Abstract
Delayed processing of peripheral blood or peripheral blood mononuclear cell isolation and cryopreservation can lead to the detection of somewhat higher levels of CD31 expression on naïve CD4 T cells by flow cytometry. These observations should be considered in the planning of multicenter clinical trials and in the interpretation of the results of functional studies.
Antibodies to CD31 (anti-PECAM-1) recognize the platelet/endothelial cell adhesion molecule-1 (PECAM-1) (2). Six extracellular immunoglobulin-like domains of the C2 group compose the CD31 antigen. The CD31 antigen is a vascular cell adhesion molecule involved in leukocyte migration through the intercellular junctions of vascular endothelial cells (1). The expression of CD31 changes during the T-cell maturation of CD4 T cells and is lost after triggering of the T-cell receptor and T-cell activation even before conversion to a memory phenotype (3). The loss of CD31 on naïve CD4+ T cells can thus allow recognition of those naïve cells that have undergone homeostatic proliferation and have diluted their receptor excision circle (TREC) content. It has been shown in humans that naïve CD4 T cells express higher levels of CD31 than memory CD4+ T cells and that naïve T cells expressing CD31 are enriched in their TREC contents (5). The study of CD31 expression may be of particular interest in settings with high rates of CD4 T-cell turnover, the loss of naïve T cells, and/or functional abnormalities in naïve CD4 T cells, such as human immunodeficiency virus (HIV) infection or T-cell recovery after stem cell transplantation or chemotherapy (7, 8).
In this study, the timing of processing of peripheral blood or the timing of lymphocyte separation and cryopreservation procedures was evaluated with blood from both HIV-negative (HIV−) and HIV-positive (HIV+) participants to determine what effect there might be on the flow cytometric measurement of the percentage of CD4+ CD45RO− CD27+ (naïve) T cells expressing CD31. Centers for Disease Control and Prevention (CDC) guidelines for CD4+ T-cell determinations with CD45 gating for HIV-infected persons state that peripheral blood can be held for up to 72 h, a practice that can allow testing of the blood at a centralized laboratory in multicenter trials (9). Previous studies have also shown that density gradient separation and cryopreservation do not lead to the loss of either CD4+ or CD8+ T cells but that measurable losses in specific T-cell subsets can be observed (4, 10-12). A cohort of 54 participants, 17 of whom were HIV− and 37 of whom were HIV+, was recruited after informed consent for participation in institutional review board-approved protocols was obtained.
The 17 HIV− study participants had a median age of 50 years (range, 37 to 60 years). The median CD4 T-cell count for the HIV− cohort was 753 cells/μl (range, 352 to 1,225 cells/μl). The 37 HIV+ participants had a median age of 45 years (range, 23 to 64 years). The median CD4 T-cell count of the HIV+ cohort was 679 cells/μl (range, 212 to 1,289 cells/μl). Twenty-two patients (61%) had viral loads of <50 copies/ml. The median viral load of the remaining patients was 13,304 copies/ml (range, 144 to 35,763 copies/ml). One patient was not receiving antiretroviral therapy at the time of the study.
Immunophenotyping of peripheral blood drawn into tubes containing EDTA was done at 4, 24, 48, and 72 h after the blood was drawn, according to the manufacturer's instructions, by using a modification of the CDC guidelines in a Clinical Laboratory Improvement Act-certified laboratory (6, 9, 13). The cells were lysed after they were stained with Optilyse C (Beckman Coulter, Hialeah, FL), washed twice, and resuspended in 500 μl of phosphate-buffered saline (Cambrex, Walkersville, MD). The samples were immediately analyzed on a Becton Dickinson FacsCanto flow cytometer (BD Biosciences). The antibodies used were CD27-fluorescein isothiocyanate (FITC) (clone M-T271), CD31-phycoerythrin (PE) (clone L133.1), CD4-peridinin chlorophyll protein (clones SK3 and SK4), CD41-FITC (clone HIP8) (which was used to exclude the potential effect of platelets on CD31 binding), and CD45RO-allophycocyanin (APC) (clone UCHL1), all from BD/Pharmingen (San Jose, CA). Gating on CD4+ CD27+ CD45RO− (naïve) T cells was used to determine the level of expression of CD31 (Fig. 1). The CD31+ population was defined by the use of an isotype control (mouse immunoglobulin G1 PE, clone X40). In addition, peripheral blood was collected in a heparinized syringe, and peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque separation (13). An aliquot from the heparinized blood was stained to assess the effect of different anticoagulants (EDTA and acid-citrate-dextrose), and no significant differences were observed (data not shown). A fraction of the PBMCs was processed immediately after separation for immunophenotyping, and the remaining PBMCs were cryopreserved. After 1 week, the cryopreserved PBMCs were thawed in a 37°C water bath, washed once in complete medium, counted, and assessed for viability with propidium iodide before they were immunophenotyped. The intra-assay and interassay variabilities were found to be low, with a coefficient of variation of <5%.
