Abstract
Microscopy, the gold standard for the detection and quantification of malaria parasites in blood, is in many aspects deficient for this purpose. The method is poorly reproducible and can be inaccurate because Plasmodium falciparum parasites sequester for a portion of each asexual cycle. Due to these deficiencies, biomarkers such as P. falciparum histidine-rich protein 2 (PfHRP2) are increasingly being used. In this study, we evaluated the use of a commercial PfHRP2 enzyme-linked immunosorbent assay (ELISA) kit with some procedural modifications. We determined the linear range of the assay, including the lower limits of detection and quantitation, using recombinant PfHRP2 (rPfHRP2). In 10 repeat experiments, the linear range of optical densities (ODs) at 450 to 650 nm was from 0.05 ± 0.002 to 2.28 ± 0.042, corresponding to 3.91 to 250 ng/ml of rPfHRP2. The coefficient of variation (CV) at each target concentration ranged from 1.93 to 8.07%. Using cultured parasites, we confirmed the linear range of ODs as well as the association between the PfHRP2 ELISA results and the microscopic parasite densities. For whole-blood samples spiked with cultured, washed, ring-stage-infected red blood cells (iRBCs), the linear range was 11.7 to 750 iRBCs/μl, with CVs of 0.29 to 7.56%. The same spiked samples evaluated by microscopists had similar sensitivities, but the CVs were unacceptably high (20.7 to 161.6%). Stock rPfHRP2 was stable through four freeze-thaw cycles (P < 0.05; paired t test). When different patient sample types at different concentrations within the linear range of the assay are compared, the recoveries of PfHRP2 from blood and serum were within ±20%, whereas the recoveries from plasma ranged between +35 and −41%. We conclude that PfHRP2 ELISA using whole-blood and serum samples is a suitable adjunct to microscopy and could ultimately benefit malaria intervention trials.
Efficacy assessment of malaria intervention studies still relies on microscopy for quantitation of malaria parasites in blood, despite increasing evidence that its reliability is questionable (1, 10, 18, 21, 22). The major attributes of malaria parasite microscopy are its cost effectiveness and simplicity, which in resource-poor countries are important considerations. The major disadvantages of microscopy for Plasmodium falciparum parasite quantitation include poor reproducibility, variable sensitivity, and unacceptably high false-positive rates. In addition, the sequestration of parasites for a portion of each asexual cycle makes mature trophozoite and schizont stages unavailable in the peripheral circulation (9).
The parasite biomarkers of choice for quantitative estimates of the burden of infection would be those that are detectable in whole blood or in its separated components, i.e., serum and plasma, irrespective of the location of the parasite. Good candidates are histidine-rich protein 2 (HRP2), found only in P. falciparum, and glycolytic lactate dehydrogenase (LDH) and Plasmodium aldolase, both of which are found in all Plasmodium species (19). Available evidence indicates that P. falciparum HRP2 (PfHRP2)-based assays are more sensitive for the detection of P. falciparum than LDH- and aldolase-detecting tests (13). In addition, PfHRP2 has been proven to be useful in detecting the presence of parasites in cases of placental malaria (16).
PfHRP2 is a histidine- and alanine-rich protein with repetitive epitopes that is synthesized by both the asexual and early sexual stages of the parasite and, thereafter, is exported through the erythrocyte cytoplasm and the surface membrane to accumulate in the extracellular plasma (12, 25). Although the amount of PfHRP2 released continues to increase throughout the erythrocytic cycle, most of it is released during schizont rupture (6, 12). In in vitro assays, the antigen can be detected in culture supernatants of synchronized parasites as early as 2 to 8 hours after ring development (12). PfHRP2 has a long half-life and persists in the circulation for up to 3 weeks, even after successful treatment (17). While the long half-life may reduce its utility for the diagnosis of an active infection, in a clinical trial setting, the persistence of HRP2 could serve as an indicator of the magnitude of current or recent infection.
Previous studies have shown that PfHRP2 is present in the plasma of persons who are infected with P. falciparum (7, 23), is produced by all natural strains and isolates of P. falciparum tested (25), and in spite of some polymorphism, is apparently substantially antigenically invariant (30). An additional attribute of PfHRP2 is that the antigen contains multiple B-cell epitopes that are arranged in tandem repeats of AHHAAD interspersed with AHH and AHHAA (26) that allow easy detection by an antigen capture assay (26, 29).
This paper describes the partial characterization of a modified commercial PfHRP2 enzyme-linked immunosorbent assay (ELISA) that could be useful in evaluating the efficacy of interventional antimalaria products.
MATERIALS AND METHODS
Cultured parasites.
