Abstract
Accurate diagnosis of tuberculosis (TB) infection is critical for the treatment, prevention, and control of TB. Conventional diagnostic tests based on purified protein derivative (PPD) do not achieve the required diagnostic sensitivity. Therefore, in this study, we have evaluated the immunogenic properties of Rv1168c, a member of the PPE family, in comparison with PPD, which is routinely used in the tuberculin test, and Hsp60 and ESAT-6, well-known immunodominant antigens of Mycobacterium tuberculosis. In a conventional enzyme immunoassay, the recombinant Rv1168c protein displayed stronger immunoreactivity against the sera obtained from patients with clinically active TB than did PPD, Hsp60, or ESAT-6 and could distinguish TB patients from Mycobacterium bovis BCG-vaccinated controls. Interestingly, Rv1168c antigen permits diagnosis of smear-negative pulmonary TB as well as extrapulmonary TB cases, which are often difficult to diagnose by conventional tests. The immunodominant nature of Rv1168c makes it a promising candidate to use in serodiagnosis of TB. In addition, our studies also show that Rv1168c is a potent T-cell antigen which elicits a strong gamma interferon response in sensitized peripheral blood mononuclear cells obtained from TB patients.
Tuberculosis (TB) remains a significant global public health concern and is a major cause of death in adults by a single bacterial agent (39). The increasing global health burden of tuberculosis is further aggravated by the alarming increase in human immunodeficiency virus infection as well as the emergence of drug-resistant strains of Mycobacterium tuberculosis (16, 18). One of the best prognoses for tuberculosis comes with early diagnosis of the infection and immediate implementation of appropriate treatment regimens. The diagnosis of the majority of TB cases in developing countries like India relies on acid-fast staining of sputum or positive cultures of M. tuberculosis in conjunction with assessment of clinical symptoms and radiographic evidence (6, 29, 38). However, these evaluations are usually expensive, tedious, and time-consuming. The most common method employed for detection of M. tuberculosis infection is the purified protein derivative (PPD) or tuberculin skin test, but PPD is a crude and poorly defined mixture of mycobacterial antigens, many of which are shared with proteins from the vaccine strain Mycobacterium bovis bacillus Calmette-Guérin (BCG) and from nontuberculous environmental mycobacteria (20, 22). Therefore, the clinical relevance of the tuberculin test with PPD is not highly reliable (17, 30).
Although several new and rapid tests for the diagnosis of TB have been developed in recent years (7, 25, 33), they must be performed in laboratories and involve costly equipment and reagents. Further, most of the antigens in these tests have poor sensitivity and specificity to diagnose TB cases with smear-negative sputum samples and are not yet considered standard practice (4, 8). A serological test to detect antibodies to M. tuberculosis has the immense potential to make a diagnostic test for TB optimal and low-cost in developing countries, especially under field conditions (10). In recent years, numerous M. tuberculosis antigens that are capable of generating specific antibody titers in TB patients have been identified, but no single antigen appears to be ideal for serodiagnostic assays (13, 21, 26). Therefore, identification of an appropriate M. tuberculosis antigen suitable for serodiagnosis that can offer high specificity, ease of detection, and sensitivity that can distinguish active tuberculosis patients from BCG-vaccinated controls is highly desirable for developing suitable control measures and early treatment of the disease.
A significant portion (∼10%) of the Mycobacterium tuberculosis genome encodes two unique protein families, the PE and PPE families, with no known apparent functions (11). The PE/PPE genes are expressed upon various environmental cues during infection, and many of the PPE proteins have been found to be strongly immunogenic (10, 12, 15, 32). Recently, studies have shown that mycobacterial PPE antigen Rv1168c (PPE 17) is associated with ESAT-6 gene cluster region 5 (ESX-5) (19), which is predicted to encode a novel secretory apparatus (1, 19). It has also been shown that this cluster is conserved among the various pathogenic mycobacteria, but not in the saprophytic species Mycobacterium smegmatis (1). Further, a BLAST analysis suggested that no genes that are strongly homologous to Rv1168c are present in the non-TB mycobacterial species that have been sequenced. The absence of homologues in other mycobacterial species, such as Mycobacterium avium, makes this protein a potential candidate for serological diagnosis of TB, which is further corroborated by its high antigen index, as calculated with the Kyte-Doolittle algorithm. Interestingly, the recent microarray and proteome studies have shown that Rv1168c is up-regulated under microaerophilic and anaerobic conditions (2, 31), nutrient starvation (5), and in the presence of palmitic acid (35), which simulates the features of the phagosomal environment. This information provides a strong justification for the expression of this gene during M. tuberculosis infection. Therefore, in the present study we have examined whether Rv1168c provides a highly sensitive means of diagnosing patients with active TB.
MATERIALS AND METHODS
Cloning, expression, and purification of recombinant Rv1168c and Hsp60 protein.
