Abstract
Neutralizing antibodies to Bacillus anthracis protective antigen (PA), a component of anthrax toxin, mediate protection against anthrax. PA is antigenically complex and can elicit protective and nonprotective antibodies. Furthermore, vaccinated individuals demonstrate considerable variability in their antibody responses to PA. To explore the relationship between PA structure and antigenicity, we produced Escherichia coli strains expressing full-length PA (PA1-4), domains 2 to 4 (PA2-4), domain 1, (PA1), and domain 4 (PA4) and evaluated the immunogenicities and protective efficacies of the protein fractions in four mouse strains (strains A/J, BALB/c, C57BL/6, and Swiss Webster). Immunization with PA1-4 resulted in significantly higher lethal toxin-neutralizing antibody titers than immunization with any recombinant protein (rPA) fraction of PA. The magnitude and neutralizing capacity of the antibody response to full-length PA and its fragments varied depending on the mouse strain. We found no correlation between the antibody titer and the neutralizing antibody titer for A/J and Swiss Webster mice. In C57BL/6 mice, antibody titers and neutralization capacity correlated for two of four rPA domain proteins tested, while BALB/c mice displayed a similar correlation with only one rPA. By correlating the reactivity of immune sera with solvent-exposed linear peptide segments of PA, we tentatively assign the presence of four new linear B-cell epitopes in PA amino acids 121 to 150, 143 to 158, 339 to 359, and 421 to 440. We conclude that the genetic background of the host determines the relative efficacy of the antitoxin response. The results suggest that the variability observed in vaccination studies with PA-derived vaccines is a result of host heterogeneity and implies a need to develop other antigens as vaccine candidates.
Bacillus anthracis is the causative agent of anthrax. Three forms of anthrax, cutaneous, gastrointestinal, and inhalational, have been described in humans. The most serious form is inhalational anthrax, because patients with this form have a rate of high mortality and it is more common than gastrointestinal anthrax. The ability of this microbe to make spores suitable for airborne dissemination makes it a potent bioterrorism threat. B. anthracis produces anthrax toxin, a tripartite toxin that possesses the ability to impair innate and adaptive immune responses (5), which enhances susceptibility to bacterial infection. After acute symptoms have appeared, antibiotics may kill the organisms but do not destroy the powerful tripartite exotoxin that has already been formed, and many individuals succumb rapidly (7). Anthrax toxin, one of the two virulence factors of B. anthracis, is composed of protective antigen (PA) plus either lethal factor (LF) or edema factor (EF). Initial stages of host cell intoxication require PA to promote the entry of the two enzymes that mediate intracellular cell damage, LF and EF. The combination of PA with LF or EF produces lethal toxin (LeTx) and edema toxin, respectively. The crystal structure of PA revealed that the molecule is folded into four distinct and functionally independent domains (4, 29, 32). Each domain is necessary for a specific step in the intoxication process. Domain 1 (amino acids [aa] 1 to 258) contains a furin protease cleavage site, which leads to the release of an amino-terminal fragment (PA20) and heptamerization of the remainder of the protein (PA63) through monomeric interactions on the cell surface (25). Furin cleavage exposes a surface in PA63 that is involved in the binding of LF or EF (32). Domain 2 (aa 259 to 487) contributes to the binding of PA63 monomers; in addition, a loop from this domain has contact with the host cell receptor binding site (19). Domain 2 along with domain 3 (aa 488 to 595) form the heptameric pore on the cell surface that allows LF or EF binding, thus leading the toxin complex to undergo receptor-mediated endocytosis into the cell (28, 30). Domain 4 (aa 596 to 735) contains the host cell receptor binding site (22). Once they are in the cytosol, LF and EF are able to carry out their respective damage-inducing processes (39). LF is a zinc metalloprotease that cleaves several mitogen-activated protein kinase kinases. EF is a calmodulin-dependent adenylate cyclase that causes local edema and that impairs neutrophil function (24).
PA has been the chief target for immune inactivation of the anthrax toxins in vaccine preparations. It is known that antibodies to PA are essential for immunity to B. anthracis (10, 14, 16, 20, 25), and the binding sites of several monoclonal antibodies (MAbs) have been mapped to key domains on the PA molecule (21, 36). Anthrax vaccine adsorbed is the current licensed anthrax vaccine for humans in the United States and consists of the cell-free culture filtrate of an attenuated strain of B. anthracis combined with an aluminum adjuvant (15, 44). Studies show that PA is the principal immunogen of anthrax vaccine adsorbed. There are a number of drawbacks associated with this vaccine, which include an uncertain chemical composition, difficulty in standardization, side effects, transient immunogenicity, and the need for multiple doses to achieve serum antibody titers (44).
The description that some antibodies to PA can enhance toxicity (31) suggests that some individuals may be at increased vulnerability to anthrax as a result of PA immunization. Given the possibility that disease-enhancing antibodies may be present and the fact that not all MAbs to PA are neutralizing, there is a need to understand the relationship between the immunogenicity of PA domains and the efficacy of antibody responses to those domains. There are widespread variations in immune responses to vaccines, and this heterogeneity of immune response presumably arises from differences in immune response genes. Immune responsiveness to the same antigen has been proved to depend upon differences in the haplotypes of the major histocompatibility complex (23, 40, 42, 43, 47); therefore, the production of antibodies to an antigen was shown to be different in the various strains studied, suggesting that antibody responses to protein immunization are strain dependent in mice. A prior study has shown that PA domains vary in their immunogenicities and functional efficacies in one strain of mice (8). In addition, it has previously been shown that immunization with domain 4 generates antibodies that are sufficient to provide protection against spore and toxin challenge in mice (8, 26). Also, there are other neutralizing epitopes in domains 1 to 3 (12, 34). In this study, we evaluated the antibody response and the titer that correlated with toxin neutralization in four strains of mice. Furthermore, we have carried out fine specificity mapping of the antibody responses by using linear peptides representing segments of solvent-exposed protein. Here we show that antibodies generated against PA1-4 and PA1 can be correlated with toxin neutralization in vitro. Overall, the results indicate that the genetic background of the host has a great influence on the immunogenicity of the domain and the toxin-neutralizing capacity of the antibodies elicited.
MATERIALS AND METHODS
Construction of PA plasmids and protein preparations.
