Abstract
This report evaluated systemic inflammatory and immune biomarkers in a cohort of Macaca mulatta (rhesus monkeys) maintained as a large family social unit, including an age range from <1 year to >24 years. We hypothesized that the systemic host responses would be affected by the age, gender, and clinical oral presentation of the population, each contributing to inflammatory and immune responses that would reflect chronic oral infections. The results demonstrated that the prevalence and severity of periodontitis, including missing teeth, increased significantly with age. Generally, minimal differences in clinical parameters were noted between the genders. Systemic inflammatory mediators, including acute-phase reactants, prostaglandin E2 (PGE2), cytokines/chemokines, and selected matrix metalloproteinases (MMP), demonstrated significant differences among the various age groups of animals. Levels of many of these were increased with age, although PGE2, RANTES, bactericidal permeability-inducing factor (BPI), MMP-1, and MMP-9 levels were significantly increased in the young group (∼1 to 3 years old) relative to those for the older animals. We observed that in the adult and aged animals, levels of the systemic inflammatory mediators related to gingival inflammation and periodontal tissue destruction were significantly elevated. Serum antibody levels in response to a battery of periodontal pathogens were generally lower in the young animals, <50% of those in the adults, and were significantly related to aging in the cohort. The levels of antibodies, particularly those to Porphorymonas gingivalis, Fusobacterium nucleatum, and Tannerella forsythia, were most significantly elevated in animals with periodontal disease, irrespective of the age of the animal. These results provide a broad description of oral health and host responses in a large cohort of nonhuman primates from very young animals to the aged of this species. The findings afford a base of data with which to examine the ontogeny of host responses at mucosal sites, such as the gingival tissues.
Periodontal disease is the predominant chronic inflammatory disease of humanity (37, 38, 78, 82) and has been noted to occur naturally with increasing age in humans and nonhuman primates (36, 63, 69, 88). This oral disease is an outcome of complex oral infections, chronic immunoinflammatory responses, and resulting destruction of soft and hard tissues of the periodontium (37, 78, 80, 82, 84). In both humans and nonhuman primates, the extent of disease is predicted to be controlled by the quality and quantity of the host response and likely is modulated by systemic disease (48), environmental stressors (6, 76, 85), and the genetic backgrounds of the individuals (3, 70, 84).
The oral microbial characteristics of subgingival biofilms in younger and older individuals demonstrate differences in composition and complexity, which have been suggested to contribute directly to the microbial infections that trigger the destructive disease of oral tissues that occurs during aging (4, 35, 49, 53, 67, 83). It is clear that levels of gram-negative periodontal pathogens increase with age, although studies of young humans and nonhuman primates demonstrate that many microorganisms associated with periodontal pathogenesis are acquired early in life and become integrated into the commensal autochthonous oral microbial ecology (9, 29, 30, 56). However, it remains unclear how the age of the host impacts recognition of and response to these oral microorganisms.
Increasing evidence also suggests that these microorganisms can translocate from the oral cavity into the systemic circulation, enabling routine stimulation of the reticuloendothelial and immune systems, albeit generally in the absence of clinical symptoms of bacteremia (17, 19, 58, 65, 74, 77). Recent studies have provided clear data that the oral cavity can function as a nidus for a variety of potential medical problems (33, 42, 75). Bacterial infections frequently provide a strong stimulus for a systemic acute-phase response manifested by the increased production of some 25 plasma proteins (18, 22). Increased levels of acute-phase proteins have been identified in adult periodontitis patients and appear to reflect both the infection and the acute and chronic inflammation that exists in the periodontium (18, 39, 55). At the same time, it is clear that a serum antibody response to these localized infections exists and that it results from specific elicitation of antibody to an infecting microorganism (19, 24, 40, 41, 46, 79).
Periodontal disease has been effectively used as a model of host-bacterium interactions, inflammation, and chronic inflammatory diseases, particularly for the ability to longitudinally describe bacterial and host factors in the oral cavity and to correlate changes in these factors with pathological changes in the juxtaposed host tissues. The nonhuman primate model has provided a model with which to critically define the interaction of the subgingival microbiota with the host inflammatory/immune response in the maintenance of gingival homeostasis or the exacerbation of a chronic inflammatory process, leading to progression of the disease (20, 22, 59, 62, 68). This study described the characteristics of systemic inflammatory mediators and serum antibody responses to oral bacteria in nonhuman primates as functions of age and in relation to clinical measures of periodontitis. The accessibility of oral tissues and the development of chronic inflammation in the oral cavity in response to microbial biofilms will provide tools for examining the ontogeny of inflammatory/immune processes as related to disease expression in this animal model.
MATERIALS AND METHODS
Animals and diet.
Rhesus monkeys (Macaca mulatta) (n = 208), housed at the Caribbean Primate Research Center (CPRC) at Sabana Seca, Puerto Rico, were used in these studies; 112 of these animals were females, and 66 were males. The age of the animals ranged from ∼0.75 to >25 years, and they have been housed in a large community representing 3 or 4 generations with many individual family units based on a matriarchal family lineage. An additional group of 30 animals (age range, 0.8 to 2.8 years) raised under specific-pathogen-free (SPF) conditions was also evaluated and included 24 female and 6 male monkeys. The CPRC's SPF Program is a source of rhesus monkeys free of B virus (herpesvirus simiae or cercopithecine herpesvirus type 1), simian type D retrovirus, simian immunodeficiency virus, and simian T-lymphotropic virus 1. The monkeys are fed a 20% protein, 5% fat, and 10% fiber commercial monkey diet (diet 8773, Teklad NIB primate diet modified; Harlan Teklad). The diet is supplemented with fruits and vegetables, and water is provided ad libitum in an enclosed corral setting. This protocol was approved by the Institutional Animal Care and Use Committee of the University of Puerto Rico.
Oral clinical parameters.