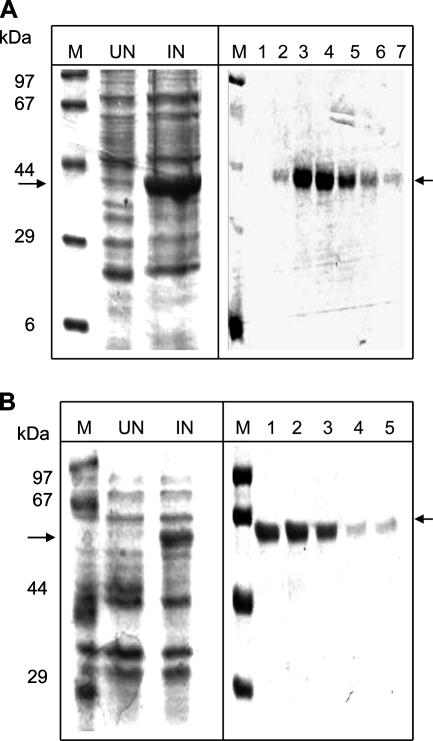
The open reading frame corresponding to Rv1168c was PCR amplified from the genomic DNA of H37Rv. XhoI and HindIII restriction sites were incorporated in the 5′ ends of the forward and reverse primers, respectively. The primers were as follows: forward, GACTCGAGATGGATTTCACAATTTTT; reverse, GCAAGCTTCTAGCCGGCGGCGGGTGACCGCAGT. The parameters for thermal cycle amplification were as follows: 94°C for 12 min; 10 cycles of 94°C for 30 s, 42°C for 30 s, and 72°C for 1 min; 20 cycles of 94°C for 30 s, 37°C for 30 s, and 72°C for 1 min; and a final step at 72°C for 30 min. The size of the amplicon generated was approximately 1,041 bp. The PCR product was first directly cloned in intermediate pGEM-T Easy vectors (Promega, Madison, WI), followed by subcloning in the bacterial expression vector pRSET-A (Invitrogen, Carlsbad, CA) in frame with an N-terminal six-histidine tag using XhoI and HindIII. The clones were validated by sequencing with the T7 promoter primer on an Applied Biosystems Prism 377 DNA sequencer. The pRSET-A clone was then transformed in an Escherichia coli BL21(DE3)pLys expression system. The transformed cells were grown in Terrific broth containing ampicillin (100 μg/ml) and chloramphenicol (35 μg/ml) at 37°C on a shaker to an optical density at 600 nm (OD600) of 0.4 to 0.6, induced with 1 mM isopropyl-β-d-thiogalactopyranoside (Sigma-Aldrich), and further grown at 37°C for 3 to 4 h. The cells were lysed, and induction of Rv1168c was checked (Fig. 1A). Polyhistidine-tagged recombinant protein was purified using Talon resin (BD Biosciences Clontech) according to the manufacturer's recommendations for purification of protein under native conditions. The purity of the protein was confirmed by loading onto a 10% sodium dodecyl sulfate gel. A single protein band with a molecular mass of ∼42 kDa corresponding to Rv1168c protein was observed. The yield of protein was 6 mg/liter culture and appeared to be 98% pure (Fig. 1A). The mycobacterial heat shock protein 60 (Hsp60) was purified (Fig. 1B) as described previously (34). The purified recombinant early secretory antigenic target 6 (ESAT-6) protein of M. tuberculosis was a kind gift from Pawan Sharma, ICGEB, New Delhi, India. Protein concentrations were estimated using the bicinchoninic acid method (Micro BCA protein assay kit; Pierce, Rockford, IL). To remove endotoxin contamination, purified Rv1168c, Hsp60, or ESAT-6 protein was incubated with 10% (vol/vol) polymyxin B-agarose (Sigma-Aldrich; binding capacity, 200 to 500 μg of lipopolysaccharide from Escherichia coli serotype O128:B12/ml) for 1 h at 4°C, and the protein preparation was used to assess the B-cell or T-cell response.
FIG. 1.
Expression and purification of M. tuberculosis proteins Rv1168c (A) and Hsp60 (B). The recombinant protein was expressed in strain BL21 of Escherichia coli and was purified to homogeneity using the Ni-nitrilotriacetic acid protein purification kit. Results shown are for Coomassie blue-stained sodium dodecyl sulfate gels showing uninduced (UN) and induced (IN) cell lysates, a protein molecular size marker (M), and different lanes showing different elution fractions containing purified protein (lanes 1 to 7 in panel A and lanes 1 to 5 in panel B) obtained during purification of the respective proteins. The arrow on the right indicates the position of the Rv1168c protein (∼42 kDa) or Hsp60 protein (∼60 kDa).
Study population.
The study population (n = 109) was comprised of pulmonary (n = 77) and extrapulmonary (n = 32) tuberculosis patients diagnosed at the DOTS (directly observed treatment, short course) Clinic of Mahavir Hospital and Research Centre, Hyderabad, India. The diagnosis of the patients with pulmonary TB was based on the results of a sputum smear examined for the presence of acid-fast bacilli, radiographic examination, and clinical symptoms as per the Revised National TB Control Programme guidelines, Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India (http://www.tbcindia.org). The extrapulmonary cases were confirmed by tissue biopsy, clinical symptoms, and radiographic evidence (http://www.tbcindia.org). All the subjects were found to be negative for human immunodeficiency virus. Sera were collected from the patients just before initiation of chemotherapeutic regimen. All the patients responded to the DOTS regimen, and at the time of preparation of the manuscript, the patients were considered cured based on relief from clinical symptoms, absence of the acid-fast bacillus in the sputum, and radiographic examination. Control sera (n = 20) were collected from volunteers from regions endemic for TB. All the control subjects were BCG vaccinated and had no clinical symptoms of TB at the time of sample collection. The bioethics committee of Mahavir Hospital and Research Centre and CDFD approved the present study, and written informed consent was obtained from all the subjects.
EIA.
