Abstract
The highly sensitive gamma interferon (IFN-γ) enzyme-linked immunosorbent spot (ELISPOT) assay permits the investigation of the role of cell-mediated immunity (CMI) in the protection of young children against influenza. Preliminary studies of young children confirmed that the IFN-γ ELISPOT assay was a more sensitive measure of influenza memory immune responses than serum antibody and that among seronegative children aged 6 to <36 months, an intranasal dose of 107 fluorescent focus units (FFU) of a live attenuated influenza virus vaccine (CAIV-T) elicited substantial CMI responses. A commercial inactivated influenza virus vaccine elicited CMI responses only in children with some previous exposure to related influenza viruses as determined by detectable antibody levels prevaccination. The role of CMI in actual protection against community-acquired, culture-confirmed clinical influenza by CAIV-T was investigated in a large randomized, double-blind, placebo-controlled dose-ranging efficacy trial with 2,172 children aged 6 to <36 months in the Philippines and Thailand. The estimated protection curve indicated that the majority of infants and young children with ≥100 spot-forming cells/106 peripheral blood mononuclear cells were protected against clinical influenza, establishing a possible target level of CMI for future influenza vaccine development. The ELISPOT assay for IFN-γ is a sensitive and reproducible measure of CMI and memory immune responses and contributes to establishing requirements for the future development of vaccines against influenza, especially those used for children.
The role of the cellular immune system in contributing to protection of humans against culture-confirmed clinical influenza remains poorly defined. While studies have measured cell-mediated immunity (CMI) against influenza virus in humans (9, 12, 28, 31, 39, 40, 45, 57), the role of CMI in protection against clinical influenza has not been established in the field, due to the technical difficulties of using these complex assays. Therefore, influenza vaccine development strategies rarely address the role of CMI in vaccine design, and investigators continue to fail to determine the role of CMI in protection of humans (44).
Instead, serum antibody, as most commonly determined using the hemagglutination inhibition (HAI) assay with protective levels established in experimental human influenza virus challenge studies, is most frequently used as a surrogate (52). However, serum HAI responses have not been proven to be predicative of efficacy, especially for live influenza virus vaccines, which might induce immune responses not elicited by conventional inactivated virus vaccines (7, 37), including inducing CMI and production of the antiviral cytokines gamma interferon (IFN-γ) and IFN-α (11, 31, 58). In human challenge studies, protection against influenza virus may occur in the absence of a detectable antibody response (64), and subjects may be protected despite the lack of a measurable antibody response to vaccination. From studies characterizing the immune response following intranasal administration of monovalent live attenuated influenza virus vaccines, CMI has been considered to have a role in protection in adults and children that could not be entirely explained by mucosal or serum antibody responses (7, 41).
Young children are a recognized high-risk group for influenza virus infection and clinical disease as well as being a viral reservoir during influenza seasons (32, 38, 47, 55, 56). To date, little reliable efficacy information is available for trivalent inactivated influenza virus vaccines (TIV) for this age group, despite recommendations for the routine use of such vaccines (56, 70). Two recent clinical trials with young children have reported the superiority of live attenuated influenza virus vaccines (LAIV) over inactivated influenza virus vaccines in the prevention of influenza (2, 5). Further, there is a paucity of data characterizing the immune responses elicited by TIV in children, and there are no data linking responses to actual protective efficacy against culture-confirmed disease.
In two studies reported here, the induction by influenza vaccination of CMI responses in young children and the association between CMI responses and protection by a LAIV against culture-confirmed influenza in the field were investigated using a highly sensitive IFN-γ enzyme-linked immunosorbent spot (ELISPOT) assay (33, 36, 60, 61).
MATERIALS AND METHODS
Ethics committees' and institutional review boards' approvals.
Approvals for the study protocols and any amendments were obtained from all human ethics committees, institutional review boards, and any regional or national ethics committees at participating centers as applicable, prior to the commencement of any protocol-related activities.
Vaccine and placebo. (i) TIV.
A commercially available TIV (FluShield; Wyeth Laboratories Inc., Marietta, PA) was used. Each adult dose contained 15 μg of hemagglutinin (HA) antigens matched to the 2001-2002 influenza vaccine composition recommendations of the Vaccines and Related Biologicals Advisory Committee of the U.S. Food and Drug Administration, consisting of an influenza virus A/H1N1/A/New Caledonia/20/99-like virus, A/H3N2/Panama/2007/99 (an influenza virus A/H3N2/Moscow/10/99-like virus), and a B/Sichuan/379/99-like virus (15). In the exploratory immunogenicity study, TIV was administered according to the manufacturer's instructions as an intramuscular injection of 0.25 ml to young children.
(ii) CAIV-T.
The reassortant virus strains of the live attenuated trivalent influenza virus vaccine (CAIV-T) were supplied by MedImmune Vaccines (Mountain View, CA). The liquid formulation of CAIV-T and the saline placebo were manufactured and release tested by Wyeth (Marietta, PA). The reassortant vaccine strains were grown in specific-pathogen-free eggs, and the allantoic fluid, which contained the virus, was harvested, concentrated, and purified. The vaccine was formulated with sucrose-phosphate-glutamate, acid-hydrolyzed porcine gelatin, and arginine as stabilizers. The vaccine contained no preservatives and had a pH of 7.2 ± 0.5. The placebo consisted of sterile physiological saline manufactured by Wyeth (Marietta, PA). Approximately 0.1 ml of vaccine or placebo was administered into each nostril, for a total volume of 0.2 ml (63).
CAIV-T and the placebo were frozen and shipped to the study sites, where they were stored at 2°C to 8°C until just before intranasal administration using a spray applicator. Both CAIV-T doses and placebo were supplied in identically packaged sprayers; neither the study subjects, their parents or guardians, or the clinical personnel were aware of the treatment being administered.
The CAIV-T <105 preparations used in the exploratory immunogenicity study were formulated at 1.0 × 107 fluorescent focus units (FFU) of each of three reassortant vaccine virus strains and were subsequently heat treated at 45°C for 60 min in a water bath. The limit of detection of the release assay was determined to be 1 × 102.1 FFU/ml. Therefore, the actual dose was <1 × 101.4 FFU of each live vaccine virus strain. One fluorescent focus unit is equivalent to 1.0 50% tissue culture infective dose.
The CAIV-T dose forms used in the field efficacy evaluation were filled and formulated to the actual delivered dose (107.0 ± 0.5 FFU, 106.0 ± 0.5 FFU, or 105.0 ± 0.5 FFU) and were then shipped and stored at or below −20°C until they were dispensed at the site.
For the efficacy evaluation, the HA and neuraminidase (NA) antigens of the wild-type influenza virus strains used to generate the type A/H1N1, A/H3N2, and B vaccine reassortants for the CAIV-T formulation were antigenically representative of virus recommendations by the World Health Organization (WHO) for the 2001-2002 Northern Hemisphere influenza season. The WHO-recommended strains for that season were A/H1N1/New Caledonia/20/99-like, A/H3N2/Moscow/10/99-like, and B/Sichuan/379/99-like viruses. The A/Panama strain used in the vaccine was A/H3N2/Moscow/10/99-like. The B antigen used in the vaccine, B/Victoria/504/2000, was considered a B/Sichuan/379/99-like virus (69).
The 107.0 ± 0.5-FFU dose has previously been established to elicit a consistently high level of protective efficacy in children aged 6 to 36 months (2, 53, 57), including those in the Philippines and Thailand (63).
HAI assay.
In the preliminary immunogenicity study, blood samples were obtained prevaccination and again 28 days postvaccination. Samples were assayed for antibodies to influenza virus A/H1N1, A/H3N2, and B strains by an HAI assay. Seroconversion was defined as a ≥4-fold increase in the antibody titer (14).
IFN-γ ELISPOT determination.
Blood samples were taken before the first dose, 7 to 10 days after the first dose, and 7 to 10 days after the second dose for enumeration of IFN-γ spot-forming cells (SFC) by the ELISPOT assay.
Peripheral blood mononuclear cells (PBMC) were isolated at the study site via Ficoll Hypaque gradient centrifugation using Accuspin tubes (Sigma-Aldrich Corp., St. Louis, MO) and cryopreserved in aliquots of 5 × 106 cells/ml in cell culture freezing medium with dimethyl sulfoxide (GIBCO, Grand Island, NY). Cells were frozen using a rate-controlled freezing container (Nalgene, Rochester, NY) and were subsequently transferred to liquid nitrogen containers. The interval between the drawing of the blood and PBMC separation was 6 to 8 h. All PBMC samples were shipped to a central testing laboratory via MVE IATA cryoshippers (Princeton Cryotech, Whitehouse, NJ) and upon arrival were transferred to liquid nitrogen freezers and stored in the vapor phase until testing.
Qualified ELISPOT assays were conducted for the enumeration of PBMC secreting IFN-γ following a 20- to 24-h in vitro stimulation with the inactivated monovalent vaccine components (influenza A/H1N1, A/H3N2, and B viruses) that corresponded to the vaccine strains administered (1, 28). Phytohemagglutinin and allantoic fluid served as positive and negative controls in these assays. Spots were enumerated via image analysis using a series 1 ImmunoSpot image analyzer (Cellular Technology, Ltd., Cleveland, OH).
Field efficacy evaluation.