All animals were examined, while anesthetized, by the same periodontal investigator in this study. The periodontal examination was conducted using a Maryland probe (William's markings) on the facial, mesiobuccal, and distobuccal aspects of all teeth, excluding the canines and third molars. The examination included probing pocket depth (PD), clinical attachment level (CAL), plaque index, and gingival bleeding index (bleeding on probing [BOP]) (13). The plaque index characterizes the extent and quantity of tooth-associated bacterial plaque. PD and CAL measures were made using a calibrated probe and are measures of the extent and severity of periodontal pathology. The presence and degree of bleeding upon gentle periodontal probing provided a measure of the presence of inflammation within the periodontal tissues.
Serum analyses.
Blood was collected from all animals, serum was prepared, and levels of immunoglobulin G (IgG) antibodies to seven oral bacteria were evaluated using an enzyme-linked immunosorbent assay (ELISA) as we have described previously (14, 15). Briefly, Campylobacter rectus, Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans, Prevotella intermedia, Treponema denticola, Tannerella forsythia, and Porphyromonas gingivalis were grown under anaerobic conditions in mycoplasma broth base with the addition of appropriate additives as we have reported previously (23). The bacteria were harvested by centrifugation, formalin killed, washed, and stored at −20°C for use as antigens (14, 15).
Selected systemic inflammatory biomarkers were quantified using ELISA procedures developed in our laboratory (C-reactive protein [CRP] [21]). Luminex Beadlyte technology was used for interleukin-8 (IL-8), monocyte chemoattractant protein 1 (MCP-1), and RANTES (Upstate, Temecula, CA) and for matrix metalloproteinase 1 (MMP-1), MMP-2, and MMP-9 (R&D Systems, Minneapolis, MN). Commercial ELISA kits were used for prostaglandin E2 (PGE2) (Assay Design, Ann Arbor, MI), lipopolysaccharide binding protein (LBP; Cell Sciences, Canton, MA), and bactericidal permeability-inducing factor (BPI; Cell Sciences, Canton, MA) in serum samples from all animals.
Statistical analyses.
An analysis of variance (ANOVA) with post hoc testing was used for the various continuous variables, including clinical parameters and serum analytes. This was accomplished using a one-way ANOVA and a Holm-Sidak test for parametric values, and a Kruskal-Wallis ANOVA with Dunn's method for multiple comparisons of variables not normally distributed. An α value of <0.05 was accepted as the level of significant difference in comparing the various parameters.
RESULTS
Systemic inflammatory mediators.
The levels of various systemic inflammatory mediators were determined in serum samples from each animal and segregated based on the age of the animal: young (<3 years), adolescent (3 to 8 years), adult (8 to 15 years), or aged (>15 years). Figure 1 summarizes the levels of the various inflammatory mediators with aging in this cohort of animals. CRP, LBP, and MCP-1 levels were significantly decreased in the young animals, and MCP-1 levels were elevated in the aged group. BPI, RANTES, MMP-1, and MMP-9 levels were all significantly elevated in the young and adolescent animals compared to adult and aged monkeys.
FIG. 1.
Acute-phase reactants and inflammatory mediators in serum samples from nonhuman primates of different age groups: young (<3 years) (n = 76), adolescent (≥3 to 8 years) (n = 61), adult (8 to 15 years) (n = 32), and aged (>15 years) (n = 10). Bars represent group means; error bars, 1 standard deviation. Statistical differences among the groups are displayed.
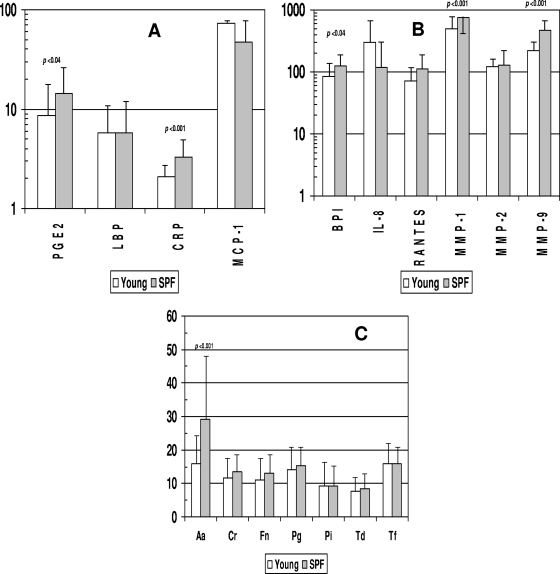
Figure 2A and B show comparisons of the levels of these inflammatory mediators in the sera of young animals within the large group cohort versus those detected in the sera of animals of similar ages maintained under SPF conditions. The results showed elevated levels of PGE2, CRP, BPI, MMP-1, and MMP-9 in the sera of the SPF animals compared to the young animals raised under standard housing conditions.
FIG. 2.
Acute-phase reactants (A), inflammatory mediators (B), and antibodies (C) in serum samples from nonhuman primates <3 years old raised under standard housing conditions (young) (n = 76) or under SPF conditions (n = 30). Bars represent mean levels of each mediator; error bars, 1 standard deviation. Statistical differences between the groups are shown. Aa, Aggregatibacter actinomycetemcomitans; Cr, Campylobacter rectus; Fn, Fusobacterium nucleatum; Pg, Porphyromonas gingivalis; Pi, Prevotella intermedia; Td, Treponema denticola; Tf, Tannerella forsythia. For panels A and B, PGE2, BPI, RANTES, MMP-2, and MMP-9 are measured in ng/ml; LBP and CRP are measured in μg/ml; and IL-8, MCP-1, and MMP-1 are measured in pg/ml (as in Fig. 1). For panel C, antibody levels are presented in ng/ml.
Systemic antibody responses to oral bacteria.
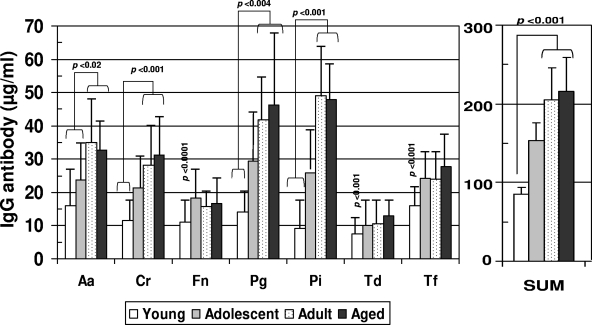
Figure 3 shows the levels of serum IgG antibodies to a group of oral bacteria commonly associated with periodontal disease (1, 34, 44) in the various age groups of nonhuman primates. The results show significantly lower antibody levels in the group of young animals. The adult and aged animals routinely demonstrated significantly elevated levels of antibodies to the individual species, with minimal differences between these age groups.
