Abstract
We developed a replication-defective reporter virus pseudotyped with the envelope glycoprotein of equine infectious anemia virus (EIAV). The in vitro host range and neutralization phenotype of EIAV Env-pseudotyped virus were similar to those of replication-competent virus. An EIAV Env pseudovirus will improve antigenic characterization of viral variants and evaluation of lentivirus vaccines.
The ability of lentiviruses to continually evolve and to escape immune control is a central impediment in developing an effective vaccine for human immunodeficiency virus type 1 (HIV-1) and other lentiviruses. Despite enormous effort, vaccines that elicit antibodies that neutralize diverse HIV-1 isolates, resulting in consistent protection against challenge with heterologous pathogenic viruses, have not been developed. Equine infectious anemia virus (EIAV) is a macrophage-tropic lentivirus that causes a lifelong persistent infection in horses (13, 16, 17). Horses infected with EIAV generally experience a clinically variable disease course that is demarcated by acute, chronic, and inapparent stages of infection. As for other lentiviral infections, an adaptive immune response is critical both in controlling acute EIAV infection and in maintaining the inapparent stage (5, 10, 15, 20). Importantly, EIAV-infected horses are able to mount broadly reactive neutralizing-antibody responses that reduce levels of replicating virus during long-term inapparent infection (1, 5, 6, 19). The identification of viral epitopes targeted by broadly reactive neutralizing antibody could facilitate the design of effective vaccines for EIAV and other lentiviruses, including HIV-1.
Pseudotyped viruses have been successfully used to characterize neutralizing antibodies and to identify broadly neutralizing epitopes of many viruses, including HIV, hepatitis C virus, severe acute respiratory syndrome virus, and Venezuelan equine encephalitis virus (2, 8, 11, 12, 21, 22). In this study, we developed an EIAV Env-pseudotyped reporter virus using the EIAV-based gene transfer vector developed by John Olsen (14). The EIAV Env pseudovirus readily transduced equine cells and was amenable to high-throughput assays for the analysis of EIAV broadly neutralizing antibodies. The EIAV Env pseudovirus may be a useful tool for the identification of neutralizing epitopes, the assessment of vaccine candidates, and the characterization of EIAV-receptor interactions.
The overall goal of these studies was to generate a replication-defective reporter virus pseudotyped with EIAV Env that would facilitate an immunological characterization of EIAV envelope glycoproteins. To overcome the instability of EIAV envelope expression in bacterial cells, the pSPEIAV19 surface (SU) and transmembrane (TM) envelope sequences (GenBank accession number EIU01866) were codon optimized by GenScript (Piscataway, NJ), cloned into the low-copy-number vector pLG338/30 (4), and grown in MAX Efficiency Stbl2 competent cells (Invitrogen, Carlsbad, CA). This plasmid was designated pLGcoSUTM. The combined effect of codon optimization and amplification in the low-copy-number plasmid resulted in a threefold increase in the stability of EIAV env. Protein expression was confirmed by Western blotting using EIAV convalescent-phase sera (not shown). EIAV Env pseudovirus stocks were generated by cotransfection of human embryonic kidney 293T/17 cells (293T; ATCC CRL 11268) with three plasmids: pEV53B, which encodes the viral core (14); the luciferase reporter plasmid pSIN6.1ClucW (14); and the EIAV19 codon-optimized envelope plasmid pLGcoSUTM, described above. For a control, vesicular stomatitis virus glycoprotein (VSV-G)-pseudotyped virus was generated by using the envelope plasmid pCI-VSV-G (7). Large-scale plasmid purification was performed with an EndoFree plasmid maxikit (Qiagen, Valencia, CA). Human embryonic kidney 293T/17 producer cells were transiently transfected with 7.5 μg envelope plasmid, 15 μg pEV53B, and 15 μg pSIN6.1ClucW using the ProFection mammalian transfection system (Promega, Madison, WI). The next day, 10 mM sodium butyrate was added in fresh media. Supernatant was collected at 48 and 72 h posttransfection, clarified by centrifugation, aliquoted, and stored at −80°C.