All children participating in the randomized, double-blind, placebo-controlled efficacy trial of CAIV-T were required to be in good health as determined by medical history, physical examination, and clinical judgment. Exclusion criteria included any serious chronic disease, including progressive neurologic disease, Down syndrome, or other cytogenetic disorder; any known or suspected disease of the immune system or receipt of immunosuppressive therapy, including systemic corticosteroids; receipt of any blood products, including immunoglobulin, in the 6 months before enrollment through the study's conclusion; immunosuppression or the presence of an immunocompromised household member; receipt of any commercial or investigational influenza vaccine before enrollment; a documented history of hypersensitivity to egg or egg protein or any other components of CAIV-T or the placebo; clinically confirmed respiratory illness with wheezing within the past 2 weeks; the receipt of aspirin (acetylsalicylic acid) or aspirin-containing products within the past 2 weeks; the receipt of another live virus vaccine within 1 month of vaccination in this study; or any medical condition(s) that in the opinion of the investigator might interfere with interpretation of the study results.
Each child was randomized to receive two sequential doses 35 ± 7 days apart of either one of three dose levels (107.0 ± 0.5 FFU, 106.0 ± 0.5 FFU, or 105.0 ± 0.5 FFU) of CAIV-T or saline placebo. Subjects were monitored for influenza through one subsequent influenza season, with surveillance concluding on 30 November 2002.
Surveillance and criteria for influenza illness.
In the evaluation for vaccine efficacy in young children, the criteria for obtaining a nasal swab sample were based on those previously reported (8, 63, 67). Surveillance for influenza-like illness began on the 11th day following receipt of the first vaccine dose and consisted of phone contacts, clinic visits, or home visits, as applicable. Nasal swab viral cultures were required if subjects had at least one of the following: fever (≥38°C rectal or ≥37.5°C axillary temperature), shortness of breath, pulmonary congestion, pneumonia, ear infection (acute otitis media, suspected or diagnosed), or wheezing. Nasal swab viral cultures were also required if subjects showed two or more of the following: runny nose or nasal congestion (rhinorrhea), sore throat (pharyngitis), cough, muscle aches, chills, headache, irritability, decreased activity, or vomiting. Cultures could also be obtained at the investigators' clinical discretion.
Nasal swab processing, viral culture, and influenza virus isolate characterization.
All isolates were cultured and typed by a central laboratory (Chinese University of Hong Kong, Hong Kong SAR, China) for influenza as previously described (2, 63).
Influenza virus isolates were identified by the Centers for Disease Control and Prevention (CDC, Atlanta, GA) using serological techniques. Identification assays, which included PCR and HA sequencing for the serotype, were also performed by Wyeth (Pearl River, NY). All efficacy analyses were based on PCR identification of cultured influenza viruses, with confirmation by serotyping if possible (16).
Statistical methods.
The post-second-dose value for determination of CMI in the field efficacy evaluation study was selected based on the observation from the preliminary study that this was the time point postvaccination with consistently higher responses.
In order for a value to be considered valid, the number of SFC obtained (before control background subtraction) had to be at least twice that for the appropriate background control (allantoic fluid). Additionally, values obtained on a specific sampling day had to be at least twice their appropriate day zero value to be considered a positive response. If the median of the stimulated counts minus the median of the medium counts was >1, then the value used in the analysis was the difference. Otherwise, the value used was 1. For the correlate analysis, inclusion of the medium counts was found not to add to or detract from the fit of the models, and thus the assay value was taken to be log[max(h3,)], where h3 represents the median count of stimulated SFC from the day 13 sample.
A scaled logit model was used to model the relationship between assay values (SFC/106 PBMC) and the occurrence of disease (21). The probability of disease was modeled as
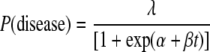 |
where t represents the assay value after logarithmic transformation. The value of log(½) was assigned if no SFC were observed; models were fitted by maximum likelihood. If it is assumed that both exposure and susceptibility are necessary for disease to occur, and that the ELISPOT fully captures the degree of protection, then λ̂ may be interpreted as an estimate of exposure and the expression 1/[1 + exp(α̂ + β̂t)] as an estimate of susceptibility, decreasing with increasing assay values. The expression 1 − {1/[1 + exp(α̂ + β̂t)]} is an estimate of protection, and a graph of this against assay values represents a “protection curve” (see Fig. 2). α is the location parameter of the protection curve, determining its left-right position, and β is the scale parameter, determining its steepness.
FIG. 2.
Estimated curve of protection against culture-confirmed clinical infection with wild-type influenza virus versus cell-mediated immune responses for all subjects (pooled). P(protection) is the estimated probability of being protected against clinical culture-confirmed influenza virus infection. The estimated protection curve (solid line) is charted with 95% CI (dashed lines) against the magnitude of the CMI response as determined using the number of IFN-γ SFC per 106 PBMC/ml.
The exposure parameter and protection curve were first estimated for all subjects taken together. The consistency of the exposure parameters and protection curves was examined when separate models were fitted for males and females, for each of the two countries, and for each of the four treatment groups. Confidence intervals (95%) for exposure parameters and protection curves were based on the observed Fisher information and the asymptotic normality of maximum-likelihood estimators. The goodness of fit of models was compared with likelihood ratio χ2 tests. The consistency of exposure parameters and protection curves was evaluated with Wald-type F tests.
RESULTS
Exploratory study. (i) HAI GMTs, GMFRs, and geometric mean antibody ratios.
Tables 1 and 2 summarize the pre- and postvaccination HAI geometric mean antibody titers (GMTs) as well as the pre- to postvaccination geometric mean fold rises (GMFRs) with 95% confidence intervals (95% CI) for each vaccine virus strain, i.e., A/H1N1/New Caledonia/20/99, A/H3N2/Panama/2007/99, and B/Yamanashi/166/98, of all subjects and seronegative subjects, respectively.
TABLE 1.
Serum HAI antibody titers and GMFRs in response to the A/H1N1, A/H3N2, and B virus strains in all subjects
| Strain and treatment | nb | Geometric meana HAI titer (95% CI)
|
GMFR (95% CI) | |
|---|---|---|---|---|
| Prevaccination | Postvaccination | |||
| A/H1N1 | ||||
| CAIV-T 107 | 40 | 3.86 (2.39, 6.24) | 6.96 (3.94, 12.30) | 1.8 (1.19, 2.74) |
| CAIV-T <105 | 40 | 4.84 (2.85, 8.23) | 4.84 (2.83, 8.27) | 1.00 (0.74, 1.35) |
| TIV | 42 | 5.56 (3.21, 9.63) | 13.34 (5.75, 30.95) | 2.40 (1.62, 3.54) |
| Placebo | 40 | 5.10 (3.01, 8.64) | 5.01 (2.99, 8.39) | 0.98 (0.68, 1.42) |
| A/H3N2 | ||||
| CAIV-T 107 | 40 | 4.07 (2.50, 6.63) | 28.34 (17.03, 47.16) | 6.96 (3.95, 12.27) |
| CAIV-T <105 | 40 | 3.93 (2.41, 6.42) | 4.68 (2.79, 7.83) | 1.19 (0.88, 1.61) |
| TIV | 42 | 4.00 (2.45, 6.52) | 6.90 (3.90, 12.19) | 1.72 (1.34, 2.21) |
| Placebo | 40 | 3.93 (2.41, 6.40) | 4.68 (2.76, 7.91) | 1.19 (0.91, 1.56) |
| B | ||||
| CAIV-T 107 | 40 | 5.76 (3.58, 9.27) | 16.00 (10.51, 24.35) | 2.78 (1.46, 5.29) |
| CAIV-T <105 | 39 | 6.28 (4.17, 9.45)c | 7.72 (4.79, 12.45) | 1.28 (0.88, 1.88) |
| TIV | 42 | 5.38 (3.60, 8.05) | 10.42 (5.49, 19.76) | 1.94 (1.25, 3.00) |
| Placebo | 39 | 5.51 (3.66, 8.29) | 7.45 (4.86, 11.43) | 1.35 (0.82, 2.22) |
Geometric mean, antilog of the mean of the log-transformed titer or fold rise.
n, number of subjects in the calculation.
A total of 40 subjects were included for this calculation.
TABLE 2.
Serum HAI antibody titers and GMFRs in response to the A/H1N1, A/H3N2, and B virus strains in seronegative subjectsa
| Strain and treatment | nc | Geometric meanb HAI titer (95% CI)
|
GMFR (95% CI) | |
|---|---|---|---|---|
| Prevaccination | Postvaccination | |||
| A/H1N1 | ||||
| CAIV-T 107 | 33 | 2.04 (1.96, 2.13) | 3.92 (2.68, 5.73) | 1.92 (1.31, 2.81) |
| CAIV-T <105 | 29 | 2.00 (2.00, 2.00) | 2.31 (1.72, 3.10) | 1.15 (0.86, 1.55) |
| TIV | 30 | 2.00 (2.00, 2.00) | 2.89 (2.27, 3.69) | 1.45 (1.14, 1.84) |
| Placebo | 30 | 2.00 (2.00, 2.00) | 2.52 (1.85, 3.42) | 1.26 (0.93, 1.71) |
| A/H3N2 | ||||
| CAIV-T 107 | 33 | 2.13 (1.98, 2.29) | 23.35 (13.91, 39.20) | 10.96 (6.63, 18.13) |
| CAIV-T <105 | 34 | 2.13 (1.98, 2.28) | 2.72 (1.98, 3.73) | 1.28 (0.92, 1.77) |
| TIV | 35 | 2.04 (1.96, 2.12) | 3.35 (2.58, 4.34) | 1.64 (1.27, 2.12) |
| Placebo | 34 | 2.17 (2.00, 2.35) | 2.72 (2.01, 3.66) | 1.25 (0.92, 1.70) |
| B | ||||
| CAIV-T 107 | 27 | 2.27 (2.04, 2.53) | 15.59 (9.18, 26.48) | 6.86 (3.98, 11.83) |
| CAIV-T <105 | 21 | 2.14 (1.94, 2.35) | 3.74 (2.19, 6.40) | 1.75 (1.01, 3.04) |
| TIV | 26 | 2.23 (3.01, 2.47) | 3.60 (2.23, 5.81) | 1.62 (1.06, 2.45) |
| Placebo | 24 | 2.31 (2.05, 2.61) | 4.62 (2.77, 7.70) | 2.00 (1.18, 3.38) |
A subject was seronegative to a particular strain if his or her prevaccination HAI titer to that strain was ≤1:4.
