Abstract
This study was designed to examine the use of the QuantiFERON-TB Gold assay as an aid in the diagnosis of active pulmonary tuberculosis (TB) in Brazilian patients. Using the receiver operating characteristic curve, the cutoff was adjusted to ≥0.20 IU/ml. The sensitivity increased to 86%, with 100% specificity. All TB patients with negative sputum smear microscopy and negative culture results were positive using this test.
Tuberculosis (TB), an infectious disease caused by Mycobacterium tuberculosis, remains a major public health problem throughout the world. In 2005, it was estimated that there were 8.8 million new cases of TB and 1.6 million deaths worldwide (about 4,400 people/day) (15). Early diagnosis and prompt treatment of patients with active pulmonary disease are the most important factors in reducing the morbidity, mortality, and incidence of TB. Diagnosis is presently based on clinical suspicion, radiological findings, and laboratory confirmation of bacilli through microbiological methods (smear microscopy for acid-fast bacillus [AFB] and/or culture) (3, 8).
Smear microscopy for AFB is still the principal method for the detection of pulmonary TB in patients, mainly in regions where TB is highly endemic (9, 11). However, its sensitivity is only 40 to 60% under field conditions, 50% in paucibacillary subjects, and 35 to 70% in children and adults with extrapulmonary TB (7, 10, 14). Limitations of these traditional microbiological methods have led to the development of additional methods, such as the gamma interferon (IFN-γ) release assay, as an aid in clinical and laboratory investigations of cases of human TB.
Tests that detect T cells producing IFN-γ in response to early secreted antigenic target 6 (ESAT-6) and culture filtrate protein (CFP-10) now available on the market include the QuantiFERON-TB Gold (Cellestis Ltd., Carnegie, Victoria, Australia) and T SPOT-TB (Oxford Immunotec, Oxford, United Kingdom) assays. The QuantiFERON-TB Gold test has been approved by the U.S. Food and Drug Administration for the diagnosis of either latent or active TB infection, meeting the requirements of the World Health Organization (WHO) regarding the sensitivity and specificity for immunological methods (greater than 80% and 95%, respectively) (2, 13).
In this study, we examined the use of the QuantiFERON-TB Gold assay as an aid in the diagnosis of pulmonary TB in 32 patients attending the TB outpatient department of the Hospital das Clinicas de São Paulo. Twenty-nine patients were confirmed to have active pulmonary TB based on a sputum culture positive for M. tuberculosis (n = 22) or based on clinical, radiological, and/or laboratory criteria (n = 7); three patients with clinical symptoms such as cough, fever, and night sweats were consistently negative by microbiological methods. The initial hypothesis of TB was ruled out after a thorough clinical, radiological, and laboratory investigation. Twenty-three healthy adult subjects who were tuberculin skin test negative, with no previous contact with pulmonary TB patients and no clinical symptoms of pulmonary TB, were enrolled as controls. All the participants in this study were vaccinated with BCG according to the National Program of the Health Minister. The study was approved by the local ethical committee of the Hospital das Clinicas de São Paulo. Written informed consent was obtained from each participant.
The QuantiFERON-TB Gold test was performed according to the manufacturer's recommendations. A blood sample was collected from each participant by venipuncture into lithium-heparin tubes and processed within 12 h. All blood samples were collected before the beginning of the treatment. Four 1-ml aliquots of whole blood from each participant were incubated on sterile cell culture plates (TPP, Switzerland) at 37°C in a wet chamber for 18 h with M. tuberculosis-specific antigens (ESAT-6 and CFP-10) and negative and positive controls (nil and mitogen). After incubation, the supernatants were harvested and frozen at −20°C for subsequent analysis. The concentration of IFN-γ was determined using a sensitive enzyme-linked immunosorbent assay, and the results were expressed as IU/ml based on the standard curve generated for each plate.
Of the 29 patients with active pulmonary TB, 62% were male and 38% were female. The mean age was 38 years. The mean time from the first symptoms to diagnosis was 3 months. Fifty-two percent of the patients had cavitary lesions and 31% had pulmonary infiltrates visible by chest X ray. Thirty-five percent of the patients had previous contact with tuberculosis patients, 24% reported hemoptysis, and 38% had some comorbidities such as diabetes mellitus, hypothyroidism, Sjögren's syndrome, chronic lymphocytic leukemia, rheumatoid arthritis, alcoholism, and gastritis. Sixteen patients were positive by AFB smear microscopy and sputum culture, six patients were positive by culture only, and seven patients with low bacterial load and negative results by smear microscopy and sputum culture had the diagnosis confirmed later by microscopy and/or culture of a bronchoalveolar lavage sample or biopsy. All patients were human immunodeficiency virus negative.