Pseudotyped virus stocks were assessed for supernatant reverse transcriptase activity (18) and transduction of equine dermal (ED) cells (ATCC CCL 57). The levels of pseudovirus reverse transcriptase activity were comparable to those of virus stocks produced from cells infected with replication-competent virus, 2 × 106 to 5 × 106 cpm/μl supernatant. For the titration of pseudovirus, ED cells were seeded in 96-well culture plates at 104 ED cells/well in Dulbecco's modified Eagle medium supplemented with antibiotics and heat-inactivated fetal calf serum. The next day, the cells were inoculated with serial dilutions of pseudotyped virus stock in the presence of 8 μg/ml Polybrene. The culture media were changed the following day, and the cells were assayed with the Bright-Glo luciferase assay system (Promega, Madison, WI) at 72 h posttransduction. Luminescence was detected with a MicroBeta Trilux luminometer (PerkinElmer, Boston, MA), and titers are reported in relative light units (RLU) per milliliter.
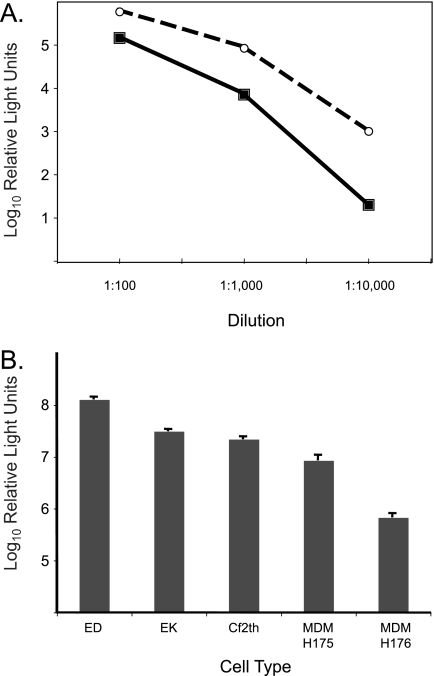
Due to its wide host cell range, the VSV-G envelope is widely used for pseudovirus production and has become a standard for evaluating other pseudotyped viruses (3). Therefore, we initially compared the EIAV Env pseudovirus titers to that of VSV-G pseudovirus. Both pseudovirus stocks readily transduced ED cells, with titers exceeding 107 RLU/ml. With both VSV-G and EIAV Env pseudoviruses, we observed a linear relationship between luciferase activity and the amount of pseudovirus used for inoculation (Fig. 1A). The in vitro host range of EIAV Env pseudovirus was investigated by the transduction of additional cell types known to be permissive for EIAV replication, including fetal equine kidney (EK) cells (10), canine fibroblast (Cf2Th) cells (ATCC CRL 1430), and equine monocyte-derived macrophages (MDM) (18). Each of these cell types was susceptible to transduction by the EIAV Env pseudovirus (Fig. 1B). Titers in EK and Cf2Th cells were greater than 107 RLU/ml, similar to that obtained in ED cells. The transduction of MDM was less efficient and more variable than that of other cell types (Fig. 1B), similar to what has been reported for the replication-competent virus (18, 19). These results indicate that the EIAV Env pseudovirus can be a useful tool for the molecular characterization of EIAV infectivity as well as targeted gene delivery to equine cells.
FIG. 1.
Infectivity and cell tropism of EIAV Env-pseudotyped viruses. (A) Linear relationship of luciferase activity and dilution of EIAV Env-pseudotyped viruses (open circles) and VSV-G-pseudotyped viruses (filled squares) in ED cells. (B) EIAV Env-pseudotyped viruses can transduce cells permissive to replication-competent EIAV. Mean titers (RLU/ml) and standard errors of EIAV Env-pseudotyped viruses in ED cells, EK cells, Cf2Th cells, and MDM are shown, with titers comparable to levels in ED cells. MDM were isolated from two horses, H175 and H176. Results represent the means ± standard errors of results for at least two independent experiments, each performed in triplicate.