Plasmid pET22b-PA (2) was used as a template to generate truncated versions of the PA domain proteins for immunization studies. The primers used for the amplification of the specific domains are listed in Table 1. DNA encoding these PA domains represented fragments encoding aa 1 to 258 (domain 1), 259 to 735 (domains 2 to 4), 596 to 735 (domain 4), and 1 to 735 (domains 1 to 4). High-purity, gst-tagged recombinant PAs (rPAs) were generated by cloning amplified PCR products into the XhoI and BamHI sites of the vector pGEX-KG and transformed into Escherichia coli DH5α. Cells were grown overnight at 37°C in rotary shaker set at 225 rpm. Larger cultures of bacterial cells were grown until the optical density reached 1 to 1.2, and these cells were then induced with 0.2 mM isopropyl-β-d-thiogalactopyranoside for 1 h for expression of rPA domain proteins. The cultures were centrifuged at 10,000 rpm for 10 min at 4°C, and the pellets were resuspended in 8 ml of phosphate-buffered saline (PBS) containing protease inhibitors. Glass beads were used to lyse the cells for 30 s, the glass beads and cells were placed on ice during lysis. The supernatants were treated with 50% glutathione agarose beads and incubated at room temperature (RT) for 1 h. Protein was collected after centrifugation at 3,200 rpm for 5 min at 4°C. The pellets were resuspended with thrombin to cleave off the gst tag and incubated at RT for 2 to 4 h. The supernatant was collected and stored at −80°C. To confirm that the rPA domain proteins were of the expected molecular weights, they were analyzed on sodium dodecyl sulfate (SDS)-10% polyacrylamide gels. SDS-polyacrylamide gel electrophoresis revealed fragments of the expected sizes with no breakdown products (data not shown). The protein concentration was determined with a protein assay kit (Bio-Rad, Hercules, CA) with bovine serum albumin (BSA) as a standard.
TABLE 1.
Oligonucleotides used for construction of PA domain proteins
| Name | Sequence |
|---|---|
| Domain 1 for | 5′-TTAAGTCTAGACGAAGTTAAACAGGAGAACCG-3′a |
| Domain 1 rev | 5′-TTAATGTCGACTAGCTGCCACAAGGGGGTG-3′b |
| Domain 2 for | 5′-TTAAGTCTAGACCCTTGTGGCAGCTTATCCG-3′a |
| Domain 4 for | 5′-TTAAGTCTAGACTGATAGAAATAACATAGCAG-3′a |
| Domain 4 rev | 5′-TTAATGTCGACTTTATCCTATCTCATAGCCTTT-3′b |
Underlined sequences represent XbaI, used for cloning.
Underlined sequences represent SalI, used for cloning.
Vaccination.
Female A/J, BALB/c, C57BL/6, and Swiss Webster mice (ages, 6 to 8 weeks; NCI, Bethesda, MD) were immunized intraperitoneally with 1 μg of the expressed rPA domain proteins in complete Freund's adjuvant on day 1 of the study, 1 μg of the expressed rPA domain proteins in incomplete Freund's adjuvant on day 30, and 10 μg of the expressed rPA domain proteins in incomplete Freund's adjuvant on day 110. Blood was collected before the first injection (preimmune serum) and at 4 weeks after the first injection and 2 weeks after both the second and the third injections. Serum was separated from the blood cells and was stored at −20°C.
Serum antibody analysis.
PA antigen-specific serum antibody titers were determined by endpoint dilution enzyme-linked immunosorbent assays (ELISAs) with sera obtained from individual mice of each group 2 weeks after both the second and the third immunizations. Polystyrene plates (Costar) were coated with 1 μg of rPA domain proteins in PBS per well, and the plates were incubated at 37°C for 1 h. The wells were blocked with 200 μl of 1% BSA in PBS at 37°C for 1 h. After the wells were washed with 0.1% Tween 20 in PBS (PBST), twofold serial dilutions of sera in 1% BSA in PBS were added to the wells, and the plates were incubated at 37°C for 1 h. The plates were washed three times with PBST and then incubated at 37°C for 1 h; bound antibodies were detected with 50 μl of alkaline phosphatase-labeled goat anti-mouse immunoglobulin M (IgM), IgA, and IgG (heavy and light chains) (Southern Biotechnology, Birmingham, AL) diluted in 1% BSA in PBS (1:1,000). The wells were washed five times with PBST, an alkaline phosphatase substrate was added to each well, the plates were developed at RT for 20 min, and the absorbance at 405 nm was measured. The endpoint titers were defined as the highest serum dilutions that resulted in an absorbance value twice as high as that for the preimmune sera with a cutoff value of 0.1. Antibody titers were expressed as the reciprocal endpoint dilution.
The concentration of serum IgG antibodies to PA was estimated by ELISA as described above for each immunization group relative to that of an MAb standard. IgG concentrations were calculated from a standard curve of an MAb to PA over a concentration range from 0.05 to 10 μg/ml. Although MAb standards have been used to estimate the amount of specific antibody in immune sera in prior studies (6), it is important to note that the calculated amount is directly dependent on the affinity of the MAb reagent and the reactivity of the secondary antibody for that MAb. Hence, the calculated numbers are best used as relative values within a study and should be considered estimates of the total specific amount. The total IgG antibody concentrations in each serum, expressed in μg per ml, are the means of triplicate determinations.
Detection of isotype-specific antibodies to PA.
ELISAs were used to determine the isotypes and subclass specificities of antibodies to PA. Plates were coated with the rPA domain proteins as described above, and serial twofold dilutions of sera from immunized mice were added. Detection was performed with alkaline phosphatase-labeled goat anti-mouse IgM, IgA, IgG1, IgG2a, and IgG2b at a 1:1,000 dilution (Southern Biotechnology).
Mouse macrophage protection assay.
LeTx-neutralizing antibody titers were determined by their ability to prevent PA- and LF-induced killing of J774 macrophage-like cells. Briefly, 24 h before the assay, cells were seeded in 96-well plates at a density of 3 × 104 cells/well in 200 μl of growth medium. The cells were incubated at 37°C in 5% CO2 for 18 h or until >90% confluence had been achieved. The antisera to be analyzed were serially diluted twofold in cell culture medium. Then, 100 μl of the cell supernatant was removed from wells containing J774 cells and replaced with 100 ng of PA and LF alone or in combination with antisera (50 μl per well). After incubation at 37°C in 5% CO2 for 4 h, 25 μl of a 5-mg/ml stock solution of 3(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was added to each well, the plates were incubated at 37°C for 2 h, 100 μl of extraction buffer (12% SDS, 45% dimethyl formamide) was added, and the cells were incubated at 37°C in 5% CO2 overnight. Cell viability was inferred from the colorimetric change measured by determination of the absorbance at 580 nm. Eight wells containing medium only were used as untreated controls, and eight wells containing PA and LF without serum served as toxin-treated controls. The ratio of viability between LeTx-treated cells and untreated control cells, expressed as a percentage of cell viability, was calculated for each dilution and plotted. The percent protection (P) conferred by mouse antisera was calculated as follows: {1 − [(untreated control mean − sample mean)/(untreated control mean − toxin treated mean)]} × 100. The LeTx-neutralizing anti-PA IgG antibody concentrations were quantified, as described above, from each dilution of antisera measured by this MTT assay and calculated by linear and logarithmic regression analyses.