Using a cutoff value for IFN-γ of ≥0.35 IU/ml, as recommended by the manufacturer, 22 out of 29 patients with active pulmonary TB were positive, giving a sensitivity of 76%. All 23 blood samples from healthy subjects were negative (specificity of 100%). This sensitivity (76%) was lower than that recommended by the WHO; however, it is in accordance with studies conducted previously by other investigators (4, 5, 12).
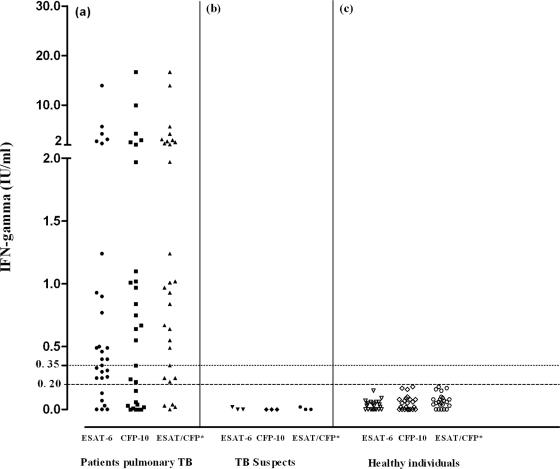
When we analyzed the levels of IFN-γ in healthy individuals, we observed that they were significantly lower than those observed in patients with active pulmonary TB. After drawing a receiver operating characteristic (ROC) curve, we defined IFN-γ levels of ≥0.20 IU/ml as being the cutoff level for the diagnosis of TB, resulting in a sensitivity of 86% (confidence interval, 73.6% to 98.7%) and a specificity of 100% (confidence interval, 100% to 100%) (Fig. 1). Using IFN-γ levels of ≥0.20 IU/ml, the test was positive for 25 out of 29 patients and negative for the 23 healthy controls. This observation shows that different epidemiological situations and population exposure levels to different microorganisms should be taken into account in the standardization, application, and analysis of results from new methods intended for use in disease diagnosis (1).
FIG. 1.
Individual response of IFN-γ released after stimulation with ESAT-6 and CFP-10 antigens. (a) IFN-γ responses in 29 patients with active pulmonary TB. IFN-γ responses in three patients negative by conventional microbiological tests (b) and in 23 healthy subjects (c). The dotted line represents the cutoff of 0.35 IU/ml recommended by the manufacturer; the dashed line represents the cutoff of 0.20 IU/ml defined in our laboratory by ROC curve analysis. *, data for the antigen (ESAT-6 or CFP-10).
Of 16 samples that were positive by sputum smear microscopy, 12 (75%) were positive and 4 were negative by the QuantiFERON-TB Gold assay. All 13 samples that were negative by sputum smear microscopy were positive by this test. Of the 22 samples with a positive sputum culture for M. tuberculosis, 18 (82%) were positive and 4 were negative by the QuantiFERON-TB Gold assay. The clinical and laboratory data for the four patients with false-negative results by the QuantiFERON-TB Gold assay showed the presence of comorbidities such as chronic lymphocytic leukemia, alcoholism, moderate lymphocytopenia, the presence of cavitary lesions and/or infiltrate on chest X ray, and hemoptysis. Evaluation of the QuantiFERON-TB Gold test for use with immunocompromised subjects and for patients with more advanced disease is crucial to determine the true clinical value of this assay in clinical practice. Of six patients with negative smear microscopy and positive sputum culture results, 100% were positive by the QuantiFERON-TB Gold assay, and seven cases with negative results by microbiological methods were positive by the test (Table 1).
TABLE 1.
Results from 29 patients with active pulmonary TB determined by AFB smear microcopy, culture, and the QuantiFERON-TB Gold assay
| QuantiFERON-TB Gold result | No. of patients with test result by:
|
|||||
|---|---|---|---|---|---|---|
| AFB smear microscopy
|
Culture
|
|||||
| Positive | Negative | Total | Positive | Negative | Total | |
| Positivea | 12 | 13 | 25b | 18 | 7 | 25b |
| Negative | 4 | 0 | 4 | 4 | 0 | 4 |
| Total | 16b | 13 | 29 | 22b | 7 | 29 |
Positive IFN-γ levels of ≥0.20 IU/ml defined by ROC curve analysis.
Differences in sensitivity between QuantiFERON-TB Gold and microscopy or culture were calculated using the McNemar test. A P value of <0.05 was considered to be significant.
