Abstract
The role of innate immunity in the host response to Bacillus anthracis is poorly understood. We found that normal human serum contains an antitoxin mechanism that is capable of protecting macrophages in vitro from B. anthracis lethal toxin-mediated killing. This protective activity was limited to defined amounts of toxin and was lost by heat treatment or serum dilution. Some person-to-person variation in the protective activity of serum was noted, especially with higher concentrations of lethal toxin. A similar protective activity was found in murine serum, though human serum consistently neutralized more toxin than did murine serum. The protective activities of both murine and human sera correlated with cleavage of the protective antigen into two fragments with approximate molecular sizes of 20 and 50 kDa that were recognized by the monoclonal antibodies 7.5G and 10F4, respectively. This pattern of fragmentation is consistent with cleavage at multiple sites, including the furin-susceptible site. Cleavage was abolished by heat treatment and calcium chelation. These findings highlight a potential role for serum proteases in protection against the lethal toxin of B. anthracis.
The lethal toxin (LeTx) is essential for Bacillus anthracis virulence. Injection of LeTx in susceptible animals results in many of the clinical manifestations of anthrax, including hypotension, pulmonary edema, and death (1, 5, 9). LeTx is composed of two components, protective antigen (PA) and lethal factor (LF). PA is an 83-kDa protein (PA83) that undergoes furin-mediated digestion into two fragments, PA63 and PA20. PA63 subsequently forms a heptamer, which binds LF. The resulting complex is taken up by the cell, where it exerts its toxic effects.
Both adaptive and innate elements of the immune response against LeTx play a central role in anthrax pathogenesis. Adaptive immunity in the form of antibody can protect against anthrax. Several groups have shown that PA-specific antibodies confer protection against anthrax in animal models, and many vaccine strategies are PA based (2, 8). In contrast, the innate immune system (e.g., macrophages and dendritic cells) is typically viewed as the target of B. anthracis infection and LeTx activity. Exposure of susceptible macrophages and dendritic cells to LeTx results in rapid cell lysis. Little, however, is known about the protective nature of the innate immune response against LeTx. In this report, we describe protective activity of nonimmune human serum against LeTx, which depends upon the proteolytic cleavage of PA by a serum protease. These findings are consistent with recent reports of proteolytic activity of murine serum against PA (11).
MATERIALS AND METHODS
Sera.
Sera from seven healthy laboratory workers were obtained and stored at −80°C with approval from the Committee of Clinical Investigations at Albert Einstein College of Medicine. In some studies, serum was heat treated at 56°C for 30 min. Serum was also subjected to ammonium sulfate precipitation followed by dialysis against 0.9% NaCl. Serum was fractionated by centrifugation using Microcon (Millipore, Billerica, MA) centrifugal filter devices with various membrane pore sizes from 3,000 to 100,000 per the manufacturer's recommendations. Serum was also obtained from BALB/c, C57BL/6, and SCID mice housed at the animal facility at our institution. We chose to examine these strains of mice because of their known differences in susceptibility to LeTx (for BALB/c and C57BL/6 mice) and the absence of antibody in SCID mice.
LeTx.
PA and LF were obtained from Wadsworth Laboratories (New York State Department of Health, Albany). PA and LF were mixed at a 1:1 ratio. For furin digests, 10 μg of PA was incubated in 20 μl of 1 mM CaCl2, 1 mM β-mercaptoethanol, 0.5% Triton X-100, 100 mM HEPES, and furin (25 U/ml; Sigma, St. Louis, MO) for 1 h at 30°C.
Cell viability.