Peptide ELISA.
Each of the peptides utilized in this study was synthesized according to the guidelines of the manufacturer (Invitrogen Corporation, Carlsbad, CA). The identity of each of the peptides was confirmed by mass spectral analysis. The peptides were >98% pure, as assessed by high-pressure liquid chromatography analysis. Each peptide was supplied as a white powder soluble in water. All peptides were stored at a concentration of 1 mg/ml. See Table 3 for the peptides representing segments of the PA molecule.
TABLE 3.
Reactivities of mouse sera with PA synthetic peptides
| Mouse serum | Reactivity with the following synthetic peptidea:
|
Neutralizationc | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PA26-29 | PA50-61 | PA91-114 | PA121-150 | PA143-158 | PA339-359 | PA421-440 | PA509-516 | PA645-657 | PA678-697 | PA711-715 | Positive controlb | ||
| A/J PA1 | − | − | − | − | − | − | − | − | − | − | − | + | No |
| A/J PA4 | − | − | − | − | − | − | − | − | − | + | + | + | No |
| A/J PA2-4 | − | − | − | − | − | − | − | − | − | − | − | + | No |
| A/J PA1-4 | − | − | − | − | − | − | − | − | − | − | − | + | Yes |
| BALB/c PA1 | − | − | − | + | − | − | − | − | − | − | − | +++ | No |
| BALB/c PA4 | − | − | − | − | − | − | − | − | − | − | − | + | No |
| BALB/c PA2-4 | − | − | − | − | − | − | − | − | − | − | − | + | No |
| BALB/c PA1-4 | + | + | − | +++ | ++ | ++ | +++ | − | − | ++ | − | +++ | Yes |
| C57BL/6 PA1 | − | − | − | +++ | − | − | − | − | − | − | − | +++ | Yes |
| C57BL/6 PA4 | − | − | − | − | − | − | − | − | + | + | + | + | No |
| C57BL/6 PA2-4 | − | − | − | − | − | − | − | − | − | − | − | + | No |
| C57BL/6 PA1-4 | + | − | − | ++ | − | − | − | − | − | − | − | +++ | Yes |
| Swiss Webster PA1 | + | − | − | − | − | − | − | − | − | − | − | + | No |
| Swiss Webster PA4 | − | − | − | − | − | − | − | − | + | + | − | + | No |
| Swiss Webster PA2-4 | − | − | − | − | − | − | − | − | − | − | − | + | No |
| Swiss Webster PA1-4 | − | − | − | − | − | − | − | − | − | − | − | + | Yes |
Plus and minus signs indicate the relative reactivities of the various immune sera for the peptide as measured by ELISA, ranging from none (−) to weak (+), moderate (++), and strong (+++).
The relative reactivities of the various immune sera to PA were measured by ELISA, as described in Materials and Methods.
Neutralization capacity of anti-PA antibodies in immune sera to protect cells against LeTx in vitro (from Fig. 2).
For ELISA determinations, microtiter plates were coated with 2 μg of streptavidin/ml and then incubated with 5 μg/ml of biotinylated peptide at 37°C for 1 h. The plates were blocked with 1% BSA in PBS at 37°C for 1 h. After an overnight incubation at 4°C, the levels of binding of the mouse sera and MAbs 7.5G and 10F4 were detected with alkaline phosphatase-conjugated goat anti-mouse IgM, IgA, and IgG (heavy and light chains). MAb 7.5G has been shown to bind to a region in domain 1, while MAb 10F4 binds to a region in domain 4 (35). The plates were developed with p-nitrophenyl phosphatase substrate, and the absorbance of the samples was measured at 405 nm.
B-cell isolation.
Mice that had received three immunizations were used for B-cell isolation. These mice were killed with CO2 2 weeks after the immunization, and the spleens were removed from each mouse. Briefly, leukocytes were isolated from the spleen by the balloon technique (injection of organ insufflation medium with a syringe and a needle) and teased, depleted of erythrocytes by use of NH4Cl lysis buffer, and washed with cold Dulbecco modified Eagle medium. Aliquots of the cells were counted with trypan blue by use of a hemocytometer. On average, the balloon technique yielded 1 × 108 leukocytes per spleen (range, 0.9 × 108 to 2.1 × 108 leukocytes per spleen).
Spot ELISA.
The spot ELISA was performed as reported previously (51), with some minor modifications. Briefly, polystyrene plates were coated with 1 μg of rPA domain proteins in coating buffer (20 mM K2HPO4, 10 mM KH2PO4, 1 mM sodium EDTA, 0.8% NaCl, 0.01% NaN3) for 2 h at 37°C in 10% CO2 and blocked overnight at 4°C with 2% BSA in Tris-buffered saline (TBS). Control wells were coated without rPA. The plates were washed three times with TBS with 0.1% Tween 20 (TBS-T) and rinsed two times with Dulbecco modified Eagle medium. Spleen leukocytes (2.5 × 107) were added to the first well, and 1-log dilutions were plated across the plates. The cells were incubated at 37°C in 10% CO2 for 16 to 18 h. The plates were washed with TBS-T to remove the cells; and a cocktail of biotin-conjugated goat anti-human IgM, IgA, IgG1, IgG2a, IgG2b, and IgG3 antibodies (Southern Biotechnology) diluted 1:500 in 2% BSA in TBS was used as the secondary antibody. The plates were incubated overnight at 4°C. Thirty minutes before the plates were washed, a Vectastain avidin phosphatase amplification system (Vector Laboratories, Burlingame, CA) was prepared in PBS with 0.1% Tween 20. The plates were incubated with the Vectastain system for 30 min at room temperature. The plates were washed and incubated with 5-bromo-4-chloro-3-indoylphosphate (Sigma, St. Louis, MO) in AMP buffer (0.2 mg/ml Cl2, 0.01% Triton X-405, 9.6% 2-amino-2-methyl-1-propanol, pH 9.8) for 3 h at room temperature until a blue color developed. The plates were washed twice with distilled H2O. The blue spots were counted by use of an inverted light microscope, and the results were expressed as the number of spot-forming cells per 2.4 × 104 spleen leukocytes. The assays were done in quadruplicate for each mouse. Most of the control wells (i.e., cells from nonimmunized mice) showed no spots.
Statistical analysis.
The correlation between the PA-specific IgG antibody levels and the neutralization capacity was analyzed by linear and logarithmic regression analyses. All tests were performed with Prism software (version 5 for Windows; GraphPad Software, San Diego, CA).
RESULTS
Antibody response to PA domains in different mouse strains.