For the enzyme immunoassay (EIA), 96-well microtiter plates (Costar, Corning, NY) were coated with 0.5 μg/well recombinant Rv1168c, ESAT-6, or Hsp60 protein or PPD (diluted in 0.1 M carbonate buffer, pH 9.5, with 50 μl added to each well) (10). Plates were incubated overnight at 4°C, washed three times with phosphate-buffered saline (PBS), and blocked with 100 μl of blocking buffer (PBS containing 2% bovine serum albumin) for 2 h at 37°C. After washing the plates three times with PBS containing 0.05% Tween 20 (PBS-T; Sigma-Aldrich), sera (diluted 200-fold in blocking buffer) from various study groups were added (50 μl) to antigen-coated wells in duplicate and incubated for 1 h at 37°C. The plates were washed three times with PBS-T and incubated with 50 μl of anti-human immunoglobulin G (IgG)-horseradish peroxidase (HRP; Sigma-Aldrich) conjugate (1:8,000 dilution in blocking buffer) for 1 h at 37°C. The plates were washed two times with PBS-T, and a final wash was carried out with PBS. The HRP activity was detected using a chromogenic substance, o-phenylenediamine tetrahydrochloride (Sigma-Aldrich) in citrate-phosphate buffer (pH 5.4) and H2O2 (Merck, Germany) as substrate (1 μl/ml). Reactions were terminated using 1 N H2SO4, and the absorbance values were measured at 492 nm with an EIA reader (Bio-Tek Instruments Inc., VT).
Cytokine assay.
The peripheral blood mononuclear cells (PBMCs) from TB patients (n = 35) and BCG-vaccinated controls (n = 10) were isolated using density gradient centrifugation in Ficoll-Hypaque solution (Sigma-Aldrich) as described elsewhere (9) and prepared at 2.5 × 106 cells/ml in RPMI 1640 medium (Invitrogen, Grand Island, NY) containing 10% fetal bovine serum (Invitrogen) and antibiotics (RPMI-10). Cell suspensions (200 μl/well) were dispensed into 96-well, flat-bottom microtiter plates (Nunc, Roskilde, Denmark) and maintained at 37°C in a 5% CO2 incubator. PBMCs from various groups were treated with a fixed concentration of Rv1168c (3 μg/ml) or PPD, and after 4 days culture supernatants were harvested for estimating gamma interferon (IFN-γ) and interleukin-5 (IL-5) cytokine levels secreted in the culture supernatants using the EIA. The cytokine was quantified by a two-site sandwich EIA (BD Biosciences Pharmingen, San Diego, CA) following the manufacturer's protocol as described by us previously (24). Briefly, 96-well polyvinyl chloride microtiter plates were coated with purified anticytokine antibody at a 2-μg/ml concentration. The plates were blocked with 2% bovine serum albumin in PBS and incubated with various culture supernatants followed by incubation with biotin-conjugated anticytokine antibody and streptavidin-HRP. The HRP activity was detected using o-phenylenediamine tetrahydrochloride, and absorbance was read at 492 nm. A standard curve for the cytokine was obtained using the IFN-γ or IL-5 recombinant standard protein provided by the manufacturer.
Statistical analysis.
For evaluation of antibody responses, cutoff values were calculated for each antigen as the means of OD492 values obtained with the sera from 20 BCG-vaccinated controls plus 6 standard deviations (SD) (27, 28). Data were analyzed with Student's t test or analysis of variance, as indicated below. A P level of <0.05 was considered significant.
RESULTS
Rv1168c shows a strong immunoreactivity toward TB patient sera compared to that of BCG-vaccinated controls.
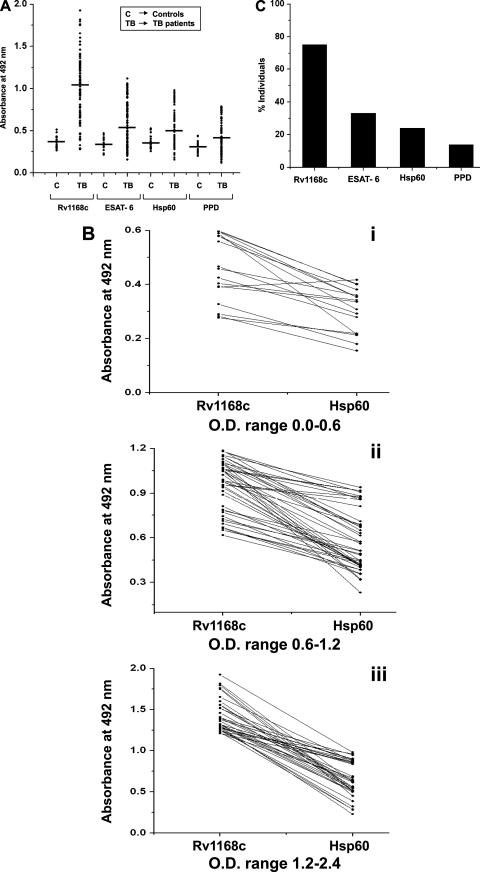
Based on its predominant expression under the conditions that mimic the in vivo phagosomal environment (2, 5, 31, 35) and its high antigenicity index, we expected Rv1168c to induce a strong B-cell response in people with active TB infection. Therefore, we examined specific antibody reactivity in responses to Rv1168c protein in sera from TB patients and compared this with the BCG-vaccinated controls. The sensitivity and specificity of Rv1168c immunoreactivity were compared with the responses elicited by ESAT-6, Hsp60, and PPD. The antibody titers (Fig. 2A) against Rv1168c were found to be significantly higher (absorbance at 492 nm [OD492] ± SD, 1.05 ± 0.381 [mean ± SD]) in TB patients compared to that of the BCG-vaccinated control sera (0.373 ± 0.066; P < 0.0001).
FIG. 2.
The PPE protein Rv1168c is more sensitive for discriminating TB patients from the BCG-vaccinated controls. The scatter plot shows the serum cross-reactivities by EIA to the mycobacterial recombinant Rv1168c, mycobacterial recombinant ESAT-6, Hsp60, and PPD with serum from either active tuberculosis patients or BCG-vaccinated controls. The horizontal line indicates the mean of the absorbance values (A). The EIA antibody responses of individual patients whose results are shown in panel A were compared between Rv1168c and Hsp60 (B, panels i to iii). Responders to Rv1168c were compared with that of ESAT-6, Hsp60, and PPD by calculating the percentage of TB patients showing absorbance value greater than or equal to the cutoff value, calculated as the mean OD492 of control sera plus 6 SD (C). Mean OD492 (SD) values used for cutoff determinations were as follows: Rv1168c, 0.376 (0.066); ESAT-6, 0.343 (0.07), Hsp60, 0.359 (0.08); PPD, 0.295 (0.071). Statistical significance was determined with Student's t test.