A laboratory strain of P. falciparum, NF54, was cultured in normal O-positive red blood cells (RBCs) from healthy donors and maintained in vitro using continuous-culture conditions (27). Parasites at an initial parasitemia of 2% were enriched for ring stages by using d-sorbitol (15). Briefly, 6 ml of culture at 2% parasitemia of mainly young rings (10 to 12 h postinvasion) was spun at 600 × g, and the pellet was resuspended in 6 ml 5% d-sorbitol. After 10 min of incubation at room temperature, the cells were washed twice in RPMI 1640 medium (pH 7.2) containing 25 mM HEPES and 0.2% sodium bicarbonate (all from Sigma-Aldrich Corporation, St. Louis, MO), diluted to 5% hematocrit, and cultured as described above. This treatment was repeated every 48 h until >98% of the parasites were synchronized in the ring stage, as confirmed by microscopy.
Patient blood samples.
Whole-blood, serum, and plasma samples were obtained from patients in a case-controlled severe-malaria study at Kisumu District Hospital under approved protocol 1145. Scientific and ethical approval for the use of these patient samples was obtained from the Ethical Review Committee of the Kenya Medical Research Institute, Nairobi, Kenya, and the Walter Reed Army Research Institute of Human Use Research Committee, Silver Spring, MD.
Negative samples.
Aliquots of 42 whole-blood and 43 serum samples, devoid of any patient-identifying information, that were malaria negative were obtained from the regional blood transfusion center in the Nyanza province of Kenya. The blood bank uses microscopy examination of Giemsa-stained blood smears to screen the donors and thereby preclude retention of infected samples. Samples were additionally determined to be negative for PfHRP2 by a separate PfHRP2-detecting rapid diagnostic (dipstick) test (Cellabs Pty. Ltd., Brookvale, New South Wales, Australia).
Assay procedure and linear range of the HRP2 ELISA.
A commercial PfHRP2 ELISA kit (Malaria Ag CELISA; Cellabs Pty. Ltd., Brookvale, New South Wales, Australia) was used with modifications (H. G. Rajasekariah, personal communication). The kit uses two different monoclonal antibodies that target different epitopes of the PfHRP2 antigen. The plates are supplied precoated with anti-P. falciparum immunoglobulin M monoclonal capture antibody. We deviated from the kit instructions by first mixing the enzyme-secondary-antibody conjugate (immunoglobulin G) and the test sample 1:1 by volume (100 μl each) and incubating this mixture at room temperature for 15 min before transferring it to the coated plates. Pilot studies had indicated that preincubation of the sample and the secondary antibody reduced the incubation times and wash steps without affecting the readout (H. G. Rajasekariah, personal communication). For generation of the recombinant PfHRP2 (rPfHRP2) standard curve, the recombinant antigen (designated clone HB3) was serially diluted in 100 μl of 0.1 M phosphate-buffered saline (PBS) containing 0.01% Tween 20 (PBS/T), in duplicate, starting from 1 μg/ml. The stock 10-μg/ml standard is stored at −80°C (see below). The secondary antibody (100 μl) conjugated to horseradish peroxidase was then added, and the mixture was incubated at room temperature for 15 min. The mixture was then transferred to the precoated plates and incubated at 37°C for 1 h. The plate wells were washed eight times with the PBS/T supplied in the kit. Finally, 100 μl of the tetramethyl benzidine substrate supplied with the kit was then added, and the mixture was incubated for 30 min at room temperature in the dark. The reaction was stopped by adding 50 μl of 2 M sulfuric acid, and the results were read spectrophotometrically at optical densities (ODs) at 450 to 650 nm using the SoftMax Pro 4.8 microplate software and reader (Molecular Devices, Sunnyvale, CA). The performance of the assay took 2 h. Ten replicate assays, performed over a period of 3 weeks, were used to generate standard curves. The mean, standard deviation (SD), and coefficient of variation (CV) for each HRP2 concentration were calculated from all 10 replicate assays. A line was drawn for best fit, using the single-line option in the nonlinear analysis package in the GraphPad Prism 5.0 software. The limit of detection (LOD) was taken as the lowest concentration at which the mean OD is just distinguishable from the background. The lower limit of quantitation (LOQ) was calculated as the point at which the response is no longer linear. For confirmation that our LOD and lower LOQ were biologically relevant, background values were derived by analyses of a series of negative blood and serum samples from the same geographic area as that of the patient samples. The samples were analyzed in duplicate for the presence of PfHRP2 in five separate assays over a 3-week period at a dilution of 1:2.
Stability of the rPfHRP2 antigen.
The rPfHPR2 antigen (designated HB3) lacks the secretory leader sequence and has a predicted molecular weight of 25,000. Details of its nucleotide and amino acid sequences can be found in the GenBank database (accession number EF535881). Since recombinant antigens may be sensitive to multiple freeze-thaw cycles, thus affecting the reproducibility of the standard curve, we evaluated the stability of 10-μg/ml aliquots after up to 10 freeze-thaw cycles. Aliquots were frozen at −80°C and allowed to thaw to room temperature (25°C) for 30 min for each cycle, followed by simultaneous assay of all 10 aliquots.