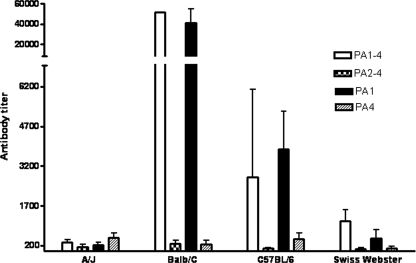
The immunogenicities of intact and truncated fragments of the PA gene product in four genetically different strains of mice were evaluated. Female A/J, BALB/c, C57BL/6, and Swiss Webster mice were immunized intraperitoneally with PA1-4, PA2-4, PA1, and PA4. These fragments include domain 1, which encompasses aa 1 to 258 and which contains the furin cleavage site; domains 2 to 4, which encompass aa 259 to 735 and which represent the PA63 fragment, which is known to be biologically functional (1, 11, 28); and domain 4, which encompasses aa 596 to 735 and which contains the site of PA binding to the host cell receptor. Serum titers were analyzed 4 weeks after the first immunization (data not shown) and 2 weeks after the second immunization (Fig. 1). No antibodies to PA were present in the serum of any mouse strain before immunization (data not shown). All mice immunized with PA1-4 and PA1 elicited specific antibodies to PA, although there were significant interstrain differences in the magnitude of the response. The levels of IgG antibody to PA in the sera from each treatment group varied widely. The amounts of specific IgG made in response to immunization with PA1-4 were, on average, 209 μg/ml in BALB/c mice and 25 μg/ml in C57BL/6 mice, while for the A/J and Swiss Webster mice, the amounts produced were very low (0.15 μg/ml and 0.54 μg/ml, respectively), as was expected because of the low immune responses produced by these two mouse strains. BALB/c and C57BL/6 mice immunized with PA1 produced larger amounts of PA-specific IgG antibody, 450 μg/ml and 67 μg/ml, respectively. All mice mounted very low antibody responses after immunization with PA2-4 or PA4. These results demonstrate that the immunogenicity of the PA domain is a function of the genetic background of the mouse. BALB/c mice made quantitatively greater antibody responses following immunization with PA, and the relative ability of the four mouse strains to respond to immunization with PA was BALB/c > C57BL/6 > Swiss Webster > A/J.
FIG. 1.
Antibody responses in vaccinated mice of different strains. Sera were collected from immunized mice 2 weeks after the second immunization with 1 μg of expressed rPA domain proteins and were tested by ELISA for the presence of PA-specific antibodies. The values represent the geometric means ± standard deviations from five mice per treatment group.
Isotype analysis.
Sera from mice immunized with the various rPA domain proteins that were immunogenic were evaluated for specific IgM, IgG subclass, and IgA antibodies to PA (Table 2). For this analysis we used sera collected 2 weeks after the second immunization, and the results obtained represent the isotype composition of a mature secondary antibody response. The predominant subclass of IgG elicited by immunization with PA1-4 in all mouse strains was IgG1. In contrast, mice immunized with PA1 had a more varied isotype response to PA that included IgG1, IgG2a, and IgG2b antibodies. A/J mice immunized with PA4 contained IgG1 antibodies to PA (results not shown). Hence, the isotype response to PA was also dependent on the genetic background of the host.
TABLE 2.
Isotype analysis following vaccination with recombinant PA domain proteins
| Strain | Titera
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Anti-PA1-4
|
Anti-PA1
|
|||||||||
| IgG1 | IgG2a | IgG2b | IgM | IgA | IgG1 | IgG2a | IgG2b | IgM | IgA | |
| A/J | 200 | 25 | 25 | 25 | 25 | 200 | 50 | 50 | 25 | 25 |
| BALB/c | 32,000 | 25 | 25 | 50 | 25 | 19,200 | 1,800 | 1,000 | 25 | 25 |
| C57BL/6 | 3,200 | 25 | 25 | 25 | 25 | 3,200 | 25 | 1,600 | 25 | 400 |
| Swiss Webster | 1,600 | 25 | 25 | 25 | 25 | 2,000 | 300 | 75 | 50 | 25 |
The IgG, IgM, and IgA isotype titers of sera collected 2 weeks after two immunizations were determined by ELISA. The results represent those for a pool of sera derived from five mice per treatment group. The endpoint was defined as the highest serum dilution that consistently showed a twofold increase in absorbance over that obtained with the preimmune serum sample (i.e., the value of 25 was equal to the background). The reactive titers were expressed as the reciprocal endpoint dilution. Anti-PA1-4, mice immunized with PAD1-4; Anti-PA1, mice immunized with PA1.
Toxin neutralization in vitro.
The LeTx-neutralizing capacity of immune sera from all vaccinated groups was evaluated with J774 cells. Cell viability was measured by the MTT method after 4 h incubation. The extent of cell death was expressed relative to that for a control containing LeTx. For all mouse strains, immune sera from mice immunized with PA1-4 conferred protection against the cytotoxic effects of LeTx, with significant protection conferred up to a dilution of 1/1,000 (Fig. 2A). Sera from the majority of mouse strains immunized with the PA1, PA2-4, and PA4 antigens had considerably less LeTx-neutralizing capacity than sera from mice immunized with PA1-4 (data not shown); but sera from C57BL/6 mice immunized with PA1 displayed a significant neutralizing capacity (Fig. 2B). We analyzed the relationship between PA-specific IgG antibodies and the neutralization capacity by regression analysis (Fig. 3). A strong, highly significant correlation was observed between IgG antibody concentrations and the LeTx-neutralizing capacity with C57BL/6 mice immunized with PA1-4 (R2 = 0.9841; P < 0.001) or PA1 (R2 = 0.9886; P < 0.001). These linear regression plots indicated that 50% protection was achieved when the concentrations of IgG antibodies elicited by PA1-4 and PA1 were 15 μg/ml and 47 μg/ml, respectively (Fig. 3A and B, respectively). By logarithmic regression analysis, a correlation (R2 = 0.9456; P < 0.001) was also shown for BALB/c mice, in which a half-maximal effective IgG antibody concentration of 22 μg/ml was achieved (Fig. 3C). However, immune sera from A/J and Swiss-Webster mice did not show a correlation between antibody titer and protective efficacy, possibly due to the very low antibody titers (data not shown).
FIG. 2.
Neutralization of LeTx by sera from immunized mice. Dilutions of sera from individual mice vaccinated two times with expressed rPA domain proteins were incubated with J774 cells and LeTx for 4 h, and cell viability was measured by the MTT assay. All mouse strains immunized with PA1-4 (A) and C57BL/6 mice immunized with PA1 (B) produced antibodies that neutralized LeTx-mediated cytotoxicity. Data are represented as the geometric means ± standard deviations from five mice per treatment group. MTT assays were done twice, with similar results obtained each time. Sera collected prior to any mouse injections or immunization of the mice with the other rPA domain proteins conferred no protection (data not shown).
