 levels (Fig. 7A).
FIG. 7.
Dynamic of IL-10 and IL-12p70 response induced by LPS or by ssRNA during the DC differentiation process. Freshly isolated blood monocytes were cultured in the presence of GM-CSF and IL-4 to allow DC differentiation. Cells cultured for different periods of time (x axis) were stimulated with 10 ng/ml LPS (A) or 500 ng/ml ssRNA (B). Twenty-four hours after stimulation, released IL-10 and IL-12p70 were quantified on culture supernatants by ELISAs. The IL-12p70 response by 5-day-differentiated DCs was significantly higher than the response by DCs in earlier differentiation states (indicated by an asterisk [P < 0.05]).
In contrast, IL-12p70 production in response to ssRNA was observed from the beginning of DC differentiation, and the response levels of IL-10 and IL-12p70 were constant during the first 3 days of DC differentiation (Fig. 7B). On day 5 of differentiation, the ssRNA-induced IL-12p70 production was significantly higher (Fig. 7B). These results show that at early DC differentiation times, the IL-12 response is accessible but only to certain stimuli, such as ssRNA, whereas for other stimuli, i.e., LPS, the IL-12p70 response is initiated only after a period of differentiation in the presence of GM-CSF and IL-4. Additionally, following stimulation with LPS, the cytokine response was uneven throughout DC differentiation, so that at different times LPS induced variable levels of IL-10 and IL-12p70, which would result in qualitatively different effector potentials of the DCs at different stages of differentiation.
Taken together, our results show that the presence of ssRNA throughout DC differentiation selectively alters the sensitivity of DCs to further TLR4-mediated stimuli: upregulation of the allostimulatory capacity is maintained but dissociated from the Th1-polarizing capacity, which is lost in response to a TLR4-mediated signal.
DISCUSSION
Integration of different pathogenic signals during DC differentiation modifies their immunological outcome (13), and ligands for TLR4 or TLR9 have already been reported to modify DC function without impeding differentiation (11, 22, 34). We recently reported that repeated addition of a synthetic TLR7/8 agonist led to the generation of monocyte-derived DCs with phenotypic modifications and production of inflammatory cytokines (3). In particular, it was shown that repeated addition of ssRNA led to phenotypic maturation (decreased CD1 and loss of induction of CXCR1) and prevented the further phenotypic maturation usually observed after addition of a TLR4 ligand (3). Functional evidence for TLR 7/8-driven maturation was provided by the decreased capacity for antigen internalization after differentiation in the presence of ssRNA (3). In the present study, we demonstrate that phenotypic maturation induced by a single addition of a TLR7 ligand does not reflect antigen presentation function, since ssRNA-DCs induced T-cell alloproliferation at levels similar to those induced by conventional monocyte-derived iDCs. Consistent with our previous study, we show that a TLR4 stimulus did not alter the phenotype of ssRNA-DCs. The cytokine response of ssRNA-DCs to LPS was much reduced in comparison with that of conventional DCs, whereas the capacity for antigen presentation was clearly improved. Although this phenomenon of uncoupled DC phenotypic maturation and DC effector capacities has not been explained, it has been observed under certain conditions of activation (27), including virus infection (7), leading to an expression of high levels of costimulatory molecules which does not translate into a good capacity to stimulate T cells.
The detriment of polarizing ability correlated with IL-12 suppression in ssRNA-DCs and LPS-DCs at late differentiation times. Both the late IL-12 suppression and the loss of Th1-polarizing capacity have been previously observed in DCs differentiated in the presence of alpha IFN or beta IFN (19) and in other myeloid cells (human monocytes and murine macrophages) activated with LPS or via FcR (2, 32). Our results support the suggestion that myeloid cells, including DCs, share IL-12 suppression as a common mechanism of limiting the immune response and consequent tissue injury in the case of long-lasting or repeated infectious and inflammatory stimuli. Indeed, IL-12 promotes the differentiation of Th1 cells and the production of IFN-γ, which enhances the cytotoxic activity of CD8 T cells and NK cells (31).