Comparison of parasite density determinations by microscopy and by PfHRP2 ELISA.
Cultured synchronized ring stages were chosen for use in these studies involving only RBCs in order to emulate the situation in infected human blood samples, where mature forms are rarely present due to sequestration. At 2 to 3% ring parasitemia, as determined on thin blood film by microscopy, the total number of RBCs in the cultures was counted by Coulter analysis (Coulter AC·T 5diff CP; Beckman Coulter, Inc., Miami, FL). The lack of white blood cells in the cultures precluded the use of thick films. The malaria culture was then washed to remove secreted PfHRP2. The washed cells were resuspended at a concentration of 1,500 ring-stage-infected RBCs (iRBCs)/μl (approximately 5 × 105 total RBCs/μl) and then carefully diluted serially in uninfected whole blood (at approximately 4 × 106 to 5 × 106 RBCs/μl) to <1 iRBC/μl. Thin blood films for microscopy were made from each dilution after careful suspension. As the original parasitemia measurement was made from thin films, all subsequent parasitemia measurements were made from thin films. The microscopists first scanned 100 high-power fields and thereafter determined the number of iRBCs out of a total of 5,000 RBCs. The slides were read by two experienced microscopists in a blind study according to published methodology (21), and the percent CVs were calculated from the mean and SD at each target concentration. The rest of the samples were frozen at −80°C to lyse the cells and release the total sample PfHRP2. An equivalent volume of the uninfected blood was frozen for use as a negative control. For the assay, the frozen samples were thawed at room temperature, and duplicate samples (50 μl) of the hemolysates were added to tubes containing 50 μl of PBS/T. Conjugated secondary antibody (100 μl) was then added and the assay continued as described in the assay procedure. The entire assay, including the synchronization of cultures and the preparation of the lysates, was repeated five times over a period of 1 week.
Assay linearity in different patient sample types.
Three patients' samples were chosen for evaluation because initial screening demonstrated that each one contained either a low (33.0 ng/ml), a middle (124.0 ng/ml) or a high (269.7 ng/ml) level of PfHRP2. For each of these three patients, whole-blood (freeze-thawed to lyse), serum, and plasma samples were evaluated. To ensure that each of the samples gave a signal across several points within the previously determined linear range of the assay (ODs of 0.05 to 2.28); they were initially diluted at 1:5 (low sample), 1:500 (medium sample), or 1:1,000 (high sample) in PBS/T. These diluted samples were then further serially diluted at 1:2, 1:4, and 1:8 in PBS/T. Analyte recovery (14) was assessed by comparing observed values at different serial dilutions against the values obtained from the initial dilution.
RESULTS
Linear range of the standard curve.
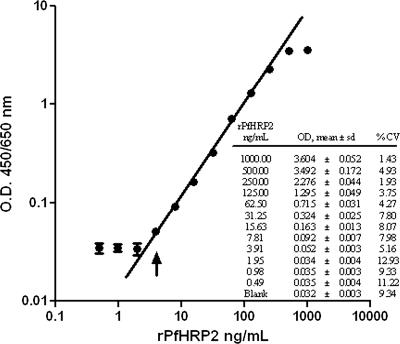
We first assessed the working linear range of the assay using various concentrations of rPfHRP2 in PBS/T. A best-fit linear calibration curve, based on the mean absorbance derived from 10 assays run independently on 10 different days, is shown in Fig. 1. The dose response is linear up to an OD of 2.28, which corresponds to 250 ng/ml rPfHRP2. Beyond this upper LOQ, the response is nonlinear. The lower LOD for this assay was at an OD of 0.052 (3.91 ng/ml rPfHRP2) (Fig. 1, inset), below which the OD values are not distinguishable from the blank. The background ODs for more than 40 negative-control whole-blood and serum samples, assayed in duplicate on five separate days, averaged 0.042 (SD, 0.006) and 0.023 (SD, 0.002), respectively. A reasonable negative biological threshold for the assay would be the average background level plus 3 SDs, which produces an OD of 0.060 for whole-blood samples and of 0.030 for serum samples. The lower LOQ was also 3.91 ng/ml. The CVs within the working linear range (Fig. 1, inset) ranged between 1.93 and 8.07%, indicating an acceptable level of variability in individual measurements. The biological and assay lower thresholds are thus very similar, and we have chosen to use an OD of 0.060 as a threshold for both accurate detection and quantitation. Therefore, OD values below 0.060 for serum or whole-blood samples are considered negative, OD values between the lower LOQ and upper LOQ can be used to extrapolate PfHRP2 concentrations from the standard curve, and OD values above the upper LOQ are meaningless. Samples with values higher than the upper LOQ must be further diluted in order to be measured within the linear range of the assay.