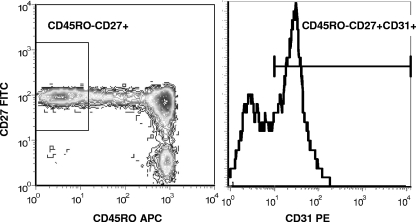
FIG. 1.
Example of gating strategy used to identify naïve CD4 T cells expressing CD31. Naïve cells were gated as CD45RO− and CD27+. The histogram of CD31 expression on naïve CD4 T cells is shown.
The effects of the delayed processing are summarized in Table 1, which reports the mean values and interquartile ranges (IQRs) for the HIV− and the HIV+ cohorts separately. A small but statistically significant overestimation of the percentage of CD31 expression on naïve CD4+ T cells was observed with delayed processing in both the HIV− study participants (+1.8 [P = 0.08] at 24 h compared to the level at 4 h, +3.3 [P = 0.003] at 48 h compared to the level at 4 h and +6.2 [P < 0.0001] at 72 h compared to the level at 4 h) and the HIV+ study participants (+1.6 [P = 0.03] at 24 h compared to the level at 4 h, +3.1 [P = 0.0002] at 48 h compared to the level at 4 h and +5.4 [P < 0.0001] at 72 h compared to the level at 4 h). Virtually all participants had an increased reading at 72 h compared to that at 4 h. From these data, it appears that samples could be held for 24 h or for up to 48 h for centralized processing in multisite trials with minimal overestimation of the level of CD31 expression on naïve CD4 T cells. Similar results were obtained when we compared the percent changes from the baseline (data not shown).
TABLE 1.
Percentage of naïve CD4+ T cells expressing CD31 in peripheral blood at 4, 24, 48, and 72 h
| Cell source | % (IQR) naïve CD4+ T cells in peripheral blood expressing CD31 at:
|
Difference in % (IQR) naïve CD4+ T cells in peripheral blood at 4 ha from the following time points:
|
|||||
|---|---|---|---|---|---|---|---|
| <4 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| HIV− subjects (n = 17) | 51.5 (44-57) | 53.2 (45-62) | 54.8 (42-62) | 57.7 (48-65) | −1.8 (−4.0 to 1.0)b | −3.3 (−7.0 to −1.0)c | −6.2 (−9.0 to −4.0)d |
| HIV+ subjects (n = 37) | 49.5 (41-57) | 51.2 (41-59) | 52.7 (42-60) | 55.0 (45-64) | −1.6 (−3.0 to 2.0)e | −3.1 (−5.0 to 1.0)f | −5.4 (−8.0 to −2.0)d |
Paired comparisons with the results for freshly isolated peripheral blood at <4 h were done by the paired t test.
P = 0.08.
P = 0.003.
P < 0.001.
P = 0.03.
P = 0.0002.
The effects of the PBMC isolation and cryopreservation are shown in Table 2, with the mean values and the IQR ranges reported for the HIV− and the HIV+ participants. There was no significant difference between the measurements for PBMCs in EDTA (peripheral blood stored for <4 h) and freshly isolated PBMCs, but the measurements for cryopreserved PBMCs were significantly greater than those for both peripheral blood in EDTA and freshly isolated PBMCs for the HIV+ participants. The effects of PBMC isolation and cryopreservation varied significantly from person to person (data not shown). One plausible explanation may be that when the cells are separated and cryopreserved, there is an enrichment of the pool of naïve T cells (CD4+ CD27+ CD45RO−), with higher levels of expression of CD31. The results were the same for all 54 participants, including the HIV+ and the HIV− participants.
TABLE 2.
Percentage of naïve CD4+ T cells expressing CD31 in peripheral blood, freshly isolated PBMCs, and cryopreserved PBMCs and absolute differences in percentages in PBMCs or cryopreserved PBMCs from that in peripheral blooda
| Cell source | Mean % (IQR) naïve CD4+ T cells expressing CD31 in:
|
Difference (IQR) between % of CD31-expressing naïve CD4+ T cells in PB and that inb:
|
P value Cryopreserved PBMCs
|
||||
|---|---|---|---|---|---|---|---|
| PB | PBMCs | Cryopreserved PBMCs | PBMCs | Cryopreserved PBMCs | PB vs freshly isolated PBMCs | PB vs cryopreserved PBMCs | |
| HIV− subjects (n = 17) | 51.5 (44-57) | 50.7 (43-58) | 54.2 (44-65) | 0.8 (−1.0 to 4.0) | −2.7 (−8.0 to 0.0) | 0.5 | 0.18 |
| HIV+ subjects (n = 37) | 49.5 (41-57) | 47.7 (34-61) | 54.8 (41-68) | 1.8 (−3.0 to 7.0) | −5.3 (−11.0 to −1.0) | 0.4 | 0.003 |
PB, peripheral blood.
Values are absolute differences of peripheral blood (tested at <4 h after collection) from PBMCs or cryopreserved PBMCs.
c P values from paired t test comparing peripheral blood (tested at <4 h after collection) and PBMCs or cryopreserved PBMCs.