Geometric mean, antilog of the mean of the log-transformed titer or fold rise.
n, number of subjects in the calculation.
In general, the antibody levels and GMFRs in response to the A/H3N2 and B virus strains were higher among subjects who received CAIV-T 107 than among CAIV-T <105, TIV, and placebo recipients. For recipients of CAIV-T 107, the highest mean fold rises were observed in response to the A/H3N2 strain (6.96; 95% CI, 3.95, 12.3), followed by the B strain (2.78; 95% CI, 1.46, 5.29) and then the A/H1N1 strain (1.80; 95% CI, 1.19, 2.74).
Among all treatment groups, subjects who received TIV as treatment had the highest GMFRs in response to the A/H1N1 strain only (2.40; 95% CI, 1.62, 3.54), higher than those of CAIV-T 107 recipients. Among TIV recipients, the GMFR was 1.94 (95% CI, 1.25, 3.00) for the B strain and 1.72 (95% CI, 1.34, 2.21) for the A/H3N2 strain.
Immunological responses among seronegative subjects to each of the vaccine virus strains generally followed a pattern similar to that seen in all subjects (Table 2). Geometric mean antibody ratios of the CAIV-T 107 group to the other treatment groups showed that levels of antibody were higher in CAIV-T107 recipients than in all other treatment groups for the A/H3N2 and B virus strains. For the A/H1N1 strain, CAIV-T 107 recipients had higher levels of antibody than subjects receiving CAIV-T <105 or placebo but did not have higher antibody levels than TIV recipients.
Among seronegative subjects, the levels of HAI antibody to each viral strain following vaccination, including the A/H1N1 strain, were higher in CAIV-T 107 recipients than in all other treatment groups.
(ii) HAI seroconversion to CAIV-T virus strains.
Table 3 summarizes the number and proportion of subjects by serostatus and treatment group who seroconverted (defined as a rise in the HAI antibody titer greater than or equal to fourfold) to each of the three influenza vaccine virus strains, A/H1N1, A/H3N2, and B, following vaccination. The proportions of subjects who seroconverted following vaccination differed by serostatus, strain, and treatment group but followed the pattern observed for GMTs. Seroconversion rates were higher among subjects who received CAIV-T 107 as treatment than among those who received CAIV-T <105, TIV, or placebo, except for the seroconversion rate of TIV recipients to the A/H1N1 viral strain. Among CAIV-T 107 recipients, seroconversion rates were 70.0%, 52.5%, and 20.0% for the A/H3N2 strain, B strain, and A/H1N1 strain, respectively; the seroconversion rate of TIV recipients to A/H1N1 was 35.7%.
TABLE 3.
Serum HAI antibody seroconversion rates to the A/H1N1, A/H3N2, and B influenza virus strains
| Serostatus,b strain, and treatment | nc | Serum HAI seroconversiona
|
|
|---|---|---|---|
| No. (%) | Significanced | ||
| All subjects | |||
| A/H1N1 | |||
| CAIV-T 107 | 40 | 8 (20.0) | N/A |
| CAIV-T <105 | 40 | 2 (5.0) | 0.087 |
| TIV | 42 | 15 (35.7) | 0.143 |
| Placebo | 40 | 3 (7.5) | 0.193 |
| A/H3N2 | |||
| CAIV-T 107 | 40 | 28 (70.0) | N/A |
| CAIV-T <105 | 40 | 3 (7.5) | <0.001 |
| TIV | 42 | 10 (23.8) | <0.001 |
| Placebo | 40 | 4 (10.0) | <0.001 |
| B | |||
| CAIV-T 107 | 40 | 21 (52.5) | N/A |
| CAIV-T <105 | 39 | 7 (17.9) | 0.002 |
| TIV | 42 | 12 (28.6) | 0.042 |
| Placebo | 39 | 7 (17.9) | 0.002 |
| Seronegative subjects | |||
| A/H1N1 | |||
| CAIV-T 107 | 33 | 7 (21.1) | N/A |
| CAIV-T <105 | 29 | 1 (3.4) | 0.057 |
| TIV | 30 | 5 (16.7) | 0.754 |
| Placebo | 30 | 3 (10.0) | 0.308 |
| A/H3N2 | |||
| CAIV-T 107 | 33 | 27 (81.8) | N/A |
| CAIV-T <105 | 34 | 3 (8.8) | <0.001 |
| TIV | 35 | 7 (20.0) | <0.001 |
| Placebo | 34 | 4 (11.8) | <0.001 |
| B | |||
| CAIV-T 107 | 27 | 19 (70.4) | N/A |
| CAIV-T <105 | 21 | 5 (23.8) | 0.003 |
| TIV | 26 | 5 (19.2) | <0.001 |
| Placebo | 24 | 6 (25.0) | 0.002 |
Seroconversion to a particular virus strain was defined as a ≥1:4-fold rise from baseline in the titer of HAI antibody to that strain.
A subject was seronegative to a particular strain if his or her prevaccination HAI titer to that strain was ≤1:4.
Number of subjects with complete data.
P values based on comparison with the CAIV-T 107 treatment group were calculated by a two-sided Fisher exact test.
Seroconversion rates among seronegative subjects to each of the vaccine virus strains generally followed a pattern similar to that of all subjects. Comparisons of the seroconversion rates between the CAIV-T 107 group and all other treatment groups showed statistically significantly higher seroconversion rates among CAIV-T 107 recipients to the A/H3N2 and B virus strains (P ≤ 0.042). The difference in seroconversion rates to the A/H1N1 strain was not statistically significant.
(iii) Median number of PBMC secreting IFN-γ (SFC/106) as measured by an ELISPOT assay in response to influenza virus, and MFRs.
An ELISPOT assay was performed for samples collected on days 0, 6, and 13. Table 4 presents the median number of PBMC secreting IFN-γ as measured by the number of SFC per million PBMC pre- and postvaccination in response to stimulation with the A/H1N1, A/H3N2, or B strain as measured by the IFN-γ ELISPOT assay for samples collected on days 0, 6, and 13. Table 5 presents the corresponding pre- to postvaccination median fold rise (MFR) following stimulation of these cells with the respective vaccine strains, as measured by IFN-γ.
TABLE 4.
Median number of PBMC secreting IFN-γ (SFC/106) in response to influenza virus strains as measured at days 0, 6, and 13 by the ELISPOT assay
| Strain, serostatus,a and treatment | Day 0b
|
Day 6
|
Day 13
|
|||
|---|---|---|---|---|---|---|
| nc | Median SFC/106 PBMC (95% CI) | n | Median SFC/106 PBMC (95% CI) | n | Median SFC/106 PBMC (95% CI) | |
| A/H1N1 | ||||||
| All | ||||||
| CAIV-T 107 | 8 | 1 (1, 10) | 8 | 13 (1, 303) | 5 | 55 (37, 230) |
| CAIV-T <105 | 11 | 1 (1, 1) | 7 | 1 (1, 20) | 11 | 1 (1, 1) |
| TIV | 9 | 1 (1, 3) | 10 | 1 (1, 122) | 9 | 3 (1, 17) |
| Placebo | 8 | 1 (1, 2) | 8 | 1 (1, 23) | 9 | 1 (1, 13 |
| Seronegative | ||||||
| CAIV-T 107 | 6 | 1 (1, 3) | 6 | 8 (1, 303) | 5 | 55 (37. 230) |
| CAIV-T <105 | 9 | 1 (1, 1) | 6 | 1 (1, 1) | 10 | 1 (1, 1) |
| TIV | 5 | 1 (1, 1) | 6 | 1 (1, 1) | 5 | 1 (1, 3) |
| Placebo | 7 | 1 (1, 2) | 5 | 1 (1, 1) | 6 | 1 (1, 1) |
| A/H3N2 | ||||||
| All | ||||||
| CAIV-T 107 | 7 | 1 (1, 17) | 7 | 3 (1, 200) | 5 | 67 (23, 193) |
| CAIV-T <105 | 10 | 1 (1, 1) | 7 | 1 (1, 2) | 9 | 1 (1, 1) |
| TIV | 9 | 1 (1, 3) | 8 | 1 (1, 85) | 6 | 3 (1, 124) |
| Placebo | 7 | 1 (1, 1) | 7 | 1 (1, 1) | 6 | 1 (1, 10) |
| Seronegative | ||||||
| CAIV-T 107 | 6 | 1 (1, 3) | 6 | 2 (1, 30) | 4 | 104 (23, 193) |
| CAIV-T <105 | 10 | 1 (1, 1) | 7 | 1 (1, 2) | 8 | 1 (1, 1) |
| TIV | 5 | 1 (1, 1) | 5 | 1 (1, 3) | 3 | 1 (1, 2) |
| Placebo | 7 | 1 (1, 1) | 7 | 1 (1, 1) | 6 | 1 (1, 10) |
| B | ||||||
| All | ||||||
| CAIV-T 107 | 7 | 1 (1, 2) | 6 | 3 (1, 33) | 5 | 130 (1, 237) |
| CAIV-T <105 | 7 | 1 (1, 3) | 5 | 1 (1, 23) | 5 | 1 (1, 7) |
| TIV | 5 | 1 (1, 10) | 5 | 1 (1, 3) | 5 | 1 (1, 63) |
| Placebo | 5 | 1 (1, 13) | 4 | 1 (1, 1) | 5 | 1 (1, 15) |
| Seronegative | ||||||
| CAIV-T 107 | 4 | 1 (1, 2) | 3 | 3 (1, 33) | 3 | 213 (130, 237) |
| CAIV-T <105 | 3 | 1 (1, 1) | 1 | 1d | 3 | 1 (1, 1) |
| TIV | 4 | 1 (1, 1) | 4 | 1 (1, 3) | 3 | 1 (1, 1) |
| Placebo | 2 | 1 (1, 1) | 2 | 1 (1, 1) | 2 | 1 (1, 1) |
Subjects were defined as seronegative to a particular virus strain if their prevaccination HAI antibody titer to that strain was ≤1:4.