We also compared the serological reactivity of Rv1168c to that elicited by two other mycobacterial immunodominant antigens, ESAT-6 (which is a better-studied antigen and has progressed to use commercially [7, 12]) and Hsp60 (3), and the conventionally used PPD. The levels of anti-PPD antibodies were found to be low in TB patients (OD492, 0.415 ± 0.184), indicating that PPD was not very sensitive in diagnosis of active TB, which was in agreement with other reports (10, 28). Although ESAT-6 (OD492, 0.612 ± 0.264) was a better discriminatory antigen than PPD, the sera of the patients reacted much more strongly against Rv1168c than ESAT-6 (Fig. 2A). Similarly, Hsp60, although it showed a better response (OD492, 0.571 ± 0.230) than PPD, had a lower reactivity than Rv1168c in the majority of the TB patients (Fig. 2B, panels i to iii). The sera of the patients reacted very strongly against Rv1168c compared to ESAT-6, Hsp60, and PPD (P < 0.0001 for all cases). Therefore, the Rv1168c protein appeared to be more efficient in discriminating active tuberculosis patients from the BCG-vaccinated controls, compared to Hsp60 and PPD. When the proportion of highly reactive sera (antibody levels greater than or equal to the mean OD492 of BCG-vaccinated control sera plus 6 SD) among responders to each antigen was calculated, it was observed that Rv1168c elicited high-level antibody responses in the majority (75.2%) of responders, compared to PPD (14%), Hsp60 (24%), and ESAT-6 (33.1%) (Fig. 2C). Thus, it appears that Rv1168c is more immunodominant and serologically more sensitive than PPD, Hsp60, and ESAT-6.
Rv1168c can be used to diagnose extrapulmonary TB in patients serologically.
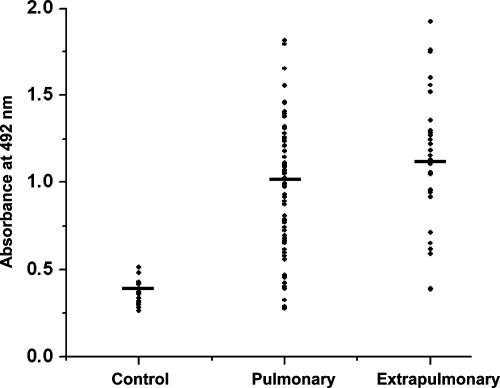
Since the recombinant Rv1168c protein was found to be seroreactive against most of the TB patients, we compared the antibody titers specific to Rv1168c in well-defined clinical categories, such as pulmonary and extrapulmonary cases. Due to limitations of the current array of diagnostic methods, diagnosis of extrapulmonary cases (since most are sputum negative) is more difficult than for pulmonary TB. Therefore, a diagnostic method with potential to identify patients with extrapulmonary TB would be highly valuable. We found that Rv1168c elicited stronger antibody responses in extrapulmonary TB cases, as well as in the pulmonary TB cases, than did the BCG-vaccinated controls (Fig. 3) (P < 0.0001 in both cases). The mean absorbance values for Rv1168c in control, pulmonary, and extrapulmonary groups were 0.373, 1.01, and 1.15, respectively (Fig. 3). As shown in Table 1, when the immunogenicities of Rv1168c versus ESAT-6, Hsp60, and PPD were compared, the mean reactivity of Rv1168c was significantly higher than those of ESAT-6 (P < 0.0001), Hsp60 (P < 0.0001), and PPD (P < 0.0001) in both pulmonary and extrapulmonary TB patient sera. When expressed as percentages of high-level responders showing antibody levels greater than or equal to cutoff values (mean OD492 of BCG-vaccinated control sera plus 6 SD), the majority of the pulmonary (73%) and extrapulmonary (81.3%) TB cases showed antibody levels greater than the cutoff value against Rv1168c antigen, whereas only 37.6 and 21.9% responders had higher levels against ESAT-6, 27.2 and 16% against Hsp60, and 14.3 and 12.5% against PPD, respectively (Table 1). As with extrapulmonary TB, diagnosis of smear-negative pulmonary TB cases is also tedious and difficult. We, therefore, checked whether anti-Rv1168c antibody titers were higher in the smear-negative pulmonary TB. Interestingly, we also found that Rv1168c was more sensitive than ESAT-6, Hsp60, and PPD in diagnosing the smear-negative pulmonary TB patients. It was found that in smear-negative TB patients (n = 24), serum samples from 75% of the patients had antibodies to Rv1168, whereas only 45.8% of patients had antibodies to ESAT-6, 25% of patients had antibodies to Hsp60, and 21% of patients had antibodies to PPD (Table 1). In the cohort of smear-positive TB patients (n = 53), 71.6% possessed Rv1168c-specific antibodies, 34% had ESAT-6-specific antibodies, 28.3% had Hsp60-specific antibodies, and 9.4% had PPD-specific antibodies (Table 1). These results indicate that Rv1168c can potentially be used in the diagnosis of all the categories of TB cases, including smear-negative pulmonary, smear-positive pulmonary, and extrapulmonary TB cases, with higher sensitivity than ESAT-6, Hsp60, or PPD.
FIG. 3.
Rv1168c is a better antigen to diagnose pulmonary as well as extrapulmonary TB cases. The EIA absorbance values at 492 nm shown in Fig. 2A were replotted to compare Rv1168c-specific immune responses of pulmonary and extrapulmonary TB patients with that of BCG-vaccinated controls. Statistical significance was determined with an analysis of variance.