FIG. 3.
Serum IgG antibodies to individual oral bacteria and total antibodies to this battery (SUM) in nonhuman primates categorized on the basis of age (see the legend to Fig. 1). Bars represent mean antibody levels; error bars, 1 standard deviation. Statistical differences are depicted on the graph. For bacterium abbreviations, see the legend to Fig. 2.
Figure 2C provides an analysis of the serum antibody levels in the young animals housed under standard conditions compared to the young SPF animals. There were few differences in serum antibody levels between these groups; the SPF animals had levels at least as high as those of the standard group of animals.
Systemic responses and clinical parameters.
No clinical differences were observed between the male and female animals, although significant increases in disease parameters of BOP (0.75 ± 0.2 versus 0.95 ± 0.15 units; P < 0.05), PD (2.50 ± 0.10 versus 3.15 ± 0.40 mm; P < 0.04), and CAL (0.25 ± 0.05 versus 0.55 ± 0.15 mm; P < 0.05) were noted in the aged animals. The younger groups of animals demonstrated negligible plaque, inflammation, or gingival tissue changes. We stratified the adult and aged animals into two groups based on the bleeding index (mean, ≥0.9 or <0.9 units) or on mean pocket depth (≥3 mm or <3 mm). When the animals were grouped based on these clinical parameters, the differences in systemic responses were greater in animals with poorer oral health.
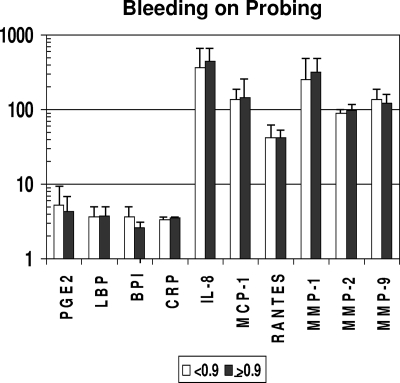
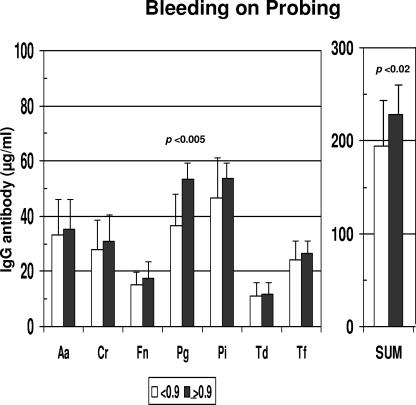
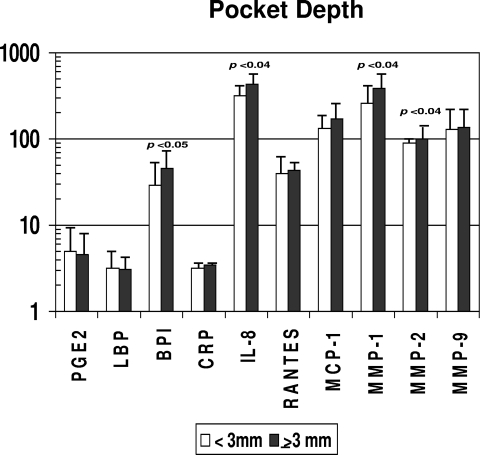
Consistent with the lack of differences in clinical presentation related to gender, no differences in serum inflammatory mediators or serum antibody levels were observed between the genders (data not shown). The results in Fig. 4 show the differences in inflammatory mediator levels between the groups stratified by gingival bleeding. No significant differences in these serum response analytes were observed between the groups. However, in Fig. 5, it can be noted that levels of antibody to P. gingivalis and the sum of antibodies to the seven target bacteria were significantly increased with greater gingival bleeding. In contrast, Fig. 6 and 7 depict significant differences in multiple inflammatory mediators (BPI, IL-8, MMP-1, MMP-2) and serum antibodies to F. nucleatum, P. gingivalis, T. forsythia, and the sum of antibodies with more-severe destructive disease, i.e., greater pocket depth.
FIG. 4.
Acute-phase reactants and inflammatory mediators in serum samples from nonhuman primates stratified on the basis of a low (<0.9) (n = 32) or high (≥0.9) (n = 9) mean index score for mouth bleeding upon probing. Bars represent group means; error bars, 1 standard deviation. PGE2, BPI, RANTES, MMP-2, and MMP-9 are measured in ng/ml; LBP and CRP are measured in μg/ml; and IL-8, MCP-1, and MMP-1 are measured in pg/ml (as in Fig. 1).
FIG. 5.
Serum IgG antibodies to individual oral bacteria and total antibodies to this battery (SUM) in nonhuman primates stratified on the basis of a low (<0.9) or high (≥0.9) index score for mean bleeding upon probing. Bars represent mean antibody levels; error bars, 1 standard deviation. Statistical differences are depicted on the graph. For bacterium abbreviations, see the legend to Fig. 2.
FIG. 6.
Acute-phase reactants and inflammatory mediators in serum samples from nonhuman primates stratified on the basis of a low (<3 mm) (n = 25) or high (≥3 mm) (n = 16) mean pocket depth in the mouth. Bars represent group means; error bars, 1 standard deviation. Statistical differences are depicted on the graph. PGE2, BPI, RANTES, MMP-2, and MMP-9 are measured in ng/ml; LBP and CRP are measured in μg/ml; and IL-8, MCP-1, and MMP-1 are measured in pg/ml (as in Fig. 1).
FIG. 7.
Serum IgG antibodies to individual oral bacteria and total antibodies to this battery (SUM) in nonhuman primates stratified on the basis of a low (<3 mm) or high (≥3 mm) mean pocket depth in the mouth. Bars represent mean levels; error bars, 1 standard deviation. Statistical differences are depicted on the graph. For bacterium abbreviations, see the legend to Fig. 2.