These data suggest that cryopreservation can result in small but systematic overestimations of the proportion of naïve T cells expressing CD31 in both HIV+ and HIV− persons. Since many functional T-cell assays and TREC measurements are performed with separated and/or cryopreserved PBMCs, this should be taken into consideration, particularly when freshly isolated cells are being compared with cryopreserved cells. Finally, although optimal results were obtained with immediate processing after the blood was drawn, the overestimation of CD31 expression on naïve CD4+ T cells would be acceptable for most laboratories for up to 48 h, which would allow off-site flow cytometry to be performed for multicenter clinical trials.
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract NO1-CO-12400.
The content of this publication does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Footnotes
Published ahead of print on 30 April 2008.
REFERENCES
- 1.DeLisser, H. M., P. J. Newman, and S. M. Albelda. 1994. Molecular and functional aspects of PECAM-1/CD31. Immunol. Today 15:490-495. [DOI] [PubMed] [Google Scholar]
- 2.DeLisser, H. M., P. J. Newman, and S. M. Albelda. 1993. Platelet endothelial cell adhesion molecule (CD31). Curr. Top. Microbiol. Immunol. 184:37-45. [DOI] [PubMed] [Google Scholar]
- 3.Demeure, C. E., D. G. Byun, L. P. Yang, N. Vezzio, and G. Delespesse. 1996. CD31 (PECAM-1) is a differentiation antigen lost during human CD4 T-cell maturation into Th1 or Th2 effector cells. Immunology 88:110-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Paoli, P., M. Reitano, S. Battistin, C. Castiglia, and G. Santini. 1984. Enumeration of human lymphocyte subsets by monoclonal antibodies and flow cytometry: a comparative study using whole blood or mononuclear cells separated by density gradient centrifugation. J. Immunol. Methods 72:349-353. [DOI] [PubMed] [Google Scholar]
- 5.Kimmig, S., G. K. Przybylski, C. A. Schmidt, K. Laurisch, B. Mowes, A. Radbruch, and A. Thiel. 2002. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J. Exp. Med. 195:789-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landay, A. L., and K. A. Muirhead. 1989. Procedural guidelines for performing immunophenotyping by flow cytometry. Clin. Immunol. Immunopathol. 52:48-60. [DOI] [PubMed] [Google Scholar]
- 7.Langford, D., A. Grigorian, R. Hurford, A. Adame, R. J. Ellis, L. Hansen, and E. Masliah. 2004. Altered P-glycoprotein expression in AIDS patients with HIV encephalitis. J. Neuropathol. Exp. Neurol. 63:1038-1047. [DOI] [PubMed] [Google Scholar]
- 8.Luciano, A. A., M. M. Lederman, A. Valentin-Torres, D. A. Bazdar, and S. F. Sieg. 2007. Impaired induction of CD27 and CD28 predicts naive CD4 T cell proliferation defects in HIV disease. J. Immunol. 179:3543-3549. [DOI] [PubMed] [Google Scholar]
- 9.Mandy, F. F., J. K. Nicholson, and J. S. McDougal. 2003. Guidelines for performing single-platform absolute CD4+ T-cell determinations with CD45 gating for persons infected with human immunodeficiency virus. MMWR Recommend. Rep. 52:1-13. [PubMed] [Google Scholar]
- 10.Read, S. W., J. Higgins, J. A. Metcalf, R. A. Stevens, A. Rupert, M. C. Nason, H. C. Lane, and I. Sereti. 2006. Decreased CD127 expression on T cells in HIV-1-infected adults receiving antiretroviral therapy with or without intermittent IL-2 therapy. J. Acquir. Immune Defic. Syndr. 42:537-544. [DOI] [PubMed] [Google Scholar]
- 11.Reimann, K. A., M. Chernoff, C. L. Wilkening, C. E. Nickerson, A. L. Landay, and the ACTG Immunology Advanced Technology Laboratories. 2000. Preservation of lymphocyte immunophenotype and proliferative responses in cryopreserved peripheral blood mononuclear cells from human immunodeficiency virus type 1-infected donors: implications for multicenter clinical trials. Clin. Diagn. Lab. Immunol. 7:352-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romeu, M. A., M. Mestre, L. Gonzalez, A. Valls, J. Verdaguer, M. Corominas, J. Bas, E. Massip, and E. Buendia. 1992. Lymphocyte immunophenotyping by flow cytometry in normal adults. Comparison of fresh whole blood lysis technique, Ficoll-Paque separation and cryopreservation. J. Immunol. Methods 154:7-10. [DOI] [PubMed] [Google Scholar]
- 13.Stevens, R. A., R. A. Lempicki, V. Natarajan, J. Higgins, J. W. Adelsberger, and J. A. Metcalf. 2006. General immunologic evaluation of patients with human immunodeficiency virus infection, p. 848-861. In B. Detrick, R. G. Hamilton, and J. D. Folds (ed.), Manual of molecular and clinical laboratory immunology. ASM Press, Washington, DC.