Prevaccination.
n, number of subjects in the calculation.
The 95% CI could not be calculated.
TABLE 5.
IFN-γ responses of children aged 6 to <36 months to influenza virus following vaccination
| Vaccine | A/H1N1
|
A/H3N2
|
B
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 6
|
Day 13
|
Day 6
|
Day 13
|
Day 6
|
Day 13
|
|||||||
| na | MFRb (95% CI) | n | MFR (95% CI) | n | MFR (95% CI) | n | MFR (95% CI) | n | MFR (95% CI) | n | MFR (95% CI) | |
| CAIV-T 107 | 8 | 7.15 (1.00, 303.0) | 5 | 55.0 (37.0, 227.0) | 7 | 1.00 (1.00, 30.0) | 4 | 36.5 (3.94, 193.0) | 6 | 3.00 (1.00, 20.0) | 4 | 73.5 (1.00, 237.0) |
| CAIV-T <105 | 7 | 1.00 (1.00, 1.18) | 10 | 1.00 (1.00, 1.00) | 7 | 1.00 (0.67, 1.00) | 7 | 1.00 (1.00, 1.00) | 3 | 1.00 (1.00, 10.0) | 4 | 1.00 (1.00, 7.00) |
| TIV | 7 | 1.00 (1.00, 29.0) | 9 | 3.00 (1.00, 5.67) | 6 | 2.00 (1.00, 3.00) | 6 | 2.50 (1.00, 41.3) | 2 | 1.00 (1.00, 1.00) | 2 | 1.00 (1.00, 1.00) |
| Placebo | 6 | 1.00 (1.00, 1.00) | 6 | 1.00 (0.50, 1.00) | 6 | 1.00 (1.00, 1.00) | 4 | 1.00 (1.00, 1.00) | 3 | 1.00 (0.08, 1.00) | 3 | 1.00 (1.00, 1.00) |
Number of subjects in the analysis. This number was largely dependent on the number of subjects with sufficient blood volume drawn to enable the conduct of the assay.
MFR, median fold rise in the postvaccination compared with the prevaccination sample.
Overall, the IFN-γ responses observed for each of the three virus strains after vaccination were minimal for TIV, CAIV-T <105, and placebo recipients. For subjects who received CAIV-T 107, the levels of response were substantial; responses were greatest on day 13 and with use of the inactivated viral antigen test stimulus. Specifically, the MFRs among CAIV-T 107 recipients using inactivated test stimulus on day 13 were highest in response to the B strain (73.5; 95% CI, 1.00, 237), followed by A/H1N1 (55.0; 95% CI, 37.0, 227) and then A/H3N2 (36.5; 95% CI, 3.94, 193). Weak responses were evident among some of the TIV recipients.
IFN-γ responses to each of the vaccine virus strains among seronegative subjects generally followed patterns similar to those of all subjects.
A high proportion of children tested seronegative by HAI, especially for both influenza A virus strains: 75.3% for influenza virus A/H1N1 (range, 71.4% to 82.5%), 84.0% for influenza virus A/H3N2 (range, 83.3% to 85.0%), and 61.0% for influenza virus B (range, 52.5% to 67.5%). While some children had low background levels of IFN-γ SFC prior to vaccination, the median value for all three influenza virus strains was 1.00 SFC/106 PBMC. Further, it was observed that while CAIV-T 107 elicited substantial IFN-γ SFC in those children considered seronegative by HAI assay titers, the few children responding to TIV were confined to those with some previous exposure to the applicable or related influenza virus as determined by a detectable HAI titer.
Field evaluation of children.
For 6 weeks from 21 February 2002, 2,172 children aged 6 to <36 months (mean, 21.1 months; range, 6.0 to 35.9 months) were enrolled at multiple centers in the Philippines (1,194 subjects) and Thailand (978 subjects).
A clear statistical trend in vaccine efficacy against culture-confirmed influenza illness caused by wild-type influenza virus antigenically similar to the vaccine strains was established across each of the dose levels of 107.0 ± 0.5 FFU, 106.0 ± 0.5 FFU, 105.0 ± 0.5 FFU, and saline placebo (Fig. 1). Maximum efficacy was achieved at the previously established dose of 107.0 ± 0.5 FFU.
FIG. 1.
Rates of attack by culture-confirmed wild-type influenza virus A/H3N2, antigenically similar to the vaccine virus, for young children by treatment group. n, total number of evaluable children in each study group. CAIV-T 10^7, 107.0 ± 0.5 FFU per dose; CAIV-T 10^6, 106.0 ± 0.5 FFU per dose; CAIV-T 10^5, 105.0 ± 0.5 FFU per dose. One fluorescent focus unit is approximately equal to 1 50% tissue culture infective dose. Saline was used as the placebo.
The predominant influenza virus associated with clinical illness was influenza virus A/H3N2, accounting for 96.4% of all community-acquired wild-type influenza virus isolates that were antigenically similar to the vaccine. Therefore, in evaluating the relationship between CMI and protection, only the relationship between IFN-γ SFC responses to influenza A/H3N2 virus from samples taken 7 to 10 days after the second dose and subsequent culture-confirmed illness due to influenza virus A/H3N2 was investigated.
Post-second-dose ELISPOT assay results for influenza A/H3N2 virus were obtained for 1,836 of the 2,107 subjects who met the criteria for inclusion in the primary analysis population. Of these, 227 subsequently developed clinical disease caused by community-acquired wild-type influenza A/H3N2 virus.
The estimated protection curve for all subjects is shown in Fig. 2, with a 95% confidence band. A significant level of contribution of CMI to protection against clinical influenza virus infection in young children, as measured by the IFN-γ ELISPOT assay, can be determined from Fig. 2. The exposure parameter was estimated at 0.201 (CI, 0.150, 0.253). There was a significant improvement in goodness of fit relative to logistic regression (P = 0.003). The estimated protection curve from this investigation indicated that only a small proportion of infants and young children with ≤10 SFC/106 PBMC could be considered “protected” against influenza, while the majority of subjects with ≥100 SFC/106 PBMC were protected.
A major potential use for this assay is as a correlate of protection. A desirable property of a correlate of protection is that its relationship to the occurrence of disease should be independent of other factors (54). The consistency of the relationship between assay values and subsequent influenza illness across gender, geographic location, and treatment groups was investigated.
There were no significant differences in the protection curves or exposure parameters across gender (P = 0.722 and P = 0.633, respectively).
Separate statistical models for each country indicated substantial differences in exposure rates (Fig. 3), estimated to be 0.287 in the Philippines and 0.149 in Thailand (P = 0.077). Separate protection curves also indicated that a difference in protection across countries could not be excluded (P = 0.163); the apparent difference was most marked between 10 and 1,000 SFC/106 PBMC. In this range, Thai children appeared to have slightly more protection than Filipino children, possibly due to higher exposure to influenza virus earlier in life. This may be attributable to the lack of seasonality of influenza in Thailand compared with the Philippines, resulting in a more continuous natural exposure to circulating wild-type influenza virus in Thailand, supported by the observation in this trial that both the length and the pattern of the influenza season differed between the countries (63). The duration of the influenza season was 7.2 months in Thailand, while in the Philippines there were two influenza seasons of less than 6 weeks each. The first season was associated with circulating influenza A/H3N2 virus. The second, later season was associated with circulating influenza B virus.
FIG. 3.
Estimated curves of protection against culture-confirmed clinical infection with wild-type influenza virus versus cell-mediated immune responses by country. P(protection) is the estimated probability of being protected against clinical culture-confirmed influenza virus infection. The estimated protection curves for subjects from each of the two participating countries, Thailand and the Philippines, are charted against the magnitude of the CMI response as determined using the number of IFN-γ SFC per 106 PBMC/ml.
There remains a level of protection conferred by CAIV-T that cannot be accounted for by CMI alone, as determined using IFN-γ ELISPOT assays. Separate protection curves for the four treatment groups were markedly consistent, as shown in Fig. 4 (P = 0.878), but differences between groups were observed in the estimated exposure parameters, which increased from 0.101 among subjects in the CAIV-T 107 group to 0.229 among placebo recipients (P = 0.054). Since subjects were randomly assigned to treatment groups, their exposure to influenza would have been approximately equal; the differences in exposure parameters thus may be taken to reflect effects of treatment not captured by assay values, or “extra-assay protection.” The magnitude of the difference in estimated exposure parameters suggests considerable extra-assay protection among vaccinated subjects, increasing with increasing dosage.