The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was used to determine toxin toxicity. Approximately 5 × 104 J774 cells were plated in 96-well culture plates and then treated with 24 μl serum in 50 μl of Dulbecco modified Eagle medium for 1 h at 37°C. LeTx was then directly added to cell cultures. For most studies, 200 ng of LeTx, consisting of 100 ng of PA and 100 ng of LF, was used. A 25-μl volume of a 5-mg/ml stock solution of MTT (Sigma, St. Louis, MO) was added to each well, and after 2 h of incubation of 37°C, 100 μl of the extraction buffer (12.5% sodium dodecyl sulfate [SDS] and 45% dimethylformamide) was added and cells were incubated overnight at 37°C. Optical densities were measured at 570 nm (Labsystem Multiskan, Franklin, MA). All sera were tested on at least two separate occasions, and the absorbance values were averaged. The protective index (PI) was calculated as the ratio of MTT absorbance values for cells treated with serum and toxin to those for cells treated with serum alone.
Cell death.
Serum-treated cells were incubated with LeTx for 3 h as described above for the MTT assay. Trypan blue (0.4%) was added directly to duplicate wells, and the proportion of blue cells was determined. Approximately 200 cells per well were counted. The average percent dead cells was then calculated.
MAbs.
The murine monoclonal antibodies (MAbs) 10F4 and 7.5G were used for immunoblotting studies (12). 10F4 is an immunoglobulin G1 (IgG1) that has been shown to selectively react with domain 4 of PA, the region of PA that binds host cell receptors. 7.5G is an IgG2a that selectively recognizes domain 1 of PA, the region of PA that is cleaved from PA83 by host cell furin.
Immunoblotting.
PA or LF (2.5 μg of each) was incubated with 25 μl of serum, medium, or furin at 37°C for 1 h. In some experiments, serum was heat treated as described above or incubated with EDTA (20 mM) or EGTA (20 mM) prior to incubation with serum. PA and LF were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Membranes were blocked with 5% milk and then incubated with primary antibody. The following MAbs were used to characterize PA cleavage: 10F4 and 7.5G. For LF detection MAb 12H (IgG1) was used. All MAbs were used at a concentration of 0.25 μg/ml. Primary antibody was detected with horseradish peroxidase-labeled goat isotype-specific antibody at a dilution of 1:25,000. Proteins were visualized by development with the ECL chemiluminescence kit (Pierce, Rockford, IL).
Heptamer formation assay.
One microgram of PA was incubated with 250 μl of serum or heat-inactivated serum for 1 h at 37°C. Serum-treated toxin was incubated with approximately 3 × 105 cells in a 24-well plate for 30 min. Medium was then removed, and the cells were washed three times with phosphate-buffered saline. Cells were lysed with modified RIPA lysis buffer (50 mM Tris-Cl, pH 7.4, 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 μg/ml protease inhibitors). Protein extracts were quantified by bicinchoninic acid assay and separated in an SDS 4 to 12% gradient gel (Bio-Rad, Hercules, CA). Proteins were then transferred to a nitrocellulose membrane, and the PA fragment was detected with 10F4 as described above.
Statistics.
All data were analyzed by the Student t test (SigmaStat, Chicago, IL). A P value of <0.05 was considered statistically significant.
RESULTS
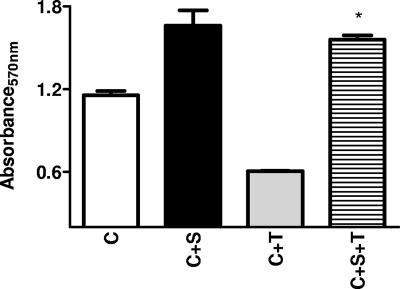
Treatment of J774 cells with 200 ng of LeTx resulted in a dramatic reduction of MTT signal indicative of cell death, which was prevented by treatment of cells with serum (Fig. 1). Small and variable effects of serum on MTT values were observed. Serum from all seven individuals consistently protected J774 cells from LeTx (200 ng)-induced death (Fig. 2) with PIs ranging from 0.74 to 1.01. The protective activity of serum for cells treated with 400 ng of LeTx, however, was more variable (Fig. 2). Furthermore, protective activity of human serum was lost by heat inactivation (e.g., treatment at 56°C for 30 min). Dilution of serum by more than a factor of 4 resulted in a loss of protective activity (not shown). Protection was also observed in commercially obtained serum (Sigma, St. Louis, MO) with preserved complement activity with an average PI of 0.86 ± 0.07. Simultaneous addition of serum and toxin resulted in protection of cells similar to that with a 1-h pretreatment of cells (PIs, 0.95 ± 0.15 versus 1.03 ± 0.04, respectively). Serum was also effective in protecting primary alveolar and peritoneal macrophages from BALB/c mice against LeTx-induced death (not shown).