FIG. 1.
Log versus log scale plot of a standard curve for results from 10 independent assay repetitions using rPfHRP2 in PBS/T. The dose response is linear for concentrations up to 250 ng/ml rPfHRP2, which corresponds to an OD of 2.28, above which the dose response becomes nonlinear. The lower LOD, i.e., the point at which the analysis becomes just feasible, is 3.91 ng/ml (arrow). The curve fitted to a true line without any data transformation.
Linearity of washed iRBCs.
Using cultured and washed iRBCs diluted in uninfected whole blood (from a blood bank), we also determined the linear relationship between PfHRP2 ODs and the numbers of iRBCs. A best-fit linear calibration curve, based on the mean absorbances from iRBCs at different concentrations, measured in five separate assays on five different days, is shown in Fig. 2. The upper range of the dose response remained linear up to an OD of 1.97 or 750 iRBCs/μl whole blood. The lower LOD for this assay, defined as the OD at which the signal could not be distinguished from the background, was 0.053, or 5.86 iRBCs/μl (Fig. 2, inset table). However, the curve was linear to the value corresponding to 11.7 parasites/μl (OD, 0.065), so this is the lower LOQ (Fig. 2). Within the linear range, the CV at each target concentration tested was <10% (Fig. 2, inset table). To calculate the quantities of PfHRP2 in spiked samples, ODs obtained within the linear range were extrapolated from those for rPfHRP2 (Fig. 2, inset graph). The results are linear across the range of the assay and correspond to an average value of 1.012 fg PfHRP2 per parasite, which is within the range of previously published reports (6).
FIG. 2.
Relationship of ODs to numbers of parasites plotted on a log versus log scale of results from five assay repetitions using washed iRBCs diluted in uninfected whole blood. The upper LOQ measured is 750 iRBCs/μl, which corresponds to an OD of 1.97. The lower LOD is 5.86 iRBCs/μl (inset table, horizontal arrow), while the lower LOQ, i.e., the point at which the analysis becomes linear and remains reproducible, is 11.7 iRBCs/μl (vertical arrow). The inset graph shows the quantities of PfHRP2 in spiked samples.
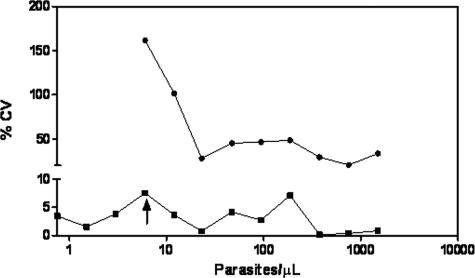
Comparison of parasite densities by PfHRP2 ELISA and microscopy.
The performance of microscopy was evaluated against that of the PfHRP2 ELISA using the same spiked and washed iRBCs. The percent CVs for repeat measures of densities ranging from 1 to 1,000 parasites per microliter for thin-film microscopy and PfHRP2 ELISA are shown in Fig. 3. Although the two assays had similar LODs (5.8 iRBCs/μl), the reproducibility of microscopy was exceedingly poor (CVs, 20.7 to 161.6%) compared to that of the PfHRP2 ELISA (CVs, <10%). A Bland-Altman analysis (4) of the parasite counts predicted by dilution versus the microscopy results (data not graphed) showed a very high bias of 270. In almost all cases, microscopy yielded much higher parasite counts than those predicted by dilution, even in the hands of expert microscopists and as previously defined (21).
FIG. 3.
CVs of results for microscopy (•) and PfHRP2 ELISA (▪) as a function of parasite density plotted on a log scale. The LODs for both assays were similar (5.8 iRBCs/μl; arrow), but the reproducibility for microscopy results (CVs, 20.7 to 161.6%) was poor compared to that for PfHRP2 ELISA results (CVs, <10%).
Stability of antigen.
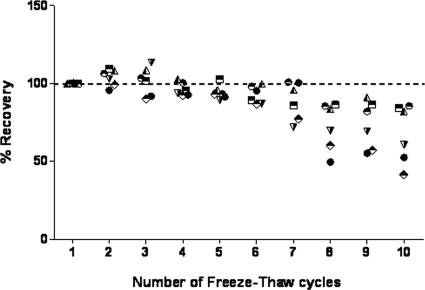
We examined the effect of subjecting the rPfHRP2 antigen to repeated freeze-thaw cycles on the assay reproducibility. Figure 4 shows the recovery of a 10-μg/ml sample of rPfHRP2 antigen, diluted in PBS/T after a series of 10 freeze-thaw cycles, expressed as a percentage of the recovery from the first cycle (Fig. 4). The antigen recovery was significantly reduced from the fifth freeze-thaw cycle onwards (P ≤ 0.05; paired t test). Diluted-antigen recovery below 62 ng/ml fell rapidly after the fifth cycle.