FIG. 3.
Correlation between anti-PA IgG levels and protection against LeTx-mediated cytotoxicity. The viability of J774 cells was plotted against the IgG antibody titer to PA (μg/ml) in sera from C57BL/6 mice vaccinated two times with PA1-4 (A) or PA1 (B) or in sera from BALB/c mice vaccinated with PA1-4 (C), as described in the legend to Fig. 2. Each point represents the average for five mice per treatment group. Regression lines and correlation coefficients (R2 values) are included for each plot.
Reactivity of immune sera with PA-derived peptides.
To gain more insight into the specificities of the antibody responses to PA, immune sera were evaluated for their ability to bind to peptides synthesized to represent PA solvent-exposed sequences. Sera from each mouse of each strain with average titers to PA ranging from >100 to <51,200 were tested in a direct binding ELISA for their ability to bind to the short linear peptides. The reactivities of the mouse sera (an average of five mice per group) with the PA peptides are summarized in Table 3. We identified four new linear B-cell epitopes in domains 1 and 2 and confirmed a previously identified epitope in domain 4 (3, 8, 9, 22, 46). Furthermore, we evaluated the reactivities to the peptides with our two previously characterized PA MAbs, 7.5G and 10F4 (35). MAb 7.5G bound to peptide PA121-150, which denotes a PA sequence in close proximity to the furin cleavage site, whereas MAb 10F4 manifested no reactivity with any peptide (data not shown). We mapped the epitopes distributed in domains 1, 2, and 4 of PA that sera recognized to show the positions of the domains and the locations of these epitopes (Fig. 4).
FIG. 4.
PA structure and locations of epitopes. The crystal structure of PA was created with the PyMOL program by using Protein Data Bank file 1ACC and shows the four domains of PA (a) and the locations of the epitopes (listed in Table 3) recognized by immune sera (b). Note that peptide PA143-158 overlaps with PA121-150.
PA-specific ASCs and serum antibody levels.
To examine whether there was a correlation in the serum titer and the frequency of spleen leukocytes producing specific antibody, B lymphocytes were isolated from the spleens of mice immunized with PA1-4 (an inducer of high levels of antibody) and PA4 (an inducer of low levels of antibody) and analyzed them for the presence of PA-specific antibody-secreting cells (ASCs) by the spot ELISA. The number of spots was then evaluated for comparison to the titers from mouse anti-PA sera collected 2 weeks after the third immunization with these PA domain proteins. Immunization of A/J mice with PA1-4 resulted in significantly higher levels of anti-PA-specific antibody titers than those detected after the prior two immunizations, and a slight increase in titers was found in sera from C57BL/6 and Swiss Webster mice (Fig. 5A). In contrast, BALB/c mice boosted a second time with PA1-4 had the same titers found in the sera collected from earlier immunizations. BALB/c, C57BL/6, and Swiss Webster mice produced the same anti-PA specific antibody titers as they did in the earlier immunizations with PA4, while A/J mice had a twofold increase in titers.
FIG. 5.
PA-specific ASCs in spleens of immunized mice. (A) Sera were collected from mice 2 weeks after the third immunization with 10 μg of PA1-4 or PA4 and were tested by ELISA for the presence of PA-specific antibodies. The values represent the geometric means ± standard deviations from five mice per treatment group. (B) Spleen leukocytes were harvested from immunized mice, and the frequency of PA-specific ASCs was determined by the spot ELISA. The data shown are a compilation of quadruplet replicates with geometric means ± standard deviations from five mice per treatment group.
PA-specific ASCs were detected in all mouse strains, even though the number of these cells detected was very low (Fig. 5B). The staining pattern observed on the spots had a homogeneously stained center with a slightly diffuse periphery. The diameter of the spots varied, probably depending upon the quantity of antibody secreted by individual ASCs. No spots developed when negative control cells (from nonimmunized mice) were added to the wells. The highest value for the number of PA-specific ASCs was approximately 15 of 104 spleen leukocytes (for Swiss Webster mice immunized with PA1-4). The lowest value for the number of PA1-4-specific ASCs was detected with A/J mice. The frequencies of PA4-specific ASCs were substantially lower in BALB/c and C57BL/6 mice and slightly lower in Swiss Webster mice, while in A/J mice the frequencies of PA4-specific ASCs found remained consistent with the frequencies of PA1-4-specific ASCs. Overall, there were no significant differences between the four mouse strains in the frequency of PA-specific ASCs. Thus, A/J and Swiss Webster mice did not elicit significant antibody responses to PA1-4 and PA4 in serum, and this result correlated with the numbers of PA-specific ASCs. However, the numbers of PA1-4-specific ASCs in the spleen correlated poorly with the antibody levels in serum (both BALB/c and C57BL/6 mice elicited significant antibody responses).
DISCUSSION
The results presented here indicate great variation in the immunogenicities of the different PA domains within and between genetically different strains of mice. All strains made significant antibody responses to full-length PA (PA1-4), but the responses to the other domains were variable. Full-length PA also elicited consistent titers of neutralizing antibodies in all mouse strains. Interestingly, immunization with PA1 was highly immunogenic in C57BL/6 mice and elicited LeTx-neutralizing antibodies. This result confirms the observation that domain 1 contains epitopes that can elicit neutralizing antibody responses (8, 35, 41). Immunization with protein containing domains 2 to 4 or domain 4 was significantly less effective in eliciting high-titer or neutralizing antibody responses. The inability of the domain 4 recombinant protein to elicit a neutralizing antibody response is in contrast to the observation that many neutralizing MAbs bind to this domain (3, 21, 22). Some of the best-studied antibodies to domain 4 recognize conformational epitopes (8, 41) that may require an intact three-dimensional protein to elicit a response. Although the immunological mechanisms responsible for the differences in antigenicity among the mouse strains were not investigated, we surmised that this phenomenon might be a reflection of differences in the processing and presentation of these antigens in genetically different mice. This finding echoes the observation that there is great heterogeneity in the human individual response to PA immunization with respect to the titer elicited and the neutralizing activity of the immune serum (13).
Previous reports have shown that protection against anthrax is associated with the production of subclass IgG1 antibodies or a Th2 response (21). In our study, the antibody isotype immune response for all mouse strains was dominated by IgG1, which is consistent with the findings of prior studies with immunization of A/J mice with complete or partial domains of rPA (8, 49) and rPA in the presence of alhydrogel (48). Although domain 1 elicited IgG1 in the primary response, an additional IgG2a/IgG2b response was also stimulated. These findings may reflect differences in host antigenicity or in the various mechanisms of immunity to these PA domain proteins among these mouse strains. The isotype analysis revealed interstrain variation in the isotype response in mice that could contribute to protective efficacy. Furthermore, comparison of the responses to PA1-4 and PA1 revealed differences in the isotype compositions of the antibody responses.