FIG. 1.
Cell viability following LeTx exposure. MTT assay results (average absorbance values) following incubation of J774 cells with human PA in the presence or absence of a representative human serum sample. C, cells alone; C+S, cells incubated with serum; C+T, cells incubated with toxin; C+S+T, cells incubated with serum and toxin. Bars represent 1 standard deviation. *, P value of <0.05 for comparison between C+T and C+S+T values.
FIG. 2.
PIs for different human sera. Each symbol represents the PI for serum from a given individual. Closed symbols represent normal human sera, while open symbols represent heat-treated sera. Lines represent the average PIs for all sera. Amounts of LeTx are shown on the horizontal axis. The PI was calculated as the ratio of MTT absorbance values for cells treated with serum and toxin to those for cells treated with serum alone.
The protective activity of serum was retained following precipitation with 30% ammonium sulfate (PI, 0.87 ± 0.01), but it was not present in a column-purified IgG fraction (PI, 0.20 ± 0.04). The protective factor was greater than 100 kDa in size and was present in the retentate, but not the filtrate, following membrane centrifugation using a 100-kDa membrane (PI, 0.90 ± 0.07 versus 0.25 ± 0.01). Serum from BALB/c, C57BL/6, and SCID mice exhibited protective activity against LT-induced cell death with PIs of 0.81 ± 0.04, 0.94 ± 0.03, and 1.03 ± 0.05, respectively. However, this protective activity was reliably present only when 100 ng of toxin was used.
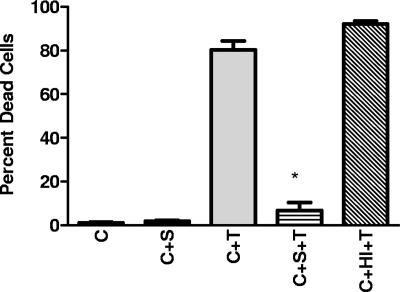
To ensure that the observed protection with an MTT assay did not represent a direct effect of serum on the MTT reaction, studies with trypan blue were done. The proportion of trypan-positive (dead) cells was dramatically reduced in association with serum treatment (Fig. 3). This reduction was not observed with heat-inactivated serum.
FIG. 3.
Cell death following LeTx exposure. Average percentages of trypan blue-positive cells are shown. C, cells alone; C+S, cells incubated with serum; C+T, cells incubated with toxin; C+S+T, cells incubated with serum and toxin; C+HI+T, cells treated with heat-inactivated serum and toxin. Bars represent 1 standard deviation. *, P value of <0.05 for comparison between C+T and C+S+T values.
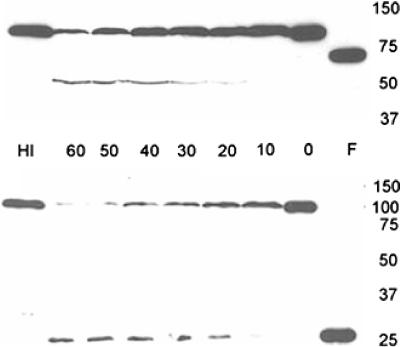
To determine if the protective effect of serum was due to its effects on PA and LF, immunoblot studies were done. Coincubation of human serum with PA resulted in loss of reactivity at 83 kDa and produced at least two distinct protein fragments, which were selectively recognized by MAbs 7.5G and 10F4. The size of the fragment recognized by MAb 7.5G was approximately 20 kDa, similar in size to the fragment generated by furin digestion (Fig. 4). The size of the fragment recognized by 10F4 was approximately 50 kDa, and the fragment appeared to be smaller than the PA63 fragment produced by furin digestion. However, this band was not always apparent following serum treatment. Digestion was greatest after 60 min of incubation (Fig. 4). A similar pattern of PA fragmentation was observed with sera from both BALB/c and SCID mice (not shown). Heat treatment and incubation of human serum in either EDTA or EGTA prevented these changes (not shown). An analysis of activity in fractions obtained from membrane centrifugation indicated that the substance conferring proteolytic activity was greater than 100 kDa (not shown). No effects of serum treatment on LF size or immunoreactivity with MAb 12HD were observed (not shown).