FIG. 4.
Effect of multiple freeze-thaw cycles on a 10-μg/ml stock of rPfHRP2 antigen. The dashed line represents 100% concentration at cycle 1. The scatter plots represent percent recoveries at target concentrations between 7.8 and 250 ng/ml. The percent recoveries were significantly different from the fifth freeze-thaw cycle onward (P ≤ 0.05; paired t test). •, 7.8;  , 15.1; ◭, 31.3; ⬘, 62.5; ⬒, 125.0; ◓, 250.0.
, 15.1; ◭, 31.3; ⬘, 62.5; ⬒, 125.0; ◓, 250.0.
Linearity of results for patient samples.
Whole-blood, serum, and plasma samples were each collected from three patients. The patients were selected because initial evaluations of their whole-blood samples had revealed them to have low (33.0 ng/ml), medium (124.0 ng/ml), or high (269.7 ng/ml) levels of PfHRP2. In order to ensure multiple data points within the previously established linear range of the assay, these samples were first diluted 1:5, 1:500, and 1:1,000 in PBS/T. The diluted samples were then further serially diluted from 1:2 to 1:8 and tested in the assay. We used the value at the highest concentration to generate expected values for comparison with results at subsequent serial dilutions, and we expressed this as “percent recovery.” Within the linear range, between the lower LOQ (3.91 ng/ml) and the upper LOQ (250 ng/ml), percent analyte recovery was within an acceptable ±20% range for whole-blood and serum samples (Fig. 5). Plasma samples, however, had poor reproducibility (CV, +35 to −41%) at concentrations of ≤25 ng/ml PfHRP2. It is not clear why the matrix effect is more pronounced in plasma samples than in the other sample types, but it is clear that the lower LOQ is considerably higher for this sample type.
FIG. 5.
Percent recovery of PfHRP2 in diluted patient samples. Sample types include whole blood (✖), plasma ( ), and serum (▪), assessed at different dilutions, and were taken from patients with low, medium, and high levels of PfHRP2. Analyte recovery was determined by comparing observed values with expected values. The precision of the assay was within the acceptable ±20% range (area between the dashed lines marked “a” and “b”) between the lower LOQ (3.91 ng/ml; arrow) and the upper LOQ (250 ng/ml) for serum and whole-blood samples. Plasma samples had higher CVs (+35 to −41%) at PfHRP2 concentrations of ≤25 ng/ml.
DISCUSSION
The use of malaria antigens for the estimation of parasite biomass (9), in rapid diagnostic tests (19, 24), and for evaluating in vitro drug (2, 20) and antibody levels (8) has been reported previously. Available evidence indicates that PfHRP2-based assays are more sensitive for the detection of P. falciparum than are LDH- and aldolase-detecting tests (11, 13). A potential advantage of PfHRP2-based detection methods for evaluating the intensity of infection in intervention trials is that PfHRP2 accumulation in the peripheral blood could be predicted to be an independent measure of the current and recent numbers of parasites, regardless of their location in the body (7).
In this study, the performance of a PfHRP2 ELISA for assessing antigen load was evaluated using a recombinant antigen and spiked iRBCs (Fig. 1 and 2) and whole-blood, serum, and plasma samples from patients. With the recombinant antigen diluted in PBS/T, the assay has a working linear range of 3.91 to 250 ng/ml, corresponding to an OD range of 0.05 to 2.28. In experiments in which iRBCs were diluted in whole blood, the working linear range was determined to be between ODs of 0.065 and 1.97, or about 11.7 to 750 iRBCs/μl, or about 6 to 200 ng/ml PfHRP2. The lower LODs for both PfHRP2 and iRBCs were comparable to those reported previously (9, 16) and to those reported for routine microscopy (21). We are of the opinion that the relative PfHRP2 concentrations from different trial cohorts could prove to be a quantitative means for reporting outcomes from clinical intervention trials.