TABLE 1.
Rv1168c can potentially be used to diagnose smear-positive and smear-negative pulmonary as well as extrapulmonary TB casesa
| Antigen | Total extrapulmonary TB cases (n = 32)
|
Total pulmonary TB cases (n = 77)
|
Smear-positive pulmonary TB cases (n = 53)
|
Smear-negative pulmonary TB cases (n = 24)
|
||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | % Responders | Mean ± SD | % Responders | Mean ± SD | % Responders | Mean ± SD | % Responders | |
| Rv1168c | 1.15 ± 0.38 | 81.3 | 1.01 ± 0.38 | 73.0 | 1.01 ± 0.36 | 71.6 | 1.03 ± 0.42 | 75.0 |
| ESAT-6 | 0.62 ± 0.22 | 21.9 | 0.61 ± 0.28 | 37.6 | 0.60 ± 0.26 | 34.0 | 0.64 ± 0.32 | 45.8 |
| Hsp60 | 0.52 ± 0.22 | 16.0 | 0.59 ± 0.23 | 27.2 | 0.61 ± 0.22 | 28.3 | 0.56 ± 0.24 | 25.0 |
| PPD | 0.41 ± 0.17 | 12.5 | 0.42 ± 0.19 | 14.3 | 0.40 ± 0.16 | 9.4 | 0.46 ± 0.23 | 21.0 |
The data from Fig. 2A were replotted to compare the antibody responses of the pulmonary and extrapulmonary TB patients against Rv1168c versus ESAT-6, Hsp60, and PPD. The percentage of responders showing absorbance values greater than or equal to the cutoff value (mean OD492 plus 6 SD, based on results with BCG-vaccinated control sera) was compared for pulmonary and extrapulmonary TB groups. The pulmonary TB cases were further categorized as smear positive and smear negative, and responders to Rv1168c were compared with those for ESAT-6, Hsp60, and PPD by calculating the percentage of individuals showing absorbance values greater than or equal to the mean OD492 plus 6 SD (obtained with BCG-vaccinated control sera).
The PPE antigen Rv1168c triggers a strong T-cell response in TB patients as opposed to BCG-vaccinated controls.
In vitro tests that measure IFN-γ production by whole blood cells have recently being used to diagnose active TB cases (14, 23). We observed that Rv1168c triggered stronger antibody responses of the IgG type in patients with active TB (Fig. 2 and 3). It is known that IgG isotype switching requires a direct interaction from T cells (14). Therefore, there is a possibility that Rv1168c can also induce stronger T-cell responses in TB patients. Therefore, we measured T-cell responses of TB patients (with pulmonary tuberculosis and extrapulmonary tuberculosis), as indicated by the amounts of cytokines secreted in vitro, and BCG-vaccinated controls. We found that IFN-γ levels in both pulmonary (mean, 287 pg/ml) and extrapulmonary (mean, 325 pg/ml) TB patients were elevated more than threefold compared with the levels (mean, 92 pg/ml) in BCG-vaccinated controls (Table 2) (P < 0.0001 in both cases). Similarly, IL-5 levels were also significantly elevated (Table 2) (P < 0.0001) in both pulmonary (mean, 147 pg/ml) and extrapulmonary (mean, 191 pg/ml) TB patients compared with the BCG-vaccinated controls (mean, 80 pg/ml). Also, it was observed that compared to PPD, Rv1168c was a potent T-cell antigen for diagnosing active TB cases (Table 2). These data indicate that T cells from TB patients respond dominantly against the mycobacterial Rv1168c protein and can distinguish TB patients from BCG-vaccinated controls.
TABLE 2.
Rv1168c mounts stronger T-cell responses in TB patients than in BCG-vaccinated controlsa
| Antigen | Controls
|
Pulmonary TB patients
|
Extrapulmonary TB patients
|
|||
|---|---|---|---|---|---|---|
| IFN-γ (pg/ml) | IL-5 (pg/ml) | IFN-γ (pg/ml) | IL-5 (pg/ml) | IFN-γ (pg/ml) | IL-5 (pg/ml) | |
| Rv1168c | 92 ± 24 | 80 ± 22 | 287 ± 70 | 147 ± 69 | 325 ± 125 | 191 ± 61 |
| PPD | 91 ± 17 | 78 ± 19 | 177 ± 52 | 81 ± 31 | 141 ± 51 | 106 ± 50 |
PBMCs collected from TB patients and BCG-vaccinated controls were stimulated in triplicate with 3 μg/ml of Rv1168c or 10 μg/ml PPD. After 4 days, levels of IFN-γ and IL-5 cytokines secreted in the culture supernatants were estimated by EIA.
DISCUSSION
There is a need to develop newer and more efficient diagnostic tests that can identify most of the TB cases with better specificity and sensitivity. Although a number of potential M. tuberculosis antigens have been studied extensively in the past, most of them are not suitable to distinguish between BCG-vaccinated controls and active TB populations with sufficient specificity due to their heterogeneous responses, especially from patients with various clinical grades of TB.
Predominant expression under conditions that mimic the in vivo environment (2, 31, 35), and expression restricted to the Mycobacterium complex, makes Rv1168c an attractive candidate for serodiagnosis to discriminate TB patients from BCG-vaccinated individuals. Therefore, in the present study we evaluated the immunological potential of Rv1168c antigen as a diagnostic marker in a cohort of clinically defined active TB patients and BCG-vaccinated controls. Our data demonstrate that compared to conventional diagnostic tests using PPD, recombinant Rv1168c is highly sensitive for distinguishing patients with active tuberculosis from BCG-vaccinated controls. In addition, recombinant Rv1168c was found to be more sensitive than ESAT-6 and Hsp60 (well-known immunodominant antigens of M. tuberculosis) for recognizing TB patients from BCG-vaccinated controls. Interestingly, although a homologue of Rv1168c is present in M. bovis, we found a negligible immunological response to this protein in BCG-vaccinated individuals, indicating that Rv1168c is probably highly expressed during the active pathogenesis of M. tuberculosis. Our results suggest that Rv1168c antigen should be considered an attractive candidate for development of new diagnostic tests that can identify people suffering from the active form of disease in regions endemic for TB.