FIG. 4.
Effects of human serum on PA. PA was incubated with human serum for various times (0, 10, 20, 30, 40, 50, and 60 min) and separated by SDS-polyacrylamide gel electrophoresis. Reduction of PA83 reactivity for both MAb 10F4 and MAb 7.5G is shown with maximum reduction at 60 min. The top blot shows results obtained using 10F4 as the primary antibody. In addition to loss of PA83 reactivity, new reactivity with a fragment of approximately 50 kDa is shown. The bottom blot shows results obtained using 7.5G as the primary antibody. In addition to loss of PA83 reactivity, new reactivity with a fragment of approximately 20 kDa is shown. Lane F represents PA treated with furin as described in Materials and Methods. Lane HI represents PA treated with heat-inactivated serum for 60 min. Molecular size in kDa is shown on the right.
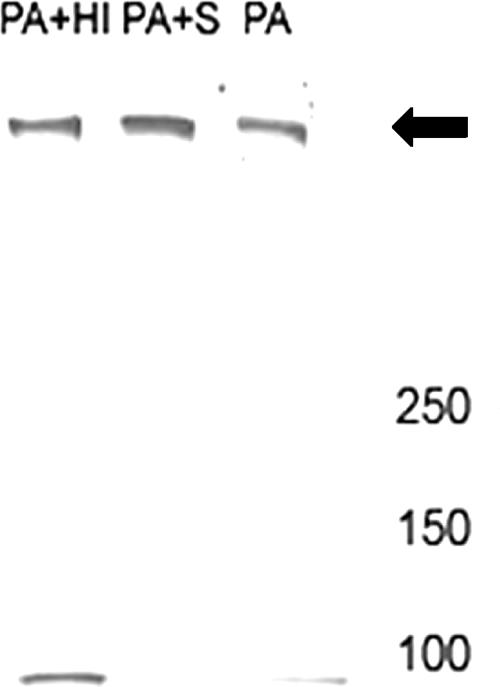
To determine if serum-mediated cleavage resulted in changes in the ability of PA to form a heptamer, PA was incubated with serum for 1 h and then incubated with J774 cells. An immunoblot of protein extract obtained from these cells indicates that serum pretreatment did not prevent heptamer formation (Fig. 5) but did decrease PA83 reactivity.
FIG. 5.
Effects of serum on heptamer formation. PA was treated with medium (PA), serum (PA+S), or heat-inactivated human serum (PA+HI) and then incubated with J774 cells. Proteins were extracted at 30 min and separated in a gradient gel. Transferred proteins were detected with MAb 10F4. Serum treatment resulted in a loss of PA83 reactivity but did not prevent heptamer formation. The arrow points to the heptamer. Molecular size in kDa is shown on the right.
DISCUSSION
In this study, we demonstrate that normal human serum contains a protease that protects against the lethal activity of LeTx in vitro by cleaving PA. PA83 contains two sites that are susceptible to proteolytic cleavage. Cleavage by cellular furin at the first site, between domains I and II, produces two fragments, PA20 and PA63. Cleavage at the second site, between domains II and III, produces two fragments of approximately 37 and 47 kDa. The second cleavage can be mediated by chymotrypsin and thermolysin and is essential for internalization of the PA/LF complex (14).