FIG. 4.
Estimated curves of protection against culture-confirmed clinical infection with wild-type influenza virus versus cell-mediated immune responses for the four treatment groups. P(protection) is the estimated probability of being protected against clinical culture-confirmed influenza virus infection. The estimated protection curves for each of the four treatment groups are charted against the magnitude of the CMI response as determined using the number of IFN-γ SFC per 106 PBMC/ml.
Role of CMI in protection against influenza viruses of distinct antigenic lineages.
Finally, the opportunity to investigate the role of CMI in cross-protection was provided through an epidemic during the surveillance period due to an influenza virus B strain that did not match the influenza B virus in the vaccine. There was no significant difference in influenza attack rates across the CAIV-T dose levels or placebo (4.38%, 5.28%, 5.6%, and 4.65%; P = 0.287), suggesting no significant difference in vaccine efficacy.
DISCUSSION
In the exploratory immunogenicity study, in agreement with the findings of other studies of children, a single dose of CAIV-T 107 generated the most consistent serum HAI antibody responses, although the level of response differed by vaccine strain and serostatus. In general, the HAI antibody levels for each of the three virus strains were higher among subjects who received CAIV-T 107 than among the other treatment groups. Specifically, among CAIV-T 107 recipients, serum HAI antibody responses to the A/H3N2 strain were greatest, followed by the B strain and then the A/H1N1 strain. TIV recipients exhibited the greatest antibody response to the A/H1N1 strain of all the treatment groups. Higher rates of seroconversion to the A/H3N2 virus strain and B strain for CAIV-T 107 recipients than for every other treatment group were statistically significant.
Levels of antibody to influenza virus in serum, as measured by the HAI assay, whether elicited by naturally acquired infection or through vaccination, have long been considered a correlate of protection against clinical influenza. More accurately, though, the studies to date investigating the role of serum antibody as a correlate have found that the protective levels of antibody have differed considerably (4, 24, 25, 46, 51). A series of studies with healthy adults vaccinated with either TIV or LAIV followed by challenge with wild-type H1N1 or H3N2 viruses demonstrated that serum HAI antibody correlated with protection against viral replication after TIV but not LAIV vaccination (18). This finding was supported by a more recent investigation that demonstrated that a LAIV containing A/Beijing/262/95(H1N1) elicited relatively low rates of seroconversion to A/New Caledonia/20/99(H1N1) virus in seronegative children, as measured by an HAI assay, that were markedly lower than the level of efficacy demonstrated in the community efficacy trial (37). Another earlier trial found an association between serum HAI antibody and protection but found that some other factor was contributing to the protection of vaccinated subjects who were seronegative (7). In that study, some vaccinated children had neither serum HAI antibody nor nasal wash immunoglobulin A, and it was postulated that an alternative immune mechanism, such as cellular immunity, may have contributed to protection by the live vaccine.
While there is no question from the previous investigations using CAIV-T or other LAIV in young children that serum HAI antibody is associated with protection against influenza, no linear correlation between protection from influenza and antibody titers has been established for any LAIV.
It is generally accepted that CMI has a significant role in recovery from clinical disease caused by influenza virus infection as well in the prevention of the development of influenza-associated complications, but it does not seem to contribute significantly to preventing infection (20, 35). However, the possible beneficial effects of the induction of T-cell-mediated immunity by vaccination in ameliorating the severity of influenza in humans remains largely unexplored (44).
CMI can be differentiated into TH1 and TH2 types based on the production of specific cytokine profiles. For TH1 and TH2 responses, the major cytokines are IFN-γ and interleukin-4, respectively (60, 65). TH1 responses, through the production of IFN-γ, mediate the killing of organisms responsible for a variety of intracellular infections (60), and as such IFN-γ is the cytokine that mediates protection. However, while NK cells of the innate immune system also are a significant source of IFN-γ (27), it has been demonstrated that for influenza A viruses, this production is dependent on the generation of influenza A virus-specific T lymphocytes (30).
IFN-γ has been measured using a variety of techniques, including flow cytometry and determination of IFN-γ concentrations in the supernatants of ex vivo-stimulated lymphocytes by enzyme-linked immunosorbent assay (29, 50, 66), but it was the emergence of the ELISPOT assay that provided a simple measure of CMI, utilizing a low volume of blood, with the potential for large-scale application in the field. The volume of blood required has been identified as a critical factor in measuring CMI in very young children and infants (62). However, there remain very few studies of CMI in very young children, and until this report, none have addressed the relationship between CMI and vaccine efficacy in a field setting.
Before proceeding with the application of the IFN-γ ELISPOT assay to the investigation of the role of CMI in the protection of very young children and infants against influenza in a community setting, we sought to confirm previous findings on the elicitation of IFN-γ in children following vaccination.
It has been observed previously that for children between the ages of 5 and 9 years, a single intranasal dose of live attenuated influenza virus vaccine (CAIV-T) elicited significant mean increases in the percentage of influenza A virus-reactive IFN-γ-positive cells in T-lymphocyte and NK cell subsets, as measured by flow cytometry following in vitro stimulation with influenza virus (31), and that a single intramuscular dose of TIV in these older children elicited lower mean CMI responses than CAIV-T.
In the exploratory study described in this paper, despite the fact that it was conducted with younger children, aged just 6 months to <36 months, and despite differences in methodology (use of an IFN-γ ELISPOT assay for CMI detection; reporting median values in defining responses), we observed similar findings. In our studies, a single intranasal dose of 107 FFU of CAIV-T consistently elicited significant CMI responses, while TIV elicited negligible responses. Although the latter observation differs from the report by He et al. in that they observed strong CMI responses to TIV in children aged 6 months to <5 years, they did not explore CMI responses elicited by CAIV-T in this younger age group (31). In both studies, the LAIV strains were provided by MedImmune, Inc., an identical single intranasal dose of CAIV-T was used, and the vaccine strains were based on the 6:2 cold-adapted temperature-sensitive reassortant influenza viruses originally described previously (8).
From the exploratory study, despite the small numbers of subjects, it was clear that CAIV-T elicited more-frequent and higher-magnitude CMI responses than TIV and as such represented the optimal vaccine for further evaluation of the relevance of these observations to vaccine efficacy.
It has been observed previously that responses elicited by LAIV, but not TIV, were inversely proportional to prevaccination levels of influenza virus-specific antibody for all age groups, suggesting a strong role of previous exposure to the antigen in eliciting IFN-γ responses upon reexposure (23, 31, 43, 53). One notable exception was that in a previous report, children aged 6 months to <5 years, with little evidence of previous exposure, were most responsive to TIV (31). There was no LAIV comparison; thus, those authors encouraged future studies to compare responses to TIV and a LAIV (e.g., CAIV-T) in this age group, as reported here.
In our study, the ability of CAIV-T to elicit CMI responses in children was similarly strongly affected by previous exposure to related or antigenically similar influenza viruses, either through vaccination or through natural exposure, as measured by prevaccination serum HAI levels.
Responses to TIV were elicited only in those children with detectable levels of preexisting antibody against influenza virus. This is consistent with the adult experience in that TIV seems to boost CMI and antibody responses only in those previously exposed to natural infection (28). In this study, the advantage of the CAIV-T 107 dose level was that it elicited strong CMI responses among children with no detectable previous exposure, who potentially had the highest susceptibility to influenza virus infection.
This difference may be explained through the known persistence of live vaccine virus in immunologically naïve children following administration of CAIV-T, which may contribute to the maintenance of memory cells (3, 26). Persistence of live influenza vaccine viruses has been observed previously: 67.4% of all children aged 6 to <36 months shed vaccine virus over a 7-day period following a single dose of CAIV-T 107 (68).
Since the frequency of T lymphocytes specific for a given infectious organism is low in children who have not previously encountered that pathogen, vaccination can cause the numbers of antigen-specific T lymphocytes to increase manyfold, leading to the formation of a population of long-lived memory T cells capable of responding rapidly to future infection in this population (10, 34). It was evident that both parenteral vaccination (TIV) and live attenuated virus vaccination could restimulate systemic CMI memory responses toward influenza virus that had initially been generated through natural infection, confirming that identifying the role of CMI in protection would prove difficult in these age groups (20). Therefore, for evaluation of the role of CMI in protection against clinically manifested, culture-confirmed influenza virus infection, very young children, who had the least prior exposure to influenza virus through vaccination or natural exposure and the maximal documented CMI responses after vaccination with CAIV-T, appeared the most appropriate candidate population. Further, the IFN-γ ELISPOT assay, as used in our exploratory study, appeared to provide comparable results across age groups, as reported previously, despite methodological differences.
For young children, we were able to demonstrate that CMI plays a significant role in protection against community-acquired clinical influenza virus infection, as determined by measuring differences in culture-confirmed clinical influenza attack rates across a range of CAIV-T doses. These ranged from a dose demonstrated to elicit suboptimal CMI (105 FFU) to the established protective dose (107 FFU). It has been reported that a certain threshold of effector TH1 CD8+ T (killer) lymphocytes may be required for protection against specific infections. For example, in a rodent malaria model, a threshold level of about 400 IFN-γ-secreting peptide-specific SFC/106 splenocytes is required to protect against sporozoite challenge (59).