One mechanism commonly presumed to be at the origin of IL-12 suppression is regulation by IL-10 (8). Although early ssRNA priming of DCs is accompanied by production of IL-10, this is certainly not the dominant suppressor mechanism, because the presence of IL-10-blocking antibodies during DC differentiation failed to restore the IL-12 production or Th-polarizing potential of ssRNA-DCs (not shown). Moreover, activation of ssRNA-DCs via CD40L did induce IL-12 production. Thus, this IL-12p70 production default is more likely to be related to a TLR-driven cross-tolerization (16). Since TLR and CD40 receptors share signaling pathways downstream of TRAF6, the mechanism controlling IL-12p70 suppression in ssRNA-DCs would act upstream of it. Downregulation of IRAK-1 expression has been previously identified as being at the origin of cytokine inhibition in LPS-nonresponding macrophages treated with TLR-7 ligands (30). However, a crucial point with ssRNA-DCs is that unresponsiveness to a late stimulus (i.e., LPS) is selective, because production of TNF-α was still possible and, more importantly, the capacity of ssRNA-DCs to stimulate CD4 T-cell proliferation was upregulated. Therefore, DC genes participating in T-cell activation must be selectively activated by the late stimulus given to ssRNA-DCs, possibly in a way similar to that recently described for genes encoding antimicrobial effectors in LPS-tolerant murine macrophages (9). Further characterization of ssRNA-DCs is required to identify such genes sustaining T-cell proliferation independently of the “mature” phenotype of ssRNA-DCs.
A particularly interesting finding of this study was that very early in DC differentiation, ssRNA but not LPS is able to induce an IL-12p70 response. Such a difference was unexpected since production of IL-12p70 by monocytes requires IFN-γ priming and since known signaling pathways through TLR7/8 are expected to be largely overlapping with TLR4. Both TLR7/8 and TLR4 receptors recruit the MyD88 adaptor protein, but TLR4 also signals via the TRIF adaptor protein (35). Yet primary human monocytes have been reported to secrete IL-12p70 with only a combined stimulation of TLR4 and TLR7/8 (4). Such an effect requires a type 1 IFN autocrine loop triggered via TLR4 (4, 10). We observed here that contrary to a TLR4 stimulus, a single TLR7/8 stimulus suffices to activate IL-12p70 production by monocytes recently engaged in DC differentiation. This, together with synergistic effects reported for TLR7/8 with other TLRs (4, 10, 17, 21), provides evidence that TLR7/8, at least for IL-12p70 production, has a particular function which is kinetically segregated from the TLR4 signaling pathway. The existence of a nonredundant role for TLR7 has been proposed to depend on its particular interaction with MyD88 (5). Moreover, quite apart from the role of monocytes as DC precursors, this IL-12p70 response to ssRNA may impinge on monocyte function per se.
Altogether, the behavior of ssRNA-DCs suggests that in the physiological context of an infection providing ligands recognized by TLR7/8, newly recruited monocytes differentiate to DCs with altered functional properties. Whereas local DCs, the first exposed to the infection, would develop conventional T-cell activating and polarizing potential, DCs differentiated from newly arrived monocytes in the presence of TLR4 ligands would have limited Th1-polarizing potential. However, the presence of TLR7/8 ligands may lead to a different outcome, since monocytes recently engaged in DC differentiation do produce IL-12p70. Thus, ssRNA-DCs could instead immediately produce IL-12, which would sustain a Th1-polarizing environment and cytotoxic responses. In animal models of pulmonary infectious disease, pretreatment with a TLR7 ligand leads to a more efficient immune response (33). However, it is difficult to extrapolate the outcome of microbial infections since infection produces a multitude of danger signals with both stimulatory and regulatory mechanisms simultaneously present. Nonetheless, in vitro-generated ssRNA-DCs could provide a means of generating phenotypically mature DCs with an inducible capacity to expand CD4+ T cells and with no Th-polarizing capacity.
In summary, our results reveal particular features of DCs differentiated in the presence of ssRNA. First, these early-activated DCs have dissociated maturation phenotypes and potential to allostimulate T cells. Second, in contrast to the response to ssRNA by fully differentiated DCs, early-activated ssRNA-DCs are unable to polarize the T-cell response toward a Th1 phenotype. Finally, we show that at very early stages of monocyte-derived DC differentiation, DCs respond to ssRNA but not to LPS by producing IL-12p70, supporting the idea of a particular role for TLR7/8 (5) in the response of monocyte-derived cells. These results are particularly relevant since TLR ligands are currently under intensive study as adjuvants for use in vaccination protocols and in treatment of immune deficiencies (14, 21).