A serum protease that cleaves PA has been hypothesized to be present in animal serum (6, 11). Moayeri et al. recently found that mouse serum contains a leupeptin-sensitive and calcium-dependent factor that is capable of cleaving PA. These authors further demonstrated that cleavage occurred at the furin site (11). The presence of a PA20 fragment which reacts with MAb 7.5G (domain I specific) in our experiment is consistent with cleavage at the furin site. However, the presence of a PA50 fragment containing domain 4 suggests that PA63 once formed by serum cleavage undergoes additional degradation, perhaps at the second cleavage site. Thus, while both human and animal sera contain protease(s) that cleaves PA, there are differences in the patterns of digestion by these proteases. In addition, human serum appears to be more effective in neutralizing LeTx activity, since human sera consistently neutralized higher amounts of LeTx in vitro than did murine sera.
Despite serum-mediated cleavage of PA, heptamer formation was not impaired. It is possible that the digestion fragments were still capable of forming a heptamer but that this complex was structurally different from that which results from normal processing. In previous studies, we have shown that an inhibition of heptamer formation is not necessary for the protective effects of a MAb which was reactive to domain I of PA (12).
The protein that mediates cleavage of PA remains to be determined, but the observed characteristics (e.g., heat lability, size, and susceptibility to Ca+2 chelation) are consistent with a complement-related protease. Complement proteins are a series of proteases that typically cleave other complement proteins. However, proteolytic cleavage of noncomplement proteins by complement (including C1s) has been described elsewhere (4, 15). We found that commercially obtained purified C3 did not protect cells or promote PA cleavage (data not shown). In addition, complement proteins typically require processing to be enzymatically active. Alternatively, it is possible that a serum protease other than complement is involved. Serum proteases, including cathepsins, have been shown to alter the antigenicity of peptides presented by class I major histocompatibility complex molecules (7, 13).
This is the first time that a protective factor against LeTx of B. anthracis has been demonstrated in human serum. These observations demonstrate an unrecognized contribution of the innate immune response to the host response to toxin exposures. Similar to the results of Moayeri et al., we found that sera from both LeTx-“susceptible” (BALB/c) and -“resistant” (C57BL/6) mouse strains were protective (11). Thus, the presence of this protective factor alone does not confer LeTx resistance in mice. Nonetheless, these findings do not exclude a protective function of serum during infection. We note that the amount of toxin neutralized by human serum is not insignificant. For example, 24 μl of human serum consistently neutralized 200 ng of LeTx. In a guinea pig model of anthrax, serum concentrations of PA at the time of death ranged from 100 to 1,700 ng/ml (10). In a macaque model of anthrax, LF concentrations on day 2 ranged from 30 to 250 ng/ml (3). Interestingly, we found that human sera exhibited some variability in protective activity with increasing LeTx concentrations. These findings highlight the possibility that this protease contributes to determining individual susceptibility to LeTx.
In summary, our results indicate that human serum, like other types of mammalian serum, contains a protease capable of neutralizing the LeTx of B. anthracis. Additional studies are warranted to determine the specific serum protease involved and its role in the host defense against B. anthracis infection.
Acknowledgments
D.L.G. and A.C. both participate in and receive support from the Northeastern Biodefense Center funded by 5U54AI057158-05.
We have no financial or commercial affiliations that pose a conflict of interest.
Footnotes
Published ahead of print on 30 April 2008.