DISCUSSION
Evaluation of inflammatory and immune responses has provided evidence of ontogenic development of the immune system (2, 5, 52, 61, 64, 71, 81), as well as alterations in various host response parameters that are affected by aging (12, 28, 60, 87). However, while changes in oral health with aging similar to those reported for humans (88) have been reported to occur in various nonhuman primate species (7), little information on the biologic underpinnings of these clinical differences has been provided. While systemic inflammation, often resulting from bacterial sepsis, is frequently related to negative clinical outcomes with both morbidity and mortality, the systemic inflammatory response can also accomplish at the whole-organism level what the local inflammatory response is designed to do: that is, utilize disparate, nonspecific effector molecules to ameliorate potential tissue damage by noxious agents, including infecting bacteria (18). However, the characteristics of this ancient response system have generally been evaluated in adult individuals and have been related to sepsis, neoplastic changes, and responses to chronic diseases (18). This study demonstrated the patterns of selected systemic inflammatory molecules in young individuals and demonstrated specific changes in these levels with aging. Of particular note were the significantly elevated levels of BPI, RANTES, and both MMP-1 and MMP-9 in the younger groups of animals. This was unexpected, with minimal previous evidence of this type of response except that reported in obese children and adolescents (86) and a general concept of a lack of systemic challenge in children in the absence of clinical changes, but it is consistent with the maturation of host responses in young animals that have to cope with a range of environmental challenges to naïve mucosal surfaces. Testing this concept, we compared these systemic responses in young animals housed under standard conditions with those in comparably aged SPF monkeys. The results showed that the SPF animals often had elevated levels of the mediators compared to normal nonhuman primates. While there are likely various explanations for these differences, one existing theory, the “hygiene hypothesis,” suggests that the increasing incidence of asthma and other allergic diseases in the human population results from a lack of sufficient ontogenic development or “training” of the immune system in the young, who are then less able to effectively distinguish a noxious challenge later in their development (27, 50, 66, 89). Irrespective of the basis, these data demonstrate significant differences in response profiles of the inflammatory and innate immune systems during aging.
Nonhuman primates have historically been utilized to evaluate infectious agents (8, 32, 47, 54) and biologic processes (10, 16, 31, 51) associated with various human diseases. This is related to homologies in a range of host responses between humans and the other primate species, as well as species tropisms for infectious agents that cross human and nonhuman primate lines (26, 43, 73). These similarities extend to the microbial ecology and host responses in the oral cavity related to microbial biofilms that trigger periodontal disease (20, 22, 45, 62, 68). We observed that levels of serum antibody to various bacteria associated with periodontal-disease biofilms were significantly lower in the youngest animals. As is noted in humans and nonhuman primates, the extent of disease was increased in the aged group, although the antibody levels were similar for the adult and aged animals. This is consistent with the early acquisition and accumulation of these species as part of the commensal microbiota of the oral cavity and an association of these bacteria as etiologic triggers of periodontal pathology related to aging. We have also characterized the effects of aging on naturally occurring periodontitis, and we use a specific ligature-induced model of specific challenge to the oral cavity to describe acute responses of mucosal tissues during aging. In addition, this model has allowed us to document gender and diet effects on local and systemic inflammatory and immune responses that are altered with aging (25).
We then addressed specific questions regarding these systemic responses and the expression of chronic periodontal infections and inflammation in the oral cavities of adult and aged animals. As is noted in humans and nonhuman primates, the extent of disease was increased in the aged group, with no gender differences in expression of disease. This is in contrast to our findings with M. mulatta raised in individual housing with ad libitum feeding. Our previous results demonstrated that aged males exhibited significant weight gain, demonstrated various biologic parameters of unhealthy aging, and exhibited significantly greater periodontal disease than similarly aged females (unpublished data). Placing the males on a calorie-restricted diet decreased the disease to a level similar to that of the female cohort. The group of animals in this study was housed in a large corral that permitted constant exercise, required food-scavenging behaviors, and permitted natural competition among the various strata of colony members. Thus, the older males were much more physically fit than the sedentary singly housed animals, which may have translated into a preservation of oral health for aging males comparable to that for females.
We also stratified the animals, irrespective of age, based on the clinical presentation of gingival bleeding, a measure of local mucosal inflammation, and on pocket depth as an indicator of local challenge resulting in destructive disease. These subgroups were then evaluated for the patterns of systemic inflammatory and antibody responses. The results demonstrated rather minimal differences in the systemic responses related to the level of gingival inflammation in these adult and aged monkeys. In contrast, multiple significant differences were observed in both the inflammatory mediators and the levels of antibodies to oral bacteria in the animals with more severe periodontitis. These data for the nonhuman primates support similar data from humans indicating that the tissue destruction associated with chronic periodontitis enhances challenge of the systemic circulation with the potential to alter the function of the vascular and/or distant tissues (11, 48, 57, 72).
The literature is generally lacking on the use of nonhuman primates to elucidate the ontogenic development of the inflammatory, innate, and adaptive immune system. The oral cavity provides a readily accessible model of these host response changes at mucosal surfaces that interface with an evolving microbial ecology. This study described oral clinical findings and systemic responses in nonhuman primates and described differences in these measures that occurred from young through aged animals. The results from these initial studies of this primate colony provide a basis for the use of this robust resource to test hypotheses regarding the local and systemic ontogeny of innate and adaptive immune responses in relationship to the acquisition, adaptation, and evolution of the microbial ecology at this mucosal surface.
Acknowledgments
This work was supported by CPRC grant 5P40 RR003640 from the NCRR (National Institutes of Health) and a UKRF grant from the University of Kentucky.
We extend our gratitude to the entire technical support group from the Caribbean Primate Research Center facility, especially Edmundo Kraiselburd, for providing support for the conduct of this study.
Footnotes
Published ahead of print on 30 April 2008.