Acknowledgments
V.M.-E. was supported by the Marie Curie Research and Training Network, Trans-Net project MRTN-CT-20046512253 (www.trans-net.org.uk). Support was also received from the Allostem project (FP6 no. 503319).
We are very grateful to Eric Assier and Alix Delaguillaumie for help and advice with preparation of DCs and to N. Dulphy for providing IL-12p70.
Footnotes
Published ahead of print on 9 April 2008.
REFERENCES
- 1.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, C. F., and D. M. Mosser. 2002. Cutting edge: biasing immune responses by directing antigen to macrophage Fc gamma receptors. J. Immunol. 168:3697-3701. [DOI] [PubMed] [Google Scholar]
- 3.Assier, E., V. Marin-Esteban, A. Haziot, E. Maggi, D. Charron, and N. Mooney. 2007. TLR 7/8 agonists impair monocyte-derived dendritic cell differentiation and maturation. J. Leukoc. Biol. 81:221-228. [DOI] [PubMed] [Google Scholar]
- 4.Bekeredjian-Ding, I., S. I. Roth, S. Gilles, T. Giese, A. Ablasser, V. Hornung, S. Endres, and G. Hartmann. 2006. T cell-independent, TLR-induced IL-12p70 production in primary human monocytes. J. Immunol. 176:7438-7446. [DOI] [PubMed] [Google Scholar]
- 5.Beutler, B., Z. Jiang, P. Georgel, K. Crozat, B. Croker, S. Rutschmann, X. Du, and K. Hoebe. 2006. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu. Rev. Immunol. 24:353-389. [DOI] [PubMed] [Google Scholar]
- 6.Cavanagh, L. L., and U. H. Von Andrian. 2002. Travellers in many guises: the origins and destinations of dendritic cells. Immunol. Cell Biol. 80:448-462. [DOI] [PubMed] [Google Scholar]
- 7.de Graaff, P. M., E. C. de Jong, T. M. van Capel, M. E. van Dijk, P. J. Roholl, J. Boes, W. Luytjes, J. L. Kimpen, and G. M. van Bleek. 2005. Respiratory syncytial virus infection of monocyte-derived dendritic cells decreases their capacity to activate CD4 T cells. J. Immunol. 175:5904-5911. [DOI] [PubMed] [Google Scholar]
- 8.Demangel, C., P. Bertolino, and W. J. Britton. 2002. Autocrine IL-10 impairs dendritic cell (DC)-derived immune responses to mycobacterial infection by suppressing DC trafficking to draining lymph nodes and local IL-12 production. Eur. J. Immunol. 32:994-1002. [DOI] [PubMed] [Google Scholar]
- 9.Foster, S. L., D. C. Hargreaves, and R. Medzhitov. 2007. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447:972-978. [DOI] [PubMed] [Google Scholar]
- 10.Gautier, G., M. Humbert, F. Deauvieau, M. Scuiller, J. Hiscott, E. E. Bates, G. Trinchieri, C. Caux, and P. Garrone. 2005. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J. Exp. Med. 201:1435-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gursel, M., D. Verthelyi, and D. M. Klinman. 2002. CpG oligodeoxynucleotides induce human monocytes to mature into functional dendritic cells. Eur. J. Immunol. 32:2617-2622. [DOI] [PubMed] [Google Scholar]
- 12.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303:1526-1529. [DOI] [PubMed] [Google Scholar]
- 13.Huang, Q., D. Liu, P. Majewski, L. C. Schulte, J. M. Korn, R. A. Young, E. S. Lander, and N. Hacohen. 2001. The plasticity of dendritic cell responses to pathogens and their components. Science 294:870-875. [DOI] [PubMed] [Google Scholar]
- 14.Krieg, A. M. 2006. Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug Discov. 5:471-484. [DOI] [PubMed] [Google Scholar]
- 15.Langenkamp, A., K. Nagata, K. Murphy, L. Wu, A. Lanzavecchia, and F. Sallusto. 2003. Kinetics and expression patterns of chemokine receptors in human CD4+ T lymphocytes primed by myeloid or plasmacytoid dendritic cells. Eur. J. Immunol. 33:474-482. [DOI] [PubMed] [Google Scholar]
- 16.Lehner, M. D., S. Morath, K. S. Michelsen, R. R. Schumann, and T. Hartung. 2001. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different Toll-like receptors independent of paracrine mediators. J. Immunol. 166:5161-5167. [DOI] [PubMed] [Google Scholar]