The estimated protection curve from this investigation of CAIV-T indicated that the majority of infants and young children with ≥100 SFC/106 PBMC were protected against clinical influenza, establishing this as a possible target level for protection in future influenza vaccine development.
The major challenge confronting those performing complex immunological studies with human infants and very young children is obtaining a sufficient volume of blood or drawing blood frequently enough to perform an expanded range of assays in order to further characterize the immune responses, including serum HAI antibody responses (13, 29, 62, 66).
In this trial, ethical considerations at the trial sites prevented additional blood draws or increased blood volume to permit characterization of the serum antibody responses. However, it had been observed previously following influenza and hepatitis B surface antigen vaccination that there is no correlation between serum antibody responses and CMI as measured by IFN-γ levels (31, 50). Further, the presence of serum HAI antibody was associated with a reduced influenza B vaccine virus take in young children, although not with a linear correlation (8). The existence of another factor contributing to protection of vaccinated subjects who were seronegative by serum HAI antibody was also postulated by the authors of that study. Given the previously documented specific antibody responses elicited by CAIV-T vaccine in young children, it is reasonable to attribute a considerable part of the unaccounted-for protection identified in this study to specific antibodies.
CAIV-T has been demonstrated to elicit cross-reactive antibody and to confer a substantial degree of protection against heterologous, drifted influenza viruses (6, 42, 63). At least four field efficacy trials have demonstrated that immunization with a LAIV can protect against antigenically drifted influenza virus strains, in addition to providing protection against homologous influenza virus strains (6, 19, 22, 48). In a pivotal efficacy field trial of CAIV-T in children, the drifted variant A/H3N2/Sydney/5/97 caused the majority of disease in year 2 of the study (6). The formulation of CAIV-T used that season contained A/Wuhan as its H3N2 antigen; Wuhan and Sydney were significantly different antigenically, as determined by ferret antisera. CAIV-T was 86% efficacious at preventing culture-confirmed influenza due to A/H3N2/Sydney/5/97. In the same year, an effectiveness study of CAIV-T in adults demonstrated significant reductions in days of work lost among vaccinated adults versus placebo recipients (48).
However, in this study, although CMI responses to the homologous (B/Yamagata-lineage) virus were elicited by the CAIV-T 107 dose level, these do not appear to be effective in contributing to protection against clinical illness with a significantly divergent influenza B virus (49). A previous efficacy trial performed with children aged 6 to <36 months attending day care demonstrated that CAIV-T showed efficacy against influenza B viruses antigenically similar to the vaccine but also showed reduced efficacy by the B/Yamagata-lineage vaccine virus against circulating wild-type B/Victoria/87-lineage viruses (B/Hong Kong/1351/02-like and B/Hong Kong/330/01-like viruses) (67).
Modern influenza B viruses are derived from one of two lineages: either B/Yamagata or B/Victoria/87. The CAIV-T formulation used in this trial contained a B/Yamagata-lineage vaccine virus. The influenza B epidemic that occurred during the trial comprised influenza B/Victoria/87-lineage viruses (B/Hong Kong/1351/02-like and B/Hong Kong/330/01-like viruses). These are not considered drift strains and as such may share fewer common antigens (17).
This investigation represents the most thorough exploration of the role of CMI in protecting humans from influenza following vaccination. The efficacy evaluation is the single largest demonstration for any infectious disease of a quantitative role of CMI in protection against culture-confirmed infection, irrespective of pathogen, especially in children. It can be concluded from these studies that the ELISPOT assay for IFN-γ is a sensitive and reproducible measure of CMI and memory immune responses in all age groups, from young children to elderly adults, with application in a field setting. Further, the assay enabled the detection of previous exposure as well as the identification of immune responses following vaccination that might not otherwise be observable.
These findings contribute significantly to our understanding of the immune correlates of protection in influenza, as well as furthering our understanding of the requirements for generating and sustaining cellular immune responses. Further, because these findings can be used to guide predictions of the likelihood of protection induced following influenza vaccination, they define the requirements for future development of vaccines against influenza, especially those for use in children.
Acknowledgments
M.R.Z.C. and T.C. are members of the CAIV-T Cellular Immunology Group. Other members of the group include the following: S. Gatchalian, Rowena Tan, Victoria Victorio, Lydia Uñaliva, Carmen Nievera, Nancy Bermal, Thelma Laot, Marina Hernandez, and Eisel Go Palestroque, Research Institute for Tropical Medicine, Muntinlupa City, Philippines; Naris Waranawat, Warunee Punpanich, and Suriyadeo Tripathi, Queen Sirikit National Institute of Child Health, Bangkok, Siriwan Sirikwin, Rujanee Soontornkhajit, Chariya Sangsajja, Napha Chiragun, Sureeporn Kobkuachaiyapong, and Visal Moolasart, Bamrasnaradura Hospital, Nonthaburi, Kulkanya Chokephaibulkit, Nirun Vanprapar, Sanay Cherskul, Rangsima Lolekha, Wanatpreeya Phongsamart, Jirasak Voradilokkul, Pimanada Chearskul, and Sanay Chearskul, Siraraj Hospital, Mahidol University, Bangkok, Bhusdee Thamanavat, Anchalee Chaovavanich, Anchalee Aramtiantamrong, Boonrod Saengungkanawin, Sadhit Santadusit, Termsang Srisuwanporn, Piyarat Suntarattiwong, and Surachai Surangsirat, Nopparatrajathanee Hospital, Bangkok, Thailand; Isaac R. Melamed, 1st Allergy and Clinical Research Center, Englewood, CO; Russell T. Bain, Babies & Beyond Pediatrics, New Port Richey, FL; Colin Marchant, Boston University School of Medicine, Boston, MA; David P. Greenberg, Children's Hospital of Pittsburgh, Pittsburgh PA; Alan R Rosenthal, Milwaukee Center for Clinical Research, Milwaukee, WI; Eric J Goldberg, Palm Beach Research Center, West Palm Beach, FL; Deane Baldwin, Physician's Group Research Clinic, Little Rock, AR; Keith S. Reisinger, Primary Physicians Research, Pittsburgh, PA; Daniel Spada, PROHEALTH Physicians, Weatogue, CT; Leticia A Cerda, Quality Assurance Research Center, San Antonio, TX; Sharon A Nachman, State University of New York at Stony Brook, Stony Brook, NY; P. Lehman, Cellular Technology Ltd., Cleveland, OH; A. Klimov, Centers for Disease Control and Prevention, Atlanta, GA; and Melanie Saville (Taplow, United Kingdom), Barbara Holzknecht, Deborah Bertero, Guiseppe Palladino, Sheau-Mei Cheng, Jonathan Skinner, and Margaret Small (Pearl River, NY), and Michelle Mineo-Kuhn (Rochester, NY), Wyeth Vaccines Research.
The studies described in this article were funded by Wyeth Research, a division of Wyeth Pharmaceuticals. The design, data collection, management, analysis, and interpretation were conducted by Wyeth Vaccines Research in collaboration with the authors. The lead author (B.D.F.) wrote the report. The decision regarding submission of the paper was made by the lead author in collaboration with Wyeth Vaccines Research, MedImmune, and the other authors listed.
Footnotes
Published ahead of print on 30 April 2008.
REFERENCES
- 1.Anthony, D. D., H. Valdez, A. B. Post, N. L. Carlson, P. S. Heeger, and P. V. Lehmann. 2002. Comprehensive determinant mapping of the hepatitis C-specific CD8 cell repertoire reveals unpredicted immune hierarchy. Clin. Immunol. 103:264-276. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi, S., A. Vertruyen, J. Arístegui, S. Esposito, D. D. McKeith, T. Klemola, J. Biolek, J. Kühr, T. Bujnowski, D. Desgrandchamps, S. M. Cheng, J. Skinner, W. C. Gruber, B. D. Forrest, and the CAIV-T Study Group. 2006. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza virus vaccine in young children with recurrent respiratory tract infections. Pediatr. Infect. Dis. J. 25:870-879. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann, M. F., T. M. Kundig, H. Hengartner, and R. M. Zinkernagel. 1997. Protection against immunopathological consequences of a viral infection by activated but not resting cytotoxic T cells: T cell memory without ‘memory T cells’? Proc. Natl. Acad. Sci. USA 94:640-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belshe, R. B. 2004. Current status of live attenuated influenza virus vaccine in the US. Virus Res. 103:177-185. [DOI] [PubMed] [Google Scholar]
- 5.Belshe, R. B., K. M. Edwards, T. Vesikari, S. V. Black, R. E. Walker, M. Hultquist, G. Kemble, E. M. Connor, and the CAIV-T Comparative Efficacy Study Group. 2007. Live attenuated versus inactivated influenza virus vaccine in infants and young children. N. Engl. J. Med. 356:685-696. [DOI] [PubMed] [Google Scholar]
- 6.Belshe, R. B., W. C. Gruber, P. M. Mendelman, I. Cho, K. Reisinger, S. L. Block, J. Wittes, D. Iacuzio, P. Piedra, J. Treanor, J. King, K. Kotloff, D. I. Bernstein, F. G. Hayden, K. Zangwill, L. Yan, and M. Wolff. 2000. Efficacy of vaccination with live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J. Pediatr. 136:168-175. [DOI] [PubMed] [Google Scholar]
- 7.Belshe, R. B., W. C. Gruber, P. M. Mendelman, H. B. Mehta, K. Mahmood, K. Reisinger, J. Treanor, K. Zangwill, F. G. Hayden, D. I. Bernstein, K. Kotloff, J. King, P. A. Piedra, S. L. Block, L. Yan, and M. Wolff. 2000. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J. Infect. Dis. 181:1133-1137. [DOI] [PubMed] [Google Scholar]
- 8.Belshe, R. B., P. M. Mendelman, J. Treanor, J. King, W. C. Gruber, P. Piedra, D. I. Bernstein, F. G. Hayden, K. Kotloff, K. Zangwill, D. Iacuzio, and M. Wolff. 1998. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N. Engl. J. Med. 338:1405-1412. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein, E., D. Kaye, E. Abtruyn, P. Gross, and M. Dorfman. 1999. Immune response to influenza vaccination in a large healthy elderly population. Vaccine 17:82-94. [DOI] [PubMed] [Google Scholar]
- 10.Bevan, M. J. 2002. Remembrance of things past. Nature 420:748-749. [DOI] [PubMed] [Google Scholar]
- 11.Blazevic, V., C. M. Trubey, and G. M. Shearer. 2000. Comparison of in vitro immunostimulatory potential of live and inactivated viruses. Hum. Immunol. 61:845-849. [DOI] [PubMed] [Google Scholar]
- 12.Boon, A. C. M., E. Fringuelli, Y. M. F. Graus, R. A. M. Fouchier, K. Sintnicolaas, A. M. Iorio, G. F. Rimmelzwaan, and A. D. M. E. Osterhaus. 2002. Influenza A virus specific T cell immunity in humans during aging. Virology 299:100-108. [DOI] [PubMed] [Google Scholar]
- 13.Carson, M. M., D. W. Spady, J. A. Beeler, M. P. Krezolek, S. Audet, and H. F. Pabst. 2005. Follow-up of infants given measles vaccine at 6 months of age: antibody and CMI responses to MMRII® at 15 months of age and antibody levels at 27 months of age. Vaccine 23:3247-3255. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. 1998. The 1998-99 WHO influenza reagent kit for the identification of influenza isolates. Centers for Disease Control and Prevention, Atlanta, GA.