REFERENCES
- 1.Aas, J. A., B. J. Paster, L. N. Stokes, I. Olsen, and F. E. Dewhirst. 2005. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 43:5721-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham, C. M., and D. R. Ownby. 2005. Ontogeny of the allergic inflammatory response. Immunol. Allergy Clin. N. Am. 25:215-229. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal, A. A., A. Kapley, R. K. Yeltiwar, and H. J. Purohit. 2006. Assessment of single nucleotide polymorphism at IL-1A+4845 and IL-1B+3954 as genetic susceptibility test for chronic periodontitis in Maharashtrian ethnicity. J. Periodontol. 77:1515-1521. [DOI] [PubMed] [Google Scholar]
- 4.Albandar, J. M., L. J. Brown, and H. Loe. 1997. Putative periodontal pathogens in subgingival plaque of young adults with and without early-onset periodontitis. J. Periodontol. 68:973-981. [DOI] [PubMed] [Google Scholar]
- 5.Annacker, O., R. Pimenta-Araujo, O. Burlen-Defranoux, and A. Bandeira. 2001. On the ontogeny and physiology of regulatory T cells. Immunol. Rev. 182:5-17. [DOI] [PubMed] [Google Scholar]
- 6.Apatzidou, D. A., M. P. Riggio, and D. F. Kinane. 2005. Impact of smoking on the clinical, microbiological and immunological parameters of adult patients with periodontitis. J. Clin. Periodontol. 32:973-983. [DOI] [PubMed] [Google Scholar]
- 7.Avery, B. E., and D. M. Simpson. 1973. The baboon as a model system for the study of periodontal disease: clinical and light microscopic observations. J. Periodontol. 44:675-686. [DOI] [PubMed] [Google Scholar]
- 8.Barry, P. A., K. M. Lockridge, S. Salamat, S. P. Tinling, Y. Yue, S. S. Zhou, S. M. Gospe, Jr., W. J. Britt, and A. F. Tarantal. 2006. Nonhuman primate models of intrauterine cytomegalovirus infection. ILAR J. 47:49-64. [DOI] [PubMed] [Google Scholar]
- 9.Bimstein, E., S. Sapir, Y. Houri-Haddad, S. Dibart, T. E. Van Dyke, and L. Shapira. 2004. The relationship between Porphyromonas gingivalis infection and local and systemic factors in children. J. Periodontol. 75:1371-1376. [DOI] [PubMed] [Google Scholar]
- 10.Black, A., and M. A. Lane. 2002. Nonhuman primate models of skeletal and reproductive aging. Gerontology 48:72-80. [DOI] [PubMed] [Google Scholar]
- 11.Bobetsis, Y. A., S. P. Barros, and S. Offenbacher. 2006. Exploring the relationship between periodontal disease and pregnancy complications. J. Am. Dent. Assoc. 137(Suppl. 2):7S-13S. [DOI] [PubMed] [Google Scholar]
- 12.Candore, G., G. Colonna-Romano, C. R. Balistreri, D. Di Carlo, M. P. Grimaldi, F. Listi, D. Nuzzo, S. Vasto, D. Lio, and C. Caruso. 2006. Biology of longevity: role of the innate immune system. Rejuvenation Res. 9:143-148. [DOI] [PubMed] [Google Scholar]
- 13.Cappelli, D., S. C. Holt, R. E. Singer, H. M. Pickrum, and J. L. Ebersole. 2000. Effects of 0.12% chlorhexidine gluconate on experimental gingivitis in non-human primates: clinical and microbiological alterations. Oral Dis. 6:124-131. [DOI] [PubMed] [Google Scholar]
- 14.Celenligil, H., and J. L. Ebersole. 1998. Analysis of serum antibody responses to periodontopathogens in early-onset periodontitis patients from different geographical locations. J. Clin. Periodontol. 25:994-1002. [DOI] [PubMed] [Google Scholar]
- 15.Celenligil-Nazliel, H., E. Kansu, and J. L. Ebersole. 1999. Periodontal findings and systemic antibody responses to oral microorganisms in Behçet's disease. J. Periodontol. 70:1449-1456. [DOI] [PubMed] [Google Scholar]
- 16.Coughlin, S. R. 2005. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J. Thromb. Haemost. 3:1800-1814. [DOI] [PubMed] [Google Scholar]
- 17.Craig, R. G., R. Boylan, J. Yip, D. Mijares, M. Imam, S. S. Socransky, M. A. Taubman, and A. D. Haffajee. 2002. Serum IgG antibody response to periodontal pathogens in minority populations: relationship to periodontal disease status and progression. J. Periodontal Res. 37:132-146. [DOI] [PubMed] [Google Scholar]
- 18.Ebersole, J. L., and D. Cappelli. 2000. Acute-phase reactants in infections and inflammatory diseases. Periodontol. 2000 23:19-49. [DOI] [PubMed] [Google Scholar]
- 19.Ebersole, J. L., D. Cappelli, and S. C. Holt. 2001. Periodontal diseases: to protect or not to protect is the question? Acta Odontol. Scand. 59:161-166. [DOI] [PubMed] [Google Scholar]
- 20.Ebersole, J. L., D. Cappelli, S. C. Holt, R. E. Singer, and T. Filloon. 2000. Gingival crevicular fluid inflammatory mediators and bacteriology of gingivitis in nonhuman primates related to susceptibility to periodontitis. Oral Microbiol. Immunol. 15:19-26. [DOI] [PubMed] [Google Scholar]
- 21.Ebersole, J. L., D. Cappelli, E. C. Mathys, M. J. Steffen, R. E. Singer, M. Montgomery, G. E. Mott, and M. J. Novak. 2002. Periodontitis in humans and non-human primates: oral-systemic linkage inducing acute phase proteins. Ann. Periodontol. 7:102-111. [DOI] [PubMed] [Google Scholar]
- 22.Ebersole, J. L., D. Cappelli, G. Mott, L. Kesavalu, S. C. Holt, and R. E. Singer. 1999. Systemic manifestations of periodontitis in the non-human primate. J. Periodontal Res. 34:358-362. [DOI] [PubMed] [Google Scholar]