A sharp dose-response of the amount of antibody and the PA neutralizing capacity was observed in C57BL/6 mice. Calculation of the concentration required for 50% neutralization revealed that it was in the range of 15 to 47 μg/ml for the mouse strains studied (Fig. 3). From a different perspective, and with full cognition of the limitations involved in estimating the amount of a specific antibody relative to that of an MAb standard, we note that the antibody/toxin molar ratio required for 50% neutralization was 3:1 when BALB/c mice where immunized with PA1-4, while for C57BL/6 mice, the ratio was 2:1. In contrast, for C57BL/6 mice immunized with protein containing domain 1, a 6.5:1 molar ratio of antibody/toxin was required for 50% neutralization. If it is assumed that one molecule of an antibody to a neutralizing epitope can interfere with the toxin action, the higher ratio for domain 1 presumably reflects the fact that this region is immunogenic and elicits a higher proportion of nonneutralizing antibodies. Other studies have also been able to show a correlation between protection and neutralizing antibody titers in different animal models (33, 34). We were able to demonstrate a clear correlation between neutralizing antibodies and protective immunity in BALB/c and C57BL/6 mice, which produces the hope that it may be possible to identify similar protective levels in humans that would be helpful in assessing immunity after vaccination.
To obtain additional insight into the differences in specificity in the antibody responses to PA among the four mouse strains, we studied the reactivity of immune sera with a set of peptides designed to represent solvent-exposed segments of PA. In addition, we investigated the reactivities of two previously characterized MAbs with the peptide set. Although we are cognizant of the fact that this approach could identify only linear epitopes and that the conformation of peptides in a polystyrene support does not necessarily reflect their conformation in the PA domain, this approach can be expected to provide additional information on serological specificity. In general, there was some correlation between the magnitude of the immune response elicited by PA or its domains and the ability of the immune sera to bind to some peptides. For example, BALB/c and C57BL/6 mice immunized with rPA mounted strong antibody responses to PA and the antibodies reacted with several peptides. Conversely, A/J and Swiss Webster mice immunized with rPA mounted weaker antibody responses and the antibodies did not react with any of the peptides.
A recent review of the antigenic determinants of PA has shown a dearth of information on B-cell epitopes (50). In this regard, the peptide reactivity study provided additional information on the linear B-cell epitopes recognized by immune sera and previously generated MAbs. MAb 7.5G is believed to protect against LeTx by slowing the proteolytic digestion of PA by furin (35). The finding that this neutralizing MAb binds to PA121-150, a sequence near the furin cleavage site, is consistent with and supportive of this proposed mechanism of action. By correlating the immune serum titer, neutralizing activity, and reactivity with peptides, we have tentatively identified four new epitopes in the PA regions comprising aa 121 to 150, aa 143 to 158, aa 339 to 359, and aa 421 to 440. The veracity of these assignments is supported by the observation that the same approach also identified a previously assigned epitope in the region of aa 678 to 697. This peptide segment includes residues in and near a solvent-exposed loop of domain 4 (aa 679 to 693) that play a critical role in receptor binding (46) and that are recognized by several neutralizing MAbs to PA (22). Furthermore, previous studies have shown that mutations in PA, including a deletion of the 2β2-2β3 loop (27) and point mutations in any of the three residues K397, D425, and F427, which confer a dominant negative phenotype (38), resulted in impaired pore formation and impaired the translocation of EF and LF across the endosomal membrane.
Antibody levels in serum correlated poorly with the numbers of spleen antigen-specific ASCs in BALB/c and C57BL/6 mice immunized with PA1-4. If one assumes that the spleen B-cell pool making antibody to PA reflects the total B-cell pool, then the low number of B cells responding to immunization with these rPA domain proteins suggests that the amount of antibody is a function of the persistence of antibody circulating in the plasma after its production. It is conceivable that PA-binding antibodies are metabolized faster in certain hosts, possibly as a result of idiotypic regulation or perhaps even reactivity with host components.
In summary, we evaluated the immunogenicities and protective efficacies of the different domains of PA in different mouse strains. Our results suggest that some mouse strains generate a more potent neutralizing antibody response than others, and the data raise important questions about the mechanisms underlying this genetic variation. The higher level of protection afforded by anti-PA1-4 sera relative to that afforded by the truncated forms of PA indicates that all four domains are required for the best level of full protection. The level of protection achieved in our study was comparable to or better than that achieved by other polyclonal antiserum or MAbs (8, 17, 22, 24, 45). The observation that host genetics makes a large contribution to the antibody response to PA with regard to the antibody amount, specificity, and isotype suggests that differences in human responses to PA-based vaccines may depend on the genetic background of the individual. If this observation can be extrapolated to humans, then universal protection may not be achievable in an outbred human population with a vaccine based on a single B. anthracis component such as PA. Consequently, the findings could be interpreted to imply a need to develop additional B. anthracis antigens for use in vaccine development. In this regard, we note encouraging reports of antibodies to the capsule elicited by a poly(gamma-d-glutamic acid)-rPA conjugate vaccine that mediate protection (18, 37).
Acknowledgments
This work was supported by National Institutes of Health grant AI 157158-04.
We thank Oscar Zaragoza, Xiabo Wang, and Carolyn Saylor for their generous help with handling the mice.
Footnotes
Published ahead of print on 14 May 2008.