In parasites cultured in vitro, the production of PfHRP2 is directly related to both the number of parasites and their stage of development (6). Many technical and biological factors prevent true reconciliation of the PfHRP2 concentration in whole blood with clinical peripheral ring-stage parasitemia on blood films. One such technical issue pertains to the multiple repetitive epitopes present in PfHRP2 (25, 30) such that 1 to 10 or more detector antibodies may bind to a single protein (3). This differs from aldolase or LDH assays which have unique capture and detector antibody epitopes producing a 1:1 molar ratio of binding. Biologically, in semi-immune individuals, immunodominant PfHRP2 antibodies may compete with either the capture or detection antibodies, which could not only reduce the signal in the ELISA but also certainly lower the correlation between the recombinant standard and the clinical samples in an assay plate. Thus, in a clinical test sample, PfHRP2 may either be (i) “free”; (ii) bound by antibody in plasma, either soluble or through large complexes; (iii) inside iRBCs; (iv) bound to uninfected RBCs as part of immune complexes via complement receptors on the surfaces of iRBCs; or (v) bound to other cells, such as leukocytes. Each of these PfHRP2 blood compartments may have separate complex kinetics which also further prevent reconciliation of the PfHRP2 concentrations with the numbers of parasites in patients, as determined by blood film microscopy, and in parasite cultures. Even though a clinical PfHRP2 whole-blood concentration theoretically could be back-calculated to a cultured parasite biomass, the biological meaning of this value and its interpretation could be clinically misleading and very well might vary among individuals, or even in the same individual, over time. Thus, the use of PfHRP2 whole-blood concentrations (25, 30) should be evaluated as an independent measure of infection intensity, without attempts to use it to calculate parasite numbers in patients.
Between-day repeatability or precision measurements, reported as CVs, are measures of the variability of the results for the same sample evaluated repeatedly in separate assay runs. Although there are no generally accepted minimal performance requirements for immunoassays, the industry standard for ELISA CV is 10 to 20% (28). Within the linear range, the PfHRP2 antigen-detecting ELISA described here has a CV of <15% for the recombinant antigen (Fig. 1), showing that the assay is reproducible with acceptable precision within the working linear range. For iRBCs diluted in whole blood (Fig. 2), equally low CVs were obtained (0.3 to 7.6%) from independently prepared parasite cultures.
Exceedingly high CVs (20.7 to 161.6%) were recorded for microscopy results (Fig. 3), and they reinforce the subjectivity and the unreliability of microscopy in providing reasonably precise measurements of parasite densities (21, 22). Evidence from other studies suggests that errors in microscopy for detection and quantification of malaria parasites can impact estimates of protective efficacy in malaria intervention trials (22). A sensitive and reliable readout for PfHRP2 could be used as both a quality control indicator for microscopy and an independent confirmation of results from negative smears.
In general, proteins are sensitive to multiple freeze-thaw cycles, and this can affect the reproducibility of a standard curve. A recent study that compared the performances of different rapid diagnostic tests for malaria showed how temperature, including repeated freeze-thaw cycles, can affect their performance (5). In the PfHRP2 ELISA reported here, the stability of the recombinant antigen used for the control curve was tested following 10 freeze-thaw cycles of a 10-μg/ml substock of the antigen. Results of a paired t test showed a significant (P < 0.05) decrease in the OD values after the fifth freeze-thaw cycle (Fig. 4). We therefore recommend that the rPfHRP2 antigen be stored in smaller aliquots for single use and that they be discarded after a maximum of four freeze-thaw cycles.
Serial-dilution experiments were performed to evaluate the assay results from biologically relevant samples to test how the assay is affected by the biological sample matrix. The demonstration of good linearity over a wide dilution range and with several sample types (whole blood, serum, plasma) would provide support for the general utility of this assay in assessing PfHRP2 from different sample types with various levels of analyte. In the assay reported here, patient samples with various levels of PfHRP2 were diluted in PBS/T to ensure that their values fell within the linear range of the standard curve. The greatest effect of the sample matrix was seen at the lower end of the linear range. The three sets of malaria parasite-infected whole-blood, serum, and plasma samples approximated ∼100% recovery at HRP2 concentrations near the upper LOQ (Fig. 5), but at concentrations closer to the lower LOQ, the precision declined. For lysed whole blood and for serum, CVs remained within ±20% within the linear assay range previously identified for the recombinant antigen. The CV for plasma deteriorated by >20% in samples with PfHRP2 concentrations of ≤25 ng/ml. Given that PfHRP2 is present in RBCs, plasma, and serum (12, 25), it is fortuitous that acceptable matrix interference characteristics were reported for whole-blood and serum samples. We therefore consider these as the sample types of choice for the PfHRP2 ELISA. However, taking into consideration the fact that most P. falciparum erythrocytic stages sequester, the fact that the amount of PfHRP2 varies throughout the erythrocytic cycle (4), and our results from the matrix experiments discussed above, we are of the opinion that any attempt to directly correlate PfHRP2 levels with detectable parasitemia will be plagued with inaccuracy.