Despite the initial clinical suspicion of TB, when a patient's sputum smear results are negative for acid-fast bacilli, the diagnosis of TB may not be made. Therefore, it is important to continue research for a rapid and reliable immunological test to diagnose the smear-negative TB cases (23, 37). The recent approaches using ESAT-6 and CFP-10 as diagnostic antigens are useful mostly as a means to diagnose either latent infection or sputum-positive pulmonary infection, and not much information is available regarding use of these antigens to diagnose smear-negative cases with higher sensitivity (14, 23, 37). We found that Rv1168c could diagnose extrapulmonary and smear-negative pulmonary TB cases with higher sensitivity than ESAT-6 or Hsp60 immunodominant antigen. A very high percentage of the serum samples obtained from the extrapulmonary and the smear-negative pulmonary TB patients had strong antibody reactivities against Rv1168c protein compared to ESAT-6, Hsp60, and PPD, indicating that Rv1168c can be used to potentially diagnose these categories of TB patients with higher sensitivity and can discriminate smear-negative pulmonary as well as extrapulmonary TB patients from the BCG-vaccinated controls. Thus, our findings are particularly significant in the context of smear-negative pulmonary and extrapulmonary TB cases, which often go undiagnosed with conventional diagnostic methods (4, 8).
Also, it is well-established that the generation of substantive antibody responses to a protein antigen is dependent on the presence of T-cell epitopes recognized by helper T cells (14). We found that Rv1168c was also a potent T-cell antigen, eliciting higher levels of IFN-γ in PBMCs obtained from TB patients in contrast to those obtained from BCG-immunized controls. Thus, Rv1168c is also a dominant T-cell antigen recognized by most of the TB patients, and this reflects that Rv1168c possibly plays an important role in certain stages of mycobacterial infection and intracellular survival.
Recently, a few PPE proteins have been studied for their suitability for use in serological diagnosis of TB patients. Interestingly, we found that Rv1168c is more potent in detecting both pulmonary and extrapulmonary TB cases than some of the earlier-studied PPE proteins, viz., Rv3425 (40), Rv2608 (9), and Rv2430 (10), in enzyme immunoassays and shows comparable immunogenicity to only Rv3872 (28). In our studies, we have used a more stringent cutoff (OD plus 6 SD) to discriminate patient sera from BCG-vaccinated control sera, and still a significantly higher percentage of TB patients (both pulmonary and extrapulmonary cases) could be diagnosed by Rv1168c compared to the above-mentioned candidate PPE proteins, for which the calculations were done using a less stringent cutoff value (OD plus 3 SD). This suggests that the Rv1168c protein is practically more sensitive for distinguishing patient sera from the BCG-immunized control sera. Interestingly, Singh et al. (36) have shown that the presence of anti-PPE55 antibodies can serve to distinguish between latent TB and incipient, subclinical TB. Though we have not evaluated the ability of Rv1168c to differentiate such cases, none of the previous studies reported the ability of PPE proteins to be used in diagnosis of smear-negative TB cases, which are difficult to diagnose with the available diagnostic methods. However, we have observed that Rv1168c can be used to diagnose almost 75% of the smear-negative cases effectively. Nonetheless, our data also suggest that Rv1168c is a potent T-cell antigen which elicits a strong IFN-γ response in sensitized PBMCs obtained from TB patients. It will be interesting to compare the IFN-γ responses from other PPE antigens with that to Rv1168c.
Acknowledgments
N.K., K.A., and S.N. were supported by fellowships from the Council of Scientific and Industrial Research, New Delhi, India. This work was supported by grants from the Indian Council of Medical Research, Government of India (48/13/2001-BMS), Department of Biotechnology, Government of India (BT/PR7890/MED/14/1171/2006), and a core grant to CDFD by the Department of Biotechnology, India.
We thank Sudip Ghosh, NIN Hyderabad, for kindly reviewing the manuscript and S. Ghousunnissa for technical assistance.
Footnotes
Published ahead of print on 9 April 2008.