REFERENCES
- 1.Benson, E. L., P. D. Huynh, A. Finkelstein, and R. J. Collier. 1998. Identification of residues lining the anthrax protective antigen channel. Biochemistry 37:3941-3948. [DOI] [PubMed] [Google Scholar]
- 2.Blaustein, R. O., T. M. Koehler, R. J. Collier, and A. Finkelstein. 1989. Anthrax toxin: channel-forming activity of protective antigen in planar phospholipid bilayers. Proc. Natl. Acad. Sci. USA 86:2209-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brossier, F., M. Levy, A. Landier, P. Lafaye, and M. Mock. 2004. Functional analysis of Bacillus anthracis protective antigen by using neutralizing monoclonal antibodies. Infect. Immun. 72:6313-6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brossier, F., M. Weber-Levy, M. Mock, and J. C. Sirard. 2000. Role of toxin functional domains in anthrax pathogenesis. Infect. Immun. 68:1781-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnett, J. C., E. A. Henchal, A. L. Schmaljohn, and S. Bavari. 2005. The evolving field of biodefence: therapeutic developments and diagnostics. Nat. Rev. Drug Discov. 4:281-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devi, S. J., R. Schneerson, W. Egan, T. J. Ulrich, D. Bryla, J. B. Robbins, and J. E. Bennett. 1991. Cryptococcus neoformans serotype A glucuronoxylomannan-protein conjugate vaccines: synthesis, characterization, and immunogenicity. Infect. Immun. 59:3700-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dixon, T. C., M. Meselson, J. Guillemin, and P. C. Hanna. 1999. Anthrax. N. Engl. J. Med. 341:815-826. [DOI] [PubMed] [Google Scholar]
- 8.Flick-Smith, H. C., N. J. Walker, P. Gibson, H. Bullifent, S. Hayward, J. Miller, R. W. Titball, and E. D. Williamson. 2002. A recombinant carboxy-terminal domain of the protective antigen of Bacillus anthracis protects mice against anthrax infection. Infect. Immun. 70:1653-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flick-Smith, H. C., E. L. Waters, N. J. Walker, J. Miller, A. J. Stagg, M. Green, and E. D. Williamson. 2005. Mouse model characterisation for anthrax vaccine development: comparison of one inbred and one outbred mouse strain. Microb. Pathog. 38:33-40. [DOI] [PubMed] [Google Scholar]
- 10.Fowler, K., B. W. McBride, P. C. Turnbull, and L. W. Baillie. 1999. Immune correlates of protection against anthrax. J. Appl. Microbiol. 87:305. [DOI] [PubMed] [Google Scholar]
- 11.Gordon, V. M., S. H. Leppla, and E. L. Hewlett. 1988. Inhibitors of receptor-mediated endocytosis block the entry of Bacillus anthracis adenylate cyclase toxin but not that of Bordetella pertussis adenylate cyclase toxin. Infect. Immun. 56:1066-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gubbins, M. J., J. D. Berry, C. R. Corbett, J. Mogridge, X. Y. Yuan, L. Schmidt, B. Nicolas, A. Kabani, and R. S. Tsang. 2006. Production and characterization of neutralizing monoclonal antibodies that recognize an epitope in domain 2 of Bacillus anthracis protective antigen. FEMS Immunol. Med. Microbiol. 47:436-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanson, J. F., S. C. Taft, and A. A. Weiss. 2006. Neutralizing antibodies and persistence of immunity following anthrax vaccination. Clin. Vaccine Immunol. 13:208-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iacono-Connors, L. C., S. L. Welkos, B. E. Ivins, and J. M. Dalrymple. 1991. Protection against anthrax with recombinant virus-expressed protective antigen in experimental animals. Infect. Immun. 59:1961-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivins, B. E., and S. L. Welkos. 1988. Recent advances in the development of an improved, human anthrax vaccine. Eur. J. Epidemiol. 4:12-19. [DOI] [PubMed] [Google Scholar]
- 16.Ivins, B. E., S. L. Welkos, G. B. Knudson, and S. F. Little. 1990. Immunization against anthrax with aromatic compound-dependent (Aro−) mutants of Bacillus anthracis and with recombinant strains of Bacillus subtilis that produce anthrax protective antigen. Infect. Immun. 58:303-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivins, B. E., S. L. Welkos, S. F. Little, M. H. Crumrine, and G. O. Nelson. 1992. Immunization against anthrax with Bacillus anthracis protective antigen combined with adjuvants. Infect. Immun. 60:662-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozel, T. R., W. J. Murphy, S. Brandt, B. R. Blazar, J. A. Lovchik, P. Thorkildson, A. Percival, and C. R. Lyons. 2004. mAbs to Bacillus anthracis capsular antigen for immunoprotection in anthrax and detection of antigenemia. Proc. Natl. Acad. Sci. USA 101:5042-5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lacy, D. B., D. J. Wigelsworth, R. A. Melnyk, S. C. Harrison, and R. J. Collier. 2004. Structure of heptameric protective antigen bound to an anthrax toxin receptor: a role for receptor in pH-dependent pore formation. Proc. Natl. Acad. Sci. USA 101:13147-13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Little, S. F., B. E. Ivins, P. F. Fellows, and A. M. Friedlander. 1997. Passive protection by polyclonal antibodies against Bacillus anthracis infection in guinea pigs. Infect. Immun. 65:5171-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Little, S. F., S. H. Leppla, and E. Cora. 1988. Production and characterization of monoclonal antibodies to the protective antigen component of Bacillus anthracis toxin. Infect. Immun. 56:1807-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Little, S. F., J. M. Novak, J. R. Lowe, S. H. Leppla, Y. Singh, K. R. Klimpel, B. C. Lidgerding, and A. M. Friedlander. 1996. Characterization of lethal factor binding and cell receptor binding domains of protective antigen of Bacillus anthracis using monoclonal antibodies. Microbiology 142(Pt 3):707-715. [DOI] [PubMed] [Google Scholar]
- 23.Masnaya, N. V., A. A. Churin, O. S. Borsuk, and E. Y. Sherstoboev. 2002. Immune reactions in different mouse strains. Bull. Exp. Biol Med. 134:376-378. [DOI] [PubMed] [Google Scholar]
- 24.Maynard, J. A., C. B. Maassen, S. H. Leppla, K. Brasky, J. L. Patterson, B. L. Iverson, and G. Georgiou. 2002. Protection against anthrax toxin by recombinant antibody fragments correlates with antigen affinity. Nat. Biotechnol. 20:597-601. [DOI] [PubMed] [Google Scholar]
- 25.McBride, B. W., A. Mogg, J. L. Telfer, M. S. Lever, J. Miller, P. C. Turnbull, and L. Baillie. 1998. Protective efficacy of a recombinant protective antigen against Bacillus anthracis challenge and assessment of immunological markers. Vaccine 16:810-817. [DOI] [PubMed] [Google Scholar]