- 15.Centers for Disease Control and Prevention. 2001. Update: influenza activity—United States and worldwide, 2000-01 season, and composition of the 2001-02 influenza vaccine. MMWR Morb. Mortal. Wkly. Rep. 50:466-470. [PubMed] [Google Scholar]
- 16.Cheng, S. M., R. Vainionpää, P. Zhao, F. Li, A. Hu, B. Forrest, and R. Rappaport. 2004. Detection of influenza B in clinical specimens: comparison of high throughput RT-PCR and culture confirmation. Virus Res. 103:85-90. [DOI] [PubMed] [Google Scholar]
- 17.Chi, X. S., T. V. Bolar, P. Zhao, R. Rappaport, and S.-M. Cheng. 2003. Cocirculation and evolution of two lineages of influenza B viruses in Europe and Israel in the 2001-2002 season. J. Clin. Microbiol. 41:5770-5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clements, M. L., R. F. Betts, E. L. Tierney, and B. R. Murphy. 1986. Resistance of adults to challenge with influenza A wild-type virus after receiving live or inactivated virus vaccine. J. Clin. Microbiol. 23:73-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clover, R. D., S. Crawford, W. P. Glezen, L. H. Taber, C. C. Matson, and R. B. Couch. 1991. Comparison of heterotypic protection against influenza A/Taiwan/86 (H1N1) by attenuated and inactivated vaccines to A/Chile/83-like viruses. J. Infect. Dis. 163:300-304. [DOI] [PubMed] [Google Scholar]
- 20.Cox, R. J., K. A. Brokstad, and P. Ogra. 2004. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live attenuated influenza vaccines. Scand. J. Immunol. 59:1-15. [DOI] [PubMed] [Google Scholar]
- 21.Dunning, A. J. 2006. A model for immunological correlates of protection. Stat. Med. 25:1485-1497. [DOI] [PubMed] [Google Scholar]
- 22.Edwards, K. M., W. D. Dupont, M. K. Westrich, W. D. Plummer, Jr., P. S. Palmer, and P. F. Wright. 1994. A randomized controlled trial of cold-adapted and inactivated vaccines for the prevention of influenza A disease. J. Infect. Dis. 169:68-76. [DOI] [PubMed] [Google Scholar]
- 23.Evans, A. E., R. L. Simmons, R. D. Ferris, S. K. Hasell, E. Letley, and D. S. Freestone. 1979. Antibody responses to vaccination with WRL 105 strain live influenza vaccine in previously vaccinated and unvaccinated volunteers. J. Hyg. (London) 82:489-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox, J. P., M. K. Cooney, C. E. Hall, and H. M. Foy. 1982. Influenza virus infections in Seattle families, 1975-1979. II. Pattern of infection in invaded households and relation of age and prior antibody to occurrence of infection and related illness. Am. J. Epidemiol. 116:228-242. [DOI] [PubMed] [Google Scholar]
- 25.Foy, H. M., M. K. Cooney, R. McMahan, E. Bor, and J. T. Grayston. 1971. Single-dose monovalent A2/Hong Kong influenza vaccine. Efficacy 14 months after immunization. JAMA 217:1067-1071. [PubMed] [Google Scholar]
- 26.Gray, D., and P. Matzinger. 1991. T cell memory is short-lived in the absence of antigen. J. Exp. Med. 174:969-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guidotti, L. G., and F. V. Chisari. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19:65-91. [DOI] [PubMed] [Google Scholar]
- 28.Guthrie, T., C. G. L. Hobbs, V. Davenport, R. E. Horton, R. S. Heyderman, and N. A. Williams. 2004. Parenteral influenza vaccination influences mucosal and systemic T cell-mediated immunity in healthy adults. J. Infect. Dis. 190:1927-1935. [DOI] [PubMed] [Google Scholar]
- 29.Habermehl, P., A. Lignitz, M. Knuf, H.-J. Schmitt, M. Slaoui, and F. Zepp. 1999. Cellular immune response of a varicella vaccine following simultaneous DTaP and VZV vaccination. Vaccine 17:669-674. [DOI] [PubMed] [Google Scholar]
- 30.He, X.-S., M. Draghi, K. Mahmood, T. H. Holmes, G. W. Kemble, C. L. Dekker, A. M. Arvin, P. Parham, and H. B. Greenberg. 2004. T cell-dependent production of IFN-γ by NK cells in response to influenza A virus. J. Clin. Investig. 114:1812-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He, X.-S., T. H. Holmes, C. Zhang, K. Mahmood, G. W. Kemble, D. B. Lewis, C. L. Dekker, H. B. Greenberg, and A. M. Arvin. 2006. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J. Virol. 80:11756-11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heikkinen, T., H. Silvennoinen, V. Peltola, T. Ziegler, R. Vainionpää, T. Vuorinen, L. Kainulainen, T. Puhakka, T. Jartti, P. Toikka, P. Lehtinen, T. Routi, and T. Juven. 2004. Burden of influenza in children in the community. J. Infect. Dis. 190:1369-1373. [DOI] [PubMed] [Google Scholar]
- 33.Helms, T., B. O. Boehm, R. J. Asaad, R. P. Trezza, P. V. Lehmann, and M. Tary-Lehmann. 2000. Direct visualization of cytokine-producing recall-antigen-specific CD4 memory T cells in healthy individuals and HIV patients. J. Immunol. 164:3723-3732. [DOI] [PubMed] [Google Scholar]
- 34.Herremans, T. M. P. T., J. H. J. Reimerink, A. M. Buisman, T. G. Kimman, and M. P. G. Koopmans. 1999. Induction of mucosal immunity by inactivated poliovirus vaccine is dependent on previous mucosal contact with live virus. J. Immunol. 162:5011-5018. [PubMed] [Google Scholar]
- 35.Karzon, D. 1996. Cytotoxic T cells in influenza immunity. Semin. Virol. 7:265-271. [Google Scholar]
- 36.Lalvani, A., R. Brookes, S. Hambleton, W. J. Britton, A. V. S. Hill, and A. J. McMichael. 1997. Rapid effector function in CD8+ memory T cells. J. Exp. Med. 186:859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee, M. S., K. Mahmood, L. Adhikary, M. J. August, J. Cordova, I. Cho, G. Kemble, K. Reisinger, R. E. Walker, and P. M. Mendelman. 2004. Measuring antibody responses to a live attenuated influenza vaccine in children. Pediatr. Infect. Dis. J. 23:852-856. [DOI] [PubMed] [Google Scholar]
- 38.Longini, I. M., and M. E. Halloran. 2005. Strategy for distribution of influenza vaccine to high-risk groups and children. Am. J. Epidemiol. 161:303-306. [DOI] [PubMed] [Google Scholar]
- 39.McElhaney, J. E., S. Gravenstein, P. Krause, J. W. Hooton, C. M. Upshaw, and P. Drinka. 1998. Assessment of markers of the cell-mediated immune response after influenza virus infection in frail older adults. Clin. Diagn. Lab. Immunol. 5:840-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McElhaney, J. E., J. W. Hooton, N. Hooton, and R. C. Bleackley. 2005. Comparison of single versus booster dose of influenza vaccination on humoral and cellular immune responses in older adults. Vaccine 23:3294-3300. [DOI] [PubMed] [Google Scholar]
- 41.McMichael, A. J., F. M. Gotch, G. R. Noble, and P. A. Beare. 1983. Cytotoxic T-cell immunity to influenza. N. Engl. J. Med. 309:13-17. [DOI] [PubMed] [Google Scholar]
- 42.Mendelman, P. M., R. Rappaport, I. Cho, S. Block, W. Gruber, M. August, D. Dawson, J. Cordova, G. Kemble, K. Mahmood, G. Palladino, M. S. Lee, A. Razmpour, J. Stoddard, and B. D. Forrest. 2004. Live attenuated influenza vaccine induces cross-reactive antibody responses in children against A/Fujian/411/2002-like H3N2 antigenic variant strain. Pediatr. Infect. Dis. J. 23:1053-1055. [DOI] [PubMed] [Google Scholar]
- 43.Minor, T. E., E. C. Dick, C. R. Dick, and S. L. Inhorn. 1975. Attenuated influenza A vaccine (Alice) in an adult population: vaccine-related illness, serum and nasal antibody production, and intrafamily transmission. J. Clin. Microbiol. 2:403-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Müllbacher, A., M. Lobigs, M. Alsharifi, and M. Regner. 2006. Cytotoxic T-cell immunity as a target for influenza vaccines. Lancet Infect. Dis. 6:255-256. [DOI] [PubMed] [Google Scholar]