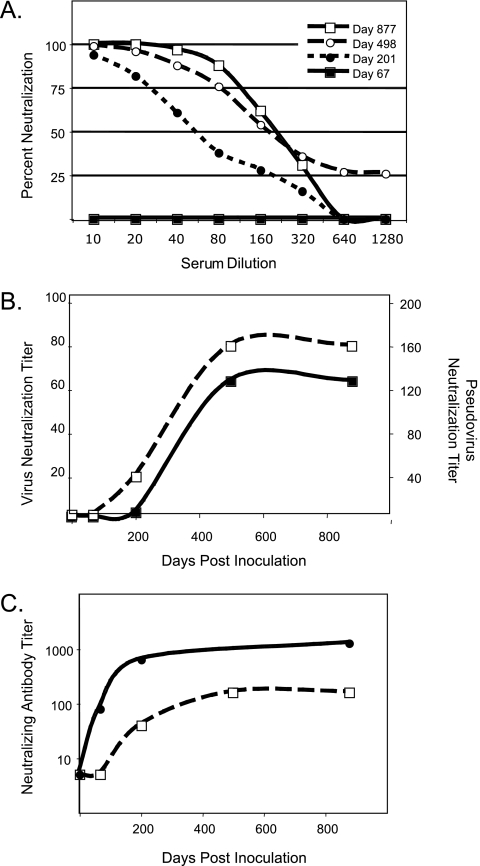
The immunological characterization of the EIAV Env pseudovirus was performed by using a well-characterized set of longitudinal serum samples obtained from an experimentally infected horse (1, 19). Serum samples collected on sequential days postinoculation were heat inactivated to destroy complement, serially diluted twofold from an initial 1:10 dilution, incubated with approximately 60,000 RLU of pseudovirus for 1 hour at 37°C, and inoculated in triplicate onto ED cells seeded in 96-well plates as described above. The cells were assayed 72 h posttransduction for luciferase activity, and the percent neutralization was calculated as a reduction in the number of RLU compared to the number in cells inoculated with virus only. The results indicated a dose-dependent decrease in neutralization with an increasing dilution of sera (Fig. 2A). In addition, we observed similar patterns in the development of neutralizing antibody to the EIAV Env pseudovirus and the replication-competent EIAV19 (Fig. 2B). The EIAV Env pseudovirus was more sensitive to neutralization, as has been reported for HIV-1-pseudotyped viruses (9).
FIG. 2.
Immunological characterization of EIAV Env-pseudotyped viruses. (A) Neutralization kinetics of EIAV Env pseudovirus by serum samples obtained from pony 524 at sequential times postinoculation. (B) Neutralizing-antibody titers against replication-competent virus (EIAV19) (filled squares) and EIAV Env-pseudotyped virus (EIAV Env19) (open squares) were determined with a focus reduction assay (19) and a luciferase assay, respectively. Titers represent the inverse of the serum dilution required to neutralize at least 75% of virus infectivity. (C) Development of neutralizing antibody to the chimeric Env pseudoviruses EIAV EnvPND-1 (filled circles) and EIAV Env19 (open squares). Neutralizing antibody titers represent the inverse of the serum dilution required to neutralize at least 75% of luciferase expression in transduced cells.
We previously developed chimeric infectious molecular clones to characterize the antigenic phenotype of SU variants that arose during the course of EIAV infection in vivo (19). To determine whether the EIAV Env pseudovirus can provide a more rapid assay system for these types of analyses, the V2 to V4 region of the codon-optimized EIAV19 envelope was replaced with the antigenic variant PND-1 sequence (GenBank accession number EF405726). The PND-1 genotype was the dominant in vivo env variant during early infection of pony 524, and neutralizing antibody to EIAVPND-1 arose before the appearance of neutralizing antibody to the heterologous EIAV19 virus (19). Consistent with the previous results using replication-competent virus, neutralizing antibody to EIAV EnvPND-1 pseudovirus was detected earlier in infection and was present at higher titers than neutralizing antibody to the EIAV Env19 pseudovirus (Fig. 2C).