This report describes a partial characterization of an ELISA used for the quantification of PfHRP2 in clinical samples. We conclude that the PfHRP2 ELISA using whole blood and serum is adequately precise and reproducible for evaluation of the intensity of infection in clinical samples as an element of intervention trials. We also conclude that any samples that have a PfHRP2 level exceeding the upper LOQ can be appropriately diluted for analysis without loss of precision. It is important to note that the PfHRP2 determined by this method constitutes antigen which may have been accumulating in the patient for a period that can extend to 3 weeks (17), and therefore, the PfHRP2 measured in biological samples includes that from both current and historical P. falciparum infections. Most other methods, including quantitative PCR, are unable to provide information regarding recent infection and sequestered parasites. The extent to which sequential episodes of malaria can be distinguished one from the other will need to be evaluated through field observations and will no doubt be influenced by the frequency, and thus the temporal proximity, of such episodes. The data analysis methods that are required for evaluation of the efficacy of an experimental intervention, such as a vaccine, also remain to be developed. Future work will focus on the experiments required to further validate the assay and on establishing correlations of patient samples with clinical status.
Acknowledgments
This work was supported by a grant from the Malaria Vaccine Development Program, United States Agency for International Development.
We thank Douglas Walsh for critical review of the manuscript. This work is published with the permission of the Director, Kenya Medical Research Institute.
The material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are our private views and are not to be construed as official, or as reflecting true views, of the U.S. Department of the Army or the U.S. Department of Defense.
Footnotes
Published ahead of print on 26 March 2008.
REFERENCES
- 1.Amexo, M., R. Tolhurst, G. Barnish, and I. Bates. 2004. Malaria misdiagnosis: effects on the poor and vulnerable. Lancet 364:1896-1898. [DOI] [PubMed] [Google Scholar]
- 2.Bacon, D. J., C. Latour, C. Lucas, O. Colina, P. Ringwald, and S. Picot. 2007. Comparison of a SYBR green I-based assay with a histidine-rich protein II enzyme-linked immunosorbent assay for in vitro antimalarial drug efficacy testing and application to clinical isolates. Antimicrob. Agents Chemother. 51:1172-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker, J., J. McCarthy, M. Gatton, D. E. Kyle, V. Belizario, J. Luchavez, D. Bell, and Q. Cheng. 2005. Genetic diversity of Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and its effect on the performance of PfHRP2-based rapid diagnostic tests. J. Infect. Dis. 192:870-877. [DOI] [PubMed] [Google Scholar]
- 4.Bland, J. M., and D. G. Altman. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet i:307-310. [PubMed] [Google Scholar]
- 5.Chiodini, P. L., K. Bowers, P. Jorgensen, J. W. Barnwell, K. K. Grady, J. Luchavez, A. H. Moody, A. Cenizal, and D. Bell. 2007. The heat stability of Plasmodium lactate dehydrogenase-based and histidine-rich protein 2-based malaria rapid diagnostic tests. Trans. R. Soc. Trop. Med. Hyg. 101:331-337. [DOI] [PubMed] [Google Scholar]
- 6.Desakorn, V., A. M. Dondorp, K. Silamut, W. Pongtavornpinyo, D. Sahassananda, K. Chotivanich, P. Pitisuttithum, A. M. Smithyman, N. P. J. Day, and N. J. White. 2005. Stage-dependent production and release of histidine-rich protein 2 by Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 99:517-524. [DOI] [PubMed] [Google Scholar]
- 7.Desakorn, V., K. Silamut, B. Angus, D. Sahassananda, K. Chotivanich, P. Suntharasamai, J. Simpson, and N. J. White. 1997. Semi-quantitative measurement of Plasmodium falciparum antigen PfHRP2 in blood and plasma. Trans. R. Soc. Trop. Med. Hyg. 91:479-483. [DOI] [PubMed] [Google Scholar]
- 8.Doderer, C., A. Heschung, P. Guntz, J.-P. Cazenave, Y. Hansmann, A. Senegas, A. W. Pfaff, T. Abdelrahman, and E. Candolfi. 2007. A new ELISA kit which uses a combination of Plasmodium falciparum extract and recombinant Plasmodium vivax antigens as an alternative to IFAT for detection of malaria antibodies. Malar. J. 6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dondorp, A. M., V. Desakorn, W. Pongtavornpinyo, D. Sahassananda, K. Silamut, K. Chotivanich, P. N. Newton, P. Pitisuttithum, A. M. Smithyman, N. J. White, and N. P. J. Day. 2005. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med. 2:e204. (Erratum, 2:390.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hänscheid, T. 2003. Current strategies to avoid misdiagnosis of malaria. Clin. Microbiol. Infect. 9:497-504. [DOI] [PubMed] [Google Scholar]