REFERENCES
- 1.Beall, F. A., and F. G. Dalldorf. 1966. The pathogenesis of the lethal effect of anthrax toxin in the rat. J. Infect. Dis. 116:377-389. [DOI] [PubMed] [Google Scholar]
- 2.Bielinska, A. U., K. W. Janczak, J. J. Landers, P. Makidon, L. E. Sower, J. W. Peterson, and J. R. Baker, Jr. 2007. Mucosal immunization with a novel nanoemulsion-based recombinant anthrax protective antigen vaccine protects against Bacillus anthracis spore challenge. Infect. Immun. 75:4020-4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyer, A. E., C. P. Quinn, A. R. Woolfitt, J. L. Pirkle, L. G. McWilliams, K. L. Stamey, D. A. Bagarozzi, J. C. Hart, Jr., and J. R. Barr. 2007. Detection and quantification of anthrax lethal factor in serum by mass spectrometry. Anal. Chem. 79:8463-8470. [DOI] [PubMed] [Google Scholar]
- 4.Busby, W. H., Jr., T. J. Nam, A. Moralez, C. Smith, M. Jennings, and D. R. Clemmons. 2000. The complement component C1s is the protease that accounts for cleavage of insulin-like growth factor-binding protein-5 in fibroblast medium. J. Biol. Chem. 275:37638-37644. [DOI] [PubMed] [Google Scholar]
- 5.Culley, N. C., D. M. Pinson, A. Chakrabarty, M. S. Mayo, and S. M. Levine. 2005. Pathophysiological manifestations in mice exposed to anthrax lethal toxin. Infect. Immun. 73:7006-7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ezzell, J. W., Jr., and T. G. Abshire. 1992. Serum protease cleavage of Bacillus anthracis protective antigen. J. Gen. Microbiol. 138:543-549. [DOI] [PubMed] [Google Scholar]
- 7.Falo, L. D., Jr., L. J. Colarusso, B. Benacerraf, and K. L. Rock. 1992. Serum proteases alter the antigenicity of peptides presented by class I major histocompatibility complex molecules. Proc. Natl. Acad. Sci. USA 89:8347-8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorse, G. J., W. Keitel, H. Keyserling, D. N. Taylor, M. Lock, K. Alves, J. Kenner, L. Deans, and M. Gurwith. 2006. Immunogenicity and tolerance of ascending doses of a recombinant protective antigen (rPA102) anthrax vaccine: a randomized, double-blinded, controlled, multicenter trial. Vaccine 24:5950-5959. [DOI] [PubMed] [Google Scholar]
- 9.Kuo, S. R., M. C. Willingham, S. H. Bour, E. A. Andreas, S. K. Park, C. Jackson, N. S. Duesbery, S. H. Leppla, W. J. Tang, and A. E. Frankel. 2007. Anthrax toxin-induced shock in rats is associated with pulmonary edema and hemorrhage. Microb. Pathog. [Epub ahead of print.] doi: 10.1016/j.micpath.2007.12.001. [DOI] [PubMed]
- 10.Mabry, R., K. Brasky, R. Geiger, R. Carrion, Jr., G. B. Hubbard, S. Leppla, J. L. Patterson, G. Georgiou, and B. L. Iverson. 2006. Detection of anthrax toxin in the serum of animals infected with Bacillus anthracis by using engineered immunoassays. Clin. Vaccine Immunol. 13:671-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moayeri, M., J. F. Wiggins, and S. H. Leppla. 2007. Anthrax protective antigen cleavage and clearance from the blood of mice and rats. Infect. Immun. 75:5175-5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivera, J., A. Nakouzi, N. Abboud, E. Revskaya, D. Goldman, R. J. Collier, E. Dadachova, and A. Casadevall. 2006. A monoclonal antibody to Bacillus anthracis protective antigen defines a neutralizing epitope in domain 1. Infect. Immun. 74:4149-4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez, G. M., and S. Diment. 1995. Destructive proteolysis by cysteine proteases in antigen presentation of ovalbumin. Eur. J. Immunol. 25:1823-1827. [DOI] [PubMed] [Google Scholar]
- 14.Singh, Y., K. R. Klimpel, N. Arora, M. Sharma, and S. H. Leppla. 1994. The chymotrypsin-sensitive site, FFD315, in anthrax toxin protective antigen is required for translocation of lethal factor. J. Biol. Chem. 269:29039-29046. [PubMed] [Google Scholar]
- 15.Yamaguchi, K., H. Sakiyama, M. Matsumoto, H. Moriya, and S. Sakiyama. 1990. Degradation of type I and II collagen by human activated C1-s. FEBS Lett. 268:206-208. [DOI] [PubMed] [Google Scholar]