The positive results for the QuantiFERON-TB Gold assay observed for patients with low bacterial loads were very important. In this situation, the test was 100% sensitive and completely consistent with the clinical data and chest X rays of the patients. Our results indicate that after adjusting the cutoff level, the QuantiFERON-TB Gold assay detected 45% more cases than did smear microscopy. The sensitivity achieved when the results from QuantiFERON-TB Gold and those from cultures were combined was 100%. Powered studies are needed to assess the real value of this new test in clinical practice in countries with different TB endemicities, mainly in areas with a high prevalence of TB infection, where the positive predictive value for a positive IFN-γ release assay will be low. However, a low positive predictive value is not bad if we consider the test's ability to increase the probability of disease (6).
Acknowledgments
This study was supported by the Fundação de Amparo a Pesquisa do Estado de São Paulo (grant no. 03/08308-2), LIM-48 Hospital das Clinicas, School of Medicine, University of São Paulo. K.A.K. was supported by a studentship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico, and A.W.F. is a recipient of a research scholarship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Footnotes
Published ahead of print on 9 April 2008.
REFERENCES
- 1.Arend, S. M., S. F. Thijsen, E. M. Leyten, J. J. Bouwman, W. P. Franken, B. F. Koster, F. G. Cobelens, A. J. van Houte, and A. W. Bossink. 2007. Comparison of two interferon-gamma assays and tuberculin skin test for tracing tuberculosis contacts. Am. J. Respir. Crit. Care Med. 175:618-627. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2005. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm. Rep. 54:49-55. [PubMed] [Google Scholar]
- 3.Chan, E. D., L. Heifets, and M. D. Iseman. 2000. Immunologic diagnosis of tuberculosis: a review. Tuber. Lung Dis. 80:131-140. [DOI] [PubMed] [Google Scholar]
- 4.Dewan, P. K., J. Grinsdale, and L. M. Kawamura. 2007. Low sensitivity of a whole-blood interferon-γ release assay for detection of active tuberculosis. Clin. Infect. Dis. 44:69-73. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara, G., M. Losi, R. D'Amico, P. Roversi, R. Piro, M. Meacci, B. Meccugni, I. M. Dori, A. Andreani, B. M. Bergamini, C. Mussini, F. Rumpianesi, L. M. Fabbri, and L. Richeldi. 2006. Use in routine clinical practice of two commercial blood tests for diagnosis of infection with Mycobacterium tuberculosis: prospective study. Lancet 367:1328-1334. [DOI] [PubMed] [Google Scholar]
- 6.Gambino, R. 1991. The misuse of predictive value—or why you must consider the odds. Ann. Ist. Super. Sanita 27:395-399. [PubMed] [Google Scholar]
- 7.Garg, S. K., R. P. Tiwari, D. Tiwari, R. Singh, D. Malhotra, V. K. Ramnani, G. B. K. S. Prasad, R. Handra, M. Fraziano, V. Colizzi, and P. S. Bisen. 2003. Diagnosis of tuberculosis: available technologies, limitations and possibilities. J. Clin. Lab. Anal. 17:155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keeler, E., M. D. Perkins, P. Small, C. Hanson, S. Reed, J. Cunningham, J. E. Aledort, L. Hillborne, M. E. Rafael, F. Girosi, and C. Dye. 2006. Reducing the global burden of tuberculosis: the contribution of improved diagnostics. Nature 444:49-57. [DOI] [PubMed] [Google Scholar]
- 9.Perkins, M. D. 2000. New diagnostic tools for tuberculosis. Int. J. Tuberc. Lung Dis. 4:182-188. [PubMed] [Google Scholar]
- 10.Perkins, M. D., G. Roscigno, and A. Zumla. 2006. Progress towards improved tuberculosis diagnostics for developing countries. Lancet 367:942-943. [DOI] [PubMed] [Google Scholar]
- 11.Ramachandran, R., and C. N. Paramasivan. 2003. What is new in the diagnosis of tuberculosis? Part I: techniques for diagnosis of tuberculosis. Ind. J. Tuber. 50:133-141. [Google Scholar]
- 12.Tsiouris, S. J., D. Coetzee, P. L. Toro, J. Austin, Z. Stein, and W. El-Sadr. 2006. Sensitivity analysis and potential uses of a novel gamma interferon release assay for diagnosis of tuberculosis. J. Clin. Microbiol. 44:2844-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. 1997. Global tuberculosis programme—report of a tuberculosis diagnostics workshop. World Health Organization, Geneva, Switzerland. http://www.who.int/tdr/publications/.
- 14.World Health Organization. 2006. Diagnostics for tuberculosis: global demand and market potential/TDR, FIND SA. World Health Organization, Geneva, Switzerland. http://www.who.int/tdr/publications/publications/pdf/tbdi/tbdi.pdf.
- 15.World Health Organization. 2007. Tuberculosis, fact sheet no. 104. World Health Organization, Geneva, Switzerland. http://www.who.int/mediacentre/factsheets/fs104/en/.