- 11.Hopkins, H., W. Kambale, M. R. Kamya, S. G. Staedke, G. Dorsey, and P. J. Rosenthal. 2007. Comparison of HRP2- and pLDH-based rapid diagnostic tests for malaria with longitudinal follow-up in Kampala, Uganda. Am. J. Trop. Med. Hyg. 76:1092-1097. [PubMed] [Google Scholar]
- 12.Howard, R. J., S. Uni, M. Aikawa, S. B. Aley, J. H. Leech, A. M. Lew, T. E. Wellems, J. Rener, and D. W. Taylor. 1986. Secretion of a malarial histidine-rich protein (Pf HRP II) from Plasmodium falciparum-infected erythrocytes. J. Cell Biol. 103:1269-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iqbal, J., N. Khalid, and P. R. Hira. 2002. Comparison of two commercial assays with expert microscopy for confirmation of symptomatically diagnosed malaria. J. Clin. Microbiol. 40:4675-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiser, M. M., and J. W. Dolan. 2004. Selecting the best curve fit. LCGC Europe 17:138-143. [Google Scholar]
- 15.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 16.Leke, R. F., R. R. Djokam, R. Mbu, R. J. Leke, J. Fogako, R. Megnekou, S. Metenou, G. Sama, Y. Zhou, T. Cadigan, M. Parra, and D. W. Taylor. 1999. Detection of the Plasmodium falciparum antigen histidine-rich protein 2 in blood of pregnant women: implications for diagnosing placental malaria. J. Clin. Microbiol. 37:2992-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayxay, M., S. Pukrittayakamee, K. Chotivanich, S. Looareesuwan, and N. J. White. 2001. Persistence of Plasmodium falciparum HRP-2 in successfully treated acute falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 95:179-182. [DOI] [PubMed] [Google Scholar]
- 18.McKenzie, F. E., J. Sirichaisinthop, R. S. Miller, R. A. Gasser, Jr., and C. Wongsrichanalai. 2003. Dependence of malaria detection and species diagnosis by microscopy on parasite density. Am. J. Trop. Med. Hyg. 69:372-376. [PMC free article] [PubMed] [Google Scholar]
- 19.Moody, A. 2002. Rapid diagnostic tests for malaria parasites. Clin. Microbiol. Rev. 15:66-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noedl, H., W. H. Wernsdorfer, R. S. Miller, and C. Wongsrichanalai. 2002. Histidine-rich protein II: a novel approach to malaria drug sensitivity testing. Antimicrob. Agents Chemother. 46:1658-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohrt, C., P. Obare, A. Nanakorn, C. Adhiambo, K. Awuondo, W. P. O'Meara, S. Remich, K. Martin, E. Cook, J.-P. Chretien, C. Lucas, J. Osoga, P. McEvoy, M. L. Owaga, J. S. Odera, and B. Ogutu. 2007. Establishing a malaria diagnostics centre of excellence in Kisumu, Kenya. Malar. J. 6:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohrt, C., Purnomo, M. A. Sutamihardja, D. Tang, and K. C. Kain. 2002. Impact of microscopy error on estimates of protective efficacy in malaria-prevention trials. J. Infect. Dis. 186:540-546. [DOI] [PubMed] [Google Scholar]
- 23.Parra, M. E., C. B. Evans, and D. W. Taylor. 1991. Identification of Plasmodium falciparum histidine-rich protein 2 in the plasma of humans with malaria. J. Clin. Microbiol. 29:1629-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piper, R., J. Lebras, L. Wentworth, A. Hunt-Cooke, S. Houze, P. Chiodini, and M. Makler. 1999. Immunocapture diagnostic assays for malaria using Plasmodium lactate dehydrogenase (pLDH). Am. J. Trop. Med. Hyg. 60:109-118. [DOI] [PubMed] [Google Scholar]
- 25.Rock, E. P., K. Marsh, A. J. Saul, T. E. Wellems, D. W. Taylor, W. L. Maloy, and R. J. Howard. 1987. Comparative analysis of the Plasmodium falciparum histidine-rich proteins HRP-I, HRP-II and HRP-III in malaria parasites of diverse origin. Parasitology 95:209-227. [DOI] [PubMed] [Google Scholar]
- 26.Taylor, D. W., and A. Voller. 1993. The development and validation of a simple antigen detection ELISA for Plasmodium falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 87:29-31. [DOI] [PubMed] [Google Scholar]
- 27.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 28.USDHHS, FDA Center for Drug Evaluation and Research. 2001. Guidance for industry: bioanalytical method validation. Center for Drug Evaluation and Research, Food and Drug Administration, Rockville, MD. http://www.fda.gov/cder/guidance/index.htm.
- 29.Voller, A., D. E. Bidwell, and P. L. Chiodini. 1994. Evaluation of a malaria antigen ELISA. Trans R Soc Trop. Med. Hyg. 88:188. [DOI] [PubMed] [Google Scholar]
- 30.Wellems, T. E., E. P. Rock, W. L. Maloy, D. W. Taylor, and R. J. Howard. 1986. Histidine-rich proteins in Plasmodium falciparum: an update and perspective. UCLA Symp. Mol. Cell. Biol. 42:47-58. [Google Scholar]