- 45.Murasko, D. M., E. D. Bernstein, E. M. Gardner, P. Gross, G. Munk, S. Dran, and E. Abrutyn. 2002. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp. Gerontol. 37:427-439. [DOI] [PubMed] [Google Scholar]
- 46.National Institutes of Health. 1973. Specific immunity in influenza B. Summary of influenza workshop III. J. Infect. Dis. 127:220-236. [Google Scholar]
- 47.Neuzil, K. M., Y. Zhu, M. R. Griffin, K. M. Edwards, J. M. Thompson, S. J. Tollefson, and P. F. Wright. 2002. Burden of interpandemic influenza in children younger than 5 years: a 25-year prospective study. J. Infect. Dis. 185:147-152. [DOI] [PubMed] [Google Scholar]
- 48.Nichol, K. L., P. M. Mendelman, K. P. Mallon, L. A. Jackson, G. J. Gorse, R. B. Belshe, W. P. Glezen, and J. Wittes for the Live Attenuated Influenza Virus Vaccine in Healthy Adults Trial Group. 1999. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. JAMA 282:137-145. [DOI] [PubMed] [Google Scholar]
- 49.Ohmit, S. E., J. C. Victor, J. R. Rotthoff, E. R. Teich, R. K. Truscon, L. L. Baum, B. Rangarajan, D. W. Newton, M. L. Boulton, and A. S. Monto. 2006. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N. Engl. J. Med. 355:2513-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ota, M. O. C., J. Vekemans, S. E. Schlegel-Haueter, K. Fielding, H. Whittle, P.-H. Lambert, K. P. W. J. McAdam, C.-A. Siegrist, and A. Marchant. 2004. Hepatitis B immunisation induces higher antibody and memory Th2 responses in new-borns than in adults. Vaccine 22:511-519. [DOI] [PubMed] [Google Scholar]
- 51.Potter, C. W., and J. S. Oxford. 1979. Determinants of immunity to influenza infection in man. Br. Med. Bull. 35:69-75. [DOI] [PubMed] [Google Scholar]
- 52.Potter, C. W., R. Jennings, K. Nicholson, D. A. Tyrrell, and K. G. Dickinson. 1977. Immunity to attenuated influenza virus WRL 105 infection induced by heterologous, inactivated influenza A virus vaccines. J. Hyg. (London) 79:321-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Powers, D. C., B. R. Murphy, L. F. Fries, W. H. Adler, and M. L. Clements. 1992. Reduced infectivity of cold-adapted influenza A H1N1 viruses in the elderly: correlation with serum and local antibodies. J. Am. Geriatr. Soc. 40:163-167. [DOI] [PubMed] [Google Scholar]
- 54.Prentice, R. L. 1989. Surrogate endpoints in clinical trials: definition and operational criteria. Stat. Med. 8:431-440. [DOI] [PubMed] [Google Scholar]
- 55.Reichert, T. A., N. Sugaya, D. S. Fedson, W. P. Glezen, L. Simonsen, and M. Tashiro. 2001. The Japanese experience with vaccinating schoolchildren against influenza. N. Engl. J. Med. 344:889-896. [DOI] [PubMed] [Google Scholar]
- 56.Rennels, M. B., H. C. Meissner, and Committee on Infectious Diseases. 2002. Technical report: reduction of the influenza burden in children. Pediatrics 110:e80. www.pediatrics.org/cgi/content/full/110/6/e80. [DOI] [PubMed] [Google Scholar]
- 57.Samdal, H. H., H. Bakke, F. Oftung, J. Holst, I. L. Haugen, G. E. Korsvold, A. C. Kristoffersen, G. Krogh, K. Nord, R. Rappuoli, A. K. Berstad, and B. Haneberg. 2005. A non-living nasal influenza vaccine can induce major humoral and cellular immune responses in humans without the need for adjuvants. Hum. Vaccines 1:85-90. [DOI] [PubMed] [Google Scholar]
- 58.Saurwein-Teissl, M., K. Zisterer, T. L. Schmitt, R. Gluck, S. Cryz, and B. Grubeck-Loebenstein. 1998. Whole virus influenza activates dendritic cells (DC) and stimulates cytokine production by peripheral blood mononuclear cells (PBMC) while subunit vaccines support T cell proliferation. Clin. Exp. Immunol. 114:271-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. Hill. 1998. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 4:397-402. [DOI] [PubMed] [Google Scholar]
- 60.Seder, R. A., and A. V. S. Hill. 2000. Vaccines against intracellular infections requiring cellular immunity. Nature 406:793-798. [DOI] [PubMed] [Google Scholar]
- 61.Speiser, D. E., M. J. Pittet, P. Guillaume, N. Lubenow, E. Hoffman, J. C. Cerottini, and P. Romero. 2004. Ex vivo analysis of human antigen-specific CD8+ T-cell responses: quality assessment of fluorescent HLA-A2 multimer and interferon-γ ELISPOT assays for patient immune monitoring. J. Immunother. 27:298-308. [DOI] [PubMed] [Google Scholar]
- 62.Svahn, A., A. Linde, R. Thorstensson, K. Karlén, L. Andersson, and H. Gaines. 2003. Development and evaluation of a flow-cytometric assay of specific cell-mediated immune response in activated whole blood for the detection of cell-mediated immunity against varicella-zoster virus. J. Immunol. Methods 277:17-25. [DOI] [PubMed] [Google Scholar]
- 63.Tam, J. S., M. R. Capeding, L. C. Lum, T. Chotpitayasunondh, Z. Jiang, L. M. Huang, B. W. Lee, Y. Qian, R. Samakoses, S. Lolekha, K. P. Rajamohanan, S. N. Narayanan, C. Kirubakaran, R. Rappaport, A. Razmpour, W. C. Gruber, B. D. Forrest, and the Pan-Asian CAIV-T Pediatric Efficacy Trial Network. 2007. Efficacy and safety of a live attenuated, cold-adapted influenza vaccine, trivalent against culture-confirmed influenza in young children in Asia. Pediatr. Infect. Dis. J. 26:619-628. [DOI] [PubMed] [Google Scholar]
- 64.Treanor, J., and P. F. Wright. 2003. Immune correlates of protection against influenza in the human challenge model. Dev. Biol. (Basel) 115:97-104. [PubMed] [Google Scholar]
- 65.Trinchieri, G. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 13:251-276. [DOI] [PubMed] [Google Scholar]
- 66.Vernacchio, L., H. Bernstein, S. Pelton, C. Allen, K. MacDonald, J. Dunn, D. D. Duncan, G. Tsao, V. LaPosta, J. Eldridge, S. Laussucq, D. M. Ambrosino, and D. C. Molrine. 2002. Effect of monophosphoryl lipid A (MPL®) on T-helper cells when administered as an adjuvant with pneumocococcal-CRM197 conjugate vaccine in healthy toddlers. Vaccine 20:3658-3667. [DOI] [PubMed] [Google Scholar]
- 67.Vesikari, T., D. M. Fleming, J. F. Arístegui, A. Vertruyen, S. Ashkenazi, R. Rappaport, J. Skinner, M. K. Saville, W. C. Gruber, B. D. Forrest, and the CAIV-T Pediatric Day Care Clinical Trial Network. 2006. Safety, efficacy, and effectiveness of live attenuated, trivalent influenza vaccine against community-acquired, culture-confirmed influenza in young children attending day care. Pediatrics 118:2298-2312. [DOI] [PubMed] [Google Scholar]
- 68.Vesikari, T., A. Karvonen, T. Korhonen, K. Edelman, R. Vainionpää, A. Salmi, M. K. Saville, I. Cho, A. Razmpour, R. Rappaport, R. O'Neill, A. Georgiu, W. Gruber, P. M. Mendelman, B. Forrest, and the CAIV-T Transmission Study Group. 2006. A randomized, double-blind study of the safety, transmissibility, and phenotypic and genotypic stability of cold-adapted influenza virus vaccine. Pediatr. Infect. Dis. J. 25:590-595. [DOI] [PubMed] [Google Scholar]
- 69.World Health Organization. 2001. Recommended composition of influenza virus vaccines for use in the 2001-2002 season. Wkly. Epidemiol. Rec. 76:58-61. [PubMed] [Google Scholar]
- 70.Zangwill, K. M., and R. B. Belshe. 2004. Safety and efficacy of trivalent inactivated influenza virus vaccine in young children: a summary for the new era of routine vaccination. Pediatr. Infect. Dis. J. 23:189-200. [DOI] [PubMed] [Google Scholar]