- 23.Ebersole, J. L., and K. S. Kornman. 1991. Systemic antibody responses to oral microorganisms in the cynomolgus monkey: development of methodology and longitudinal responses during ligature-induced disease. Res. Immunol. 142:829-839. [DOI] [PubMed] [Google Scholar]
- 24.Ebersole, J. L., M. J. Steffen, and D. Cappelli. 1999. Longitudinal human serum antibody responses to outer membrane antigens of Actinobacillus actinomycetemcomitans. J. Clin. Periodontol. 26:732-741. [DOI] [PubMed] [Google Scholar]
- 25.Ebersole, J. L., M. J. Steffen, M. A. Reynolds, G. L. Branch-Mays, D. R. Dawson, K. F. Novak, J. C. Gunsolley, J. A. Mattison, D. K. Ingram, and M. J. Novak. Differential gender effects of a reduced calorie diet on systemic inflammatory and immune parameters in nonhuman primates. J. Periodontal Res., in press. [DOI] [PMC free article] [PubMed]
- 26.Engel, G., L. L. Hungerford, L. Jones-Engel, D. Travis, R. Eberle, A. Fuentes, R. Grant, R. Kyes, and M. Schillaci. 2006. Risk assessment: a model for predicting cross-species transmission of simian foamy virus from macaques (M. fascicularis) to humans at a monkey temple in Bali, Indonesia. Am. J. Primatol. 68:934-948. [DOI] [PubMed] [Google Scholar]
- 27.Folkerts, G., G. Walzl, and P. J. Openshaw. 2000. Do common childhood infections ‘teach’ the immune system not to be allergic? Immunol. Today 21:118-120. [DOI] [PubMed] [Google Scholar]
- 28.Fulop, T., A. Larbi, N. Douziech, I. Levesque, A. Varin, and G. Herbein. 2006. Cytokine receptor signalling and aging. Mech. Ageing Dev. 127:526-537. [DOI] [PubMed] [Google Scholar]
- 29.Gafan, G. P., V. S. Lucas, G. J. Roberts, A. Petrie, M. Wilson, and D. A. Spratt. 2004. Prevalence of periodontal pathogens in dental plaque of children. J. Clin. Microbiol. 42:4141-4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashi, F., M. Okada, Y. Soda, K. Miura, and K. Kozai. 2006. Subgingival distribution of Campylobacter rectus and Tannerella forsythensis in healthy children with primary dentition. Arch. Oral Biol. 51:10-14. [DOI] [PubMed] [Google Scholar]
- 31.Henson, M. C., and V. D. Castracane. 2002. Leptin: roles and regulation in primate pregnancy. Semin. Reprod. Med. 20:113-122. [DOI] [PubMed] [Google Scholar]
- 32.Herodin, F., P. Thullier, D. Garin, and M. Drouet. 2005. Nonhuman primates are relevant models for research in hematology, immunology and virology. Eur. Cytokine Netw. 16:104-116. [PubMed] [Google Scholar]
- 33.Hu, S. W., C. H. Huang, H. C. Huang, Y. Y. Lai, and Y. Y. Lin. 2006. Transvascular dissemination of Porphyromonas gingivalis from a sequestered site is dependent upon activation of the kallikrein/kinin pathway. J. Periodontal Res. 41:200-207. [DOI] [PubMed] [Google Scholar]
- 34.Jervoe-Storm, P. M., M. Koltzscher, W. Falk, A. Dorfler, and S. Jepsen. 2005. Comparison of culture and real-time PCR for detection and quantification of five putative periodontopathogenic bacteria in subgingival plaque samples. J. Clin. Periodontol. 32:778-783. [DOI] [PubMed] [Google Scholar]
- 35.Kamma, J. J., M. Nakou, and F. A. Manti. 1995. Predominant microflora of severe, moderate and minimal periodontal lesions in young adults with rapidly progressive periodontitis. J. Periodontal Res. 30:66-72. [DOI] [PubMed] [Google Scholar]
- 36.Katancik, J. A., S. Kritchevsky, R. J. Weyant, P. Corby, W. Bretz, R. O. Crapo, R. Jensen, G. Waterer, S. M. Rubin, and A. B. Newman. 2005. Periodontitis and airway obstruction. J. Periodontol. 76:2161-2167. [DOI] [PubMed] [Google Scholar]
- 37.Kilian, M., E. V. Frandsen, D. Haubek, and K. Poulsen. 2006. The etiology of periodontal disease revisited by population genetic analysis. Periodontol. 2000 42:158-179. [DOI] [PubMed] [Google Scholar]
- 38.Kinane, D. F., and P. M. Bartold. 2007. Clinical relevance of the host responses of periodontitis. Periodontol. 2000 43:278-293. [DOI] [PubMed] [Google Scholar]
- 39.Kinane, D. F., and T. C. Hart. 2003. Genes and gene polymorphisms associated with periodontal disease. Crit. Rev. Oral Biol. Med. 14:430-449. [DOI] [PubMed] [Google Scholar]
- 40.Kinane, D. F., J. Mooney, and J. L. Ebersole. 1999. Humoral immune response to Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in periodontal disease. Periodontol. 2000 20:289-340. [DOI] [PubMed] [Google Scholar]
- 41.Kinane, D. F., M. Podmore, M. C. Murray, P. J. Hodge, and J. Ebersole. 2001. Etiopathogenesis of periodontitis in children and adolescents. Periodontol. 2000 26:54-91. [DOI] [PubMed] [Google Scholar]
- 42.Kinane, D. F., M. P. Riggio, K. F. Walker, D. MacKenzie, and B. Shearer. 2005. Bacteraemia following periodontal procedures. J. Clin. Periodontol. 32:708-713. [DOI] [PubMed] [Google Scholar]
- 43.Kodama, M., K. Murakami, R. Sato, T. Okimoto, A. Nishizono, and T. Fujioka. 2005. Helicobacter pylori-infected animal models are extremely suitable for the investigation of gastric carcinogenesis. World J. Gastroenterol. 11:7063-7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar, P. S., A. L. Griffen, J. A. Barton, B. J. Paster, M. L. Moeschberger, and E. J. Leys. 2003. New bacterial species associated with chronic periodontitis. J. Dent. Res. 82:338-344. [DOI] [PubMed] [Google Scholar]