- 26.McConnell, M. J., P. C. Hanna, and M. J. Imperiale. 2006. Cytokine response and survival of mice immunized with an adenovirus expressing Bacillus anthracis protective antigen domain 4. Infect. Immun. 74:1009-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller, C. J., J. L. Elliott, and R. J. Collier. 1999. Anthrax protective antigen: prepore-to-pore conversion. Biochemistry 38:10432-10441. [DOI] [PubMed] [Google Scholar]
- 28.Milne, J. C., D. Furlong, P. C. Hanna, J. S. Wall, and R. J. Collier. 1994. Anthrax protective antigen forms oligomers during intoxication of mammalian cells. J. Biol. Chem. 269:20607-20612. [PubMed] [Google Scholar]
- 29.Mock, M., and A. Fouet. 2001. Anthrax. Annu. Rev. Microbiol. 55:647-671. [DOI] [PubMed] [Google Scholar]
- 30.Mogridge, J., M. Mourez, and R. J. Collier. 2001. Involvement of domain 3 in oligomerization by the protective antigen moiety of anthrax toxin. J. Bacteriol. 183:2111-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohamed, N., J. Li, C. S. Ferreira, S. F. Little, A. M. Friedlander, G. L. Spitalny, and L. S. Casey. 2004. Enhancement of anthrax lethal toxin cytotoxicity: a subset of monoclonal antibodies against protective antigen increases lethal toxin-mediated killing of murine macrophages. Infect. Immun. 72:3276-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petosa, C., R. J. Collier, K. R. Klimpel, S. H. Leppla, and R. C. Liddington. 1997. Crystal structure of the anthrax toxin protective antigen. Nature 385:833-838. [DOI] [PubMed] [Google Scholar]
- 33.Pitt, M. L., S. Little, B. E. Ivins, P. Fellows, J. Boles, J. Barth, J. Hewetson, and A. M. Friedlander. 1999. In vitro correlate of immunity in an animal model of inhalational anthrax. J. Appl. Microbiol. 87:304. [DOI] [PubMed] [Google Scholar]
- 34.Reuveny, S., M. D. White, Y. Y. Adar, Y. Kafri, Z. Altboum, Y. Gozes, D. Kobiler, A. Shafferman, and B. Velan. 2001. Search for correlates of protective immunity conferred by anthrax vaccine. Infect. Immun. 69:2888-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivera, J., A. Nakouzi, N. Abboud, E. Revskaya, D. Goldman, R. J. Collier, E. Dadachova, and A. Casadevall. 2006. A monoclonal antibody to Bacillus anthracis protective antigen defines a neutralizing epitope in domain 1. Infect. Immun. 74:4149-4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosovitz, M. J., P. Schuck, M. Varughese, A. P. Chopra, V. Mehra, Y. Singh, L. M. McGinnis, and S. H. Leppla. 2003. Alanine-scanning mutations in domain 4 of anthrax toxin protective antigen reveal residues important for binding to the cellular receptor and to a neutralizing monoclonal antibody. J. Biol. Chem. 278:30936-30944. [DOI] [PubMed] [Google Scholar]
- 37.Schneerson, R., J. Kubler-Kielb, T. Y. Liu, Z. D. Dai, S. H. Leppla, A. Yergey, P. Backlund, J. Shiloach, F. Majadly, and J. B. Robbins. 2003. Poly(gamma-d-glutamic acid) protein conjugates induce IgG antibodies in mice to the capsule of Bacillus anthracis: a potential addition to the anthrax vaccine. Proc. Natl. Acad. Sci. USA 100:8945-8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sellman, B. R., M. Mourez, and R. J. Collier. 2001. Dominant-negative mutants of a toxin subunit: an approach to therapy of anthrax. Science 292:695-697. [DOI] [PubMed] [Google Scholar]
- 39.Singh, Y., S. H. Leppla, R. Bhatnagar, and A. M. Friedlander. 1989. Internalization and processing of Bacillus anthracis lethal toxin by toxin-sensitive and -resistant cells. J. Biol. Chem. 264:11099-11102. [PubMed] [Google Scholar]
- 40.Sinha, P., J. A. Snyder, E. Y. Kim, and K. D. Moudgil. 2007. The major histocompatibility complex haplotypes dictate and the background genes fine-tune the dominant versus the cryptic response profile of a T-cell determinant within a native antigen: relevance to disease susceptibility and vaccination. Scand. J. Immunol. 65:158-165. [DOI] [PubMed] [Google Scholar]
- 41.Stokes, M. G., R. W. Titball, B. N. Neeson, J. E. Galen, N. J. Walker, A. J. Stagg, D. C. Jenner, J. E. Thwaite, J. P. Nataro, L. W. Baillie, and H. S. Atkins. 2007. Oral administration of a Salmonella enterica-based vaccine expressing Bacillus anthracis protective antigen confers protection against aerosolized B. anthracis. Infect. Immun. 75:1827-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subramanian, S., J. Rao, P. Jyothi, and P. R. Adiga. 2000. Strain-dependent variability in immune response to chicken riboflavin carrier protein in mice with different haplotypes. Immunol. Investig. 29:397-409. [PubMed] [Google Scholar]
- 43.Tokunaga, Y., C. Hiramine, M. Itoh, A. Mukasa, and K. Hojo. 1993. Genetic susceptibility to the induction of murine experimental autoimmune orchitis (EAO) without adjuvant. I. Comparison of pathology, delayed type hypersensitivity, and antibody. Clin. Immunol. Immunopathol. 66:239-247. [DOI] [PubMed] [Google Scholar]
- 44.Turnbull, P. C. 1991. Anthrax vaccines: past, present and future. Vaccine 9:533-539. [DOI] [PubMed] [Google Scholar]
- 45.Turnbull, P. C., S. H. Leppla, M. G. Broster, C. P. Quinn, and J. Melling. 1988. Antibodies to anthrax toxin in humans and guinea pigs and their relevance to protective immunity. Med. Microbiol. Immunol. (Berlin) 177:293-303. [DOI] [PubMed] [Google Scholar]
- 46.Varughese, M., A. V. Teixeira, S. Liu, and S. H. Leppla. 1999. Identification of a receptor-binding region within domain 4 of the protective antigen component of anthrax toxin. Infect. Immun. 67:1860-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaz, N. M., and B. B. Levine. 1970. Immune responses of inbred mice to repeated low doses of antigen: relationship to histocompatibility (H-2) type. Science 168:852-854. [DOI] [PubMed] [Google Scholar]
- 48.Williamson, E. D., R. J. Beedham, A. M. Bennett, S. D. Perkins, J. Miller, and L. W. Baillie. 1999. Presentation of protective antigen to the mouse immune system: immune sequelae. J. Appl. Microbiol. 87:315-317. [DOI] [PubMed] [Google Scholar]
- 49.Williamson, E. D., I. Hodgson, N. J. Walker, A. W. Topping, M. G. Duchars, J. M. Mott, J. Estep, C. Lebutt, H. C. Flick-Smith, H. E. Jones, H. Li, and C. P. Quinn. 2005. Immunogenicity of recombinant protective antigen and efficacy against aerosol challenge with anthrax. Infect. Immun. 73:5978-5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zarebski, L. M., K. Vaughan, J. Sidney, B. Peters, H. Grey, K. D. Janda, A. Casadevall, and A. Sette. 2008. Analysis of epitope information related to Bacillus anthracis and Clostridium botulinum. Expert Rev. Vaccines 7:55-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang, W., P. D. Bardwell, C. J. Woo, V. Poltoratsky, M. D. Scharff, and A. Martin. 2001. Clonal instability of V region hypermutation in the Ramos Burkitt's lymphoma cell line. Int. Immunol. 13:1175-1184. [DOI] [PubMed] [Google Scholar]