- 17.Levy, O., E. E. Suter, R. L. Miller, and M. R. Wessels. 2006. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood 108:1284-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manthey, C. L., and S. N. Vogel. 1994. Elimination of trace endotoxin protein from rough chemotype LPS. J. Endotoxin Res. 1:84-91. [Google Scholar]
- 19.McRae, B. L., T. Nagai, R. T. Semnani, J. M. van Seventer, and G. A. van Seventer. 2000. Interferon-alpha and -beta inhibit the in vitro differentiation of immunocompetent human dendritic cells from CD14(+) precursors. Blood 96:210-217. [PubMed] [Google Scholar]
- 20.Naik, S. H., D. Metcalf, A. van Nieuwenhuijze, I. Wicks, L. Wu, M. O'Keeffe, and K. Shortman. 2006. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat. Immunol. 7:663-671. [DOI] [PubMed] [Google Scholar]
- 21.Napolitani, G., A. Rinaldi, F. Bertoni, F. Sallusto, and A. Lanzavecchia. 2005. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat. Immunol. 6:769-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palucka, K. A., N. Taquet, F. Sanchez-Chapuis, and J. C. Gluckman. 1999. Lipopolysaccharide can block the potential of monocytes to differentiate into dendritic cells. J. Leukoc. Biol. 65:232-240. [DOI] [PubMed] [Google Scholar]
- 23.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 24.Pulendran, B. 2005. Variegation of the immune response with dendritic cells and pathogen recognition receptors. J. Immunol. 174:2457-2465. [DOI] [PubMed] [Google Scholar]
- 25.Randolph, G. J., K. Inaba, D. F. Robbiani, R. M. Steinman, and W. A. Muller. 1999. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 11:753-761. [DOI] [PubMed] [Google Scholar]
- 26.Re, F., and J. L. Strominger. 2004. IL-10 released by concomitant TLR2 stimulation blocks the induction of a subset of Th1 cytokines that are specifically induced by TLR4 or TLR3 in human dendritic cells. J. Immunol. 173:7548-7555. [DOI] [PubMed] [Google Scholar]
- 27.Reis e Sousa, C. 2006. Dendritic cells in a mature age. Nat. Rev. Immunol. 6:476-483. [DOI] [PubMed] [Google Scholar]
- 28.Roake, J. A., A. S. Rao, P. J. Morris, C. P. Larsen, D. F. Hankins, and J. M. Austyn. 1995. Systemic lipopolysaccharide recruits dendritic cell progenitors to nonlymphoid tissues. Transplantation 59:1319-1324. [PubMed] [Google Scholar]
- 29.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato, S., O. Takeuchi, T. Fujita, H. Tomizawa, K. Takeda, and S. Akira. 2002. A variety of microbial components induce tolerance to lipopolysaccharide by differentially affecting MyD88-dependent and -independent pathways. Int. Immunol. 14:783-791. [DOI] [PubMed] [Google Scholar]
- 31.Trinchieri, G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133-146. [DOI] [PubMed] [Google Scholar]
- 32.Wittmann, M., V. A. Larsson, P. Schmidt, G. Begemann, A. Kapp, and T. Werfel. 1999. Suppression of interleukin-12 production by human monocytes after preincubation with lipopolysaccharide. Blood 94:1717-1726. [PubMed] [Google Scholar]
- 33.Wu, C. C., T. Hayashi, K. Takabayashi, M. Sabet, D. F. Smee, D. D. Guiney, H. B. Cottam, and D. A. Carson. 2007. Immunotherapeutic activity of a conjugate of a Toll-like receptor 7 ligand. Proc. Natl. Acad. Sci. USA 104:3990-3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie, J., J. Qian, S. Wang, M. E. Freeman III, J. Epstein, and Q. Yi. 2003. Novel and detrimental effects of lipopolysaccharide on in vitro generation of immature dendritic cells: involvement of mitogen-activated protein kinase p38. J. Immunol. 171:4792-4800. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301:640-643. [DOI] [PubMed] [Google Scholar]