- 45.Madden, T. E., and J. G. Caton. 1994. Animal models for periodontal disease. Methods Enzymol. 235:106-119. [DOI] [PubMed] [Google Scholar]
- 46.McArthur, W. P., C. Bloom, M. Taylor, J. Smith, T. Wheeler, and N. I. Magnusson. 1995. Antibody responses to suspected periodontal pathogens in elderly subjects with periodontal disease. J. Clin. Periodontol. 22:842-849. [DOI] [PubMed] [Google Scholar]
- 47.McMurray, D. N. 2000. A nonhuman primate model for preclinical testing of new tuberculosis vaccines. Clin. Infect. Dis. 30(Suppl. 3):S210-212. [DOI] [PubMed] [Google Scholar]
- 48.Mealey, B. L. 2006. Periodontal disease and diabetes: a two-way street. J. Am. Dent. Assoc. 137(Suppl.):26S-31S. [DOI] [PubMed] [Google Scholar]
- 49.Miura, M., T. Hamachi, O. Fujise, and K. Maeda. 2005. The prevalence and pathogenic differences of Porphyromonas gingivalis fimA genotypes in patients with aggressive periodontitis. J. Periodontal Res. 40:147-152. [DOI] [PubMed] [Google Scholar]
- 50.Moreels, T. G., and P. A. Pelckmans. 2006. The hygiene hypothesis and inflammatory bowel diseases: role of helminths. Acta Gastroenterol. Belg. 69:413-417. [PubMed] [Google Scholar]
- 51.Morton, W. R., and K. Swindler. 2005. Serendipitous insights involving nonhuman primates. ILAR J. 46:346-351. [DOI] [PubMed] [Google Scholar]
- 52.Newburg, D. S., and W. A. Walker. 2007. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr. Res. 61:2-8. [DOI] [PubMed] [Google Scholar]
- 53.Nonnenmacher, C., R. Mutters, and L. F. de Jacoby. 2001. Microbiological characteristics of subgingival microbiota in adult periodontitis, localized juvenile periodontitis and rapidly progressive periodontitis subjects. Clin. Microbiol. Infect. 7:213-217. [DOI] [PubMed] [Google Scholar]
- 54.O'Connor, C. M., and D. H. Kedes. 2007. Rhesus monkey rhadinovirus: a model for the study of KSHV. Curr. Top. Microbiol. Immunol. 312:43-69. [DOI] [PubMed] [Google Scholar]
- 55.Offenbacher, S., and J. D. Beck. 2005. A perspective on the potential cardioprotective benefits of periodontal therapy. Am. Heart J. 149:950-954. [DOI] [PubMed] [Google Scholar]
- 56.Okada, M., F. Hayashi, Y. Soda, X. Zhong, K. Miura, and K. Kozai. 2004. Intra-familial distribution of nine putative periodontopathogens in dental plaque samples analyzed by PCR. J. Oral Sci. 46:149-156. [DOI] [PubMed] [Google Scholar]
- 57.Padilla, C., O. Lobos, E. Hubert, C. Gonzalez, S. Matus, M. Pereira, S. Hasbun, and C. Descouvieres. 2006. Periodontal pathogens in atheromatous plaques isolated from patients with chronic periodontitis. J. Periodontal Res. 41:350-353. [DOI] [PubMed] [Google Scholar]
- 58.Papapanou, P. N., A. M. Neiderud, A. Papadimitriou, J. Sandros, and G. Dahlen. 2000. “Checkerboard” assessments of periodontal microbiota and serum antibody responses: a case-control study. J. Periodontol. 71:885-897. [DOI] [PubMed] [Google Scholar]
- 59.Persson, G. R. 2005. Immune responses and vaccination against periodontal infections. J. Clin. Periodontol. 32(Suppl. 6):39-53. [DOI] [PubMed] [Google Scholar]
- 60.Prelog, M. 2006. Aging of the immune system: a risk factor for autoimmunity? Autoimmun. Rev. 5:136-139. [DOI] [PubMed] [Google Scholar]
- 61.Prescott, S. 2004. Developmental immunology and vaccines: cellular immune development and future vaccine strategies. Expert Rev. Vaccines 3:339-342. [DOI] [PubMed] [Google Scholar]
- 62.Roberts, F. A., L. S. Houston, S. A. Lukehart, L. A. Mancl, G. R. Persson, and R. C. Page. 2004. Periodontitis vaccine decreases local prostaglandin E2 levels in a primate model. Infect. Immun. 72:1166-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roth, G. S., J. A. Mattison, M. A. Ottinger, M. E. Chachich, M. A. Lane, and D. K. Ingram. 2004. Aging in rhesus monkeys: relevance to human health interventions. Science 305:1423-1426. [DOI] [PubMed] [Google Scholar]
- 64.Rumbo, M., and E. J. Schiffrin. 2005. Ontogeny of intestinal epithelium immune functions: developmental and environmental regulation. Cell. Mol. Life Sci. 62:1288-1296. [DOI] [PubMed] [Google Scholar]
- 65.Sakai, Y., H. Shimauchi, H. O. Ito, M. Kitamura, and H. Okada. 2001. Porphyromonas gingivalis-specific IgG subclass antibody levels as immunological risk indicators of periodontal bone loss. J. Clin. Periodontol. 28:853-859. [DOI] [PubMed] [Google Scholar]
- 66.Schaub, B., R. Lauener, and E. von Mutius. 2006. The many faces of the hygiene hypothesis. J. Allergy Clin. Immunol. 117:969-978. [DOI] [PubMed] [Google Scholar]
- 67.Schlegel-Bregenzer, B., R. E. Persson, S. Lukehart, P. Braham, T. Oswald, and G. R. Persson. 1998. Clinical and microbiological findings in elderly subjects with gingivitis or periodontitis. J. Clin. Periodontol. 25:897-907. [DOI] [PubMed] [Google Scholar]