In summary, we developed an EIAV Env-pseudotyped reporter virus system and demonstrated that the infectivity and neutralization phenotypes of the pseudovirus recapitulate the results for the replication-competent virus. This pseudotyped virus system should facilitate studies of EIAV persistence and pathogenesis and should aid in the design and evaluation of lentivirus vaccines.
Acknowledgments
We thank Sue Pritchard, Steve Leib, Matt Littke, and Yvonne Wannemuehler for their excellent technical assistance. The pEV53B, pSIN6.1ClucW, and pCI-VSV-G plasmids were generously provided by John C. Olsen, University of North Carolina, Chapel Hill, NC.
This work was supported in part by the U.S. Public Health Service, National Institutes of Health grants AI060395, AI073101, and AI067125 (R.H.M.). R.L.T. was supported by NIH grant T32 AI007025.
Footnotes
Published ahead of print on 30 April 2008.
REFERENCES
- 1.Belshan, M., P. Baccam, J. L. Oaks, B. A. Sponseller, S. C. Murphy, J. Cornette, and S. Carpenter. 2001. Genetic and biological variation in equine infectious anemia virus Rev correlates with variable stages of clinical disease in an experimentally infected pony. Virology 279:185-200. [DOI] [PubMed] [Google Scholar]
- 2.Cham, F., P. F. Zhang, L. Heyndrickx, P. Bouma, P. Zhong, H. Katinger, J. Robinson, G. van der Groen, and G. V. Quinnan, Jr. 2006. Neutralization and infectivity characteristics of envelope glycoproteins from human immunodeficiency virus type 1 infected donors whose sera exhibit broadly cross-reactive neutralizing activity. Virology 347:36-51. [DOI] [PubMed] [Google Scholar]
- 3.Cronin, J., X. Y. Zhang, and J. Reiser. 2005. Altering the tropism of lentiviral vectors through pseudotyping. Curr. Gene Ther. 5:387-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunningham, T. P., R. C. Montelaro, and K. E. Rushlow. 1993. Lentivirus envelope sequences and proviral genomes are stabilized in Escherichia coli when cloned in low-copy-number plasmid vectors. Gene 124:93-98. [DOI] [PubMed] [Google Scholar]
- 5.Hammond, S. A., S. J. Cook, D. L. Lichtenstein, C. J. Issel, and R. C. Montelaro. 1997. Maturation of the cellular and humoral immune responses to persistent infection in horses by equine infectious anemia virus is a complex and lengthy process. J. Virol. 71:3840-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammond, S. A., F. Li, B. M. McKeon, Sr., S. J. Cook, C. J. Issel, and R. C. Montelaro. 2000. Immune responses and viral replication in long-term inapparent carrier ponies inoculated with equine infectious anemia virus. J. Virol. 74:5968-5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson, L. G., J. P. Mewshaw, H. Ni, T. Friedmann, R. C. Boucher, and J. C. Olsen. 1998. Effect of host modification and age on airway epithelial gene transfer mediated by a murine leukemia virus-derived vector. J. Virol. 72:8861-8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolokoltsov, A. A., E. Wang, T. M. Colpitts, S. C. Weaver, and R. A. Davey. 2006. Pseudotyped viruses permit rapid detection of neutralizing antibodies in human and equine serum against Venezuelan equine encephalitis virus. Am. J. Trop. Med. Hyg. 75:702-709. [PubMed] [Google Scholar]