REFERENCES
- 1.Abdallah, A. M., T. Verboom, F. Hannes, M. Safi, M. Strong, D. Eisenberg, R. J. Musters, C. M. Vandenbroucke-Grauls, B. J. Appelmelk, J. Luirink, and W. Bitter. 2006. A specific secretion system mediates PPE41 transport in pathogenic mycobacteria. Mol. Microbiol. 62:667-679. [DOI] [PubMed] [Google Scholar]
- 2.Bacon, J., B. W. James, L. Wernisch, A. Williams, K. A. Morley, G. J. Hatch, J. A. Mangan, J. Hinds, N. G. Stoker, P. D. Butcher, and P. D. Marsh. 2004. The influence of reduced oxygen availability on pathogenicity and gene expression in Mycobacterium tuberculosis. Tuberculosis 84:205-217. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee, S., A. Nandyala, R. Podili, V. M. Katoch, K. J. Murthy, and S. E. Hasnain. 2004. Mycobacterium tuberculosis (Mtb) isocitrate dehydrogenases show strong B cell response and distinguish vaccinated controls from TB patients. Proc. Natl. Acad. Sci. USA 101:12652-12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes, P. F. 1997. Rapid diagnostic tests for tuberculosis: progress but no gold standard. Am. J. Respir. Crit. Care Med. 155:1497-1498. [DOI] [PubMed] [Google Scholar]
- 5.Betts, J. C., P. T. Lukey, L. C. Robb, R. A. McAdam, and K. Duncan. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 43:717-731. [DOI] [PubMed] [Google Scholar]
- 6.Bock, N. N., J. E. McGowan, Jr., J. Ahn, J. Tapia, and H. M. Blumberg. 1996. Clinical predictors of tuberculosis as a guide for a respiratory isolation policy. Am. J. Respir. Crit. Care Med. 154:1468-1472. [DOI] [PubMed] [Google Scholar]
- 7.Brock, I., M. E. Munk, A. Kok-Jensen, and P. Andersen. 2001. Performance of whole blood IFN-gamma test for tuberculosis diagnosis based on PPD or the specific antigens ESAT-6 and CFP-10. Int. J. Tuberc. Lung Dis. 5:462-467. [PubMed] [Google Scholar]
- 8.Brugiere, O., M. Vokurka, D. Lecossier, G. Mangiapan, A. Amrane, B. Milleron, C. Mayaud, J. Cadranel, and A. J. Hance. 1997. Diagnosis of smear-negative pulmonary tuberculosis using sequence capture polymerase chain reaction. Am. J. Respir. Crit. Care Med. 155:1478-1481. [DOI] [PubMed] [Google Scholar]
- 9.Chakhaiyar, P., Y. Nagalakshmi, B. Aruna, K. J. Murthy, V. M. Katoch, and S. E. Hasnain. 2004. Regions of high antigenicity within the hypothetical PPE major polymorphic tandem repeat open-reading frame, Rv2608, show a differential humoral response and a low T cell response in various categories of patients with tuberculosis. J. Infect. Dis. 190:1237-1244. [DOI] [PubMed] [Google Scholar]
- 10.Choudhary, R. K., S. Mukhopadhyay, P. Chakhaiyar, N. Sharma, K. J. Murthy, V. M. Katoch, and S. E. Hasnain. 2003. PPE antigen Rv2430c of Mycobacterium tuberculosis induces a strong B-cell response. Infect. Immun. 71:6338-6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole, S. T. 2002. Comparative and functional genomics of the Mycobacterium tuberculosis complex. Microbiology 148:2919-2928. [DOI] [PubMed] [Google Scholar]
- 12.Demangel, C., P. Brodin, P. J. Cockle, R. Brosch, L. Majlessi, C. Leclerc, and S. T. Cole. 2004. Cell envelope protein PPE68 contributes to Mycobacterium tuberculosis RD1 immunogenicity independently of a 10-kilodalton culture filtrate protein and ESAT-6. Infect. Immun. 72:2170-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devi, K. R., K. S. Kumar, B. Ramalingam, and R. Alamelu. 2002. Purification and characterization of three immunodominant proteins (38, 30, and 16 kDa) of Mycobacterium tuberculosis. Protein Expr. Purif. 24:188-195. [DOI] [PubMed] [Google Scholar]
- 14.Dillon, D. C., M. R. Alderson, C. H. Day, T. Bement, A. Campos-Neto, Y. A. Skeiky, T. Vedvick, R. Badaro, S. G. Reed, and R. Houghton. 2000. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J. Clin. Microbiol. 38:3285-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dillon, D. C., M. R. Alderson, C. H. Day, D. M. Lewinsohn, R. Coler, T. Bement, A. Campos-Neto, Y. A. Skeiky, I. M. Orme, A. Roberts, S. Steen, W. Dalemans, R. Badaro, and S. G. Reed. 1999. Molecular characterization and human T-cell responses to a member of a novel Mycobacterium tuberculosis mtb39 gene family. Infect. Immun. 67:2941-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dye, C., M. A. Espinal, C. J. Watt, C. Mbiaga, and B. G. Williams. 2002. Worldwide incidence of multidrug-resistant tuberculosis. J. Infect. Dis. 185:1197-1202. [DOI] [PubMed] [Google Scholar]
- 17.Edwards, L. B., F. A. Acquaviva, V. T. Livesay, F. W. Cross, and C. E. Palmer. 1969. An atlas of sensitivity to tuberculin, PPD-B, and histoplasmin in the United States. Am. Rev. Respir. Dis. 99:1-132. [PubMed] [Google Scholar]
- 18.Friedland, G. 2007. Tuberculosis, drug resistance, and HIV/AIDS: a triple threat. Curr. Infect. Dis. Rep. 9:252-261. [DOI] [PubMed] [Google Scholar]
- 19.Gey van Pittius, N. C., S. L. Sampson, H. Lee, Y. Kim, P. D. van Helden, and R. M. Warren. 2006. Evolution and expansion of the Mycobacterium tuberculosis PE and PPE multigene families and their association with the duplication of the ESAT-6 (esx) gene cluster regions. BMC Evol. Biol. 6:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harboe, M. 1981. Antigens of PPD, old tuberculin, and autoclaved Mycobacterium bovis BCG studied by crossed immunoelectrophoresis. Am. Rev. Respir. Dis. 124:80-87. [DOI] [PubMed] [Google Scholar]