- 68.Schou, S., P. Holmstrup, and K. S. Kornman. 1993. Non-human primates used in studies of periodontal disease pathogenesis: a review of the literature. J. Periodontol. 64:497-508. [DOI] [PubMed] [Google Scholar]
- 69.Seto, H., M. Ninomiya, and T. Nagata. 2006. Alveolar bone resorption in animal models of periodontitis. Clin. Calcium 16:24-30. (In Japanese.) [PubMed] [Google Scholar]
- 70.Shapira, L., A. Wilensky, and D. F. Kinane. 2005. Effect of genetic variability on the inflammatory response to periodontal infection. J. Clin. Periodontol. 32(Suppl. 6):72-86. [DOI] [PubMed] [Google Scholar]
- 71.Smith, D. J., and M. A. Taubman. 1992. Ontogeny of immunity to oral microbiota in humans. Crit. Rev. Oral Biol. Med. 3:109-133. [DOI] [PubMed] [Google Scholar]
- 72.Spahr, A., E. Klein, N. Khuseyinova, C. Boeckh, R. Muche, M. Kunze, D. Rothenbacher, G. Pezeshki, A. Hoffmeister, and W. Koenig. 2006. Periodontal infections and coronary heart disease: role of periodontal bacteria and importance of total pathogen burden in the Coronary Event and Periodontal Disease (CORODONT) study. Arch. Intern. Med. 166:554-559. [DOI] [PubMed] [Google Scholar]
- 73.Stearns-Kurosawa, D. J., F. Lupu, F. B. Taylor, Jr., G. Kinasewitz, and S. Kurosawa. 2006. Sepsis and pathophysiology of anthrax in a nonhuman primate model. Am. J. Pathol. 169:433-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Takahashi, K., F. Nishimura, M. Kurihara, Y. Iwamoto, S. Takashiba, T. Miyata, and Y. Murayama. 2001. Subgingival microflora and antibody responses against periodontal bacteria of young Japanese patients with type 1 diabetes mellitus. J. Int. Acad. Periodontol. 3:104-111. [PubMed] [Google Scholar]
- 75.Takai, S., T. Kuriyama, M. Yanagisawa, K. Nakagawa, and T. Karasawa. 2005. Incidence and bacteriology of bacteremia associated with various oral and maxillofacial surgical procedures. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 99:292-298. [DOI] [PubMed] [Google Scholar]
- 76.Tanaka, H., N. Tanabe, M. Shoji, N. Suzuki, T. Katono, S. Sato, M. Motohashi, and M. Maeno. 2006. Nicotine and lipopolysaccharide stimulate the formation of osteoclast-like cells by increasing macrophage colony-stimulating factor and prostaglandin E2 production by osteoblasts. Life Sci. 78:1733-1740. [DOI] [PubMed] [Google Scholar]
- 77.Tanner, A. C., R. L. Kent, Jr., M. F. Maiden, P. J. Macuch, and M. A. Taubman. 2000. Serum IgG reactivity to subgingival bacteria in initial periodontitis, gingivitis and healthy subjects. J. Clin. Periodontol. 27:473-480. [DOI] [PubMed] [Google Scholar]
- 78.Tatakis, D. N., and P. S. Kumar. 2005. Etiology and pathogenesis of periodontal diseases. Dent. Clin. N. Am. 49:491-516. [DOI] [PubMed] [Google Scholar]
- 79.Taubman, M. A., A. D. Haffajee, S. S. Socransky, D. J. Smith, and J. L. Ebersole. 1992. Longitudinal monitoring of humoral antibody in subjects with destructive periodontal diseases. J. Periodontal Res. 27:511-521. [DOI] [PubMed] [Google Scholar]
- 80.Taubman, M. A., P. Valverde, X. Han, and T. Kawai. 2005. Immune response: the key to bone resorption in periodontal disease. J. Periodontol. 76:2033-2041. [DOI] [PubMed] [Google Scholar]
- 81.Teitelbaum, J. E., and W. Allan Walker. 2005. The development of mucosal immunity. Eur. J. Gastroenterol. Hepatol. 17:1273-1278. [DOI] [PubMed] [Google Scholar]
- 82.Timmerman, M. F., and G. A. van der Weijden. 2006. Risk factors for periodontitis. Int. J. Dent. Hyg. 4:2-7. [DOI] [PubMed] [Google Scholar]
- 83.Timmerman, M. F., G. A. Van der Weijden, F. Abbas, E. M. Arief, S. Armand, E. G. Winkel, A. J. Van Winkelhoff, and U. Van der Velden. 2000. Untreated periodontal disease in Indonesian adolescents. Longitudinal clinical data and prospective clinical and microbiological risk assessment. J. Clin. Periodontol. 27:932-942. [DOI] [PubMed] [Google Scholar]
- 84.Van Dyke, T. E., and D. Sheilesh. 2005. Risk factors for periodontitis. J. Int. Acad. Periodontol. 7:3-7. [PMC free article] [PubMed] [Google Scholar]
- 85.van Winkelhoff, A. J., C. J. Bosch-Tijhof, E. G. Winkel, and W. A. van der Reijden. 2001. Smoking affects the subgingival microflora in periodontitis. J. Periodontol. 72:666-671. [DOI] [PubMed] [Google Scholar]
- 86.Visser, M., L. M. Bouter, G. M. McQuillan, M. H. Wener, and T. B. Harris. 2001. Low-grade systemic inflammation in overweight children. Pediatrics 107:E13. [DOI] [PubMed] [Google Scholar]
- 87.Weng, N. P. 2006. Aging of the immune system: how much can the adaptive immune system adapt? Immunity 24:495-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Willershausen-Zönnchen, B., and C. Gleissner. 1998. Periodontal disease in elderly patients. Eur. J. Med. Res. 3:55-64. [PubMed] [Google Scholar]
- 89.Zavos, C., D. Vini, J. Kountouras, N. Zavos, and E. Trivara. 2007. Hygiene hypothesis and protection against asthma in infants: spending time in the countryside encountering natural allergens may boost maternal immunity. Med. Hypotheses 68:914-915. [DOI] [PubMed] [Google Scholar]