- 9.Li, M., F. Gao, J. R. Mascola, L. Stamatatos, V. R. Polonis, M. Koutsoukos, G. Voss, P. Goepfert, P. Gilbert, K. M. Greene, M. Bilska, D. L. Kothe, J. F. Salazar-Gonzalez, X. Wei, J. M. Decker, B. H. Hahn, and D. C. Montefiori. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108-10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGuire, T. C., D. B. Tumas, K. M. Byrne, M. T. Hines, S. R. Leib, A. L. Brassfield, K. I. O'Rourke, and L. E. Perryman. 1994. Major histocompatibility complex-restricted CD8+ cytotoxic T lymphocytes from horses with equine infectious anemia virus recognize Env and Gag/PR proteins. J. Virol. 68:1459-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meunier, J.-C., R. S. Russell, V. Goossens, S. Priem, H. Walter, E. Depla, A. Union, K. N. Faulk, J. Bukh, S. U. Emerson, and R. H. Purcell. 2008. Isolation and characterization of broadly neutralizing human monoclonal antibodies to the E1 glycoprotein of hepatitis C virus. J. Virol. 82:966-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nie, Y., G. Wang, X. Shi, H. Zhang, Y. Qiu, Z. He, W. Wang, G. Lian, X. Yin, L. Du, L. Ren, J. Wang, X. He, T. Li, H. Deng, and M. Ding. 2004. Neutralizing antibodies in patients with severe acute respiratory syndrome-associated coronavirus infection. J. Infect. Dis. 190:1119-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oaks, J. L., T. C. McGuire, C. Ulibarri, and T. B. Crawford. 1998. Equine infectious anemia virus is found in tissue macrophages during subclinical infection. J. Virol. 72:7263-7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen, J. C. 1998. Gene transfer vectors derived from equine infectious anemia virus. Gene Ther. 5:1481-1487. [DOI] [PubMed] [Google Scholar]
- 15.Perryman, L. E., K. I. O'Rourke, and T. C. McGuire. 1988. Immune responses are required to terminate viremia in equine infectious anemia lentivirus infection. J. Virol. 62:3073-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sellon, D. C., F. J. Fuller, and T. C. McGuire. 1994. The immunopathogenesis of equine infectious anemia virus. Virus Res. 32:111-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sellon, D. C., S. T. Perry, L. Coggins, and F. J. Fuller. 1992. Wild-type equine infectious anemia virus replicates in vivo predominantly in tissue macrophages, not in peripheral blood monocytes. J. Virol. 66:5906-5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith, T. A., E. Davis, and S. Carpenter. 1998. Endotoxin treatment of EIAV-infected horse macrophage cultures decreases production of infectious virus. J. Gen. Virol. 79:747-755. [DOI] [PubMed] [Google Scholar]
- 19.Sponseller, B. A., W. O. Sparks, Y. Wannemuehler, Y. Li, A. K. Antons, J. L. Oaks, and S. Carpenter. 2007. Immune selection of equine infectious anemia virus env variants during the long-term inapparent stage of disease. Virology 363:156-165. [DOI] [PubMed] [Google Scholar]
- 20.Zhang, W., S. M. Lonning, and T. C. McGuire. 1998. Gag protein epitopes recognized by ELA-A-restricted cytotoxic T lymphocytes from horses with long-term equine infectious anemia virus infection. J. Virol. 72:9612-9620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zolla-Pazner, S., P. Zhong, K. Revesz, B. Volsky, C. Williams, P. Nyambi, and M. K. Gorny. 2004. The cross-clade neutralizing activity of a human monoclonal antibody is determined by the GPGR V3 motif of HIV type 1. AIDS Res. Hum. Retrovir. 20:1254-1258. [DOI] [PubMed] [Google Scholar]
- 22.Zwick, M. B., R. Jensen, S. Church, M. Wang, G. Stiegler, R. Kunert, H. Katinger, and D. R. Burton. 2005. Anti-human immunodeficiency virus type 1 (HIV-1) antibodies 2F5 and 4E10 require surprisingly few crucial residues in the membrane-proximal external region of glycoprotein gp41 to neutralize HIV-1. J. Virol. 79:1252-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]