- 21.Houghton, R. L., M. J. Lodes, D. C. Dillon, L. D. Reynolds, C. H. Day, P. D. McNeill, R. C. Hendrickson, Y. A. Skeiky, D. P. Sampaio, R. Badaro, K. P. Lyashchenko, and S. G. Reed. 2002. Use of multiepitope polyproteins in serodiagnosis of active tuberculosis. Clin. Diagn. Lab. Immunol. 9:883-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huebner, R. E., M. F. Schein, Jr. J. B. Bass, R. E. Huebner, M. F. Schein, and Jr. J. B. Bass. 1993. The tuberculin skin test. Clin. Infect. Dis. 17:968-975. [DOI] [PubMed] [Google Scholar]
- 23.Jafari, C., M. Ernst, B. Kalsdorf, U. Greinert, R. Diel, D. Kirsten, K. Marienfeld, A. Lalvani, and C. Lange. 2006. Rapid diagnosis of smear-negative tuberculosis by bronchoalveolar lavage enzyme-linked immunospot. Am. J. Respir. Crit. Care Med. 174:1048-1054. [DOI] [PubMed] [Google Scholar]
- 24.Khan, N., S. S. Rahim, C. S. Boddupalli, S. Ghousunnissa, S. Padma, N. Pathak, D. Thiagarajan, S. E. Hasnain, and S. Mukhopadhyay. 2006. Hydrogen peroxide inhibits IL-12 p40 induction in macrophages by inhibiting c-rel translocation to the nucleus through activation of calmodulin protein. Blood 107:1513-1520. [DOI] [PubMed] [Google Scholar]
- 25.Lalvani, A., A. A. Pathan, H. McShane, R. J. Wilkinson, M. Latif, C. P. Conlon, G. Pasvol, and A. V. Hill. 2001. Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Am. J. Respir. Crit. Care Med. 163:824-828. [DOI] [PubMed] [Google Scholar]
- 26.Ljungqvist, L., A. B. Andersen, P. Andersen, K. Hasløv, A. Worsaae, J. Bennedsen, and I. Heron. 1990. Affinity purification, biological characterization and serological evaluation of defined antigens from Mycobacterium tuberculosis. Trop. Med. Parasitol. 41:333-335. [PubMed] [Google Scholar]
- 27.Lyashchenko, K., R. Colangeli, M. Houde, H. Al Jahdali, D. Menzies, and M. L. Gennaro. 1998. Heterogeneous antibody responses in tuberculosis. Infect. Immun. 66:3936-3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukherjee, P., M. Dutta, P. Datta, R. Pradhan, M. Pradhan, M. Kund, J. Basu, and P. Chakrabarti. 2007. The RD1-encoded antigen Rv3872 of Mycobacterium tuberculosis as a potential candidate for serodiagnosis of tuberculosis. Clin. Microbiol. Infect. 13:146-152. [DOI] [PubMed] [Google Scholar]
- 29.Murphy, G. D., R. R. Rydman, D. Batesky, and S. Hill. 1995. Clinical parameters that predict culture-positive pulmonary tuberculosis in the emergency department. Ann. Emerg. Med. 25:137. [Google Scholar]
- 30.Mustafa, A. S. 2002. Development of new vaccines and diagnostic reagents against tuberculosis. Mol. Immunol. 39:113-119. [DOI] [PubMed] [Google Scholar]
- 31.Muttucumaru, D. G., G. Roberts, J. Hinds, R. A. Stabler, and T. Parish. 2004. Gene expression profile of Mycobacterium tuberculosis in a non-replicating state. Tuberculosis (Edinburgh) 84:239-246. [DOI] [PubMed] [Google Scholar]
- 32.Okkels, L. M., I. Brock, F. Follmann, E. M. Agger, S. M. Arend, T. H. Ottenhoff, F. Oftung, I. Rosenkrands, and P. Andersen. 2003. PPE protein (Rv3873) from DNA segment RD1 of Mycobacterium tuberculosis: strong recognition of both specific T-cell epitopes and epitopes conserved within the PPE family. Infect. Immun. 71:6116-6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perkins, M. D. 2000. New diagnostic tools for tuberculosis. Int. J. Tuberc. Lung Dis. 4:S182-S188. [PubMed] [Google Scholar]
- 34.Qamra, R., V. Srinivas, and S. C. Mande. 2004. Mycobacterium tuberculosis GroEL homologues unusually exist as lower oligomers and retain the ability to suppress aggregation of substrate proteins. J. Mol. Biol. 342:605-617. [DOI] [PubMed] [Google Scholar]
- 35.Schnappinger, D., S. Ehrt, M. I. Voskuil, Y. Liu, J. A. Mangan, I. M. Monahan, G. Dolganov, B. Efron, P. D. Butcher, C. Nathan, and G. K. Schoolnik. 2003. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J. Exp. Med. 198:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh, K. K., Y. Dong, S. A. Patibandla, D. N. McMurray, V. K. Arora, and S. Laal. 2005. Immunogenicity of the Mycobacterium tuberculosis PPE55 (Rv3347c) protein during incipient and clinical tuberculosis. Infect. Immun. 73:5004-5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Streitz, M., L. Tesfa, V. Yildirim, A. Yahyazadeh, T. Ulrichs, R. Lenkei, A. Quassem, G. Liebetrau, L. Nomura, H. Maecker, H. D. Volk, and F. Kern. 2007. Loss of receptor on tuberculin-reactive T-cells marks active pulmonary tuberculosis. PLoS One 2:e735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tattevin, P., E. Casalino, L. Fleury, G. Egmann, M. Ruel, and E. Bouvet. 1999. The validity of medical history, classic symptoms, and chest radiographs in predicting pulmonary tuberculosis: derivation of a pulmonary tuberculosis prediction model. Chest 115:1248-1253. [DOI] [PubMed] [Google Scholar]
- 39.WHO. 2005. Global tuberculosis control. Surveillance, planning, financing. WHO, Geneva, Switzerland.
- 40.Zhang, H., J. Wang, J. Lei, M. Zhang, Y. Yang, Y. Chen, and H. Wang. 2007. PPE protein (Rv3425) from DNA segment RD11 of Mycobacterium tuberculosis: a potential B-cell antigen used for serological diagnosis to distinguish vaccinated controls from tuberculosis patients. Clin. Microbiol. Infect. 13:139-145. [DOI] [PubMed] [Google Scholar]